The Composite Physiological Response of Hydraulic and Photosynthetic Traits and Nonstructural Carbon in Masson Pine Seedlings to Drought Associated with High Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
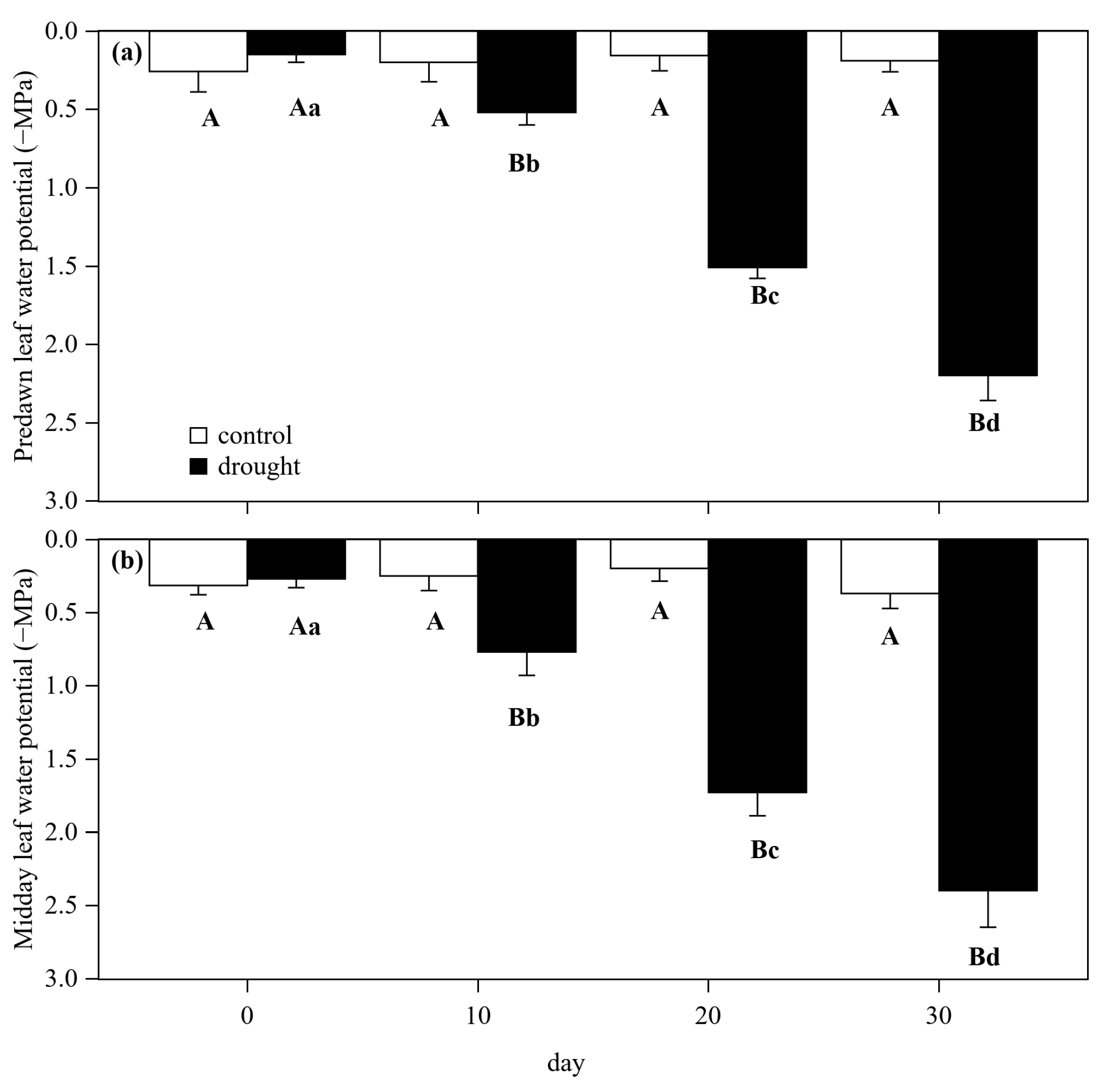
2.2. Leaf Water Potential
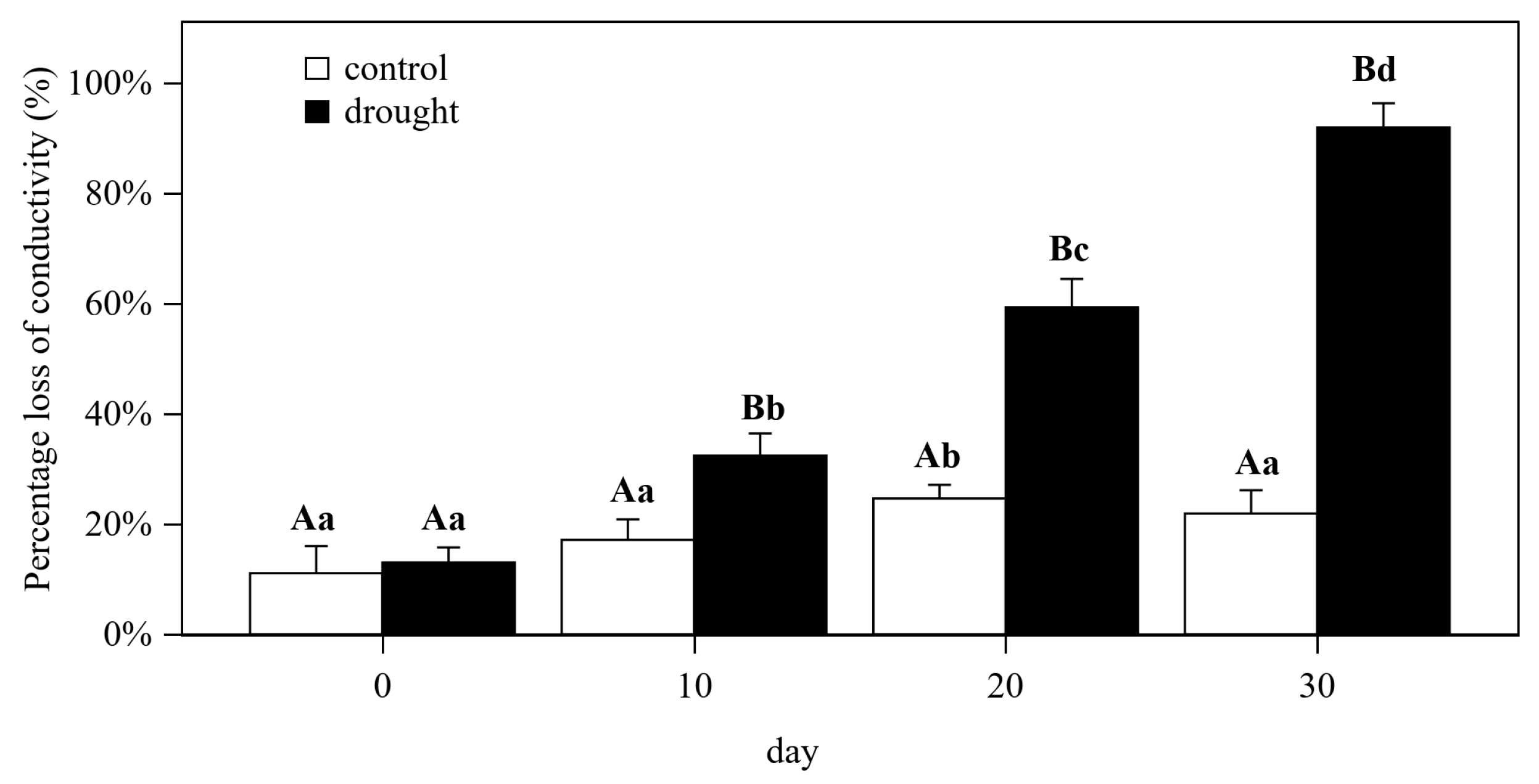
2.3. Hydraulic Conductivity
