Forest Canopy Structures and Bamboo Rhizome Internodes Impact the Appearance Quality of Bamboo Shoots
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Environmental Factors
2.3. Structural Factors of Mother Bamboos
2.4. Anatomical Structure of Bamboo Shoots
2.5. Appearance Qualities and Pigment Contents of Bamboo Shoots
2.6. Taste and Nutritional Quality of Bamboo Shoots
2.7. Statistical Analysis
3. Results
3.1. Canopy Structures, Mother Bamboo Structures, and Understory Environmental Factors
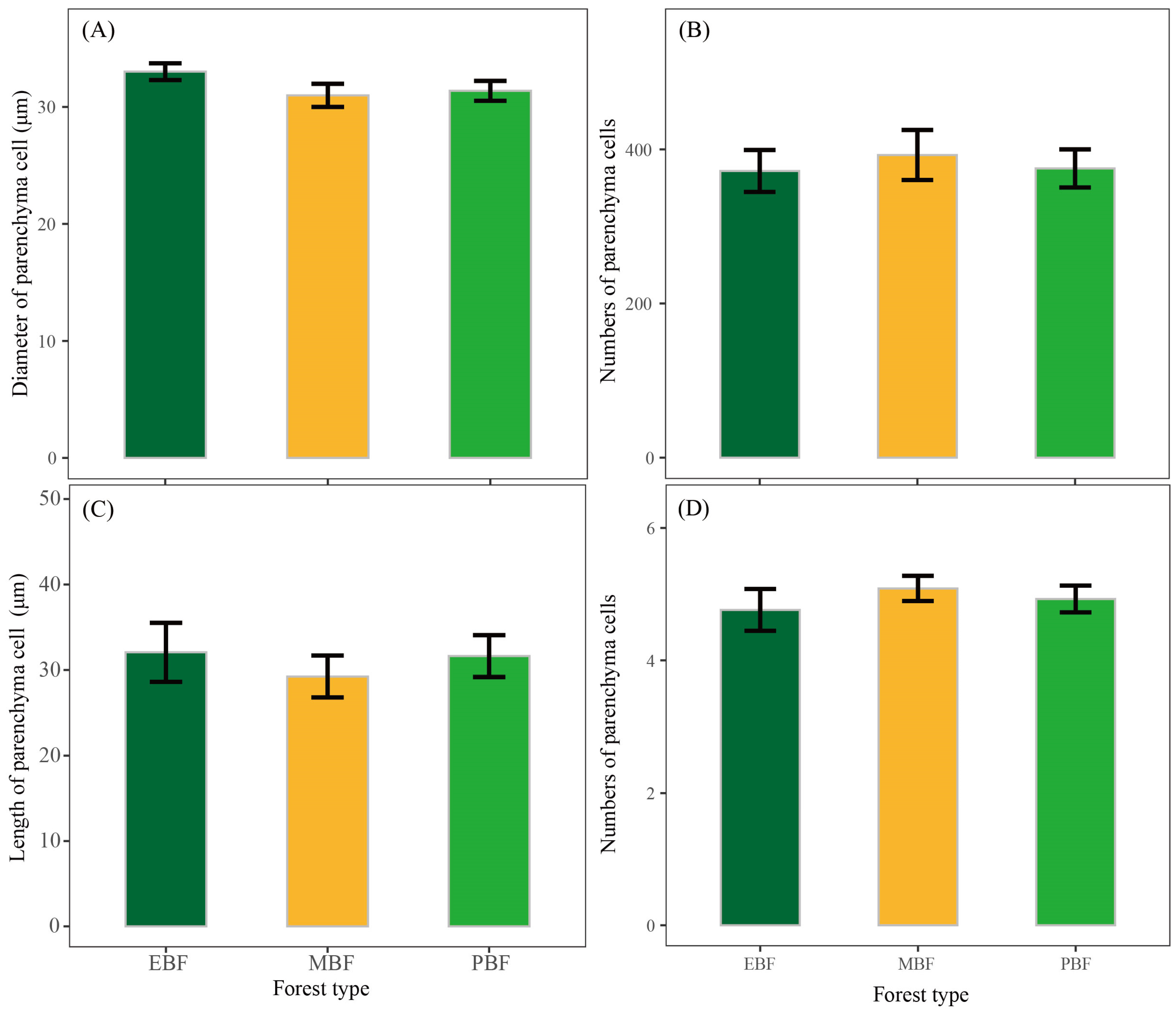
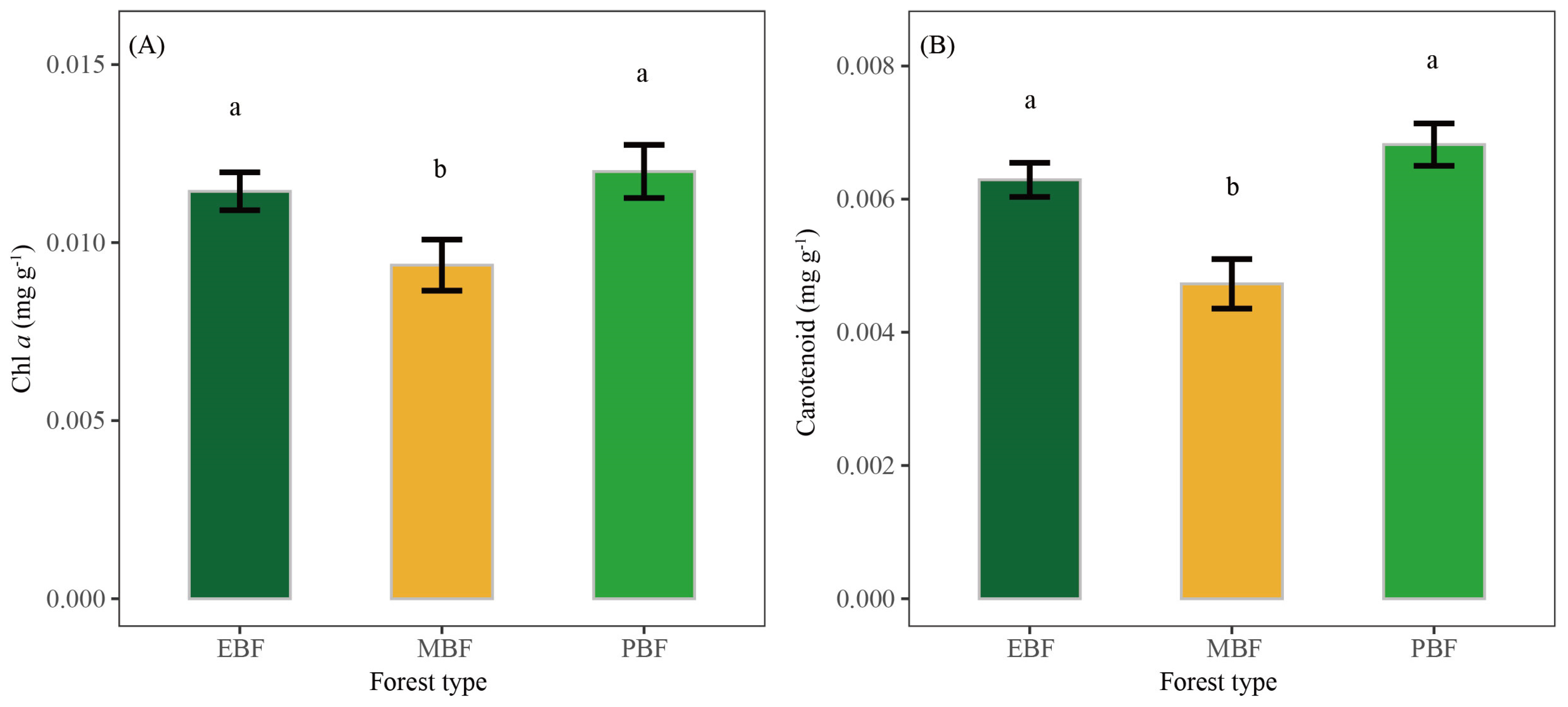
3.2. The Number and Size of Tissue Cells and Pigment Contents of Bamboo Shoots
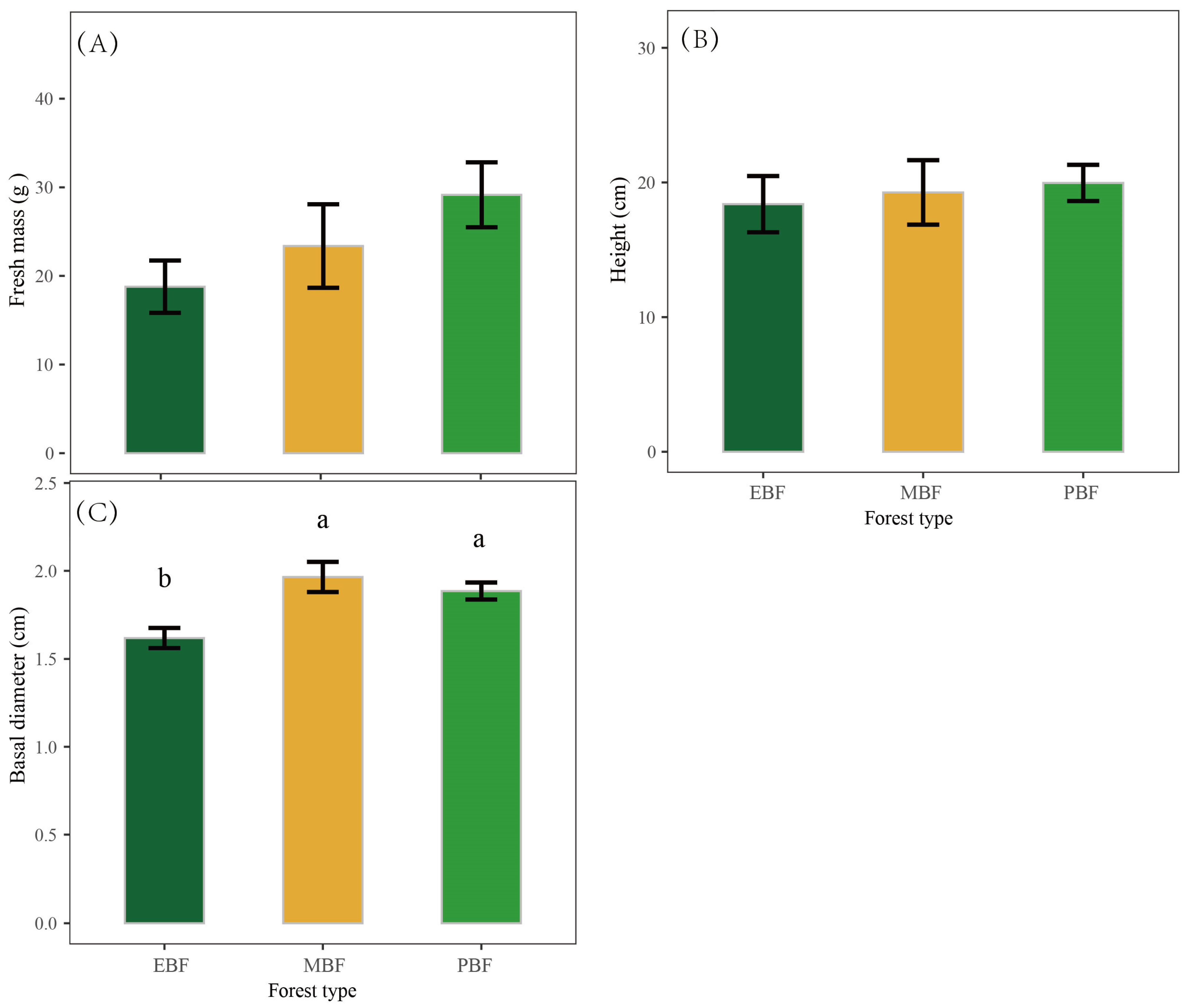
3.3. The Appearance Quality of Bamboo Shoots
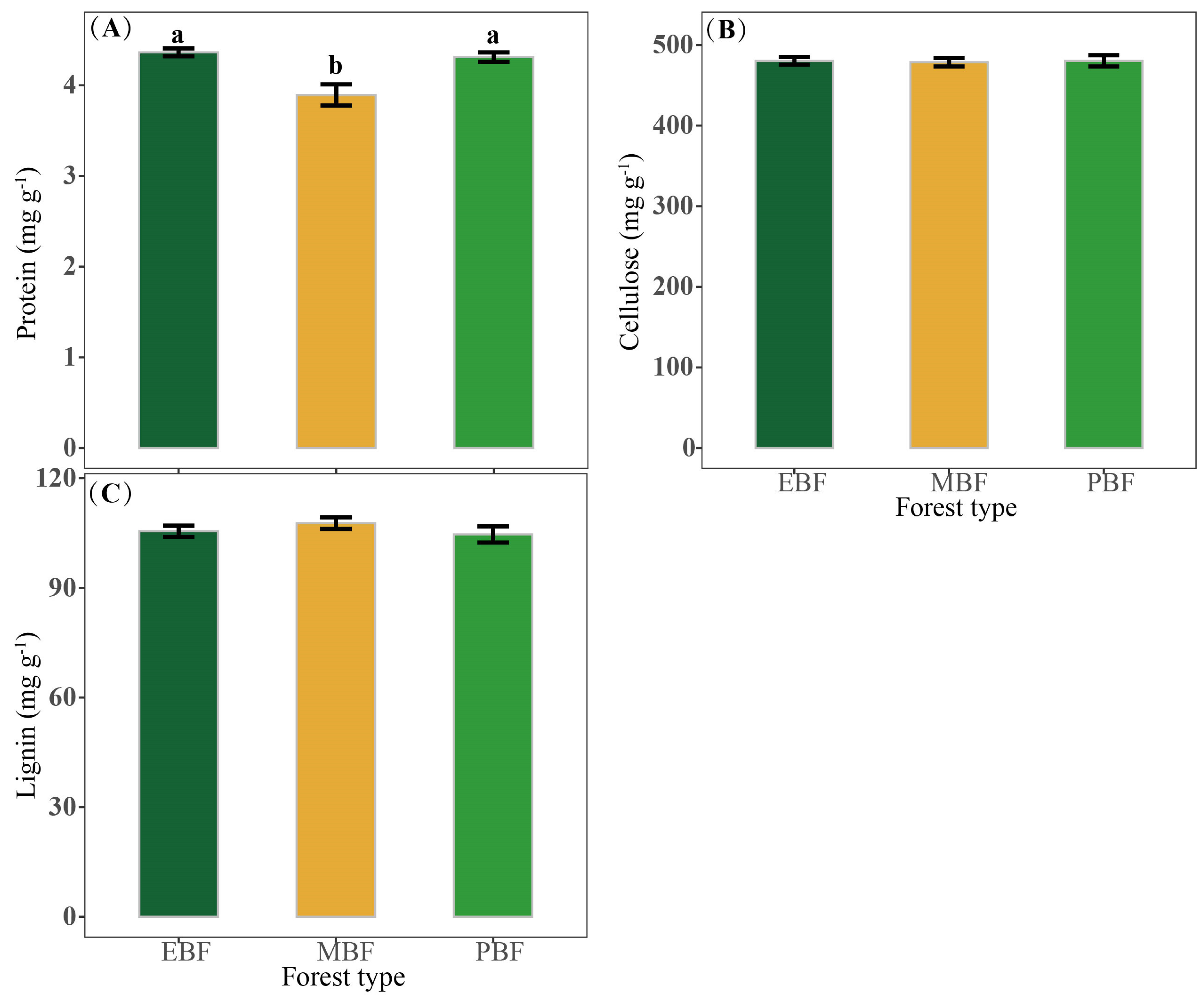
3.4. The Taste and Nutritional Quality of Bamboo Shoots
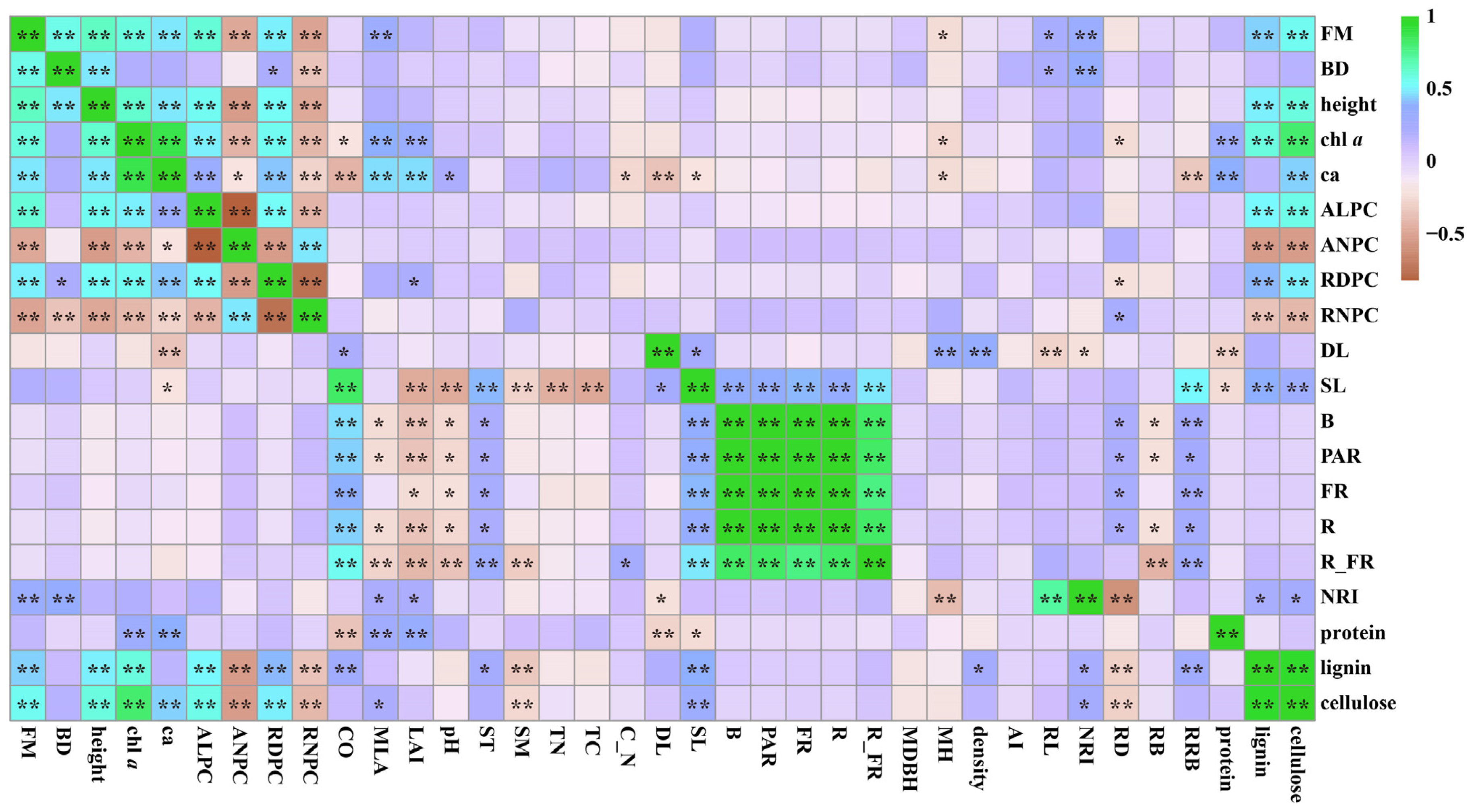
3.5. Correlations between the Appearance Quality of Bamboo Shoots and Influencing Factors
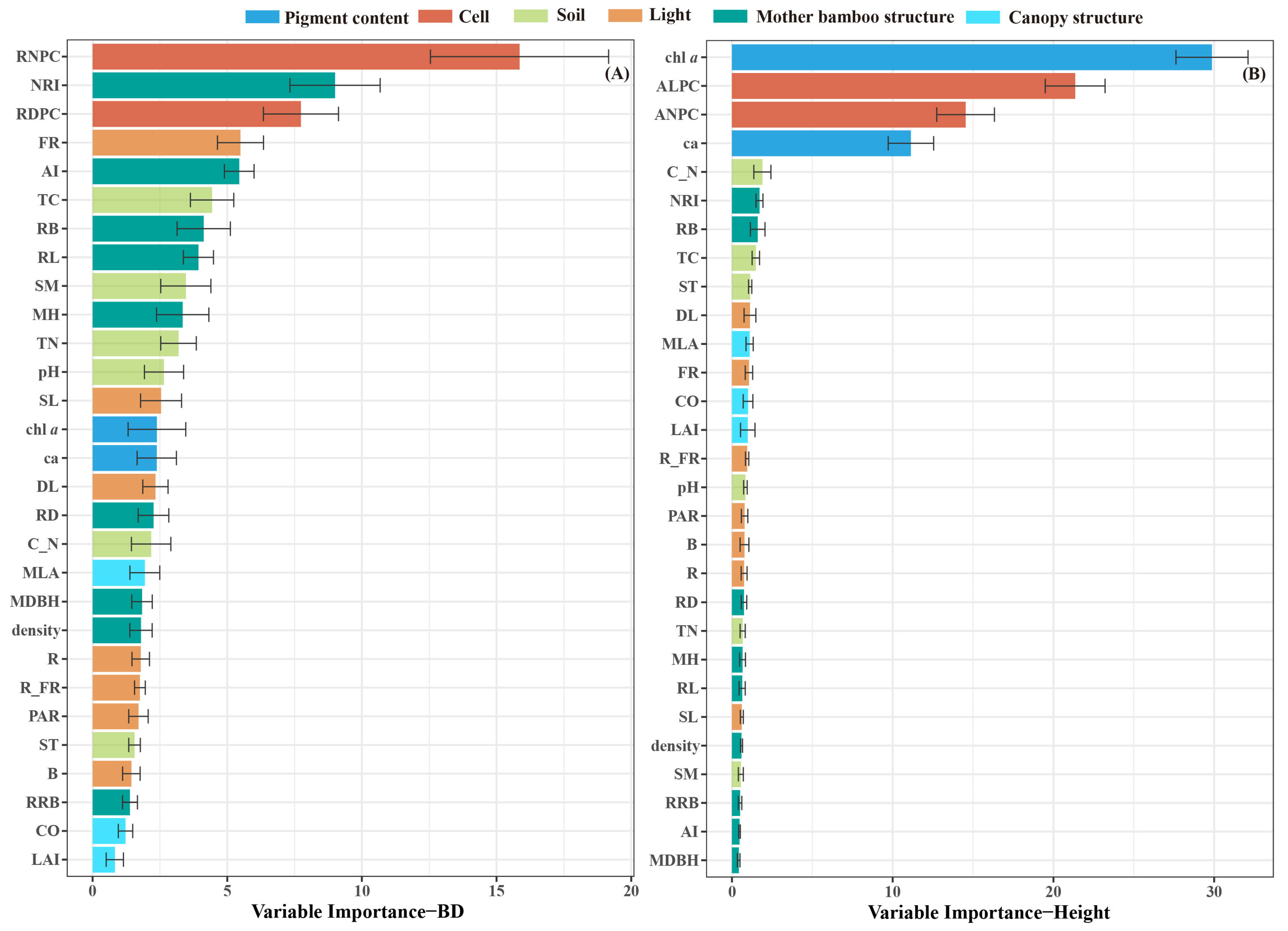
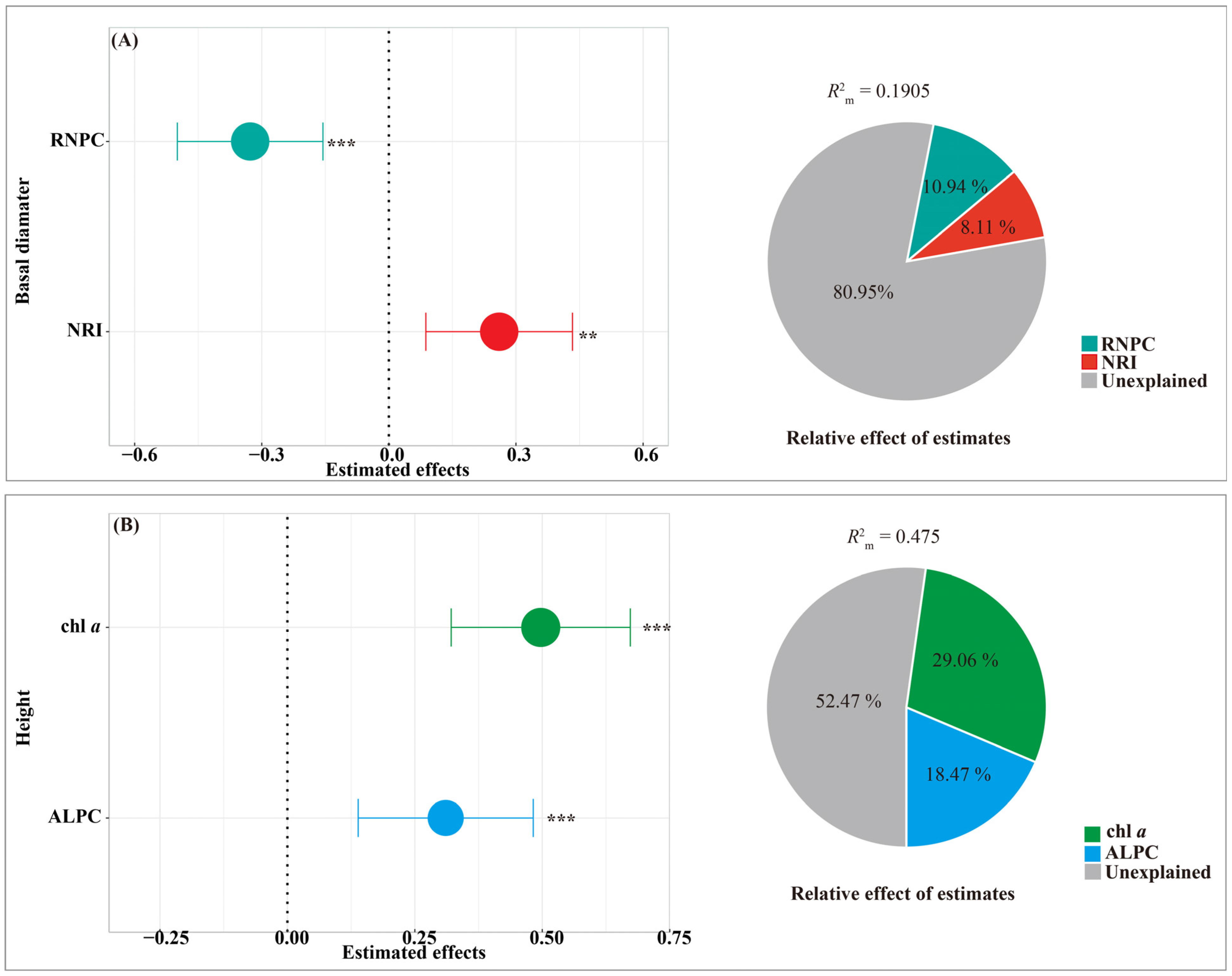
3.6. The Predictors of Bamboo Shoot Morphology
4. Discussion
4.1. The Effects of Removing Competitive Wood on the Quality of Bamboo Shoots
4.2. The Effects of Influence Factors on the Basal Diameter of Bamboo Shoots
4.3. The Effects of Influencing Factors on the Height of Bamboo Shoots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chongtham, N.; Bisht, M.S.; Haorongbam, S. Nutritional Properties of Bamboo Shoots: Potential and Prospects for Utilization as a Health Food. Compr. Rev. Food Sci. Food Saf. 2011, 10, 153–168. [Google Scholar] [CrossRef]
- Guo, Z.W.; Jiang, Z.B.; Chen, S.L.; Xu, B.; Ye, S.Y.; Li, M.L. Comparative Study on Quality and Palatability of Rhizome Shoot of Phyllostachys prominens and Phyllostachys edulis. For. Res. 2015, 28, 447–450. [Google Scholar] [CrossRef]
