Quantifying the Probability of Decline in Quality: Implications for Selection Management in Northern Hardwood Forests
Abstract
1. Introduction
2. Materials and Methods
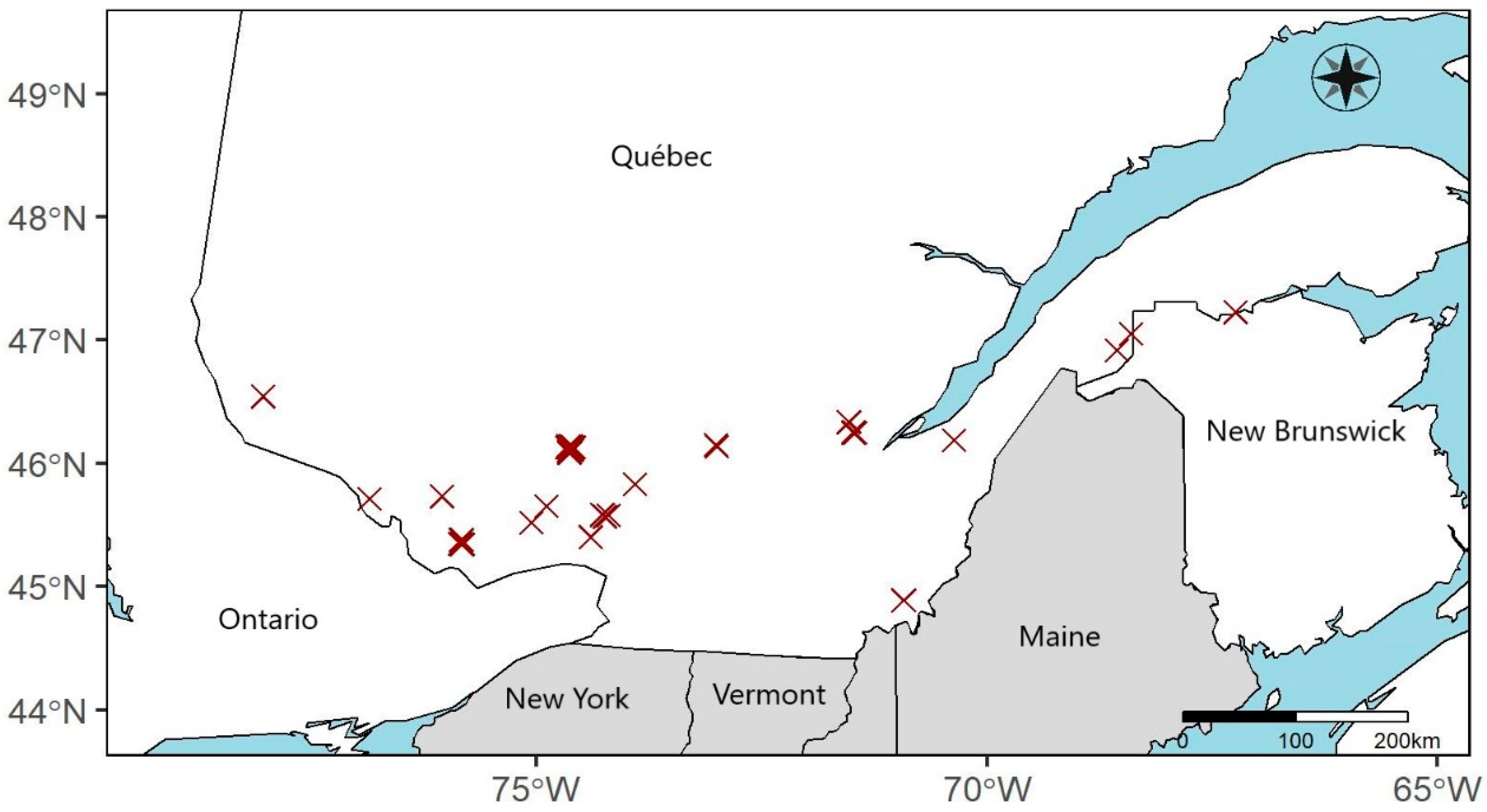
2.1. Sampling Sites
2.2. Experimental Design and Data Collection
2.3. Statistical Analysis
2.3.1. Probability of Decline in Quality
2.3.2. Combined Probability of Decline in Quality and Mortality
3. Results
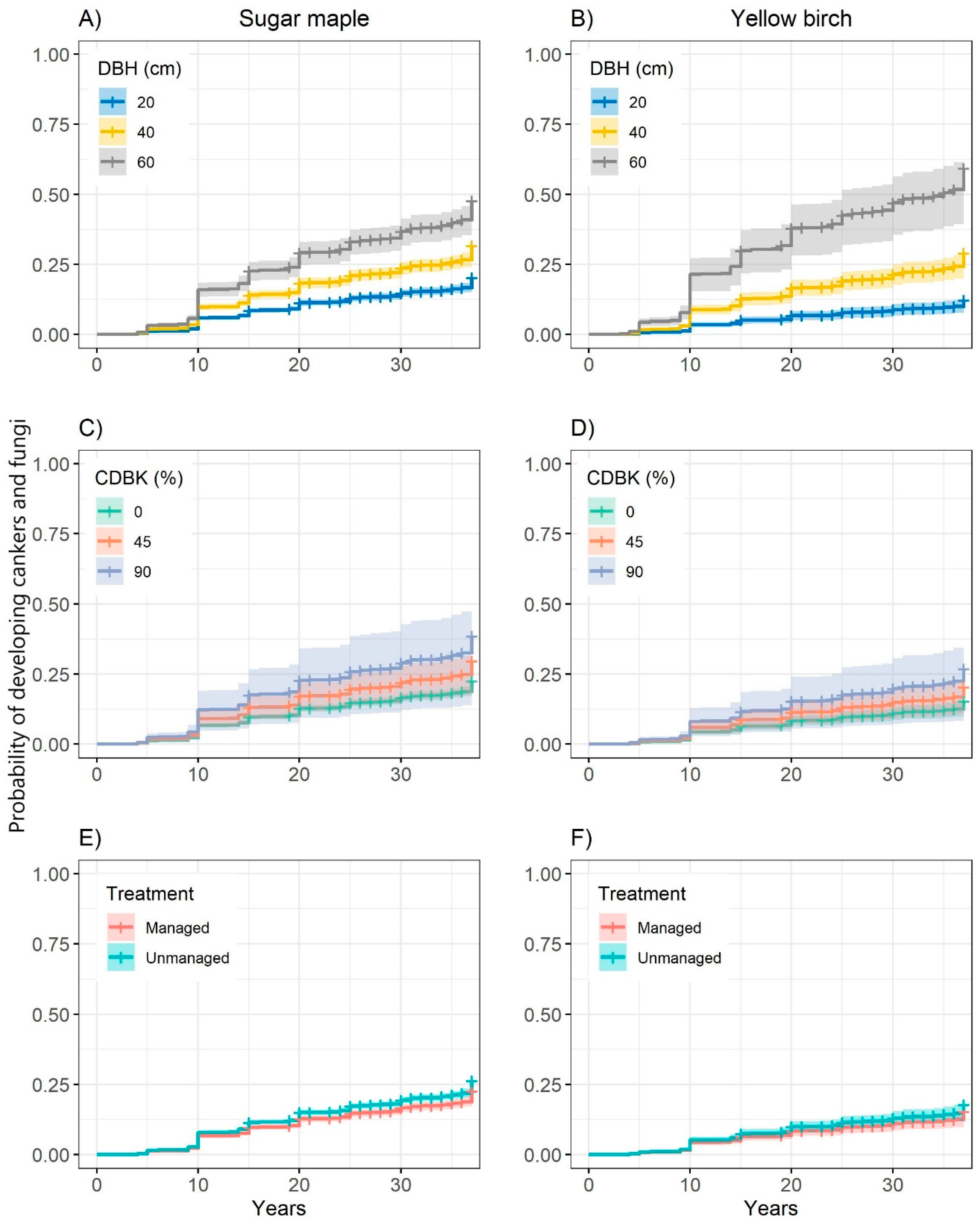
3.1. Probability of Developing Cankers and Fungi
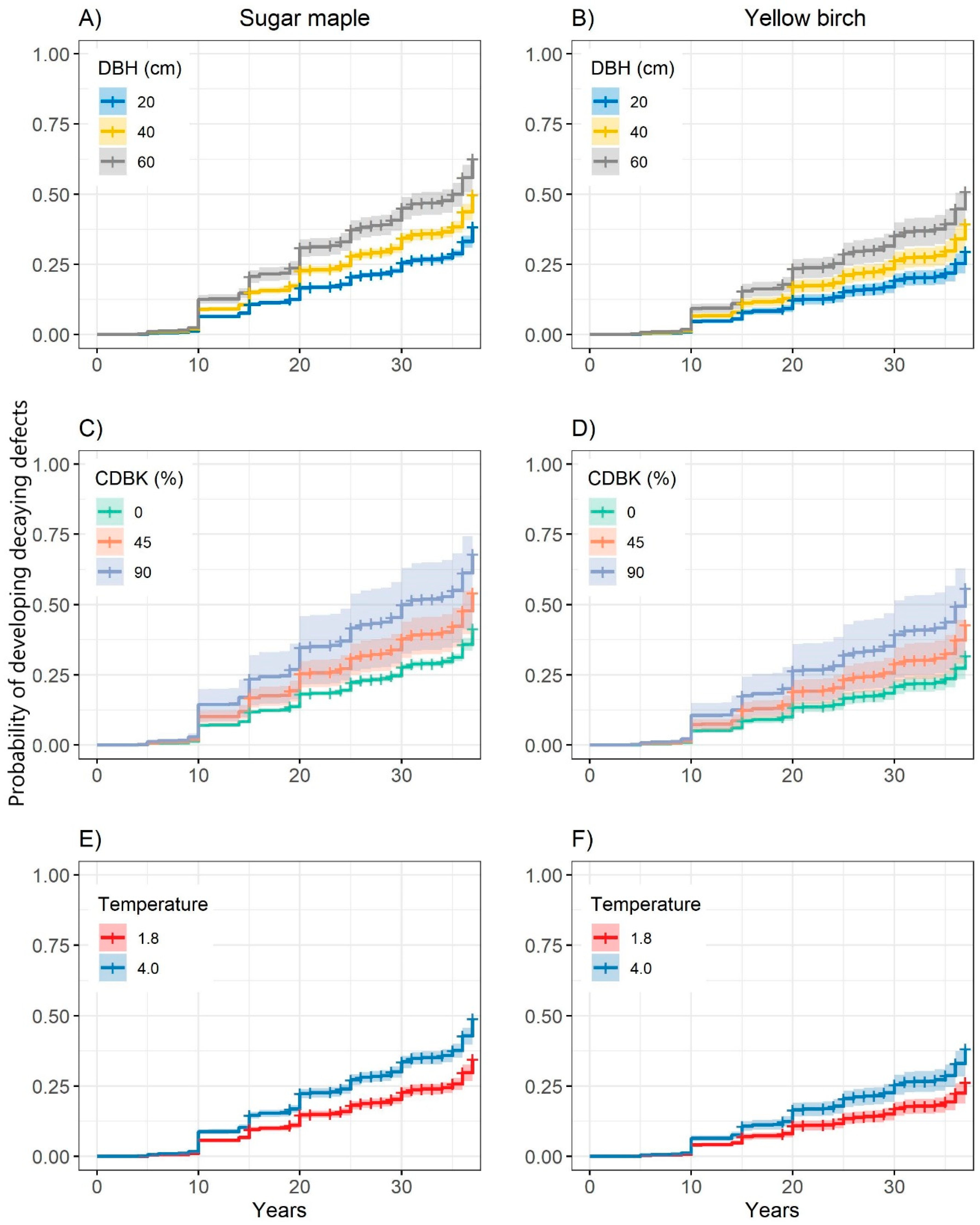
3.2. Probability of Developing Decaying Defects
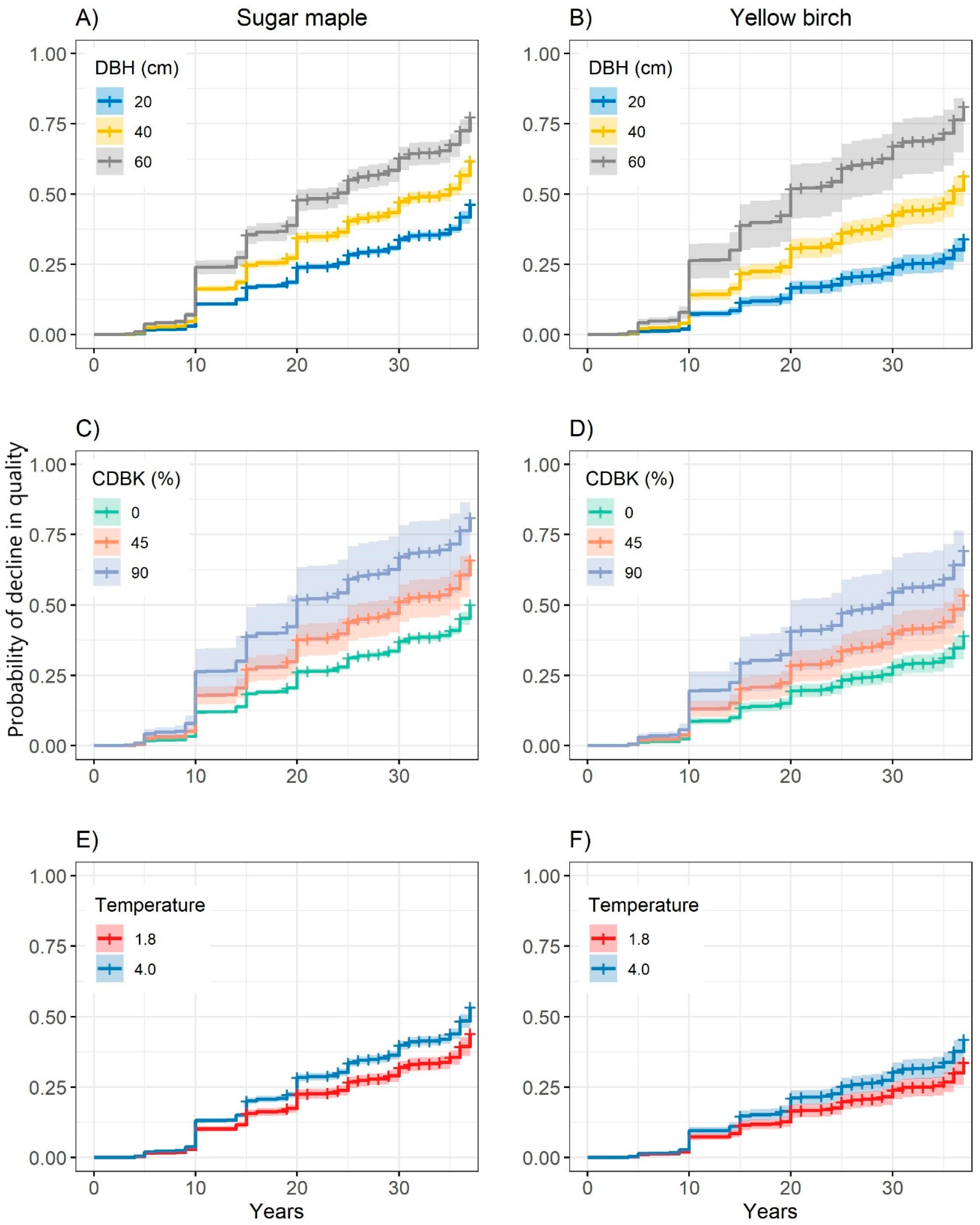
3.3. Probability of Decline in Quality
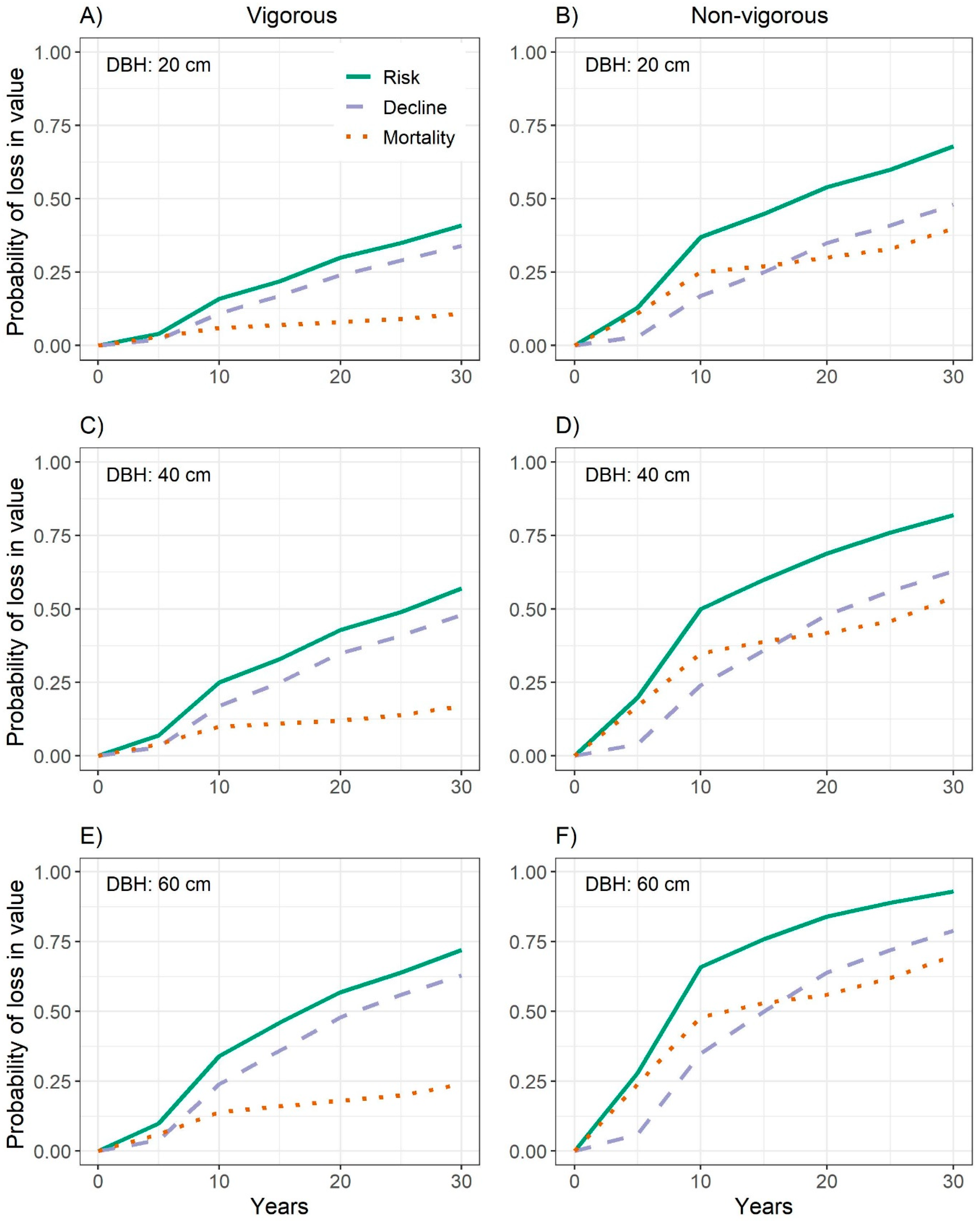
3.4. Combined Probability of Decline in Quality and Mortality
4. Discussion
Managing for Yield and Biodiversity Conservation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nyland, R.D. Selection system in northern hardwoods. J. For. 1988, 96, 18–21. [Google Scholar]
- Baral, S.K.; Schneider, R.; Pothier, D.; Berninger, F. Predicting sugar maple (Acer saccharum) discoloured wood characteristics. Can. J. For. Res. 2013, 43, 649–657. [Google Scholar] [CrossRef]
- Dey, D.C.; Dwyer, J.; Wiedenbeck, J. Relationship between Tree Value, Diameter, and Age in High-Quality Sugar Maple (Acer saccharum) on the Menominee Reservation, Wisconsin. J. For. 2017, 115, 397–405. [Google Scholar] [CrossRef]
