Tree Growth and Vegetation Diversity in Northern Idaho Forest Water Reclamation Facilities
Abstract
1. Introduction
2. Materials and Methods
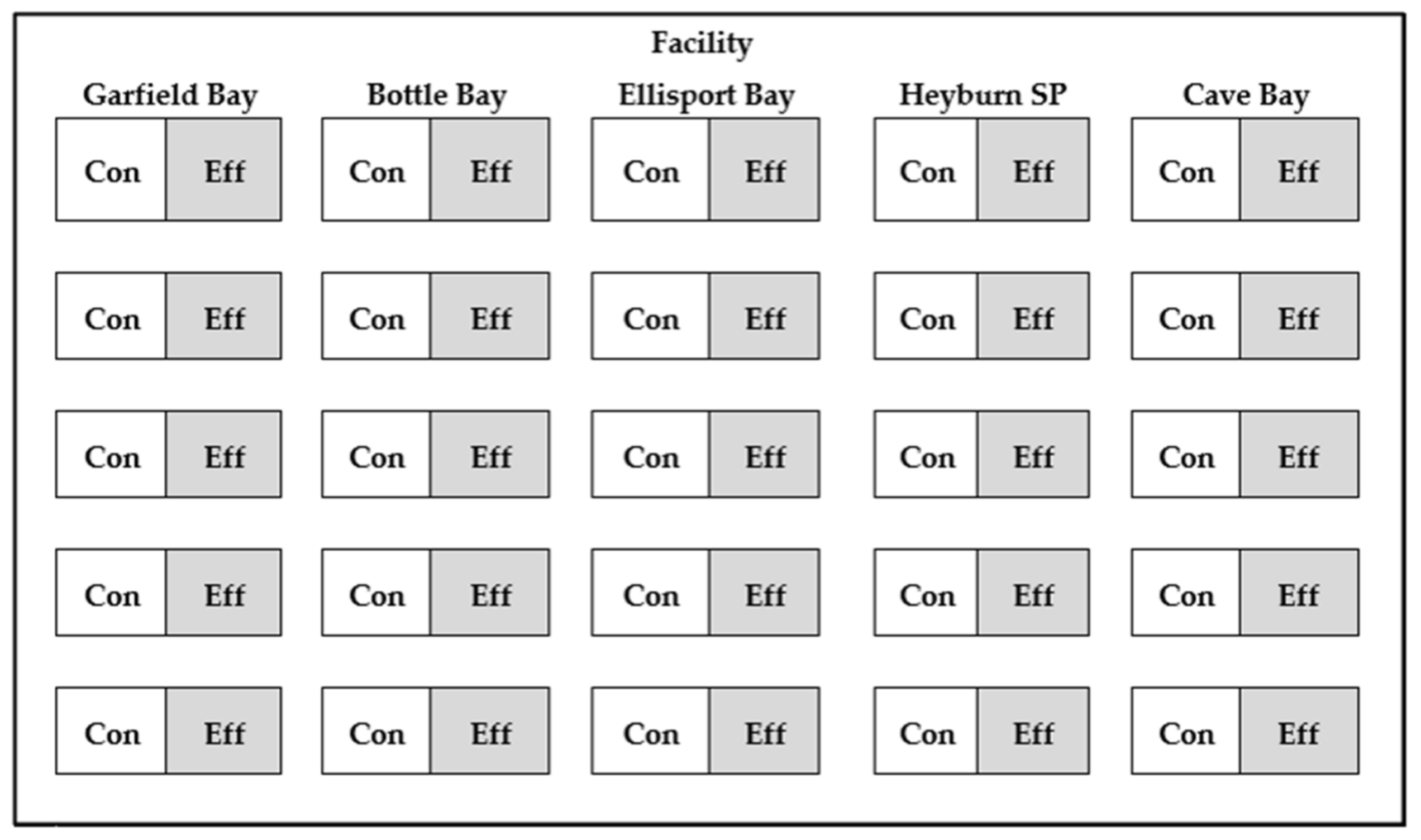
2.1. Study Facilities
2.2. Stand Parameters
2.3. Tree-Ring Width Data and Series Chronologies
2.4. Understory Vegetation Survey and Biomass
2.4.1. Understory Vegetation Survey
2.4.2. Understory Vegetation Biomass
2.5. Statistical Analysis
3. Results
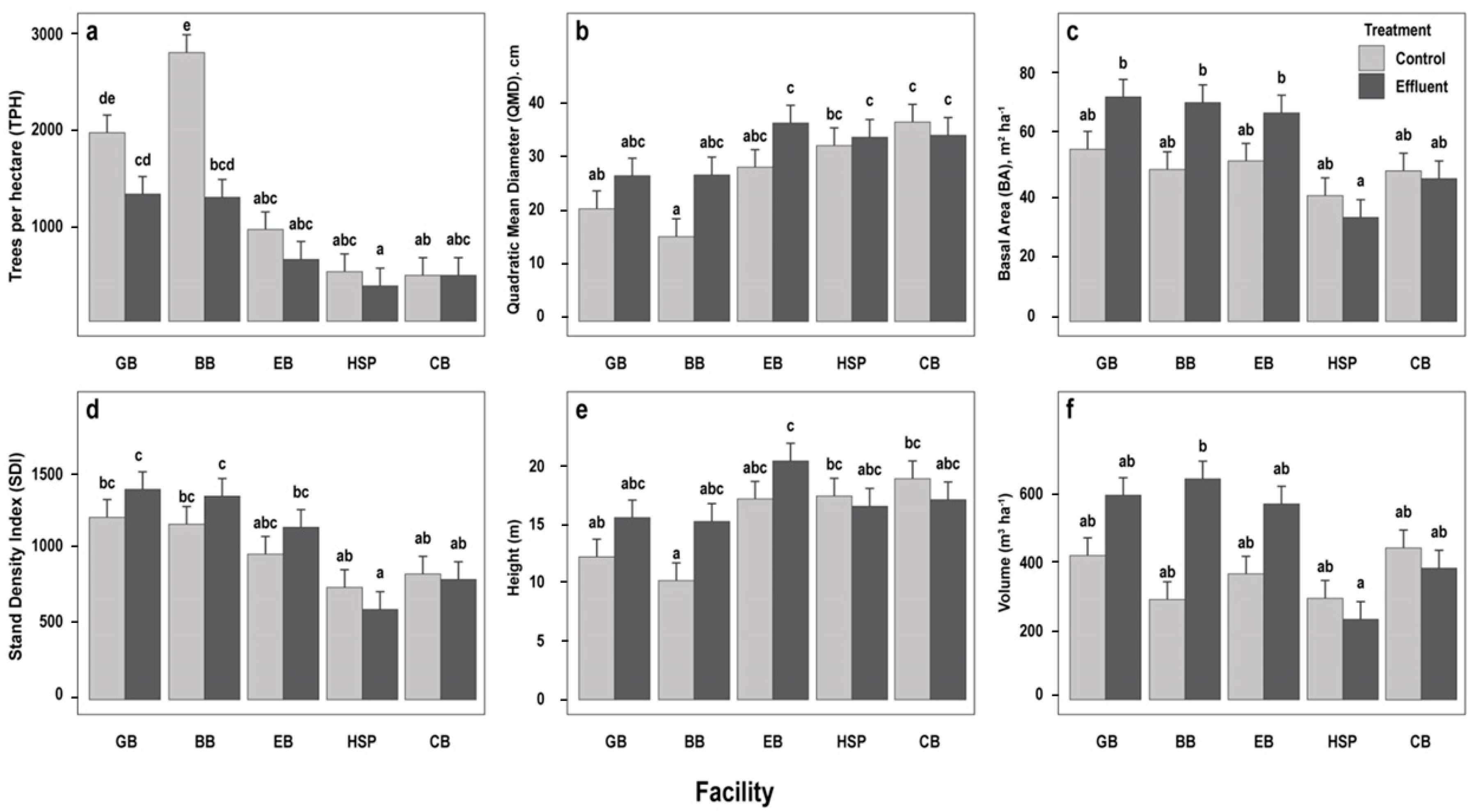
3.1. Initial Stand Parameters
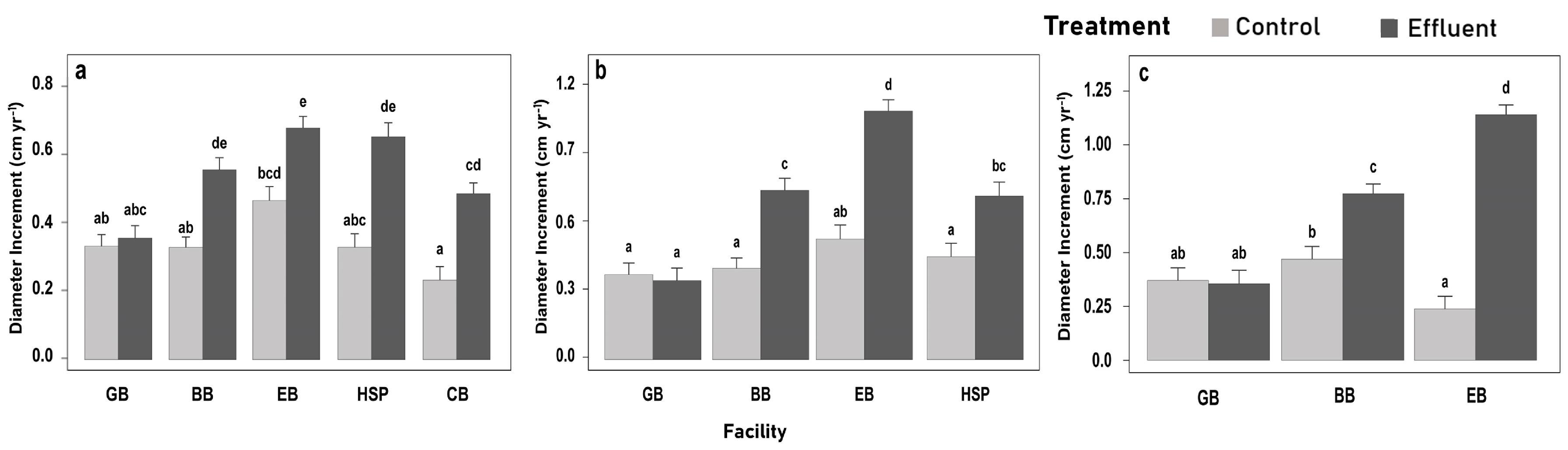
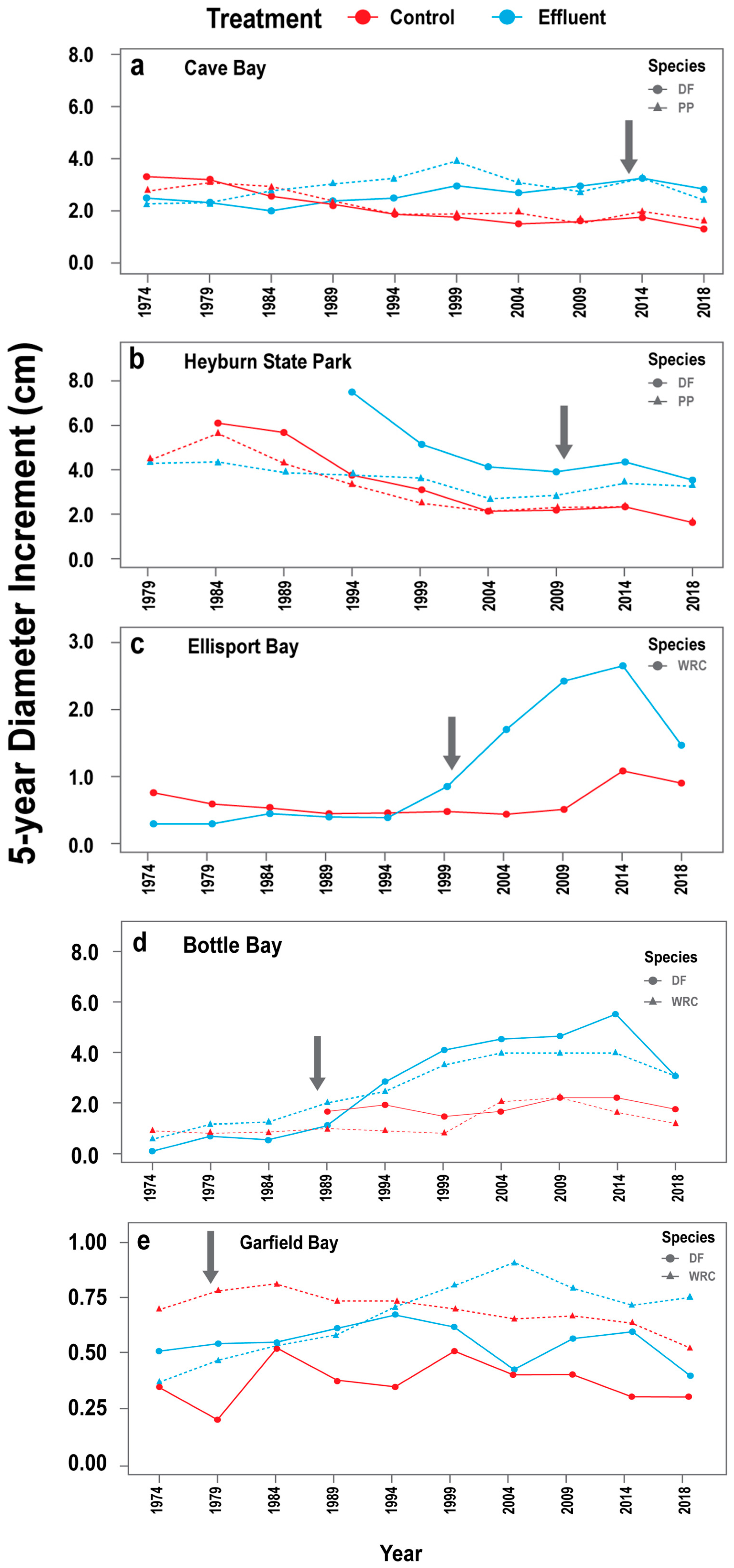
3.2. Estimated Historic Diameter Increment Responses from Tree Cores (DIc)
3.2.1. DIc Responses across Facilities
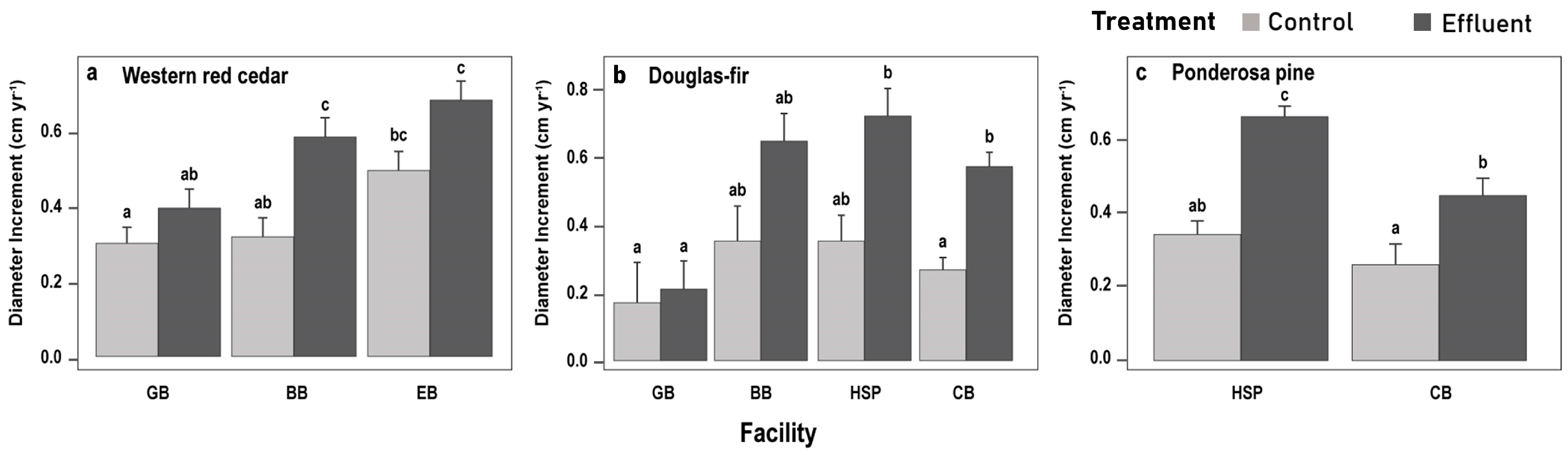
3.2.2. Species-Specific DIc Responses
3.3. Growth Increments during Study Period
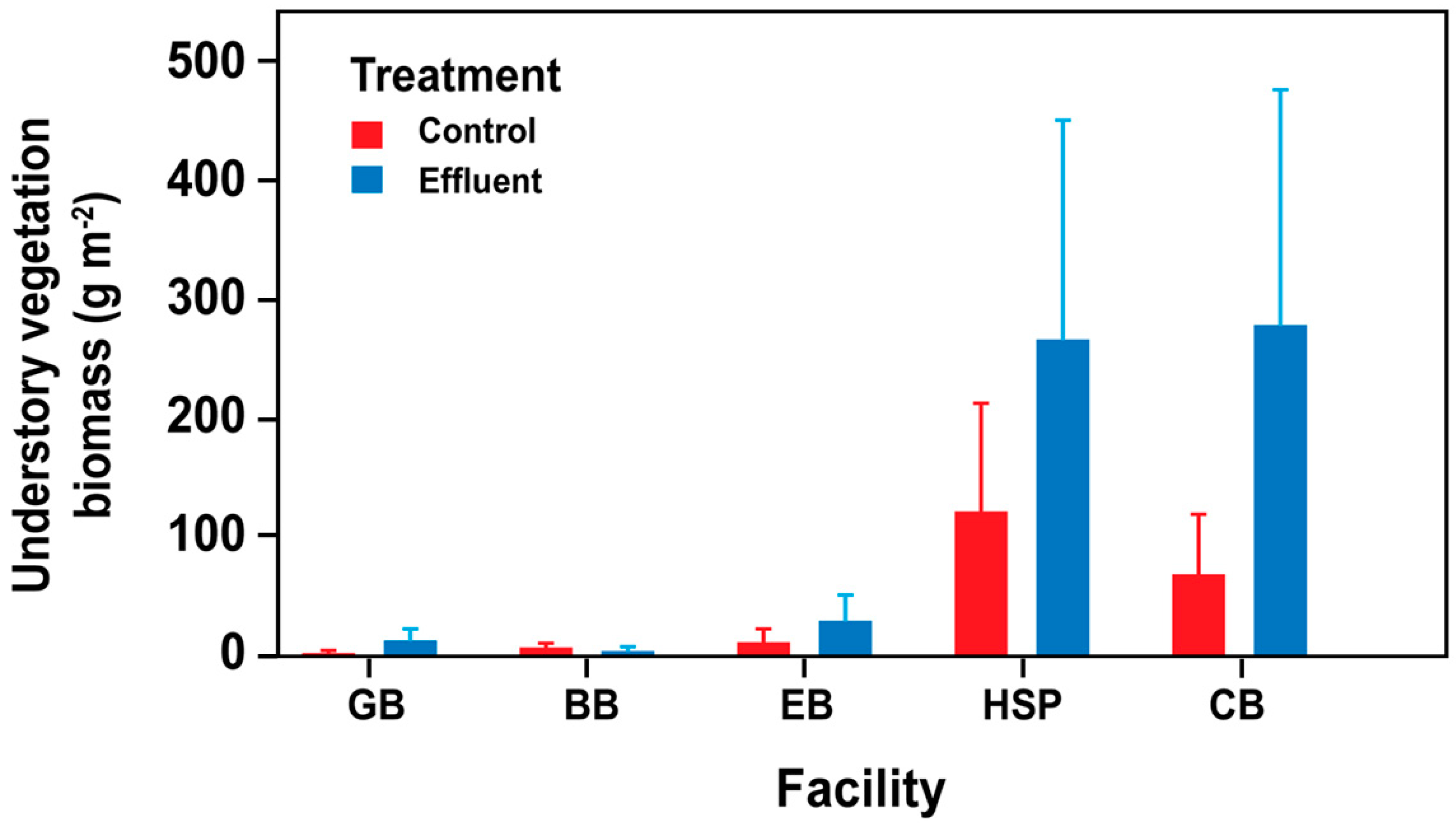
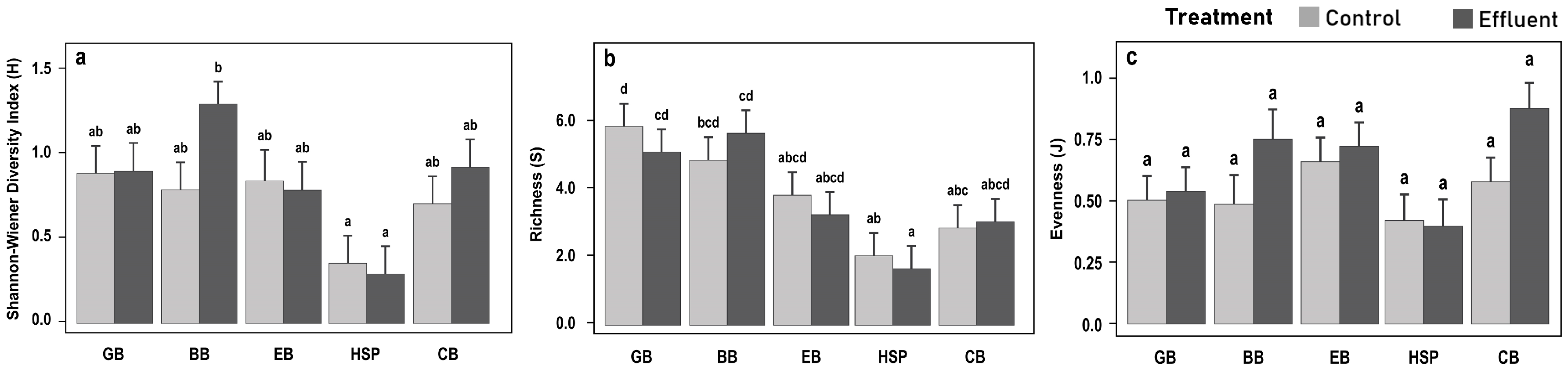
3.4. Understory Vegetation Biomass and Diversity Responses
3.5. Overstory Tree Diversity Responses
4. Discussion
4.1. Forest Growth Response
4.2. Individual Species Responses
4.3. Vegetation Diversity Responses
4.4. Time Series Study Design and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Al-Jamal, M.S.; Sammis, T.W.; Mexal, J.G.; Picchioni, G.A.; Zachritz, W.H. A Growth-Irrigation Scheduling Model for Wastewater Use in Forest Production. Agric. Water Manag. 2002, 56, 57–79. [Google Scholar] [CrossRef]
- Cole, D.; Gessel, S. Fundamentals of Tree Nutrition. In Forest Fertilization: Sustaining and Improving Nutrition and Growth of Western Forests; Institute of Forest Resources Contribution: Seattle, WA, USA, 1992; pp. 7–16. [Google Scholar]
- Gessel, S.P.; Miller, R.E.; Cole, D.W. Relative Importance of Water and Nutrients on the Growth of Coast Douglas Fir in the Pacific Northwest. For. Ecol. Manag. 1990, 30, 327–340. [Google Scholar] [CrossRef]
- Cole, D.W.; Rapp, M. 6. Elemental Cycling in Forest Ecosystems. Dyn. Prop. For. Ecosyst. 1981, 23, 341. [Google Scholar]
- Johnson, D.W.; Turner, J. Nitrogen Budgets of Forest Ecosystems: A Review. For. Ecol. Manag. 2014, 318, 370–379. [Google Scholar] [CrossRef]
