Genetic Variation among Somatic Embryo Clones of Nordmann Fir Grown as Christmas Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Test Sites
2.2. Measurements
2.2.1. Growth
- The cumulative final heights of the years 2016, 2017, 2018, 2019, and 2020 were obtained after 2, 3, 4, 5, and 6 growing seasons, respectively; thus, the annual height increments of the years 2017, 2018, and 2019 were recorded.
- The length of the current year’s new shoot growth at the uppermost whorl was measured.
- The number of branches in the uppermost whorl, which is an important characteristic reflecting tree quality and also related to growth vigor, was counted.
- The angle of branches was compared to the main stem in the uppermost whorl on a scale from 0 to 9 (0 = flat, ca. 0 degrees; 9 = vertical, ca. 90 degrees).
2.2.2. Christmas Tree Quality
- Christmas tree quality was scored on a scale from 1 (worst) to 9 (best), following the methods outlined by Nielsen et al. [7].
- Needle color was scored into categories in steps of 0.5, from pale yellow, score 1, to very dark green, score 4.
- Post-harvest needle loss of twigs was observed indoors for 10 days. One twig from a side branch was harvested on all trees in mid-November—after 6 growing seasons for Lundygaard and Ry and 5 seasons for Holstebro. Following the procedures developed by Nielsen and Chastagner [4], the twigs were immediately transported to an indoor testing facility, where they were kept for 10 days at 20 °C with a relative humidity of around 55%. After gently stroking each twig between two fingers in the direction from base to tip, needle loss was evaluated on a scale from 0–7, where 0: no needle loss, 1: <1%, 2: 1%–5%, 3: 6%–15%, 4: 16%–33%, 5: 34%–66%, 6: 67%–90%, and 7: 91%–100%.
