Impact of Temperature and Moisture on the Decomposition of Peat-Forming Plants: Results of a Two-Year Incubation Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region and Characteristics of Plant Samples
2.2. Experimental Design and Measurement of the Peat-Forming Plant Decay Rates
2.3. Thermal Analyses of Plant Samples
2.4. Data Processing and Statistical Analyses
3. Results and Discussion
3.1. Changes in Chemical Properties and Biochemical Composition of Peat-Forming Plants after the Experiment
3.2. Changes in Thermal Characteristics of Peat-Forming Plants after the Experiment
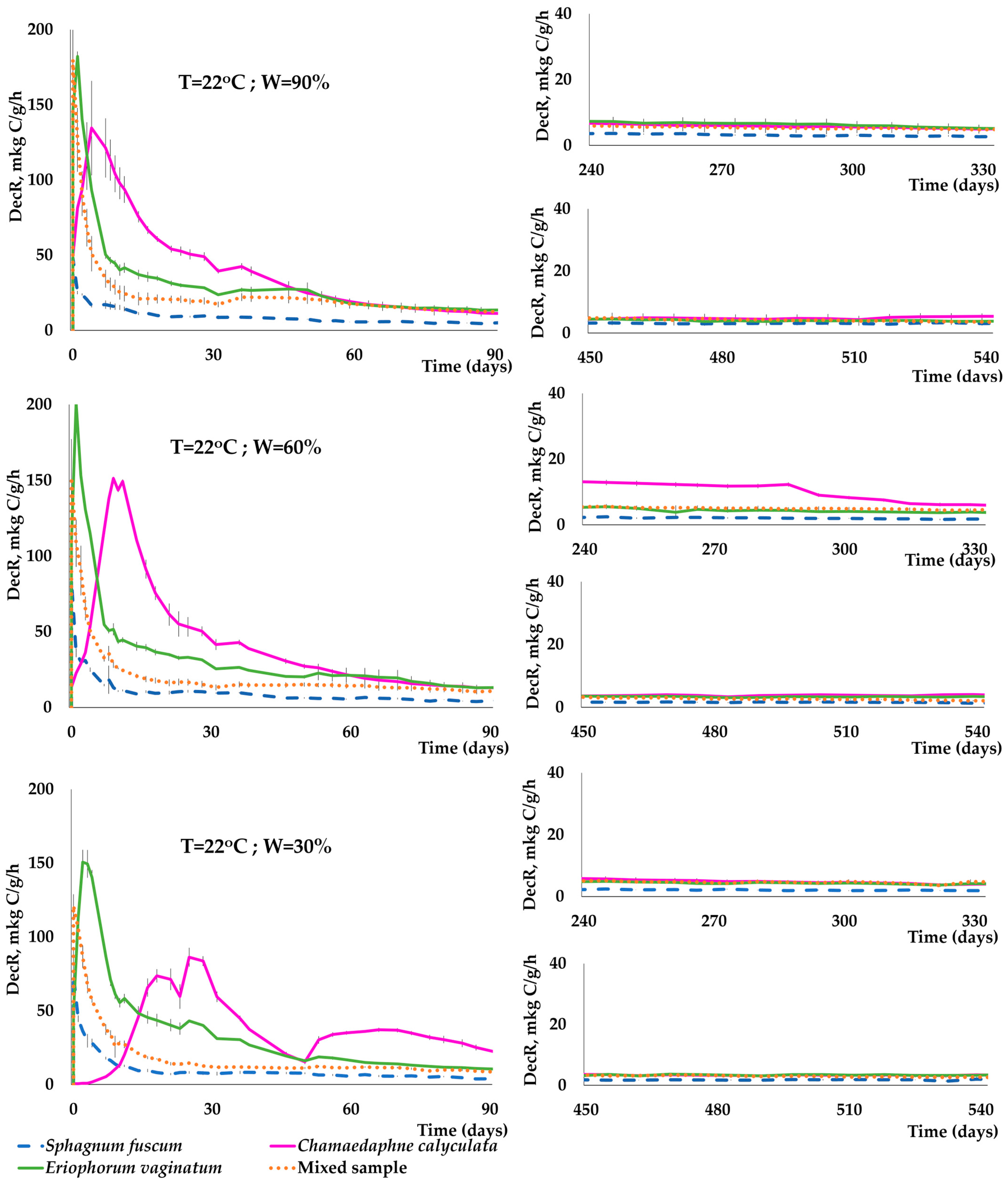
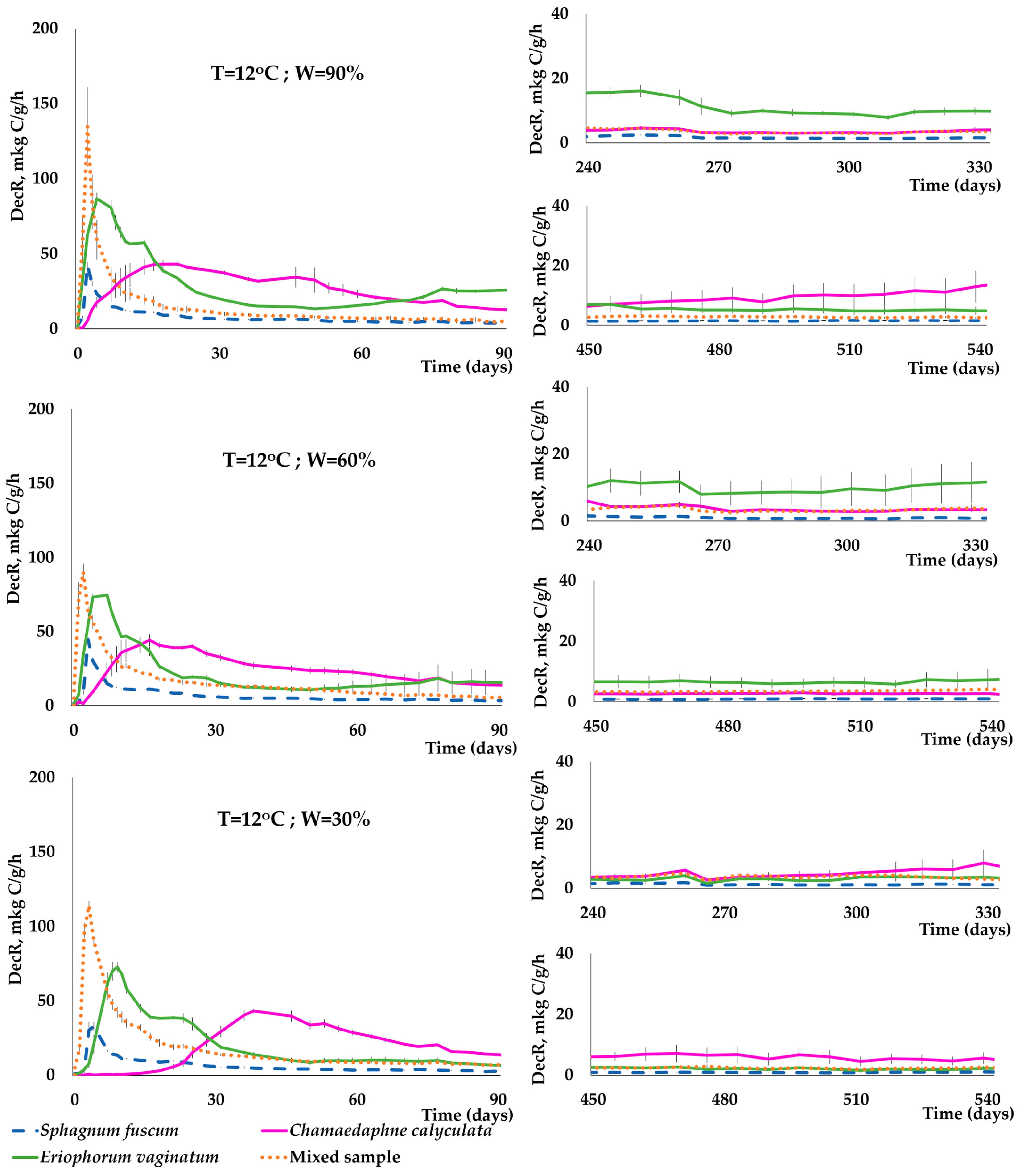
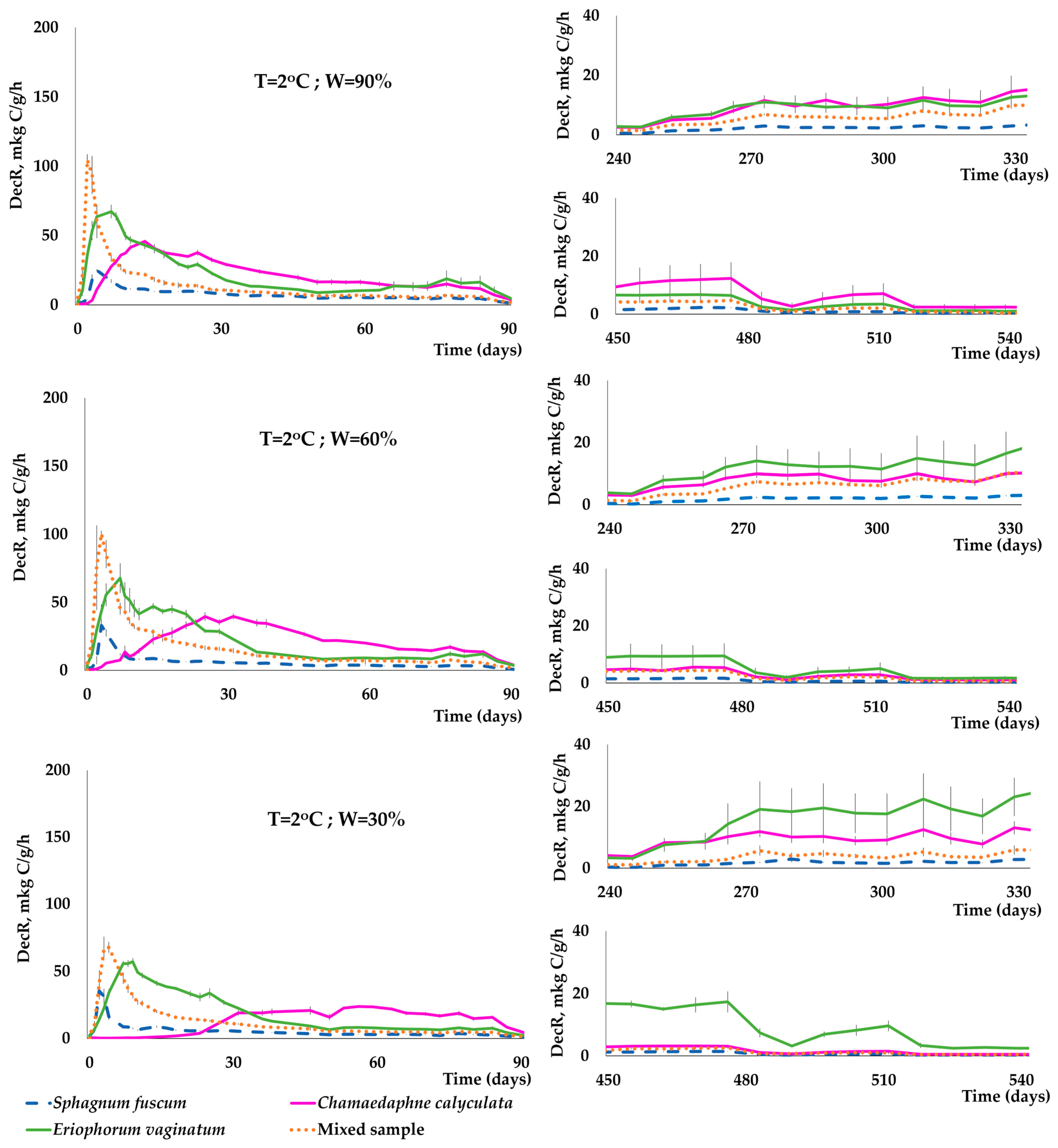
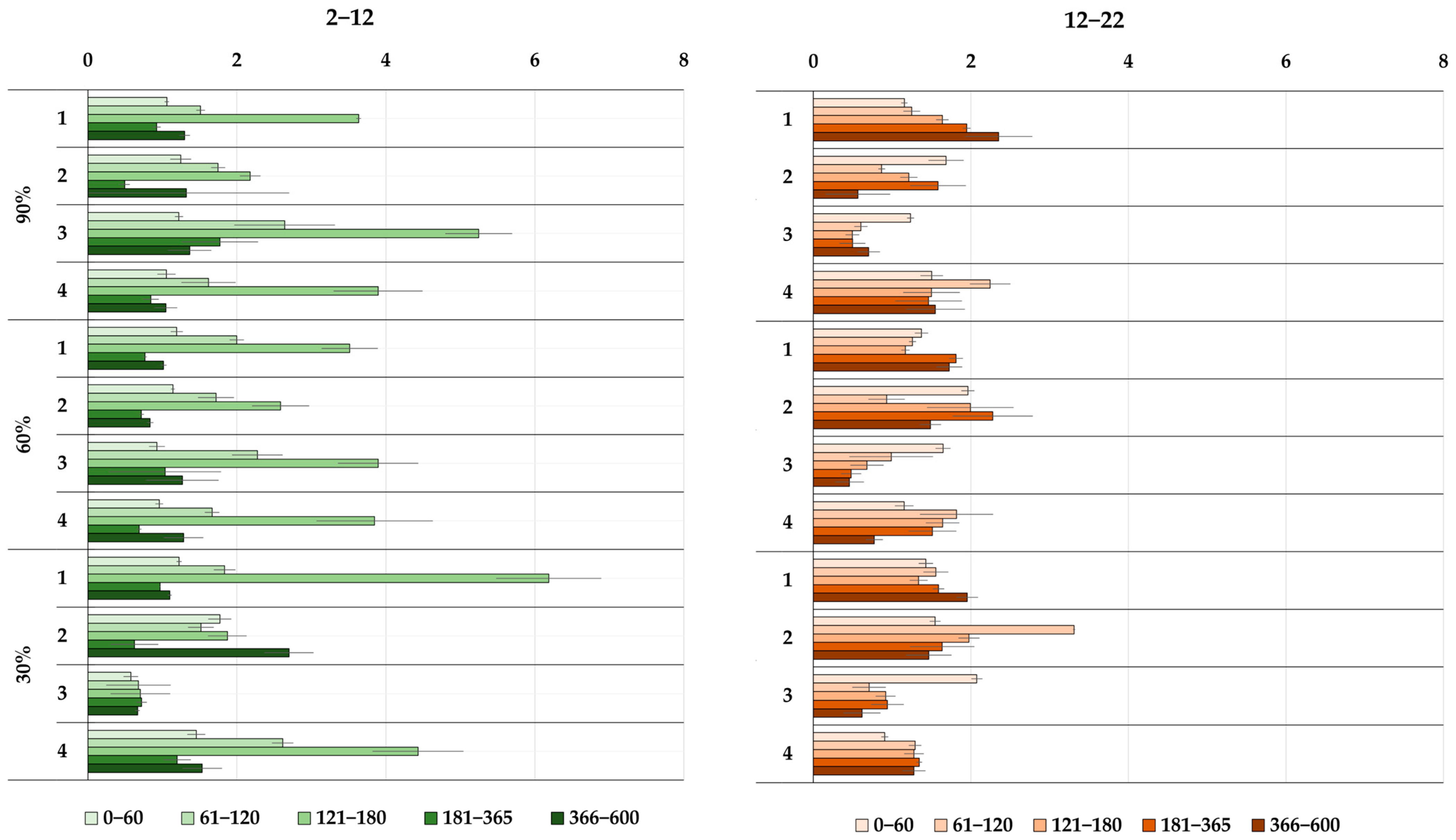
3.3. Dynamics of C(CO2) Release during the Decomposition of Peat-Forming Plants
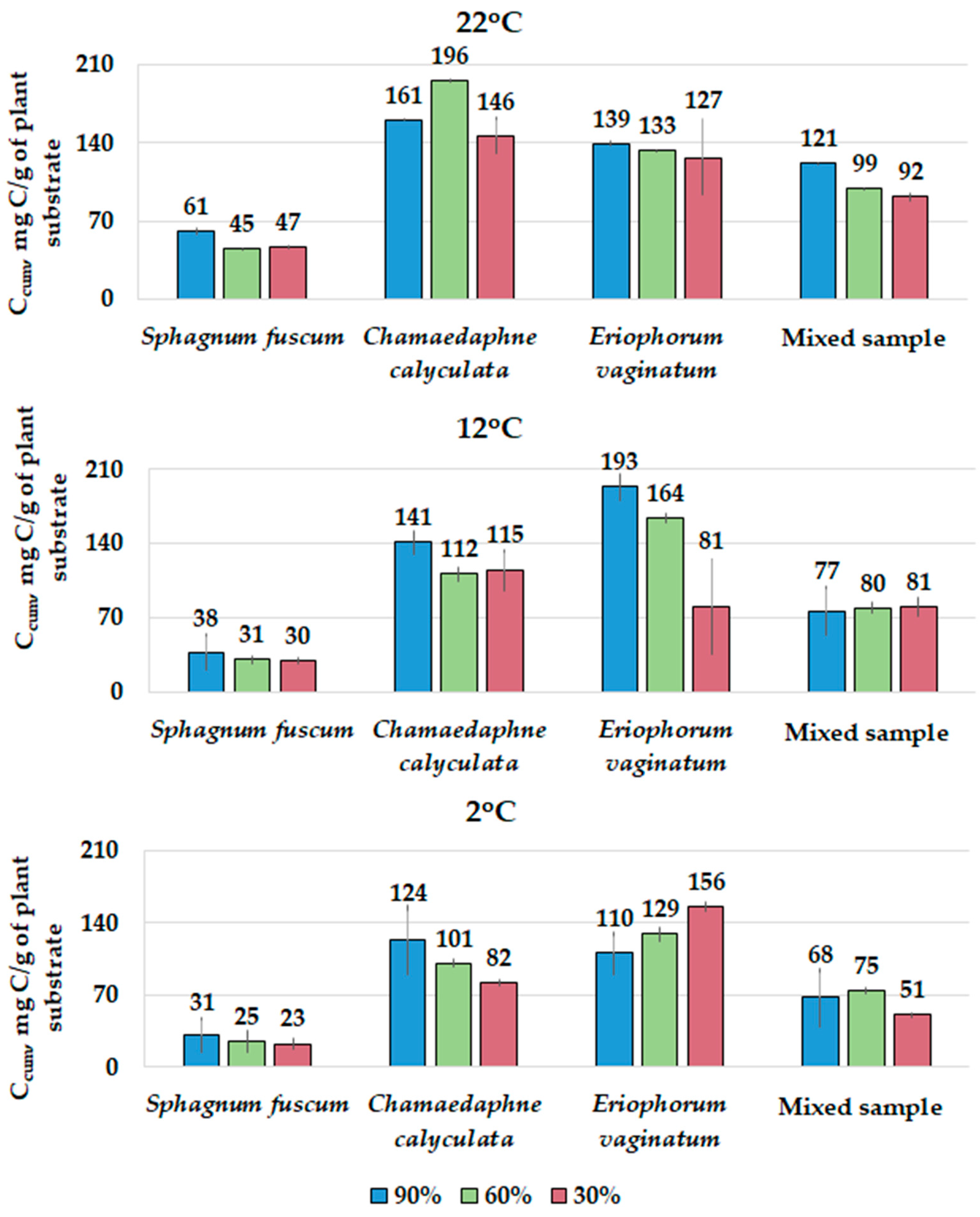
3.4. Cumulative Losses of C(CO2) during the Experiment
3.5. Decay Constants of the Main Peat-Forming Plants under Different Combinations of Abiotic Factors
3.6. Temperature Sensitivity of DecR of Peat-Forming Plants
3.7. Evaluating the Impact of Abiotic Factors on Peat-Forming Plant Decomposition in a Model Experiment
4. Conclusions
- Temperature, moisture, and the species of plant residues had a significant impact on the dynamics and intensity of C(CO2) release during a two-year incubation experiment. In the early stages of plant sample decomposition, there was a significant increase in C(CO2) release. This increase was rapid at 22 °C, occurring within the first few days of the experiment. At 2 °C, this increase took longer to become evident, typically appearing after 1–2 weeks. Conversely, the decrease in the rate of decomposition of plant residues occurred much faster at 22 °C than at 2 °C.
- Sphagnum fuscum was characterized by the lowest decay rate constant. The decay rate constant increased with temperature for all species of plant substrate, except for Eriophorum vaginatum. Furthermore, the effect of moisture on the decay rate constant of the studied peat-forming plants varied, and depending on temperature and the plant substrate species, it could have different directions. Overall, during long-term decomposition, the decay rate constant diminished.