- Lang, F.; Bauhus, J.; Frossard, E.; George, E.; Kaiser, K.; Kaupenjohann, M.; Krüger, J.; Matzner, E.; Polle, A.; Prietzel, J.; et al. Phosphorus in Forest Ecosystems: New Insights from an Ecosystem Nutrition Perspective. J. Plant Nutr. Soil Sci. 2016, 179, 129–135. [Google Scholar] [CrossRef]
- Chappell, H.N.; Cole, D.W.; Gessel, S.P.; Walker, R.B. Forest Fertilization Research and Practice in the Pacific Northwest. Fertil. Res. 1991, 27, 129–140. [Google Scholar] [CrossRef]
- Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. Forest Fertilization in Southern Pine Plantations. Better Crops 2006, 90, 12–15. [Google Scholar]
- Coyle, D.R.; Aubrey, D.P.; Coleman, M.D. Growth Responses of Narrow or Broad Site Adapted Tree Species to a Range of Resource Availability Treatments after a Full Harvest Rotation. For. Ecol. Manag. 2016, 362, 107–119. [Google Scholar] [CrossRef]
- Iivonen, S.; Kaakinen, S.; Jolkkonen, A.; Vapaavuori, E.; Linder, S. Influence of Long-Term Nutrient Optimization on Biomass, Carbon, and Nitrogen Acquisition and Allocation in Norway Spruce. Can. J. For. Res. 2006, 36, 1563–1571. [Google Scholar] [CrossRef]
- Weetman, G.F.; Prescott, C.E.; Kohlberger, F.L.; Fournier, R.M. Ten-Year Growth Response of Coastal Douglas-Fir on Vancouver Island to N and S Fertilization in an Optimum Nutrition Trial. Can. J. For. Res. 1997, 27, 1478–1482. [Google Scholar] [CrossRef]
- Ingestad, T. New Concepts on Soil Fertility and Plant Nutrition as Illustrated by Research on Forest Trees and Stands. Geoderma 1987, 40, 237–252. [Google Scholar] [CrossRef]
- Van Miegroet, H.; Cole, D.W. The Impact of Nitrification on Soil Acidification and Cation Leaching in a Red Alder Ecosystem. J. Environ. Qual. 1984, 13, 586–590. [Google Scholar] [CrossRef]
- Asano, T. Irrigation with Reclaimed Municipal Wastewater. GeoJournal 1987, 15, 273–282. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Stagnitti, F.; Xiong, X.; Kreidl, S.L.; Benke, K.K.; Maher, P. Wastewater Irrigation: The State of Play. Vadose Zone J. 2007, 6, 823–840. [Google Scholar] [CrossRef]