2.2.3. Disorders
- Bare shoulders were measured as needle loss or yellowing of last year’s needles in the second uppermost whorl of the tree. Score 0: none; score 1: yellowing of needles and minor needle loss; scores 2 and 3: moderate and severe needle loss, respectively.
- Needle loss due to unknown reasons for branches seen in the field was bivariate.
2.3. Statistical Data Analysis
2.4. Genetic Parameters
3. Results
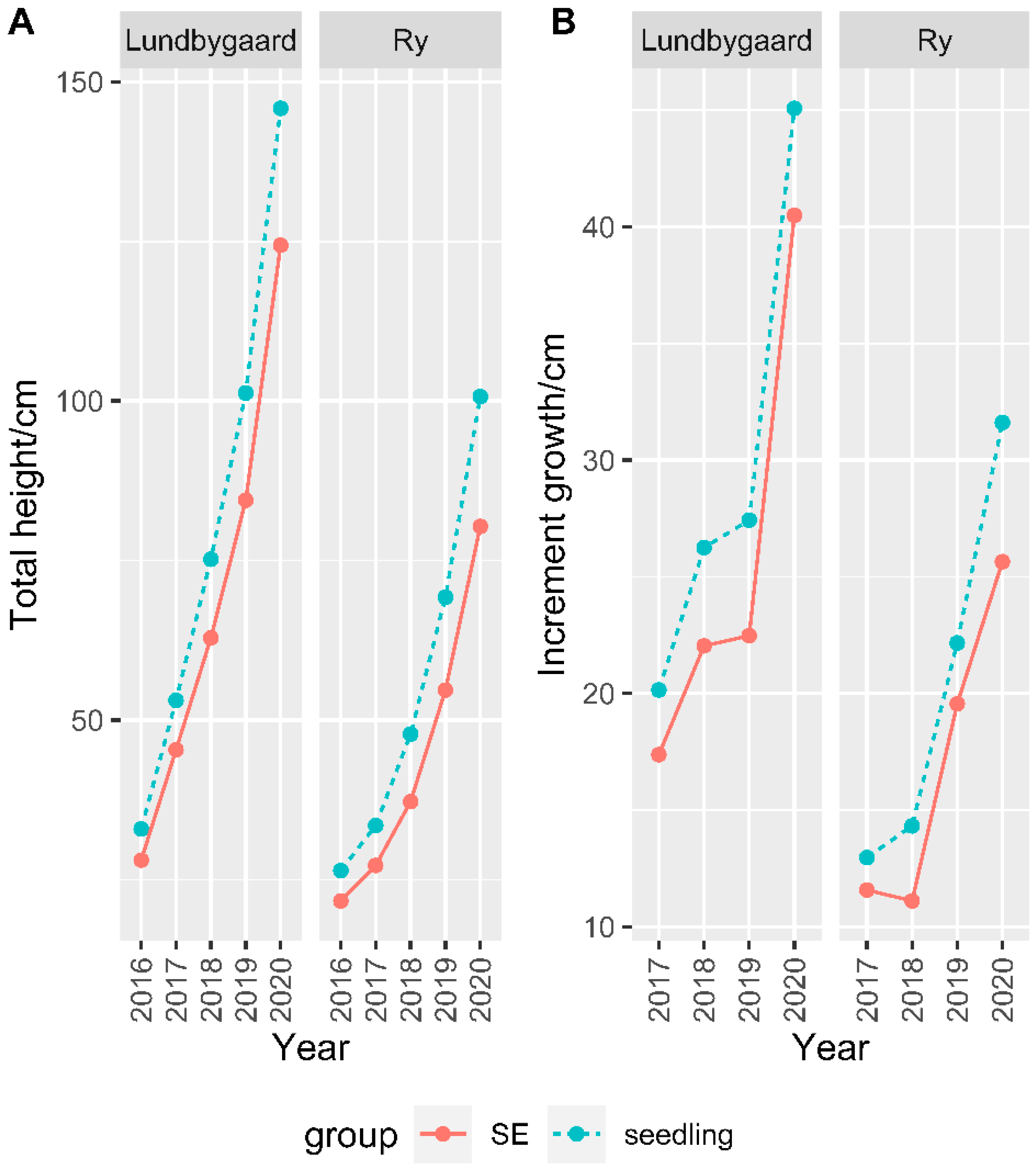
3.1. Zygotic Seedlings vs. SE Clones
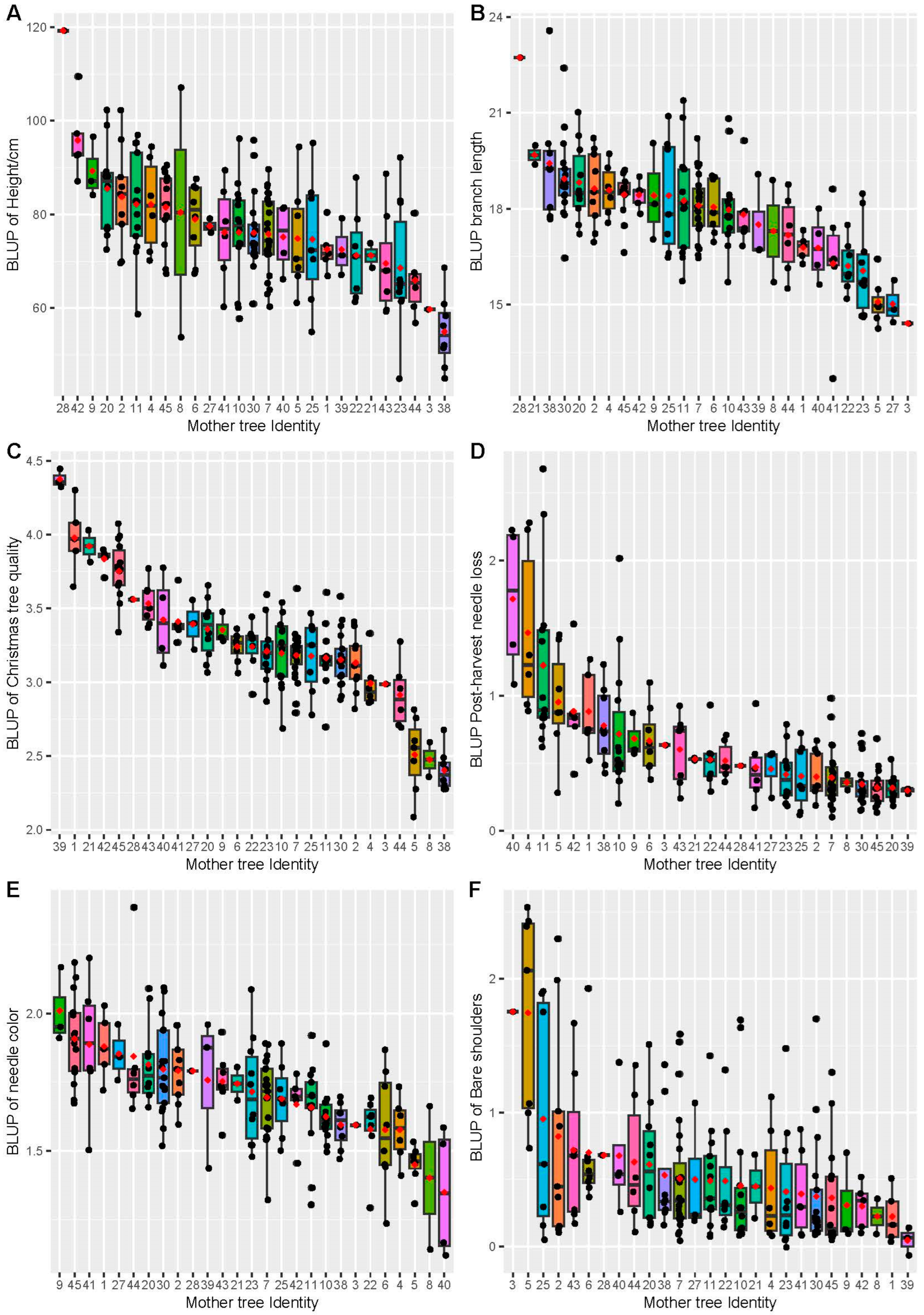
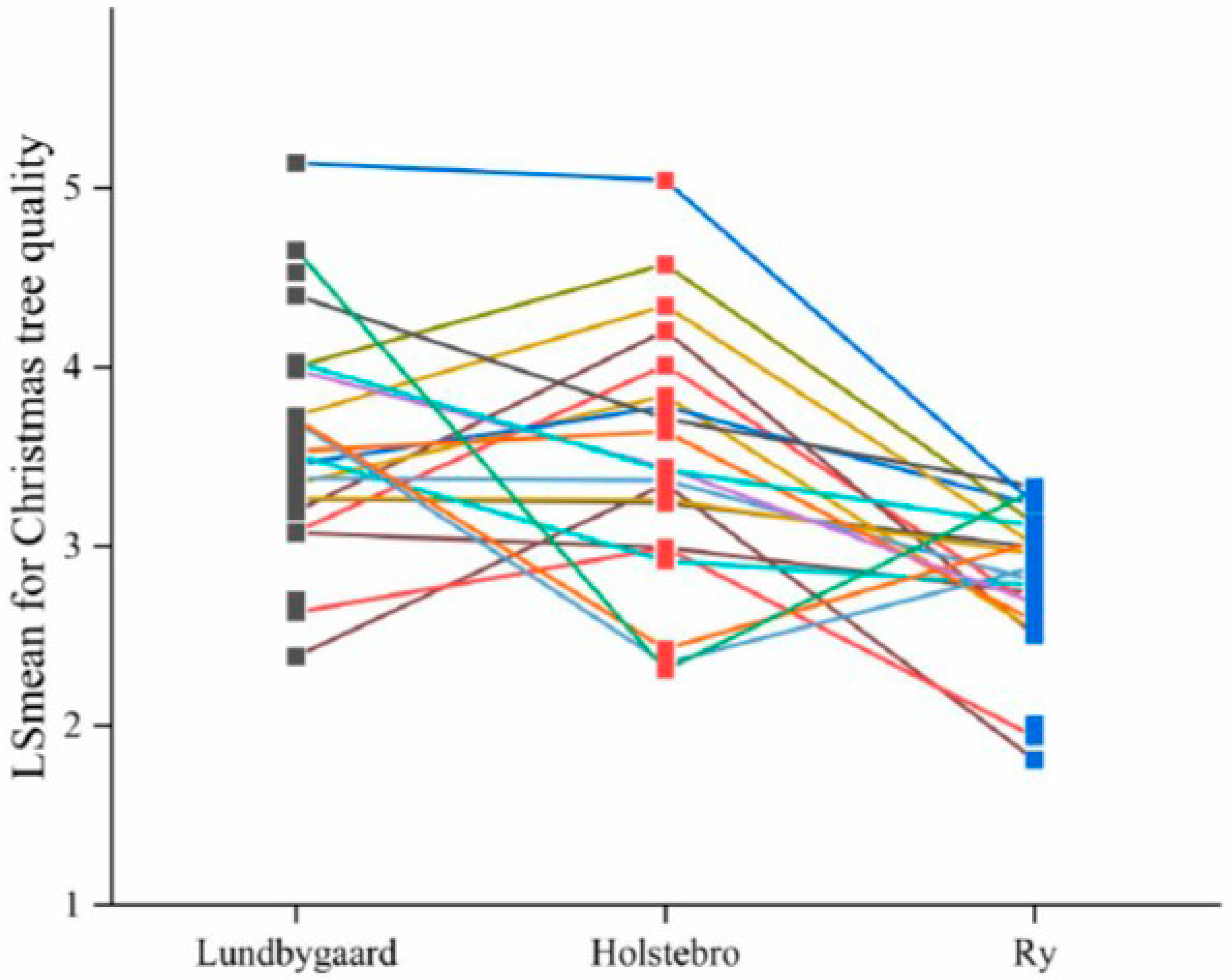
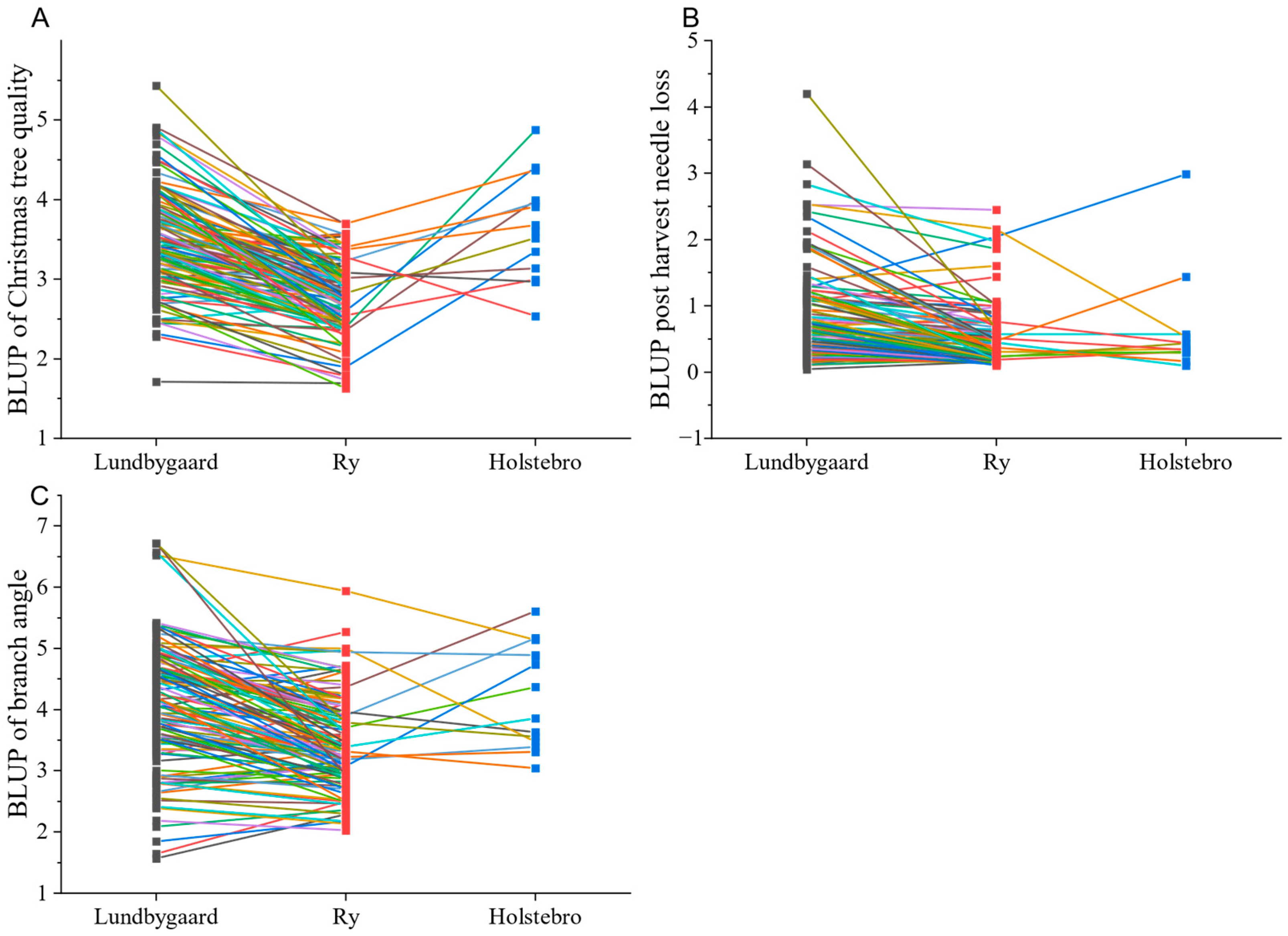
3.2. Site Means and Family Effect
3.3. Variance Components, Heritability, and Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larson, R.B. Christmas Tree Marketing: Product, Price, Promotion, and Place Tactics. J. For. 2004, 102, 40–45. [Google Scholar] [CrossRef]
- Nielsen, U.B.; Hansen, J.K.; Kromann, H.K. Impact of site and provenance on economic return in Nordmann fir Christmas tree production. Scand. J. For. Res. 2011, 26, 74–89. [Google Scholar] [CrossRef]
- Nzokou, P.; Leefers, L.A. Costs and Returns in Michigan Christmas Tree Production, 2006; Michigan State University Extension, MSUE Extension bulletin E-2999: East Lansing, MI, USA, 2007. [Google Scholar]
- Nielsen, U.B.; Chastagner, G.A. Variation in Postharvest Quality among Nordmann Fir Provenances. HortScience 2005, 40, 553–557. [Google Scholar] [CrossRef]