- Yang, L.T.; Xie, Y.Y.; Yu, W.X.; Chen, S.L.; Guo, Z.W.; Xu, S.; Gu, R. Effects of Long—Term Mulching on Appearance, Nutrition and Taste of Bamboo Shoots of Phyllostachys violascens. J. Bamboo Res. 2021, 40, 7–12. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, H.; Li, P.; Chu, C.; Li, J.; Wang, C. Physiological Mechanism of Internode Bending Growth After the Excision of Shoot Sheath in. Front. Plant Sci. 2020, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; He, C.; Zhang, J.; Duan, A.; Zeng, Y. Temporal and Spatial Profiling of Internode Elongation-Associated Protein Expression in Rapidly Growing Culms of Bamboo. J. Proteome Res. 2012, 11, 2492–2507. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid growth of Moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Tao, G.Y.; Ramakrishnan, M.; Vinod, K.K.; Yrjala, K.; Satheesh, V.; Cho, J.; Fu, Y.; Zhou, M. Multi-omics analysis of cellular pathways involved in different rapid growth stages of moso bamboo. Tree Physiol. 2020, 40, 1487–1508. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.P.S.; Randhawa, G.S.; Dhugga, K.S. Plant Cell Wall Matrix Polysaccharide Biosynthesis. Mol. Plant 2009, 2, 840–850. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, D.; Ye, F.; He, Y.; Hu, Z.; Zhao, G. A systematic review on the composition, storage, processing of bamboo shoots: Focusing the nutritional and functional benefits. J. Funct. Foods 2020, 71, 104015. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Guo, Z.; He, Y.; Yang, L.; Dong, Y.; Xie, Y.; Zhang, J. Effect of Sheath Blade Removal on Phyllostachys violascens Shoot Quality. Agriculture 2022, 12, 1396. [Google Scholar] [CrossRef]
- Xu, X.; Xie, W.; Xiang, C.; You, Q.; Tian, X. Predicting the dietary fiber content of fresh-cut bamboo shoots using a visible and near-infrared hyperspectral technique. J. Food Meas. Charact. 2023, 17, 3218–3227. [Google Scholar] [CrossRef]
- Liu, G.; Shi, P.; Xu, Q.; Dong, X.; Wang, F.; Wang, G.G.; Hui, C. Does the size-density relationship developed for bamboo species conform to the self-thinning rule? Forest Ecol. Manag. 2016, 361, 339–345. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, J.; Sun, J.; Li, Q.; Lin, X.; Song, X. Unequal nitrogen translocation pattern caused by clonal integration between connected ramets ensures necessary nitrogen supply for young Moso bamboo growth. Environ. Exp. Bot. 2022, 200, 104900. [Google Scholar] [CrossRef]