- Andrews, D.M.; Robb, T.; Elliott, H.; Watson, J.E. Impact of Long-Term Wastewater Irrigation on the Physicochemical Properties of Humid Region Soils: “The Living Filter” Site Case Study. Agric. Water Manag. 2016, 178, 239–247. [Google Scholar] [CrossRef]
- Mexal, J.G.; Zachritz, W.H.; Sammis, T.W. Trees Are the Solution to Wastewater Treatment for Small Communities. In National Proceedings: Forest and Conservation Nursery Associations—1999, 2000 and 2001; US Department of Agriculture, Forest Service, Southern Research Station: Ogden, UT, USA, 2002; pp. 327–335. [Google Scholar]
- Duan, R.; Sheppard, C.D.; Fedler, C.B. Short-Term Effects of Wastewater Land Application on Soil Chemical Properties. Water Air Soil Pollut. 2010, 211, 165–176. [Google Scholar] [CrossRef]
- Jordan, M.J.; Nadelhoffer, K.J.; Fry, B. Nitrogen Cycling in Forest and Grass Ecosystems Irrigated with 15N-Enriched Wastewater. Ecol. Appl. 1997, 7, 864–881. [Google Scholar] [CrossRef]
- Barton, L.; Schipper, L.A.; Barkle, G.F.; McLeod, M.; Speir, T.W.; Taylor, M.D.; McGill, A.C.; van Schaik, A.P.; Fitzgerald, N.B.; Pandey, S.P. Land Application of Domestic Effluent onto Four Soil Types: Plant Uptake and Nutrient Leaching. J. Environ. Qual. 2005, 34, 635–643. [Google Scholar] [CrossRef]
- Tomer, M.D.; Smith, C.T.; Thorn, A.J.; Gielen, G.J.H.P.; Barton, L. Evaluation of Treatment Performance and Processes after Six Years of Wastewater Application at Whakarewarewa Forest, New Zealand. For. Res. 2000, 155–162. [Google Scholar]
- Beedlow, P.A.; Waschmann, R.S.; Lee, E.H.; Tingey, D.T. Seasonal Patterns of Bole Water Content in Old Growth Douglas-Fir (Pseudotsuga Menziesii (Mirb.) Franco). Agric. For. Meteorol. 2017, 242, 109–119. [Google Scholar] [CrossRef]
- Marron, N. Agronomic and Environmental Effects of Land Application of Residues in Short-Rotation Tree Plantations: A Literature Review. Biomass Bioenergy 2015, 81, 378–400. [Google Scholar] [CrossRef]
- Moffat, A.J.; Armstrong, A.T.; Ockleston, J. The Optimization of Sewage Sludge and Effluent Disposal on Energy Crops of Short Rotation Hybrid Poplar. Biomass Bioenergy 2001, 20, 161–169. [Google Scholar] [CrossRef]