- Power, H.; Havreljuk, F. Predicting hardwood quality and its evolution over time in Quebec's forests. For. Int. J. For. Res. 2018, 91, 259–270. [Google Scholar] [CrossRef]
- Martin, M.; Raymond, P.; Boucher, Y. Influence of individual tree characteristics, spatial structure and logging history on tree-related microhabitat occurrence in North American hardwood forests. For. Ecosyst. 2021, 8, 27. [Google Scholar] [CrossRef]
- Ontario Ministry of Natural Ressources. Ontario Tree Marking Guide, Ver. 1.1.; Ontario Ministry of Natural Ressources: Québec, QC, Canada, 2004; 252p.
- Fortin, M.; Bédard, S.; DeBlois, J.; Meunier, S. Predicting individual tree mortality in northern hardwood stands under uneven-aged management in southern Québec, Canada. Ann. For. Sci. 2008, 65, 205. [Google Scholar] [CrossRef]
- Courbaud, B.; Larrieu, L.; Kozak, D.; Kraus, D.; Lachat, T.; Ladet, S.; Müller, J.; Paillet, Y.; Sagheb-Talebi, K.; Schuck, A.; et al. Factors influencing the rate of formation of tree-related microhabitats and implications for biodiversity conservation and forest management. J. Appl. Ecol. 2021, 59, 492–503. [Google Scholar] [CrossRef]