- Duchoslavová, J.; Jansa, J. The direction of carbon and nitrogen fluxes between ramets in Agrostis stolonifera changes during ontogeny under simulated competition for light. J. Exp. Bot. 2018, 69, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, Q.; Lei, Z.; Zhang, J.; Song, X.; Song, X. Ecological stoichiometry of nitrogen and phosphorus in Moso bamboo (Phyllostachys edulis) during the explosive growth period of new emergent shoots. J. Plant Res. 2019, 132, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, Y.; Xiao, L.; Gao, X.; Shi, Y.; Zhou, Y.; Du, H.; Zhou, G. Rhizome extension characteristics, structure and carbon storage relationships with culms in a 10-year moso bamboo reforestation period. Forest Ecol. Manag. 2021, 498, 119556. [Google Scholar] [CrossRef]
- Tachiki, Y.; Makita, A.; Suyama, Y.; Satake, A. A spatially explicit model for flowering time in bamboos: Long rhizomes drive the evolution of delayed flowering. J. Ecol. 2015, 103, 585–593. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Z.; Ma, Y.; Bai, Q.; Li, X.; Cao, Z.; Wu, Z.; Gao, J. The association of hormone signalling genes, transcription and changes in shoot anatomy during moso bamboo growth. Plant Biotechnol. J. 2018, 16, 72–85. [Google Scholar] [CrossRef]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez-Juez, E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2022, 233, 2000–2016. [Google Scholar] [CrossRef]
- Hendron, R.; Kelly, S. Subdivision of Light Signaling Networks Contributes to Partitioning of C4 Photosynthesis. Plant Physiol. 2020, 182, 1297–1309. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, J.; Wu, J.; Classen, A.T.; Li, Y.; Lou, Y.; Zhang, W.; Jing, X.; Shao, Y.; Fu, S. Bamboo forest management leads to a shift in the soil energy channel. Geoderma 2019, 353, 201–203. [Google Scholar] [CrossRef]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.S.