- Weetman, G.F.; McDonald, M.A.; Prescott, C.E.; Kimmins, J.P. Responses of Western Hemlock, Pacific Silver Fir, and Western Red Cedar Plantations on Northern Vancouver Island to Applications of Sewage Sludge and Inorganic Fertilizer. Can. J. For. Res. 1993, 23, 1815–1820. [Google Scholar] [CrossRef]
- Hesse, I.D.; Day, J.W.; Doyle, T.W. Long-Term Growth Enhancement of Baldcypress (Taxodium Distichum) from Municipal Wastewater Application. Environ. Manag. 1998, 22, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Affleck, D.L.R. Aboveground Biomass Equations for the Predominant Conifer Species of the Inland Northwest USA. For. Ecol. Manag. 2019, 432, 179–188. [Google Scholar] [CrossRef]
- Oswald, B.P.; Ott, B.; Williams, H.M.; Farrish, K.W.; Van Kley, J.E. Understory Vegetative Diversity of Post-Thinned Pine Plantations Treated with Fertilizer, Fire and Herbicide in East Texas. Int. J. For. Res. 2009, 2009, 572480. [Google Scholar] [CrossRef]
- Thomas, S.C.; Halpern, C.B.; Falk, D.A.; Liguori, D.A.; Austin, K.A. Plant Diversity in Managed Forests: Understory Responses to Thinning and Fertilization. Ecol. Appl. 1999, 9, 864–879. [Google Scholar] [CrossRef]
- Aiello, R.; Cirelli, G.L.; Consoli, S. Effects of Reclaimed Wastewater Irrigation on Soil and Tomato Fruits: A Case Study in Sicily (Italy). Agric. Water Manag. 2007, 93, 65–72. [Google Scholar] [CrossRef]
- Wallach, R.; Ben-Arie, O.; Graber, E.R. Soil Water Repellency Induced By Long-Term Irrigation with Treated Sewage Effluent. J. Environ. Qual. 2005, 34, 1910–1920. [Google Scholar] [CrossRef]
- Duan, R.; Fedler, C.B.; Sheppard, C.D. Field Study of Salt Balance of a Land Application System. Water Air Soil Pollut. 2011, 215, 43–54. [Google Scholar] [CrossRef]
- Magesan, G.N.; Williamson, J.C.; Yeates, G.W.; Lloyd-Jones, A.R. Wastewater C:N Ratio Effects on Soil Hydraulic Conductivity and Potential Mechanisms for Recovery. Bioresour. Technol. 2000, 71, 21–27. [Google Scholar] [CrossRef]
- Toze, S. Reuse of Effluent Water—Benefits and Risks. Agric. Water Manag. 2006, 80, 147–159. [Google Scholar] [CrossRef]
- Hunt, E.J.; Shure, D.J. Vegetation and Arthropod Responses to Wastewater Enrichment of a Pine Forset. Oecologia 1980, 47, 118–124. [Google Scholar] [CrossRef]