- Bédard, S.; Majcen, Z. Ten-Year Response of Sugar Maple-Yellow Birch-Beech Stands to Selection Cutting in Québec. North. J. Appl. For. 2001, 18, 119–126. [Google Scholar] [CrossRef]
- Moreau, G.; Achim, A.; Pothier, D. A dendrochronological reconstruction of sugar maple growth and mortality dynamics in partially cut northern hardwood forests. For. Ecol. Manag. 2019, 437, 17–26. [Google Scholar] [CrossRef]
- Moreau, G.; Cecil-Cockwell, M.J.; Pothier, D.; Achim, A.; Bédard, S.; Guillemette, F.; Caspersen, J. Visual assessment of tree vigour in Canadian northern hardwood forests: The need for a simplified system. For. Ecol. Manag. 2023, 529, 120720. [Google Scholar] [CrossRef]
- Puettmann, K.J.; Coates, K.D.; Messier, C. A Critique of Silviculture: Managing for Complexity; Island Press: Washington, DC, USA, 2009. [Google Scholar]
- Cockwell, M.; Caspersen, J. Sources of Variation in the Net Value of Sugar Maple Trees: Implications for Tree Selection and Operations Management. For. Prod. J. 2014, 64, 250–258. [Google Scholar] [CrossRef]
- Havreljuk, F.; Achim, A.; Auty, D.; Bédard, S.; Pothier, D. Integrating standing value estimations into tree marking guidelines to meet wood supply objectives. Can. J. For. Res. 2014, 44, 750–759. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Regnery, B.; Vandekerkhove, K. Tree related microhabitats in temperate and Mediterranean European forests: A hierarchical typology for inventory standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Larrieu, L.; Cabanettes, A.; Brin, A.; Bouget, C.; Deconchat, M. Tree microhabitats at the stand scale in montane beech–fir forests: Practical information for taxa conservation in forestry. Eur. J. For. Res. 2013, 133, 355–367. [Google Scholar] [CrossRef]
- Paillet, Y.; Archaux, F.; Boulanger, V.; Debaive, N.; Fuhr, M.; Gilg, O.; Gosselin, F.; Guilbert, E. Snags and large trees drive higher tree microhabitat densities in strict forest reserves. For. Ecol. Manag. 2017, 389, 176–186. [Google Scholar] [CrossRef]
- Raymond, P.; Royo, A.A.; Prévost, M.; Dumais, D. Assessing the single-tree and small group selection cutting system as intermediate disturbance to promote regeneration and diversity in temperate mixedwood stands. For. Ecol. Manag. 2018, 430, 21–32. [Google Scholar] [CrossRef]
- Guillemette, F.; Bédard, S.; Fortin, M. Evaluation of a tree classification system in relation to mortality risk in Québec northern hardwoods. For. Chron. 2008, 84, 886–899. [Google Scholar] [CrossRef]
- Robitaille, G.; Boutin, R.; Lachance, D. Effects of soil freezing stress on sap flow and sugar content of mature sugar maples (Acer saccharum). Can. J. For. Res. 1995, 25, 577–587. [Google Scholar] [CrossRef]