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Neinavaz, E.; Darvishzadeh, R.; Skidmore, A.K.; Groen, T.A. Measuring the response of canopy emissivity spectra to leaf area index variation using thermal hyperspectral data. Int. J. Appl. Earth 2016, 53, 40–47. [Google Scholar] [CrossRef]
- Cogliati, S.; Celesti, M.; Cesana, I.; Miglietta, F.; Genesio, L.; Julitta, T.; Schuettemeyer, D.; Drusch, M.; Rascher, U.; Jurado, P.; et al. A Spectral Fitting Algorithm to Retrieve the Fluorescence Spectrum from Canopy Radiance. Remote Sens. 2019, 11, 1840. [Google Scholar] [CrossRef]
- Mieke, D.W.; Galvao, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, H.; Chen, Y.; Xu, Y.; Shan, F.; Li, H.; Yan, C.; Ma, C. Red light regulates metabolic pathways of soybean hypocotyl elongation and thickening. Environ. Exp. Bot. 2022, 199, 104890. [Google Scholar] [CrossRef]
- Chen, Z.; Li, M.; Liu, S.; Chen, X.; Zhang, W.; Zhu, Q.; Kohnen, M.V.; Wang, Q. The Function and Photoregulatory Mechanisms of Cryptochromes from Moso Bamboo (Phyllostachys edulis). Front. Plant Sci. 2022, 13, 866057. [Google Scholar] [CrossRef]
- Mockler, T.; Yang, H.; Yu, X.; Parikh, D.; Cheng, Y.; Dolan, S.; Lin, C. Regulation of Photoperiodic Flowering by Arabidopsis Photoreceptors. Proc. Natl. Acad. Sci. USA 2003, 100, 2140–2145. [Google Scholar] [CrossRef]
- Parks, B.M. The Red Side of Photomorphogenesis. Plant Physiol. 2003, 133, 1437–1444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.; Lin, C. Mechanisms of Cryptochrome-Mediated Photoresponses in Plants. Annu. Rev. Plant Biol. 2020, 71, 103–129. [Google Scholar] [CrossRef]
- Qian, Z.; Zhuang, S.; Gui, R.; Tang, L. Effect of soil aeration treatment on the physiological and biochemical characteristics of Phyllostachys praecox under the organic material mulching. Plant Soil. 2021, 459, 357–369. [Google Scholar] [CrossRef]