- Alvarez-Clare, S.; Mack, M.C.; Brooks, M. A Direct Test of Nitrogen and Phosphorus Limitation to Net Primary Productivity in a Lowland Tropical Wet Forest. Ecology 2013, 94, 1540–1551. [Google Scholar] [CrossRef]
- Ibáñez, I.; Zak, D.R.; Burton, A.J.; Pregitzer, K.S. Chronic Nitrogen Deposition Alters Tree Allometric Relationships: Implications for Biomass Production and Carbon Storage. Ecol. Appl. 2016, 26, 913–925. [Google Scholar] [CrossRef]
- Schroth, G.; da Mota, M.D.S.S.; de Assis Elias, M.E. Growth and Nutrient Accumulation of Brazil Nut Trees (Bertholletia Excelsa) in Agroforestry at Different Fertilizer Levels. J. For. Res. 2015, 26, 347–353. [Google Scholar] [CrossRef]
- Burton, P.J.; Balisky, A.C.; Coward, L.P.; Cumming, S.G.; Kneeshaw, D.D. The Value of Managing for Biodiversity. For. Chron. 1992, 68, 225–237. [Google Scholar] [CrossRef]
- DeWald, L.E.; Mahalovich, M.F. The Role of Forest Genetics in Managing Ecosystems. J. For. 1997, 95, 12–16. [Google Scholar]
- Oliver, C.D.; Lippke, B. Managing for Multiple Values: A Proposal for the Pacific Northwest. J. For. 1993, 91, 14–18. [Google Scholar]
- Zhang, J.; Young, D.H.; Oliver, W.W.; Fiddler, G.O. Effect of Overstorey Trees on Understorey Vegetation in California (USA) Ponderosa Pine Plantations. Forestry 2016, 89, 91–99. [Google Scholar] [CrossRef]
- Shikangalah, R.; Mapani, B.; Mapaure, I.; Herzschuh, U.; Musimba, A.; Tabares, X. Growth Ring Formation of Dichrostachys Cinerea and Senegalia Mellifera in Arid Environments in Namibia. Dendrochronologia 2020, 59, 125661. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Fernández-Haeger, J.; Jordano-Barbudo, D. A Method for Tree-Ring Analysis Using Diva-Gis Freeware on Scanned Core Images. Tree-Ring Res. 2015, 71, 118–129. [Google Scholar] [CrossRef]
- McCarroll, D.; Loader, N.J. Stable Isotopes in Tree Rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Soil Survey Staff Official Soil Series Descriptions. 2019. Available online: https://soils.usda.gov/technical/classification/osd/index.html (accessed on 24 January 2023).