- Xu, J.; Nielsen, U.B.; Isik, F.; Jensen, M.; Hansen, O.K. Genetic variation and inheritance of susceptibility to Neonectria neomacrospora and Christmas tree traits in a progeny test of Nordmann fir. Ann. For. Sci. 2021, 78, 22. [Google Scholar] [CrossRef]
- Nielsen, U.B. Breeding noble fir (Abies procera Rehder) and Nordmann fir (Abies nordmanniana (Stev.) Spach) for Christmas trees and greenery in Denmark. In The Nordic Group for Tree Breeding; Forestry Commission: Edinburgh, Scotland, 1994. [Google Scholar]
- Nielsen, U.B.; Xu, J.; Hansen, O.K. Genetics in and opportunities for improvement of Nordmann fir (Abies nordmanniana (Steven) Spach) Christmas tree production. Tree Genet. Genomes 2020, 16, 66. [Google Scholar] [CrossRef]
- Xu, J.; Hansen, O.K.; Thomsen, I.M.; Nielsen, U.B. Genetic variation and genotype by environment interaction in the susceptibility of Abies nordmanniana (Steven) Spach to the fungus Neonectria neomacrospora (Booth & Samuels) Mantiri & Samuels. Ann. For. Sci. 2018, 75, 17. [Google Scholar] [CrossRef]
- Chalupa, V. Somatic embryogenesis and plantlet regeneration from cultured immature and mature embryos of Picea abies (L.). Karst. Commun. Inst. For. Cech. 1985, 14, 57–63. [Google Scholar]
- Hakman, I.; Fowke, L.C.; Von Arnold, S.; Eriksson, T. The development of somatic embryos in tissue cultures initiated from immature embryos of Picea abies (Norway spruce). Plant Sci. 1985, 38, 53–59. [Google Scholar] [CrossRef]
- Nagmani, R.; Bonga, J. Embryogenesis in subcultured callus of Larix decidua. Can. J. For. Res. 1985, 15, 1088–1091. [Google Scholar] [CrossRef]
- Nørgaard, J.; Baldursson, S.; Krogstrup, P. Genotypic differences in the ability of embryogenic Abies nordmanniana cultures to survive cryopreservation. Silvae Genet. 1993, 42, 93. [Google Scholar]