- The primary factors affecting the decomposition rate of peat-forming plants were the species of plant substrate (accounting for 76% of the total variance) and temperature (contributing 12% of the total variance). As time progressed, the influence of temperature diminished while the impact of the plant sample type increased. The moisture content of the substrate also had an effect but was not a decisive factor in bog conditions, exerting a comparatively lower impact (5%). In the case of mixed plant sample decomposition, the decay rate increased due to the non-additive effect.
- The highest temperature sensitivity of DecR of peat-forming plants was noted in the low-temperature range during the period from day 121 to day 180 of incubation and varied from 0.71 to 6.19. The highest Q10 values of DecR were observed for Sphagnum fuscum (Q10 = 6.19) and Eriophorum vaginatum (Q10 = 5.25) at 30% and 90% moisture levels, respectively.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inisheva, L.I. Torfyanye resursy i ikh kharakteristika [Peat resources and their characteristics]. In Bolota Zapadnoy Sibiri—Ikh rol’ v Biosfere [Bogs of Western Siberia—Their Role in the Biosphere], 2nd ed.; Zemtsov, A.A., Ed.; Tomsk State University SibNIIT Publisher: Tomsk, Russia, 2000; 72p. [Google Scholar]
- Guo, X.; Lu, X.; Tong, S.; Dai, G. Influence of environment and substrate quality on the decomposition of wetland plant root in the Sanjiang Plain, Northeast China. J. Environ. Sci. 2008, 20, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, K.; Strakova, P.; Fritze, H.; Iraizoz, P.A.; Pennanen, T.; Laiho, R. How water-level drawdown modified litter-decomposing fungal and actinobacterial communities in boreal peatlands. Soil Biol. Biochem. 2012, 51, 20–34. [Google Scholar] [CrossRef]
- Golovatskaya, E.A.; Nikonova, L.G. Influence of the level of bog waters on the processes of transformation of sphagnum mosses in peat soil of oligotrophic bogs. Eurasian Soil Sci. 2017, 5, 580–588. [Google Scholar] [CrossRef]
- Pastukhov, A.; Kovaleva, V.; Kaverin, D. Microbial Community Structure in Ancient European Arctic Peatlands. Plants 2022, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.C.; Vitt, D.H.; Cooper, D.J. The High-Elevation Peatlands of the Northern Andes, Colombia. Plants 2023, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Denisenkov, V.P. Osnovy Bolotovedeniya [Basics of Swamp Science]; St. Petersburg University Publisher: St. Petersburg, Russia, 2000; 224p. [Google Scholar]
- Dobrovol’skaya, T.G.; Golovchenko, A.V.; Zvyagintsev, D.G. Analysis of ecological factors limiting the destruction of high-moor peat. Eurasian Soil Sci. 2014, 47, 182–193. [Google Scholar] [CrossRef]
- Drzymulska, D. Peat decomposition–shaping factors, significance in environmental studies and methods of determination; a literature review. Geologos 2016, 22, 61–69. [Google Scholar] [CrossRef]
- Mironycheva-Tokareva, N.P.; Kosykh, N.P.; Vishnyakova, E.K. Production and destruction processes in peatland ecosystems of Vasugan region. Environ. Dyn. Glob. Clim. Change 2013, 4, 1–9. [Google Scholar]
- Vishnyakova, E.K.; Koronatova, N.G.; Mikhaylova, E.V. Transformation of carbon and macroelements compounds in mire peat deposits of different nutritional status. Interekspo Geo-Sib.—Interexpo Geo Sib. 2017, 4, 137–140. [Google Scholar]
- Koronatova, N.G. An investigation of the peat decomposition in mires using method of incubation of dry and wet samples. Environ. Dyn. Glob. Clim. Chang. 2010, 1, 77–84. [Google Scholar]
- Filippova, N.V.; Glagolev, M.V. Short-term standard litter decomposition across three different ecosystems in middle taiga zone of West Siberia. IOP Conf. Ser. Earth Environ. Sci. 2018, 138, 012004. [Google Scholar] [CrossRef]
- Domisch, T.; Finer, L.; Laine, J.; Laiho, R. Decomposition and nitrogen dynamics of litter in peat soils from two climatic regions under different temperature regimes. Eur. J. Soil Biol. 2006, 42, 74–81. [Google Scholar] [CrossRef]