- Caron, T.M.F.; Chuma, V.J.U.R.; Sandi, A.A.; Norris, D. Big Trees Drive Forest Structure Patterns across a Lowland Amazon Regrowth Gradient. Sci. Rep. 2021, 11, 3380. [Google Scholar] [CrossRef] [PubMed]
- VanderSchaaf, C.L.; Burkhart, H.E. Relationship Between Maximum Basal Aarea Carrying Capacity and Maximum Size-Density Rrelationships. Mod. Appl. Sci. 2007, 1, 3–6. [Google Scholar] [CrossRef]
- Woodall, C.W.; Miles, P.D.; Vissage, J.S. Determining Maximum Stand Density Index in Mixed Species Stands for Strategic-Scale Stocking Assessments. For. Ecol. Manag. 2005, 216, 367–377. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Swenson, N.G.; Weiser, M.D. Tree Height-Diameter Allometry across the United States. Ecol. Evol. 2015, 5, 1193–1204. [Google Scholar] [CrossRef]
- Wykoff, W.R.; Crookston, N.L.; Stage, A.R. User’s Guide to the Stand Prognosis Model (Forestry); US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1982. [Google Scholar]
- Maxwell, R.S.; Wixom, J.A.; Hessl, A.E. A Comparison of Two Techniques for Measuring and Crossdating Tree Rings. Dendrochronologia 2011, 29, 237–243. [Google Scholar] [CrossRef]
- Shikangalah, R.; Musimba, A.; Mapaure, I.; Mapani, B.; Herzschuh, U.; Tabares, X.; Kamburona-Ngavetene, C. Growth Rings and Stem Diameter of Dichrostachys Cinerea and Senegalia Mellifera along a Rainfall Gradient in Namibia. Trees For. People 2021, 3, 100046. [Google Scholar] [CrossRef]
- Bogino, S.; Fernández Nieto, M.J.; Bravo, F. Climate Effect on Radial Growth of Pinus Sylvestris at Its Southern and Western Distribution Limits. Silva Fenn. 2009, 43, 609–623. [Google Scholar] [CrossRef]
- Roversi, G.D.; Canussio, V.; Spennacchio, M. Recognition and Significance of Maternogenic Fetal Acidosis during Intensive Monitoring of Labor. J. Perinat. Med. 1975, 3, 53–67. [Google Scholar] [CrossRef]
- Yang, S.I.; Radtke, P.J. Predicting Bark Thickness with One- and Two-Stage Regression Models for Three Hardwood Species in the Southeastern US. For. Ecol. Manag. 2022, 503, 119778. [Google Scholar] [CrossRef]
- Wang, G.G.; Kemball, K.J. Effects of Fire Severity on Early Development of Understory Vegetation. Can. J. For. Res. 2005, 35, 254–262. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–243. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. 2021. Available online: http://www.R-project.org (accessed on 24 January 2023).
- Bergh, J.; Linder, S.; Lundmark, T.; Elfving, B. The Effect of Water and Nutrient Availability on the Productivity of Norway Spruce in Northern and Southern Sweden. For. Ecol. Manag. 1999, 119, 51–62. [Google Scholar] [CrossRef]
- Pereira, J.S.; Linder, S.; Araújo, M.C.; Pereira, H.; Ericsson, T.; Borralho, N.; Leal, L.C. Optimization of Biomass Production in Eucalyptus Globulus Plantations—A Case Study. In Biomass Production by Fast-Growing Trees; Springer: Berlin/Heidelberg, Germany, 1989; pp. 101–121. [Google Scholar]
- Mika, P.G.; Moore, J.A.; Brockley, R.P.; Powers, R.F. Fertilization response by interior forests: When, where, and how much. In Forest Fertilization: Sustaining and Improving Nutrition and Growth of Western Gorests Chappell; Weetman, G.F., Miller, R.E., Eds.; College of Forest Resources, University of Washington: Seattle, WA, USA, 1992; pp. 127–142. [Google Scholar]
- Cromer, R.N. Irrigation of Radiata Pine with Waste Water: A Review of the Potential for Tree Growth and Water Renovation. Aust. For. 1980, 43, 87–100. [Google Scholar] [CrossRef]
- Aubrey, D.P.; Coyle, D.R.; Coleman, M.D. Functional Groups Show Distinct Differences in Nitrogen Cycling during Early Stand Development: Implications for Forest Management. Plant Soil 2012, 351, 219–236. [Google Scholar] [CrossRef]
- Wallace, Z.P.; Lovett, G.M.; Hart, J.E.; Machona, B. Effects of Nitrogen Saturation on Tree Growth and Death in a Mixed-Oak Forest. For. Ecol. Manag. 2007, 243, 210–218. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Currie, W.S.; Nadelhoffer, K.J.; Martin, M.E.; McDowell, W.H.; Melillo, J.M.; Steudler, P. Ecosystem Response to 15 Years of Chronic Nitrogen Additions at the Harvard Forest LTER, Massachusetts, USA. For. Ecol. Manag. 2004, 196, 7–28. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G.; Barnard, H.R.; Fownes, J. Age-Related Decline in Forest Ecosystem Growth: An Individual-Tree, Stand-Structure Hypothesis. Ecosystems 2002, 5, 58–67. [Google Scholar] [CrossRef]