- Nørgaard, J.V. Somatic embryo maturation and plant regeneration in Abies nordmanniana Lk. Plant Sci. 1997, 124, 211–221. [Google Scholar] [CrossRef]
- Find, J.I. Towards industrial production of tree varieties through somatic embryogenesis and other vegetative propagation technologies: Nordmann fir (Abies nordmanniana (Steven) Spach)-from research laboratory to production. In Vegetative Propagation of Forest Trees; National Institute of Forest Science: Seoul, Republic of Korea, 2016; pp. 528–537. [Google Scholar]
- Find, J.I. Method for Growing Somatic Embryos of Conifers into Trees. US20200236884A1, 30 July 2020. [Google Scholar]
- Nielsen, U.B.; Hansen, C.B.; Hansen, U.; Johansen, V.K.; Egertsdotter, U. Accumulated effects of factors determining plant development from somatic embryos of Abies nordmanniana and Abies bornmuelleriana. Front. Plant Sci. 2022, 13, 989484. [Google Scholar] [CrossRef] [PubMed]
- Blödtner-Piske, C. Die erste klone sind da (The first clones have arrived). Nadel J. 2017, 11–12, 36–37. [Google Scholar]
- Wahid, N.; Lamhamedi, M.S.; Beaulieu, J.; Margolis, H.A.; Deblois, J. Genetic parameters and clonal variation in growth and nutritional traits of containerized white spruce somatic seedlings. Acta Bot. Gall. 2012, 159, 373–384. [Google Scholar] [CrossRef]
- Dean, C.A. Genetic Parameters of Somatic Clones of Coastal Douglas-fir at 5 1/2 -Years across Washington and Oregon, USA. Silvae Genet. 2008, 57, 4–5. [Google Scholar] [CrossRef]
- Dias, P.C.; Xavier, A.; Resende, M.D.V.d.; Barbosa, M.H.P.; Biernaski, F.A.; Estopa, R.A. Genetic evaluation of Pinus taeda clones from somatic embryogenesis and their genotype x environment interaction. Crop Breed. Appl. Biotechnol. 2018, 18, 55–64. [Google Scholar] [CrossRef]