- Régnière, J.; Saint-Amant, R.; Béchard, A. BioSIM 10–Guide de l’utilisateur. Ressour. Nat. Can., Serv. can. for., Cent. for. Laurentides, Québec (Québec). Rapp. Inf. LAU-X-137. 2014. Available online: https://cfs.nrcan.gc.ca/publications?id=34817 (accessed on 9 May 2021).
- Majcen, Z.; Richard, Y.; Ménard, M.; Grenier, Y. Choix des Tiges à Marquer Pour le Jardinage D’érablières Inéquiennes, Guide Technique; Mémoire No. 96; Ministère de l’Énergie et des Ressources du Québec, Direction de la Recherche Forestière: Québec, QC, Canada, 1990; p. 96.
- Shigo, A.L. Wounded forests, starving trees. J. For. 1985, 83, 668–673. [Google Scholar] [CrossRef]
- Monger, R. Classification des Tiges D’essences Feuillues: Normes Techniques; Ministère des Ressources Naturelles du Québec, Québec: Québec, QC, Canada, 1991.
- Boulet, B.; Landry, G. La Carie Des Arbres: Fondements, Diagnostic Et Application, 3rd ed.; Les Publications du Québec: Québec, QC, Canada, 2015; p. 347.
- Cox, D.R.; Oakes, D. Analysis of survival data, Chapman and Hall, London-New York, 201 S. Biom. J. 1984, 29, 114. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Guillemette, F.; Gauthier, M.M.; Lambert, M.C.; Bédard, S. Effets Réels Décennaux des Coupes de Jardinage Pratiquées de 1995 à 1999 Dans un Contexte Opérationnel; Mémoire de recherche forestière No 168; Ministère des Ressources Naturelles, Direction de la Recherche Forestière: Québec, QC, Canada, 2013; p. 36.
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Therneau, T. A Package for Survival Analysis in R, R Package Version 3.5-0; R Foundation: Vienna, Austria, 2023. [Google Scholar]
- Kuitunen, I.; Ponkilainen, V.T.; Uimonen, M.M.; Eskelinen, A.; Reito, A. Testing the proportional hazards assumption in cox regression and dealing with possible non-proportionality in total joint arthroplasty research: Methodological perspectives and review. BMC Musculoskelet. Disord. 2021, 22, 489. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, J.P.; Vanderwel, M.C.; Cole, W.G.; Purves, D.W. How Stand Productivity Results from Size- and Competition-Dependent Growth and Mortality. PLoS ONE 2011, 6, e28660. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.; Weiskittel, A.; Wagner, R.; Ducey, M.; Frank, J.; Pelletier, G. Variation in stem form and risk of four commercially important hardwood species in the Acadian Forest: Implications for potential sawlog volume and tree classification systems. Can. J. For. Res. 2017, 47, 1457–1467. [Google Scholar] [CrossRef]
- Anderson, H.W.; McLean, M. Value Increase Potential of Various Grades of Sugar Maple Trees; Ontario Department Lands and Forests, Research Branch: Dorset, ON, Canada, 1970.