- Foster, J.R.; D’Amato, A.W.; Bradford, J.B. Looking for Age-Related Growth Decline in Natural Forests: Unexpected Biomass Patterns from Tree Rings and Simulated Mortality. Oecologia 2014, 175, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Turnbull, M.H.; Tissue, D.T.; Lewis, J.D.; Carson, R.; Schuster, W.S.F.; Whitehead, D.; Walcroft, A.S.; Li, J.; Griffin, K.L. Age-Related Decline of Stand Biomass Accumulation Is Primarily Due to Mortality and Not to Reduction in NPP Associated with Individual Tree Physiology, Tree Growth or Stand Structure in a Quercus-Dominated Forest. J. Ecol. 2012, 100, 428–440. [Google Scholar] [CrossRef]
- Klinka, K.; Brisco, D. Silvics and Silviculture of Coastal Western Redcedar: A Literature Review; Special Report Series; Ministry of Forests and Range: Vancouver, BC, Canada, 2009. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; Wiley: New York, NY, USA, 1996; p. 520. [Google Scholar]
- Scanlin, D.C.; Loewenstein, L. Response of inland Douglas-fir and grand fir to thinning and nitrogen fertilization in northern Idaho. In Proceedings of the Forest Fertilization Conference, Union, WA, USA, 25–27 September 1979; Gessel, S.P., Kenady, R.M., Atkinson, W.A., Eds.; University of Washington, College of Forest Resources, Institute of Forest Resources: Seattle, WA, USA, 25 September 1979; pp. 82–88. [Google Scholar]
- Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. Tree Nutrition and Forest Fertilization of Pine Plantations in the Southern United States. South. J. Appl. For. 2007, 31, 5–11. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global Assessment of Nitrogen Deposition Effects on Terrestrial Plant Diversity: A Synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Binkley, D.; Giardina, C. Why Do Tree Species Affect Soils? The Warp and Woof of Tree-Soil Interactions. Biogeochemistry 1998, 42, 89–106. [Google Scholar] [CrossRef]
- Compton, J.E.; Church, M.R.; Larned, S.T.; Hogsett, W.E. Nitrogen Export from Forested Watersheds in the Oregon Coast Range: The Role of N2-Fixing Red Alder. Ecosystems 2003, 6, 773–785. [Google Scholar] [CrossRef]
- Hobbie, S.E. Effects of Plant Species on Nutrient Cycling. Trends Ecol. Evol. 1992, 7, 336–339. [Google Scholar] [CrossRef]
- VanderSchaaf, C.L.; Moore, J.A.; Kingery, J.L. The Effect of Multi-Nutrient Fertilization on Understory Plant Diversity. Northwest Sci. 2000, 74, 316–324. [Google Scholar]
- McNulty, S.G.; Aber, J.D.; Newman, S.D. Nitrogen Saturation in a High Elevation New England Spruce-Fir Stand. For. Ecol. Manag. 1996, 84, 109–121. [Google Scholar] [CrossRef]
- Dirnböck, T.; Grandin, U.; Bernhardt-Römermann, M.; Beudert, B.; Canullo, R.; Forsius, M.; Grabner, M.T.; Holmberg, M.; Kleemola, S.; Lundin, L.; et al. Forest Floor Vegetation Response to Nitrogen Deposition in Europe. Glob. Chang. Biol. 2014, 20, 429–440. [Google Scholar] [CrossRef]
- Gurmesa, G.A.; Lu, X.; Gundersen, P.; Mao, Q.; Zhou, K.; Fang, Y.; Mo, J. High Retention of 15N-Labeled Nitrogen Deposition in a Nitrogen Saturated Old-Growth Tropical Forest. Glob. Chang. Biol. 2016, 22, 3608–3620. [Google Scholar] [CrossRef]
- Walter, C.A.; Raiff, D.T.; Burnham, M.B.; Gilliam, F.S.; Adams, M.B.; Peterjohn, W.T. Nitrogen Fertilization Interacts with Light to Increase Rubus Spp. Cover in a Temperate Forest. Plant Ecol. 2016, 217, 421–430. [Google Scholar] [CrossRef]
- Scheller, R.M.; Mladenoff, D.J. Understory Species Patterns and Diversity in Old-Growth and Managed Northern Hardwood Forests. Ecol. Appl. 2002, 12, 1329–1343. [Google Scholar] [CrossRef]
- Wogan, G.O.U.; Wang, I.J. The Value of Space-for-Time Substitution for Studying Fine-Scale Microevolutionary Processes. Ecography 2018, 41, 1456–1468. [Google Scholar] [CrossRef]
- Banet, A.I.; Trexler, J.C. Space-for-Time Substitution Works in Everglades Ecological Forecasting Models. PLoS ONE 2013, 8, e81025. [Google Scholar] [CrossRef]
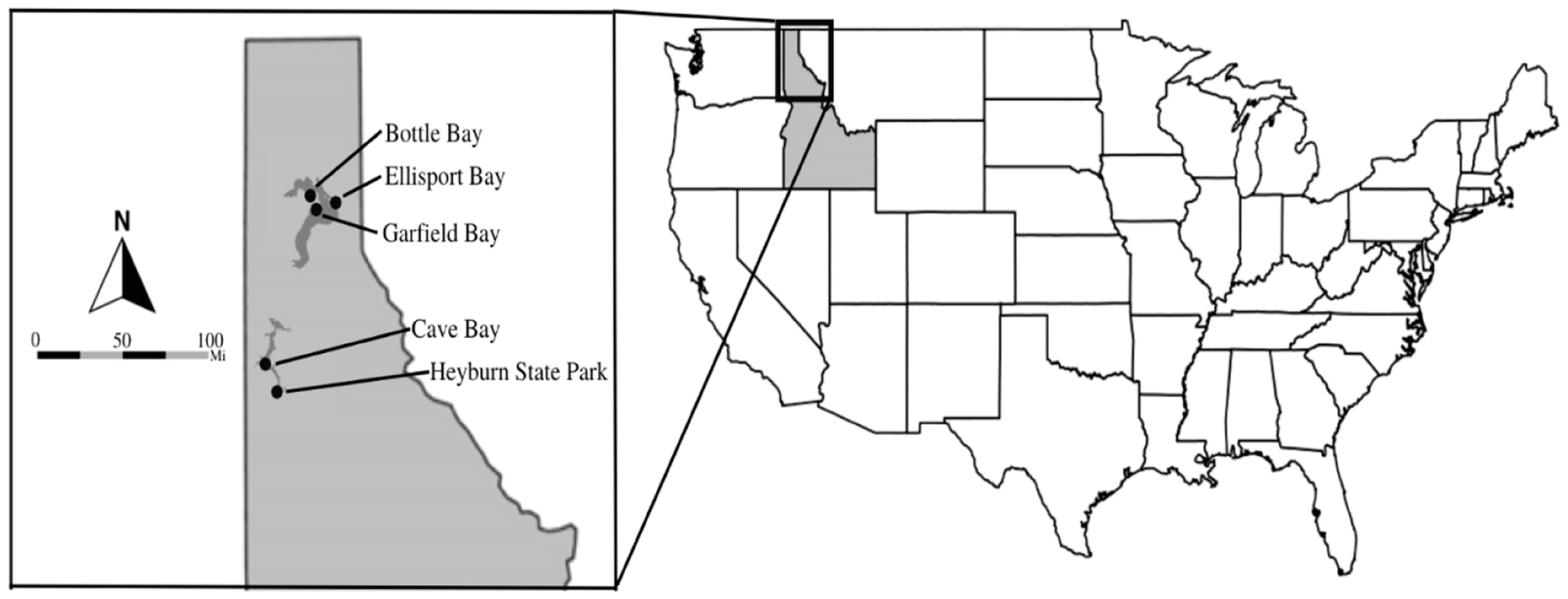



| Reuse Facility | Coordinates | Elevation (m) | Establishment Date | Mean Annual Precipitation 1 (mm) | Mean Annual Temperature 1 (°C) | Average Max Temperature 1 (°C) | Average Min Temperature 1 (°C) |
|---|---|---|---|---|---|---|---|
| Cave Bay | 47.4703° N 116.8803° W | 711 | 2013 | 534.4 | 8.4 | 19.6 | −5.1 |
| Heyburn State Park | 47.3462° N 116.7821° W | 769 | 2010 | 662.6 | 8.2 | 19.4 | −5.2 |
| Ellisport Bay | 48.2159° N 116.2696° W | 659 | 2000 | 633.1 | 7.7 | 18.8 | −5.6 |
| Bottle Bay | 48.2018° N 116.4207° W | 696 | 1989 | 752.4 | 7.4 | 27.4 | −5.7 |