- Weng, Y.H.; Park, Y.S.; Krasowski, M.J.; Tosh, K.J.; Adams, G. Partitioning of genetic variance and selection efficiency for alternative vegetative deployment strategies for white spruce in Eastern Canada. Tree Genet. Genomes 2008, 4, 809–819. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Major, J.E.; Folk, R.S. Interior spruce seedlings compared with emblings produced from somatic embryogenesis. I. Nursery development, fall acclimation, and over-winter storage. Can. J. For. Res. 1994, 24, 1376–1384. [Google Scholar] [CrossRef]
- Nsangou, M.; Greenwood, M. Physiological and morphological differences between somatic, in vitro germinated, and normal seedlings of red spruce (Picea rubens Sarg.). Can. J. For. Res. 1998, 28, 1088–1092. [Google Scholar] [CrossRef]
- Benowicz, A.; Grossnickle, S.C.; El-Kassaby, Y.A. Field assessment of Douglas-fir somatic and zygotic seedlings with respect to gas exchange, water relations, and frost hardiness. Can. J. For. Res. 2002, 32, 1822–1828. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Chamberland, H.; Bernier, P.Y.; Tremblay, F.M. Clonal variation in morphology, growth, physiology, anatomy and ultrastructure of container-grown white spruce somatic plants. Tree Physiol. 2000, 20, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Christmas Tree Grower Council of Europe CTGCE. Classification of quality standards for Nordmann Christmas trees. Available online: https://www.ctgce.com/downloads/Classification_1996_E_G_F.pdf (accessed on 6 December 2022).