- Chen, G.; Fang, C.; Ran, C.; Tan, Y.; Yu, Q.; Kan, J. Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2019, 130, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, M. Relationship between site and structure and its influence on biomass in Phyllostachys edulis forest. J. Zhejiang A F Univ. 2020, 37, 1–10. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, H.; Dong, X. Experimental Guidance of Plant Physiology; China Agricuturaral Science and Technology Press: Beijing, China, 1998; Volume 10, pp. 68–72. [Google Scholar]
- Lott, J.A.; Stephan, V.A.; Pritchard, K.J. Evaluation of the Coomassie Brilliant Blue G-250 method for urinary protein. Clin. Chem. 1983, 29, 1946–1950. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, P. Determination of Crude Polysaccharides in Pine Pollen Anthrone-Sulphuric Acid Colorimetry. J. Yunnan Norm. Univ. 2017, 37, 58–63. [Google Scholar] [CrossRef]
- Yang, K.; Li, L.; Lou, Y.; Zhu, C.; Li, X.; Gao, Z. A regulatory network driving shoot lignification in rapidly growing bamboo. Plant Physiol. 2021, 187, 900–916. [Google Scholar] [CrossRef] [PubMed]
- Wiener, A.L.A.M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. glmm.hp: An R package for computing individual effect of predictors in generalized linear mixed models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Luan, J.; Li, S.; Wang, Y.; Ding, L.; Cai, C.; Liu, S. Decomposition of diverse litter mixtures affected by drought depends on nitrogen and soil fauna in a bamboo forest. Soil Biol. Biochem. 2022, 173, 108783. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Z.; Bian, F.; Yang, C. Effects of composted bamboo residue amendments on soil microbial communities in an intensively managed bamboo (Phyllostachys praecox) plantation. Appl. Soil Ecol. 2019, 136, 178–183. [Google Scholar] [CrossRef]
- Song, X.; Li, Q.; Gu, H. Effect of nitrogen deposition and management practices on fine root decomposition in Moso bamboo plantations. Plant Soil. 2017, 410, 207–215. [Google Scholar] [CrossRef]