| Garfield Bay | 48.2287° N 116.4384° W | 707 | 1978 | 708.9 | 7.4 | 27.4 | −5.8 |
| Facility | Soil Type | Texture | pH | Bulk Density (g m−3) | Dominant Tree Species 1 | Dominant Understory Vegetation Species 2 |
|---|---|---|---|---|---|---|
| Cave Bay | Lacy | Gravelly loam | 6.9 ± 0.18 | 0.8 ± 0.04 | P. menziesii, P. ponderosa | P. malvaceus, H. discolor, S. albus |
| Heyburn SP | Carlinton | Silt loam | 6.5 ± 0.18 | 0.99 ± 0.05 | P. ponderosa, P. menziesii | P. malvaceus, H. discolor, S. albus |
| Ellisport Bay | Pend Oreille | Silt loam | 6.4 ± 0.13 | 0.85 ± 0.04 | T. plicata, T. heterophylla, A. grandis | P. munitum, C. alpina, B. aquifolium |
| Bottle Bay | Pend Oreille | Silt loam | 6.2 ± 0.15 | 0.68 ± 0.04 | T. plicata, P. menziesii, A. grandis | P. malvaceus, S. albus, H. discolor, B. aquifolium |
| Garfield Bay | Pend Oreille | Silt loam | 6.7 ± 0.17 | 0.71 ± 0.04 | T. plicata, P. menziesii, A. grandis | P. malvaceus, S. albus, H. discolor, B. aquifolium |
| Effect | TPH | QMD (cm) | BA (m2 ha−1) | SDI | Height (m) | Volume (m3 ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| T | 21.01 | <0.01 *** | 8.31 | <0.01 *** | 4.10 | 0.05 ** | 1.32 | 0.26 | 3.51 | <0.01 *** | 5.28 | 0.03 ** |
| F | 32.48 | <0.01 *** | 10.52 | <0.01 *** | 5.17 | <0.01 *** | 14.75 | <0.01 *** | 6.27 | 0.06 * | 2.59 | 0.05 ** |
| TxF | 5.68 | <0.01 *** | 2.04 | 0.11 | 1.71 | 0.17 | 1.15 | 0.35 | 1.93 | 0.12 | 2.26 | 0.08 * |
| Treatment | TPH | QMD | BA | SDI | Height | Volume |
|---|---|---|---|---|---|---|
| Control | 1335 ± 160 a | 26.4 ± 1.7 a | 48.3 ± 3.67 a | 968 ± 68.2 a | 15.3 ± 0.84 a | 368 ± 41.2 a |
| Effluent | 817 ± 160 b | 31.4 ± 1.7 b | 57.1 ± 3.67 a | 1044 ± 68.2 a | 17.1 ± 0.84 a | 488 ± 41.2 b |
| Effect | DIc (cm) | |||||
|---|---|---|---|---|---|---|
| 2014–2018 | 2009–2014 | 2004–2009 | ||||
| F | p | F | p | F | p | |
| T | 53.33 | <0.01 *** | 50.24 | <0.01 *** | 58.94 | <0.01 *** |
| F | 15.41 | <0.01 *** | 33.68 | <0.01 *** | 25.98 | <0.01 *** |
| Sp | 2.05 | 0.03 ** | 2.16 | 0.02 ** | 2.49 | <0.01 *** |
| TxF | 2.88 | 0.02 ** | 9.49 | <0.01 *** | 32.77 | <0.01 *** |
| D0 | 97.55 | <0.01 *** | 84.99 | <0.01 *** | 83.34 | <0.01 *** |
| Effect | DI (cm yr−1) | BAI (m2 ha−1 yr−1) | VI (m3 ha−1 yr−1) | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| T | 9.11 | <0.01 *** | 8.50 | <0.01 *** | 11.06 | <0.01 *** |
| F | 30.23 | <0.01 *** | 7.35 | <0.01 *** | 5.44 | <0.01 *** |
| TxF | 10.48 | <0.01 *** | 0.54 | 0.71 | 0.47 | 0.76 |
| Covariate | 230.23 | <0.01 *** | 1.17 | 0.29 | 4.34 | 0.04 ** |
| Test | Effect | Biomass (g m−2) | Shannon–Wiener Diversity Index (H) | Richness (S) | Evenness (J) | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| ANOVA | T | 5.3 | 0.03 ** | 2.17 | 0.15 | 0.94 | 0.34 | 0.94 | 0.34 |
| F | 20.69 | <0.01 *** | 3.08 | 0.03 ** | 3.80 | <0.01 *** | 1.94 | 0.12 | |
| TxF | 1.67 | 0.17 | 2.64 | 0.05 ** | 0.88 | 0.48 | 2.17 | 0.09 * | |
| ANCOVA | T | 0.01 | 0.91 | 1.84 | 0.18 | 0.95 | 0.34 | 0.80 | 0.38 |
| E | 58.38 | <0.01 *** | 6.88 | <0.01 *** | 12.62 | <0.01 *** | 1.88 | 0.18 | |
| TxE | 0.02 | 0.90 | 0.47 | 0.50 | 0.60 | 0.44 | 0.02 | 0.88 | |
| Test | Effect | Shannon–Wiener Diversity Index (H) | Richness (S) | Evenness (J) | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| ANOVA | T | 1.49 | 0.23 | 0.17 | 0.68 | 3.75 | 0.06 * |
| F | 5.67 | <0.01 *** | 12.32 | <0.01 *** | 2.99 | 0.03 ** | |
| TxF | 1.15 | 0.35 | 0.56 | 0.69 | 0.96 | 0.44 | |
| ANCOVA | T | 1.16 | 0.29 | 0.16 | 0.69 | 3.11 | 0.09 * |
| E | 6.43 | 0.02 ** | 38.94 | <0.01 *** | 0.18 | 0.67 | |
| TxE | 0.10 | 0.75 | 0.05 | 0.83 | 0.06 | 0.81 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, E.; Coleman, M.D. Tree Growth and Vegetation Diversity in Northern Idaho Forest Water Reclamation Facilities. Forests 2023, 14, 266. https://doi.org/10.3390/f14020266
Joshi E, Coleman MD. Tree Growth and Vegetation Diversity in Northern Idaho Forest Water Reclamation Facilities. Forests. 2023; 14(2):266. https://doi.org/10.3390/f14020266
Chicago/Turabian StyleJoshi, Eureka, and Mark D. Coleman. 2023. "Tree Growth and Vegetation Diversity in Northern Idaho Forest Water Reclamation Facilities" Forests 14, no. 2: 266. https://doi.org/10.3390/f14020266
APA StyleJoshi, E., & Coleman, M. D. (2023). Tree Growth and Vegetation Diversity in Northern Idaho Forest Water Reclamation Facilities. Forests, 14(2), 266. https://doi.org/10.3390/f14020266