- Gilmour, A.R.; Gogel, B.J.; Cullis, B.R.; Welham, S.J.; Thompson, R. ASReml User Guide Release 4.2. VSN International Ltd.: Hemel Hempstead, UK, 2021. [Google Scholar]
- Fløistad, I.S.; Nyeggen, H.; Skage, J.-O. Testing species of genus Abies for Christmas tree production in Norway. Scand. J. For. Res. 2015, 30, 653–663. [Google Scholar] [CrossRef]
- Larsen, J.B.; Larsen, B.G.; Kromann, H.K. Abies Nordmanniana provenienser til pyntegrønt og juletræer (Provenances of Abies nordmanniana for greenery and Christmas tree production). Forstl. Forsøgsv. Danm. 1984, 39, 365–382. [Google Scholar]
- Grossnickle, S.C.; El-Kassaby, Y.A. Bareroot versus container stocktypes: A performance comparison. New For. 2016, 47, 1–51. [Google Scholar] [CrossRef]
- Nielsen, U.B. Nordmannsgran proveniensforsøg-hvad betyder vækstregulering? Videnblade Pyntegrønt 2009, 3, 1–26. [Google Scholar]
- Egertsdotter, U.; Ahmad, I.; Clapham, D. Automation and scale up of somatic embryogenesis for commercial plant production, with emphasis on conifers. Front. Plant Sci. 2019, 10, 109. [Google Scholar] [CrossRef]
- Madsen, S. Provenance trial of Abies nordmanniana and Abies bornmuelleriana for Christmas tree production in North Sealand. For. Landsc. Res. 1994, 1, 143–166. [Google Scholar]
- Hansen, O.K.; Nielsen, U.B.; Edvardsen, Ø.M.; Skulason, B.; Skage, J.-O. Nordic provenance trials with Abies lasiocarpa and Abies lasiocarpa var. arizonica: Three-year results. Scand. J. For. Res. 2004, 19, 112–126. [Google Scholar] [CrossRef]
- Nielsen, U.B.; Xu, J.; Nielsen, K.N.; Talgø, V.; Hansen, O.K.; Thomsen, I.M. Species variation in susceptibility to the fungus Neonectria neomacrospora in the genus Abies. Scand. J. For. Res. 2017, 32, 421–431. [Google Scholar] [CrossRef]
- Högberg, K.-A.; Bozhkov, P.V.; Von Arnold, S. Early selection improves clonal performance and reduces intraclonal variation of Norway spruce plants propagated by somatic embryogenesis. Tree Physiol. 2003, 23, 211–216. [Google Scholar] [CrossRef]