- Vanhala, P.; Karhu, K.; Tuomi, M.; Björklöf, K.; Fritze, H.; Liski, J. Temperature sensitivity of soil organic matter decomposition in southern and northern areas of the boreal forest zone. Soil Biol. Biochem. 2008, 40, 1758–1764. [Google Scholar] [CrossRef]
- Preston, M.D.; Smemo, K.A.; McLaughlin, J.W.; Basiliko, N. Peatland microbial communities and decomposition processes in the James Bay Lowlands, Canada. Front. Microbiol. 2012, 3, 70. [Google Scholar] [CrossRef] [PubMed]
- Kluber, L.A.; Johnston, E.R.; Allen, S.A.; Hendershot, J.N.; Hanson, P.J.; Schadt, C.W. Constraints on microbial communities, decomposition and methane production in deep peat deposits. PLoS ONE 2020, 15, e0223744. [Google Scholar] [CrossRef] [PubMed]
- Mastný, J.; Bárta, J.; Kaštovská, E.; Picek, T. Decomposition of peatland DOC affected by root exudates is driven by specific r and K strategic bacterial taxa. Sci. Rep. 2021, 11, 18677. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, L.S.; Medvedeva, V.M.; Piavchenko, N.I. Dinamika Organicheskogo Veshhestva v Processe Torfoobrazovanija [Dynamics of Organic Matter in the Process of Peat Formation]; Nauka LO Publisher: Leningrad, Russia, 1978; 172p. [Google Scholar]
- Kukharenko, O.S.; Pavlova, N.S.; Dobrovol’skaya, T.G.; Golovchenko, A.V.; Pochatkova, T.N.; Zenova, G.M.; Zvyagintsev, D.G. The influence of aeration and temperature on the structure of bacterial complexes in high-moor peat soil. Eurasian Soil Sci. 2010, 43, 573–579. [Google Scholar] [CrossRef]
- Hogg, E.H.; Lieffers, V.J.; Wein, R.W. Potential carbon losses from peat profiles: Effects of temperature, drought cycles, and fire. Ecol. Appl. 1992, 2, 298–306. [Google Scholar] [CrossRef]
- Barreto, C.; Lindo, Z. Decomposition in peatlands: Who are the players and what affects them? Front. Young Minds 2022, 8, 138–146. [Google Scholar] [CrossRef]
- Larionova, A.A.; Maltseva, A.N.; Lopes de Gerenyu, V.O.; Kvitkina, A.K.; Bykhovets, S.S.; Zolotareva, B.N.; Kudeyarov, V.N. Effect of temperature and moisture on the mineralization and humification of leaf litter in a model incubation experiment. Eurasian Soil Sci. 2017, 4, 422–431. [Google Scholar] [CrossRef]
- Chernova, I.Y. (Ed.) Functioning of Microbial Complexes in Upland Peat Bogs—Analysis of the Causes of Slow Destruction of Peat; The Partnership of Scientific Publications KMK Publisher: Moscow, Russia, 2013; 128p. [Google Scholar]
- Vishnyakova, E.K.; Mironycheva-Tokareva, N.P.; Kosykh, N.P. Decomposition Dynamics of Plants of Bogs (Data: Vasyugan Bog). Tomsk. State Pedagog. Univ. Bull. 2012, 7, 88–93. [Google Scholar]
- Berg, B. Decomposition patterns for foliar litter: A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Nikonova, L.G.; Golovatskaya, E.A.; Kur’ina, I.V.; Kurganova, I.N. Decomposition rate of peat-forming plants in oligotrophic bogs of the southern taiga subzone of Western Siberia: Assessment of the effect of water table level and peat deposit temperature. Eurasian Soil Sci. 2019, 52, 1101–1111. [Google Scholar] [CrossRef]
- Leroy, F.; Gogo, S.; Buttler, A.; Bragazza, L.; Laggoun-Defarge, F. Litter decomposition in peatlands is promoted by mixed plants. J. Soils Sediments 2018, 18, 739–749. [Google Scholar] [CrossRef]
- Zhou, G.; Cao, W.; Bai, J.; Xu, C.; Zeng, N.; Gao, S.; Rees, R. Non-additive responses of soil C and N to rice straw and hairy vetch (Vicia villosa Roth L.) mixtures in a paddy soil. Plant Soil 2019, 436, 229–244. [Google Scholar] [CrossRef]
- Durynina, E.P.; Egorov, V.S. Agrochemical Analysis of Soil, Plants, Fertilizers; Moscow State University Publisher: Moscow, Russia, 1998; 113p. [Google Scholar]
- Vorob’eva, L.A.; Ladonin, D.V.; Lopukhina, O.V.; Rudakova, T.A.; Kiryushin, A.V. Chemical Soil Analysis. Questions and Answers; Moscow State University Publisher: Moscow, Russia, 2012; 186p. [Google Scholar]
- Giglev, V.Y. Physics of Soils; Perm State University Publisher: Perm, Russia, 2012; 37p. [Google Scholar]
- Kaurichev, I.S. Practical Work on Soil Science; Kolos Publisher: Moscow, Russia, 1980; 272p. [Google Scholar]
- Browning, B.L. Methods of Wood Chemistry; Intersci P Publisher: New York, NY, USA, 1967; Volume 2, 498p. [Google Scholar]