- Guillemette, F. Diamètres à Maturité Financière Pour L’érable à Sucre et le Bouleau Jaune au Québec; Note de recherche forestière No 145; Mnistère des Forêts, de la Faune et des Parcs, Direction de la Recherche Forestière: Québec, QC, Canada, 2016; p. 14.
- Kenefic, L.S.; Sendak, P.E.; Brissette, J.C. Comparison of fixed diameter-limit and selection cutting in northern conifers. North. J. Appl. For. 2005, 22, 77–84. [Google Scholar] [CrossRef]
- Martin, A.R.; Caspersen, J.P.; Fuller, M.M.; Jones, T.A.; Thomas, S.C. Temporal dynamics and causes of postharvest mortality in a selection-managed tolerant hardwood forest. For. Ecol. Manag. 2014, 314, 183–192. [Google Scholar] [CrossRef]
- Moreau, G.; Achim, A.; Pothier, D. Relevance of stem and crown defects to estimate tree vigour in northern hardwood forests. For. Int. J. For. Res. 2020, 93, 630–640. [Google Scholar] [CrossRef]
- Pothier, D.; Fortin, M.; Auty, D.; Delisle-Boulianne, S.; Gagné, L.-V.; Achim, A. Improving tree selection for partial cutting through joint probability modelling of tree vigor and quality. Can. J. For. Res. 2013, 43, 288–298. [Google Scholar] [CrossRef]
- Hartmann, H.; Beaudet, M.; Mazerolle, M.J.; Messier, C. Sugar maple (Acer saccharum Marsh.) growth is influenced by close conspecifics and skid trail proximity following selection harvest. For. Ecol. Manag. 2009, 258, 823–831. [Google Scholar] [CrossRef]
- Moreau, G.; Achim, A.; Pothier, D. Growth and survival dynamics of partially cut northern hardwood stands as affected by precut competition and spatial distribution of residual trees. For. Int. J. For. Res. 2020, 93, 96–106. [Google Scholar] [CrossRef]
- Chagnon, C.; Moreau, G.; Bombardier-Cauffopé, C.; Barrette, J.; Havreljuk, F.; Achim, A. Broad-scale wood degradation dynamics in the face of climate change: A meta-analysis. GCB Bioenergy 2022, 14, 941–958. [Google Scholar] [CrossRef]
- Laidler, K.J.; Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S.; Abyazisani, M.; et al. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Jomura, M.; Kominami, Y.; Dannoura, M.; Kanazawa, Y. Spatial variation in respiration from coarse woody debris in a temperate secondary broad-leaved forest in Japan. For. Ecol. Manag. 2008, 255, 149–155. [Google Scholar] [CrossRef]
- Liu, W.; Schaefer, D.; Qiao, L.; Liu, X. What controls the variability of wood-decay rates? For. Ecol. Manag. 2013, 310, 623–631. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, S.; Baskin, C.; Baskin, J.; Schaefer, D.; Yang, X.; Yang, L. Hollows in living trees develop slowly but considerably influence the estimate of forest biomass. Funct. Ecol. 2015, 30, 830–838. [Google Scholar] [CrossRef]
- Guillemette, F.; Bédard, S. Potential for Sugar Maple to Provide High-Quality Sawlog Trees at the Northern Edge of Its Range. For. Sci. 2019, 65, 411–419. [Google Scholar] [CrossRef]
- Frank, J.; E Castle, M.; A Westfall, J.; Weiskittel, A.R.; Macfarlane, D.W.; Baral, S.K.; Radtke, P.J.; Pelletier, G. Variation in occurrence and extent of internal stem decay in standing trees across the eastern US and Canada: Evaluation of alternative modelling approaches and influential factors. For. Int. J. For. Res. 2018, 91, 382–399. [Google Scholar] [CrossRef]
- Martin, M.; Paillet, Y.; Larrieu, L.; Kern, C.C.; Raymond, P.; Drapeau, P.; Fenton, N.J. Tree-Related Microhabitats Are Promising Yet Underused Tools for Biodiversity and Nature Conservation: A Systematic Review for International Perspectives. Front. For. Glob. Chang. 2022, 5, 136. [Google Scholar] [CrossRef]