- Högberg, K.; Varis, S. Vegetative Propagation of Norway Spruce: Experiences and Present Situation in Sweden and Finland. In Vegetative Propagation of Forest Trees; Park, Y.S., Bonga, J.M., Moon, H.-K., Eds.; National Institute of Forest Science (Nifos): Seoul, Republic of Korea, 2016; pp. 528–550. [Google Scholar]
- Lobo, A.; Find, J.I.; Hansen, J.K.; Ræbild, A.; Kjær, E.D. Effect of temperature and osmotic stress during somatic embryogenesis on phenology and physiology of abies nordmanniana emblings. For. Ecol. Manag. 2022, 514, 120212. [Google Scholar] [CrossRef]
- Jensen, M.; Korsgaard, M.; Pedersen, H.L.; Toldam-Andersen, T.B.; Sørensen, A.H.; Kjærgaard, K.B.; Bjerregaard, G.; Poulsen, J. Frugt og Bær–Gode Sorter til Haven (Fruit and Berries—Good Varieties for the Garden); Århus Universitet: Århus, Denmark, 2019. [Google Scholar]
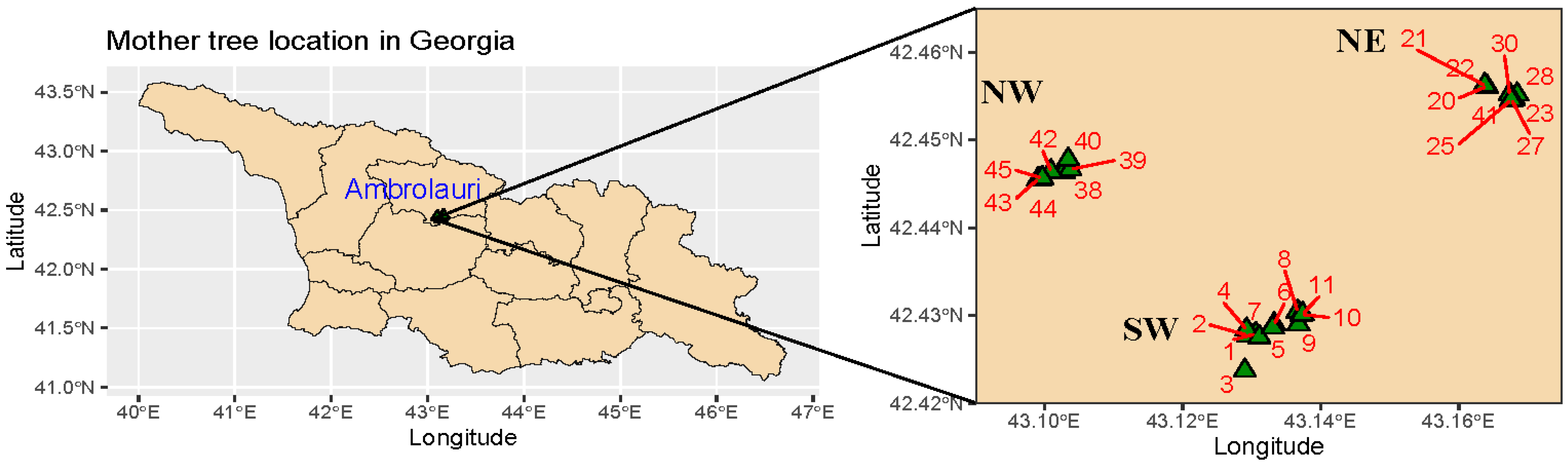
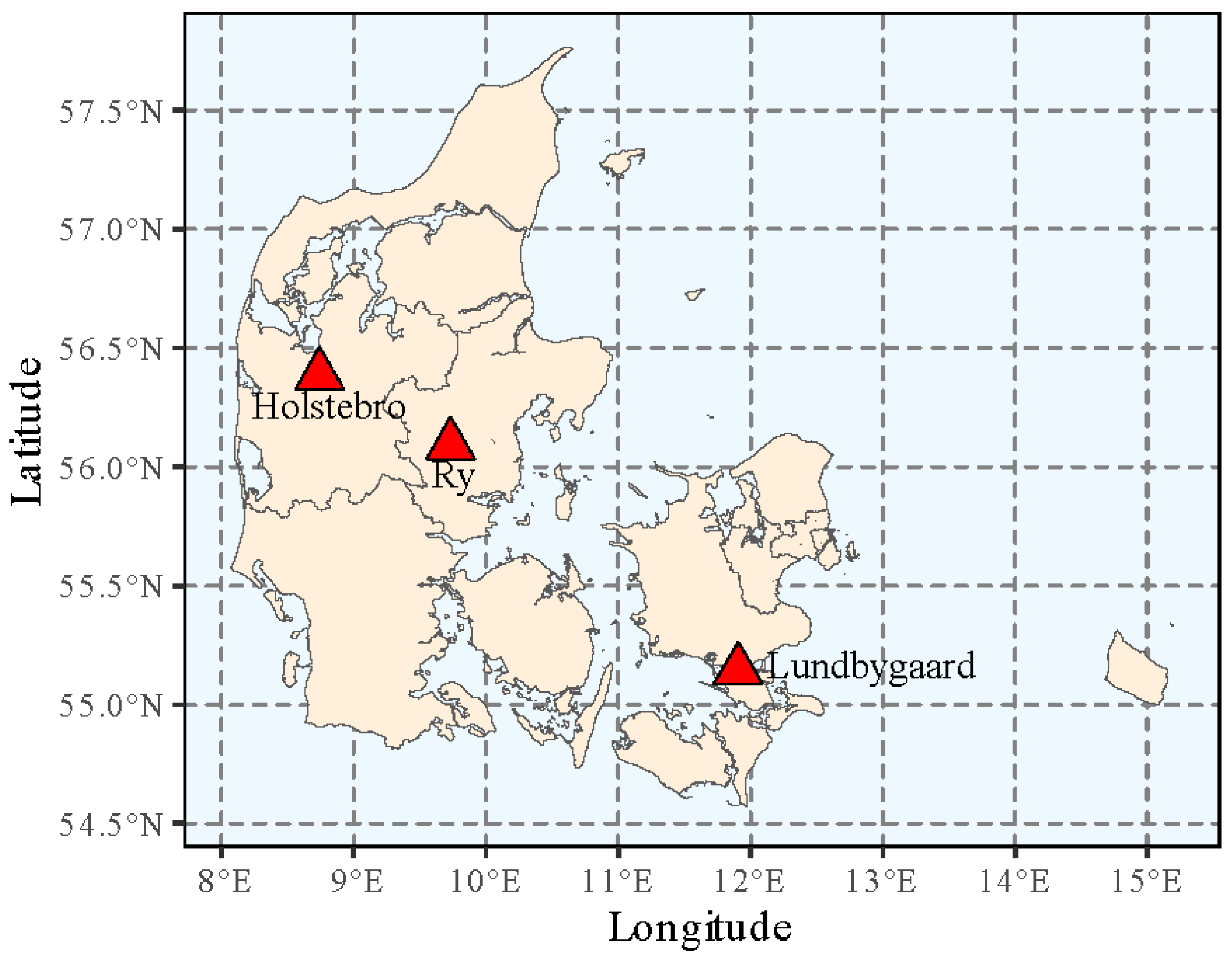



| Site Information | Lundbygaard | Ry | Holstebro |
|---|---|---|---|
| Plant year | Autumn 2014 | Autumn 2014 | Autumn 2015 |
| Soil type | Moraine | Meltwater sand | Meltwater sand |
| Spacing | 1.5 m × 1.7 m | 1.1 m × 1.1 m | 1.1 m × 1.15 m |
| No. of clones | 153 | 129 | 62 |
| No. of half-sib families | 25 | 25 | 23 |
| Average No. clones/family | 6.1 | 5.2 | 2.6 |
| Range no. clones per family | 1–18 | 1–17 | 1–6 |
| Average No. of ramets/clone | 10.8 | 8.6 | 9.4 |
| Range of No. ramets/clone | 1–72 | 2–15 | 5–20 |
| Row/Column | 38/52 | 16/100 | 6/243 |
| No. SE trees | 1636 | 1124 | 574 |
| No. seedlings | 153 | 122 | 0 |
| Branch Length 1st Whorl/cm | No. Twigs 1st Whorl | Height/cm | Christmas Tree Quality/Score | |
|---|---|---|---|---|
| p value | <0.001 | <0.001 | <0.001 | 0.03 |
| Seedling | 24.9 (0.37) | 5.1 (0.10) | 122.8 (1.80) | 3.4 (0.09) |
| SE clones | 23.0 (0.12) | 4.5 (0.03) | 102.0(0.59) | 3.2 (0.03) |
| Field Age | p Value of Fixed Effects | LSmean of Each Site | Genetic Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | Family | Hol | Ry | Lund | H2 | ||||