- Dence, C.W. The determination of lignin. In Methods of Lignin Chemistry; Springer Publisher: Berlin, Germany, 1992; pp. 33–61. [Google Scholar]
- Obolenskaya, A.V.; El’nitskaya, Z.P.; Leonovich, A.A. Laboratory Work on the Chemistry of Wood and Cellulose; Ekologiya Publisher: Moscow, Russia, 1991; 320p. [Google Scholar]
- Golovatskaya, E.A.; Nikonova, L.G. Decomposition of plant residues in peat soils of oligotrophic peatlands. Tomsk. State Univ. J. Biol. 2013, 3, 137–151. [Google Scholar]
- Nikonova, L.G.; Golovatskaya, E.A.; Terechshenko, N.N. Decomposition rate of peat-forming plants in the oligotrophic peatland at the first stages of destruction. IOP Conf. Ser. Earth Environ. Sci. 2018, 138, 012013. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Susyan, E.A.; Ryzhova, I.M.; Bocharnikova, E.O.; Stolnikova, E.V. Microbial biomass carbon and the microbial carbon dioxide production by soddy-podzolic soils in postagrogenic biogeocenoses and in native spruce forests of the southern taiga (Kostroma oblast). Eurasian Soil Sci. 2009, 42, 1029–1037. [Google Scholar] [CrossRef]
- Kurganova, I.N.; Lopes de Gerenyu, V.O.; Gallardo Lancho, J.F.; Oehm, C.T. Evaluation of the rates of soil organic matter mineralization in forest ecosystems of temperate continental, mediterranean, and tropical monsoon climates. Eurasian Soil Sci. 2012, 45, 68–79. [Google Scholar] [CrossRef]
- Kurganova, I.; Lopes de Gerenyu, V.; Galibina, N.; Kapitsa, E.; Shorohova, E. Coupled effect of temperature and mineral additions facilitates decay of aspen bark. Geoderma 2018, 316, 27–37. [Google Scholar] [CrossRef]
- Kosheleva, Y.P.; Trofimov, S.Y. Characteristics of the biochemical composition of plant litter at different stages of decomposition (according to thermal analysis data). Biol. Bull. 2008, 35, 64–69. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Kätterer, T.; Reichstein, M.; Andren, O. Temperature dependence of organic matter decomposition: A critical review using literature data analyzed with different models. Biol. Fertil. Soils 1998, 27, 258–262. [Google Scholar] [CrossRef]
- Chen, H.; Harmon, M.E.; Griffiths, R.P.; Hicks, W. Effects of temperature and moisture on carbon respired from decomposing woody roots. Ecol. Manag. 2000, 138, 51–64. [Google Scholar] [CrossRef]
- Bambalov, N.N.; Horuzhik, A.V.; Lukoshko, E.S.; Strigutsky, V.P. Transformation of dead plants in marsh biogeocenoses. In Experiment and Mathematical Modeling in the Study of Biogeocenoses of Forests and Bog; Nauka Publisher: Moscow, Russia, 1990; pp. 53–63. [Google Scholar]
- Hajek, T. Habitat and species controls on Sphagnum production and decomposition in a mountain bog. Boreal Environ. Res. 2009, 14, 947–958. [Google Scholar]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 1083–1091. [Google Scholar] [CrossRef]
- Kosyh, N.P.; Mironycheva-Tokareva, N.P.; Parshina, E.K. Phytomass, production and decomposition of plant residues in oligotrophic bogs of the middle taiga of Western Siberia. Tomsk. State Pedagog. Univ. Bull. 2009, 3, 63–68. [Google Scholar]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Vos, V.C.; van Ruijven, J.; Berg, M.P.; Peeters, E.T.; Berendse, F. Leaf litter quality drives litter mixing effects through complementary resource use among detritivores. Oecologia 2013, 173, 269–280. [Google Scholar] [CrossRef]
- Fierer, N.; Craine, J.M.; McLauchlan, K.; Schimel, J.P. Litter quality and the temperature sensitivity of decomposition. Ecology 2005, 86, 320–326. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.M.; Nault, J.R.; Trofymov, J.A.; Smyth, C. Chemical changes during 6 years decomposition of 11 litters in some Canadian forest sites. Ecosystems 2009, 12, 1053–1077. [Google Scholar] [CrossRef]
- Prescott, C. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils. Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Trofimov, S.Y.; Botner, P.; Kutu, M.M. Decomposition of organic matter of organogenic horizons of forest soils under laboratory conditions. Eurasian Soil Sci. 1998, 12, 1480–1488. [Google Scholar]




| Index | Ash, % | pH | HM, % | WHC 1, % | ||
|---|---|---|---|---|---|---|
| Plant | 1M KCl | H2O | ||||