- Holloway, G.L.; Caspersen, J.P.; Vanderwel, M.C.; Naylor, B.J. Cavity tree occurrence in hardwood forests of central Ontario. For. Ecol. Manag. 2007, 239, 191–199. [Google Scholar] [CrossRef]
- Martin, M.; Raymond, P. Assessing tree-related microhabitat retention according to a harvest gradient using tree-defect surveys as proxies in Eastern Canadian mixedwood forests. For. Chron. 2019, 95, 157–170. [Google Scholar]

| DBH Class (cm) | Sugar Maple | Yellow Birch |
|---|---|---|
| 9.1–19.0 | 4412 | 350 |
| 19.1–29.0 | 2764 | 325 |
| 29.1–39.0 | 1637 | 273 |
| 39.1–49.0 | 860 | 168 |
| ≥49.1 | 348 | 84 |
| Total | 10,021 | 1200 |
| Probability of Developing Cankers and Fungi | ||||
|---|---|---|---|---|
| Explanatory Variable | Estimate | SE | Chisq | p |
| DBH | 0.046 | 0.005 | 77.46 | <0.001 |
| Species (sugar maple) | 0.960 | 0.220 | 19.14 | <0.001 |
| CDBK | 0.007 | 0.003 | 2.92 | 0.045 |
| Treatment (managed) | −0.190 | 0.080 | 5.56 | 0.018 |
| Precipitation | 0.001 | 0.001 | 0.15 | 0.700 |
| Temperature | 0.008 | 0.001 | 1.32 | 0.250 |
| Initial plot basal area | 0.001 | 0.005 | 0.05 | 0.830 |
| DBH:Species | −0.022 | 0.005 | 14.36 | <0.001 |
| Frailty (plot variance) | 159.56 | <0.001 | ||
| Probability of developing decaying defects | ||||
| Explanatory variable | Estimate | SE | Chisq | p |
| DBH | 0.016 | 0.001 | 96.53 | <0.001 |
| Species (sugar maple) | 0.233 | 0.080 | 8.46 | <0.001 |
| CDBK | 0.010 | 0.002 | 13.49 | <0.001 |
| Treatment (managed) | 0.187 | 0.090 | 0.08 | 0.780 |
| Precipitation | −0.001 | 0.001 | 0.98 | 0.320 |
| Temperature | 0.013 | 0.006 | 4.92 | 0.026 |
| Initial plot basal area | −0.001 | 0.004 | 0.06 | 0.810 |
| Frailty (plot variance) | 191.69 | <0.001 | ||
| Probability of developing any defect related to tree quality | ||||
| Explanatory variable | Estimate | SE | Chisq | p |
| DBH | 0.037 | 0.005 | 59.61 | <0.001 |
| Species (sugar maple) | 0.680 | 0.174 | 18.89 | <0.001 |
| CDBK | 0.010 | 0.002 | 19.28 | <0.001 |
| Treatment (managed) | −0.007 | 0.085 | 0.01 | 0.900 |
| Precipitation | 0.001 | 0.001 | 0.00 | 0.980 |
| Temperature | 0.010 | 0.006 | 3.61 | 0.056 |
| Initial plot basal area | 0.001 | 0.004 | 0.01 | 0.690 |
| DBH:Species | 0.017 | 0.006 | 11.66 | <0.001 |
| Frailty (plot variance) | 235.20 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreau, G.; Cecil-Cockwell, M.J.L.; Achim, A.; Bédard, S.; Guillemette, F.; Caspersen, J. Quantifying the Probability of Decline in Quality: Implications for Selection Management in Northern Hardwood Forests. Forests 2023, 14, 280. https://doi.org/10.3390/f14020280
Moreau G, Cecil-Cockwell MJL, Achim A, Bédard S, Guillemette F, Caspersen J. Quantifying the Probability of Decline in Quality: Implications for Selection Management in Northern Hardwood Forests. Forests. 2023; 14(2):280. https://doi.org/10.3390/f14020280
Chicago/Turabian StyleMoreau, Guillaume, Malcolm J. L. Cecil-Cockwell, Alexis Achim, Steve Bédard, François Guillemette, and John Caspersen. 2023. "Quantifying the Probability of Decline in Quality: Implications for Selection Management in Northern Hardwood Forests" Forests 14, no. 2: 280. https://doi.org/10.3390/f14020280
APA StyleMoreau, G., Cecil-Cockwell, M. J. L., Achim, A., Bédard, S., Guillemette, F., & Caspersen, J. (2023). Quantifying the Probability of Decline in Quality: Implications for Selection Management in Northern Hardwood Forests. Forests, 14(2), 280. https://doi.org/10.3390/f14020280