- Yuan, N.; Wang, E.; Lv, S.; Tang, X.; Wang, T.; Wang, G.; Zhou, Y.; Zhou, G.; Shi, Y.; Xu, L. Degradation reduces greenhouse gas emissions while weakening ecosystem carbon sequestration of Moso bamboo forests. Sci. Total Environ. 2023, 877, 162915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, S.; Binkley, D.; Zhou, G.; Fang, F. The independence of clonal shoot’s growth from light availability supports moso bamboo invasion of closed-canopy forest. Forest Ecol. Manag. 2016, 368, 105–110. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Xue, G.; Peng, Y.; Song, H. Effect of clonal integration on nitrogen cycling in rhizosphere of rhizomatous clonal plant, Phyllostachys bissetii, under heterogeneous light. Sci. Total Environ. 2018, 628–629, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Wang, Y.; Luan, J.; Liu, S. Effects of nitrogen addition on clonal integration between mother and daughter ramets of Moso bamboo: A 13C-CO2 pulse labeling study. J. Plant Ecol. 2022, 15, 756–770. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Perez-Llorca, M.; Munné-Bosch, S.; Gibin, M.S.; Sato, F.; Pelozo, A.; Pattaro, M.C.; Giacomelli, M.E.; Rüggeberg, M.; et al. Cell wall structure and composition is affected by light quality in tomato seedlings. J. Photoch. Photobio. B. 2020, 203, 111745. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- He, D.A.; Peng, D.; Yang, H.; Zhang, X. The response of seedlings and saplings to canopy structure and light in different gaps in a spruce-fir mixed stand in Changbai Mountains, China. Forest Ecol. Manag. 2023, 546, 121365. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lin, M.Y.; Mitchell, C.A. Light spectral and thermal properties govern biomass allocation in tomato through morphological and physiological changes. Environ. Exp. Bot. 2019, 157, 228–240. [Google Scholar] [CrossRef]
- Liu, L.; Shao, Z.; Zhang, M.; Wang, Q. Regulation of Carotenoid Metabolism in Tomato. Mol. Plant. 2015, 8, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Sng, B.J.R.; Singh, G.P.; Vu, V.K.; Chua, N.H.; Ram, R.J.; Jang, I.C. Rapid metabolite response in leaf blade and petiole as a marker for shade avoidance syndrome. Plant Methods 2020, 16, 144. [Google Scholar] [CrossRef]