| Sphagnum fuscum | 0.74 | 2.5 | 3.4 | 13.7 | 2541 ± 241 | |
| Eriophorum vaginatum | 2.25 | 4.3 | 4.8 | 3.9 | 178 ± 3 | |
| Chamaedaphne calyculata | 2.23 | 4.5 | 4.9 | 7.6 | 244 ± 1 | |
| Mixed sample | 1.53 | 3.7 | 3.0 | 9.3 | 1203 ± 55 | |
| Index | C *, % | N *, % | C/N * | Eth-Ext 1, % | Cel 2, % | Lig 3, % | Lig/N | |
|---|---|---|---|---|---|---|---|---|
| Plant | ||||||||
| Sphagnum fuscum | 44.0 ± 0.6 | 0.37 ± 0.01 | 118 ± 1 | 3.9 | 25.3 | 12.2 | 32 | |
| Eriophorum vaginatum | 45.4 ± 0.4 | 0.93 ± 0.01 | 49 ±0.1 | 11.3 | 35.3 | 20.3 | 22 | |
| Chamaedaphne calyculata | 51.8 ± 1.1 | 1.15 ± 0.03 | 45 ± 1 | 33.4 | 12.1 | 28.5 | 24 | |
| Mixed sample | 46.2 ± 0.7 | 0.66 ± 0.01 | 70 ± 0 | 19.2 | 19.2 | 20.8 | 32 | |
| Plant | Variant | Temperature Range, °C | |||||
|---|---|---|---|---|---|---|---|
| 35–150 | 150–265 | 265–380 | 380–495 | 495–610 | 610–725 | ||
| Sphagnum fuscum | Initial | 10.39 | 14.15 | 39.55 | 32.41 | 1.21 | - |
| Post-exper. | 8.23 | 14.06 | 42.38 | 31.95 | 0.52 | - | |
| ∆, % | −2.16 | −0.09 | 2.83 | −0.46 | −0.69 | - | |
| Eriophorum vaginatum | Initial | 6.96 | 9.64 | 46.52 | 27.3 | 4.71 | 0.18 |
| Post-exper. | 5.22 | 8.42 | 47.75 | 28.28 | 5.97 | 0.07 | |
| ∆, % | −1.74 | −1.22 | 1.23 | 0.98 | 1.26 | −0.11 | |
| Chamaedaphne calyculata | Initial | 6.96 | 9.62 | 33.80 | 18.17 | 17.34 | 10.33 |
| Post-exper. | 6.51 | 7.71 | 34.74 | 22.48 | 16.58 | 4.58 | |
| ∆, % | −0.45 | −1.91 | 0.94 | 4.31 | −0.76 | −5.75 | |
| Mixed sample | Initial | 8.90 | 12.59 | 39.60 | 33.09 | 2.07 | 0.28 |
| Post-exper. | 7.98 | 11.67 | 43.79 | 29.4 | 1.8 | 0.47 | |
| ∆, % | −0.92 | −0.92 | 4.19 | −3.69 | −0.27 | 0.19 | |
| Factor | 90 Days of Incubation | 180 Days of Incubation | 360 Days of Incubation | 600 Days of Incubation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| η, % | F | p | η, % | F | p | η, % | F | p | η, % | F | p | |
| P | 61.0 | 450 | <0.0001 | 54.7 | 204 | <0.0001 | 68.8 | 125 | <0.0001 | 75.9 | 95.8 | <0.0001 |
| T | 31.2 | 230 | <0.0001 | 36.6 | 136 | <0.0001 | 20.2 | 37 | <0.0001 | 12.4 | 15.6 | <0.0001 |
| W | 2.1 | 15 | <0.0001 | 3.1 | 12 | <0.0001 | 4.0 | 7 | 0.001 | 4.6 | 5.8 | 0.004 |
| P × T | 4.3 | 32 | <0.0001 | 3.6 | 13 | <0.0001 | 3.2 | 6 | <0.0001 | 2.6 | 3.2 | 0.007 |
| P × W | 0.6 | 4 | 0.001 | 0.7 | 3 | 0.018 | 1.0 | 2 | 0.117 | 0.3 | 0.4 | 0.858 |
| T × W | 0.3 | 2 | 0.129 | 0.3 | 1 | 0.375 | 0.7 | 1 | 0.304 | 0.9 | 1.2 | 0.329 |
| P × T × W | 0.4 | 3 | 0.001 | 0.7 | 3 | 0.005 | 1.6 | 3 | 0.002 | 2.5 | 3.1 | 0.001 |
| UV | 0.1 | 0.3 | 0.6 | 0.8 | ||||||||
| Factor | Sphagnum fuscum | Chamaedaphne calyculata | Eriophorum vaginatum | Mixed Sample | ||||
|---|---|---|---|---|---|---|---|---|
| η, % | p | η, % | p | η, % | p | η, % | p | |
| T | 82.06 | <0.0001 | 73.93 | <0.0001 | 6.12 | 0.70 | 74.85 | <0.0001 |
| W | 16.31 | <0.0001 | 13.56 | 0.08 | 19.93 | 0.33 | 10.29 | 0.27 |
| T × W | 1.30 | 0.02 | 7.91 | 0.19 | 57.27 | 0.03 | 7.45 | 0.43 |
| UV | 0.33 | 4.61 | 16.68 | 7.41 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikonova, L.; Kurganova, I.; Lopes de Gerenyu, V.; Rogova, O.; Golovatskaya, E. Impact of Temperature and Moisture on the Decomposition of Peat-Forming Plants: Results of a Two-Year Incubation Experiment. Forests 2023, 14, 2355. https://doi.org/10.3390/f14122355
Nikonova L, Kurganova I, Lopes de Gerenyu V, Rogova O, Golovatskaya E. Impact of Temperature and Moisture on the Decomposition of Peat-Forming Plants: Results of a Two-Year Incubation Experiment. Forests. 2023; 14(12):2355. https://doi.org/10.3390/f14122355
Chicago/Turabian StyleNikonova, Liliya, Irina Kurganova, Valentin Lopes de Gerenyu, Olga Rogova, and Evgeniya Golovatskaya. 2023. "Impact of Temperature and Moisture on the Decomposition of Peat-Forming Plants: Results of a Two-Year Incubation Experiment" Forests 14, no. 12: 2355. https://doi.org/10.3390/f14122355
APA StyleNikonova, L., Kurganova, I., Lopes de Gerenyu, V., Rogova, O., & Golovatskaya, E. (2023). Impact of Temperature and Moisture on the Decomposition of Peat-Forming Plants: Results of a Two-Year Incubation Experiment. Forests, 14(12), 2355. https://doi.org/10.3390/f14122355