| Growth traits | |||||||||
| Height | 5–6 | <0.001 | <0.001 | 87.3 (8.69) | 72.8 (8.51) | 123.8 (8.76) | 228.55 (32.98) | 43.49 (11.66) | 0.31 (0.03) |
| Branch length 1st whorl | 5–6 | <0.001 | 0.03 | 18.2 (0.39) | 19.1 (0.33) | 25.9 (0.34) | 5.58 (0.95) | 1.42 (0.48) | 0.19 (0.03) |
| Number of branches | 5–6 | <0.001 | <0.001 | 4.8 (0.09) | 4.5 (0.09) | 4.4 (0.07) | 0.27 (0.05) | 0.10 (0.02) | |
| Quality | |||||||||
| Christmas tree quality | 6 | <0.001 | <0.001 | 3.5 (0.10) | 2.8 (0.08) | 3.6 (0.08) | 0.17 (0.05) | 0.16 (0.04) | 0.09 (0.02) |
| Branch angle | 5–6 | <0.001 | <0.001 | 3.7 (0.13) | 3.5 (0.11) | 4.0 (0.11) | 0.60 (0.10) | 0.18 (0.05) | 0.24 (0.03) |
| Needle color | 5–6 | <0.001 | <0.001 | 1.6 (0.03) | 1.6 (0.03) | 1.9 (0.03) | 0.03 (0.01) | 0.01 (0.003) | 0.15 (0.02) |
| Post-harvest needle retention | 5–6 | <0.001 | <0.001 | 0.6 (0.08) | 0.5 (0.06) | 0.8 (0.06) | 0.11 (0.03) | 0.12 (0.03) | 0.11 (0.03) |
| Disorders | |||||||||
| Bare shoulders | 6 | <0.001 | 0.01 | 0.7 (0.08) | 0.4 (0.07) | 0.7 (0.07) | 0.26 (0.04) | 0.07 (0.02) | 0.28 (0.03) |
| Needle loss | 5–6 | <0.001 | 0.009 | 0.6 (0.03) | 0.5 (0.03) | 0.5 (0.03) | 0.05 (0.01) | 0.004 (0.003) | 0.20 (0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Nielsen, U.B. Genetic Variation among Somatic Embryo Clones of Nordmann Fir Grown as Christmas Trees. Forests 2023, 14, 279. https://doi.org/10.3390/f14020279
Xu J, Nielsen UB. Genetic Variation among Somatic Embryo Clones of Nordmann Fir Grown as Christmas Trees. Forests. 2023; 14(2):279. https://doi.org/10.3390/f14020279
Chicago/Turabian StyleXu, Jing, and Ulrik Braüner Nielsen. 2023. "Genetic Variation among Somatic Embryo Clones of Nordmann Fir Grown as Christmas Trees" Forests 14, no. 2: 279. https://doi.org/10.3390/f14020279
APA StyleXu, J., & Nielsen, U. B. (2023). Genetic Variation among Somatic Embryo Clones of Nordmann Fir Grown as Christmas Trees. Forests, 14(2), 279. https://doi.org/10.3390/f14020279