- Parhizkar, P.; Sagheb-Talebi, K.; Mataji, A.; Nyland, R.; Namiranian, M. Silvicultural characteristics of Oriental beech (Fagus orientalis Lipsky) regeneration under different RLI and positions within gaps. Forestry 2011, 84, 177–185. [Google Scholar] [CrossRef]
- Prévost, M.; Raymond, P. Effect of gap size, aspect and slope on available light and soil temperature after patch-selection cutting in yellow birch–conifer stands, Quebec, Canada. Forest Ecol. Manag. 2012, 274, 210–221. [Google Scholar] [CrossRef]
- Pisek, J.; Diaz-Pines, E.; Matteucci, G.; Noe, S.; Rebmann, C. On the leaf inclination angle distribution as a plant trait for the most abundant broadleaf tree species in Europe. Agric. For. Meteorol. 2022, 323, 15. [Google Scholar] [CrossRef]
- Niinemets, U. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Zhao, W.; Ren, T.; Huang, X.; Xu, Z.; Zhou, Y.; Yin, C.; Zhao, R.; Liu, S.; Ning, T.; Li, G. Leaf shape, planting density, and nitrogen application affect soybean yield by changing direct and diffuse light distribution in the canopy. Plant Physiol. Bioch. 2023, 204, 108071. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? New Phytol. 2010, 158, 509–525. [Google Scholar] [CrossRef]

| Impact Factors | Forest Types | |||
|---|---|---|---|---|
| EBF | MBF | PBF | ||
| Canopy structures | CO (%) | 10.67 ± 0.28 b | 13.08 ± 0.35 a | 10.58 ±0.17 b |
| MLA (°) | 40.53± 2.64 b | 34.39 ± 1.98 c | 55.98 ± 1.64 a | |
| LAI | 2.34 ± 0.07 a | 1.98 ± 0.05 b | 2.47 ± 0.03 a | |
| Mother bamboo structures | MDBH (cm) | 1.59 ± 0.03 c | 1.82 ± 0.03 b | 2.01 ± 0.06 a |
| MH (m) | 3.88 ± 0.07 a | 3.88 ± 0.06 a | 3.52 ± 0.13 b | |
| Density (culms hm−2) | 42,640.00 ± 2592.98 a | 43,760.00 ± 1773.74 a | 26,066.67 ± 1978.33 b | |
| AI | 0.84 ± 0.02 b | 0.97 ± 0.01 a | 0.99 ± 0.02 a | |
| NRI (internode m−2) | 55.33 ± 1.84 | 58.02 ± 1.54 | 57.13 ± 0.78 | |
| RB (g m−2) | 430.96 ± 23.96 c | 643.08 ± 45.52 b | 924.32 ± 49.72 a | |
| RL (cm) | 38.18 ± 0.70 a | 38.16 ± 0.33 a | 36.33 ± 0.47 b | |
| RD (cm) | 0.97 ± 0.05 b | 1.11 ± 0.08 a | 1.07 ± 0.06 a | |
| RRB (g m−2) | 109.8 ± 5.92 b | 132.56 ± 8.44 a | 117.36 ± 3.36 ab | |
| Light parameters | B (μmol m−2 s−1) | 5.50 ± 1.24 b | 19.51 ± 4.76 a | 5.31 ± 0.63 b |
| R (μmol m−2 s−1) | 8.97 ± 2.18 b | 31.88 ± 8.04 a | 8.14 ± 0.99 b | |
| FR (μmol m−2 s−1) | 18.78 ± 1.70 b | 41.18 ± 6.39 a | 28.40 ± 1.28 b | |
| R/FR | 0.38 ± 0.04 b | 0.54 ± 0.06 a | 0.26 ± 0.02 b | |
| PAR (μmol m−2 s−1) | 22.54 ± 5.20 b | 79.31 ± 19.57 a | 21.21 ± 2.47 b | |
| DL (mol m−2 yr−1) | 1491.97 ± 53.05 ab | 1658.89 ± 70.21 a | 1420.82 ± 55.51 b | |
| SL (mol m−2 yr−1) | 1416.37 ± 26.57 c | 1671.50 ± 23.37 a | 1651.68 ± 29.69 b | |
| Soil factors | pH | 4.89 ± 0.09 | 4.74 ± 0.08 | 4.90 ± 0.05 |
| ST (°C) | 17.68 ± 0.15 b | 18.08 ± 0.06 a | 17.71 ± 0.11 b | |
| SM (%) | 15.92 ± 0.97 b | 15.97 ± 0.64 b | 25.31 ± 1.49 a | |
| TN (g kg−1) | 17.01 ± 1.00 a | 12.27 ± 0.85 b | 10.99 ± 0.62 b | |
| TC (g kg−1) | 200.91 ± 6.70 a | 150.95 ± 7.42 b | 134.68 ± 7.47 b | |
| C/N | 12.50 ± 0.43 | 12.80 ± 0.32 | 12.34 ± 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
She, C.; Yu, Y.; Wan, M.; Abubakar, A.A.; Luo, W.; Liu, J.; Tao, J. Forest Canopy Structures and Bamboo Rhizome Internodes Impact the Appearance Quality of Bamboo Shoots. Forests 2023, 14, 2435. https://doi.org/10.3390/f14122435
She C, Yu Y, Wan M, Abubakar AA, Luo W, Liu J, Tao J. Forest Canopy Structures and Bamboo Rhizome Internodes Impact the Appearance Quality of Bamboo Shoots. Forests. 2023; 14(12):2435. https://doi.org/10.3390/f14122435
Chicago/Turabian StyleShe, Chunyan, Yulin Yu, Maji Wan, Adamu Abdullahi Abubakar, Weixue Luo, Jinchun Liu, and Jianping Tao. 2023. "Forest Canopy Structures and Bamboo Rhizome Internodes Impact the Appearance Quality of Bamboo Shoots" Forests 14, no. 12: 2435. https://doi.org/10.3390/f14122435
APA StyleShe, C., Yu, Y., Wan, M., Abubakar, A. A., Luo, W., Liu, J., & Tao, J. (2023). Forest Canopy Structures and Bamboo Rhizome Internodes Impact the Appearance Quality of Bamboo Shoots. Forests, 14(12), 2435. https://doi.org/10.3390/f14122435