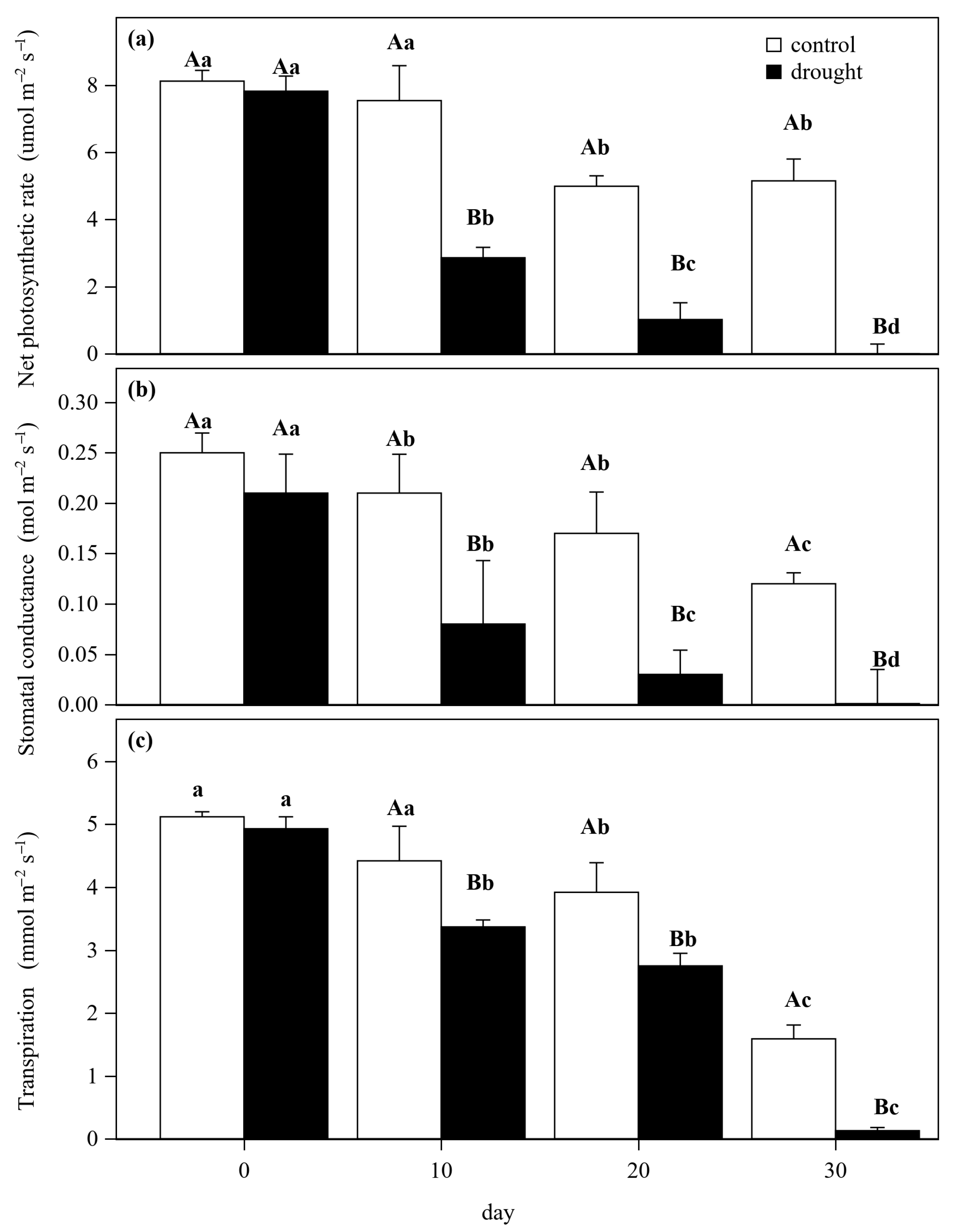
2.4. Leaf Photosynthesis Measurements
2.5. Measurement of Nonstructural Carbohydrate
2.6. Data Analysis
3. Results
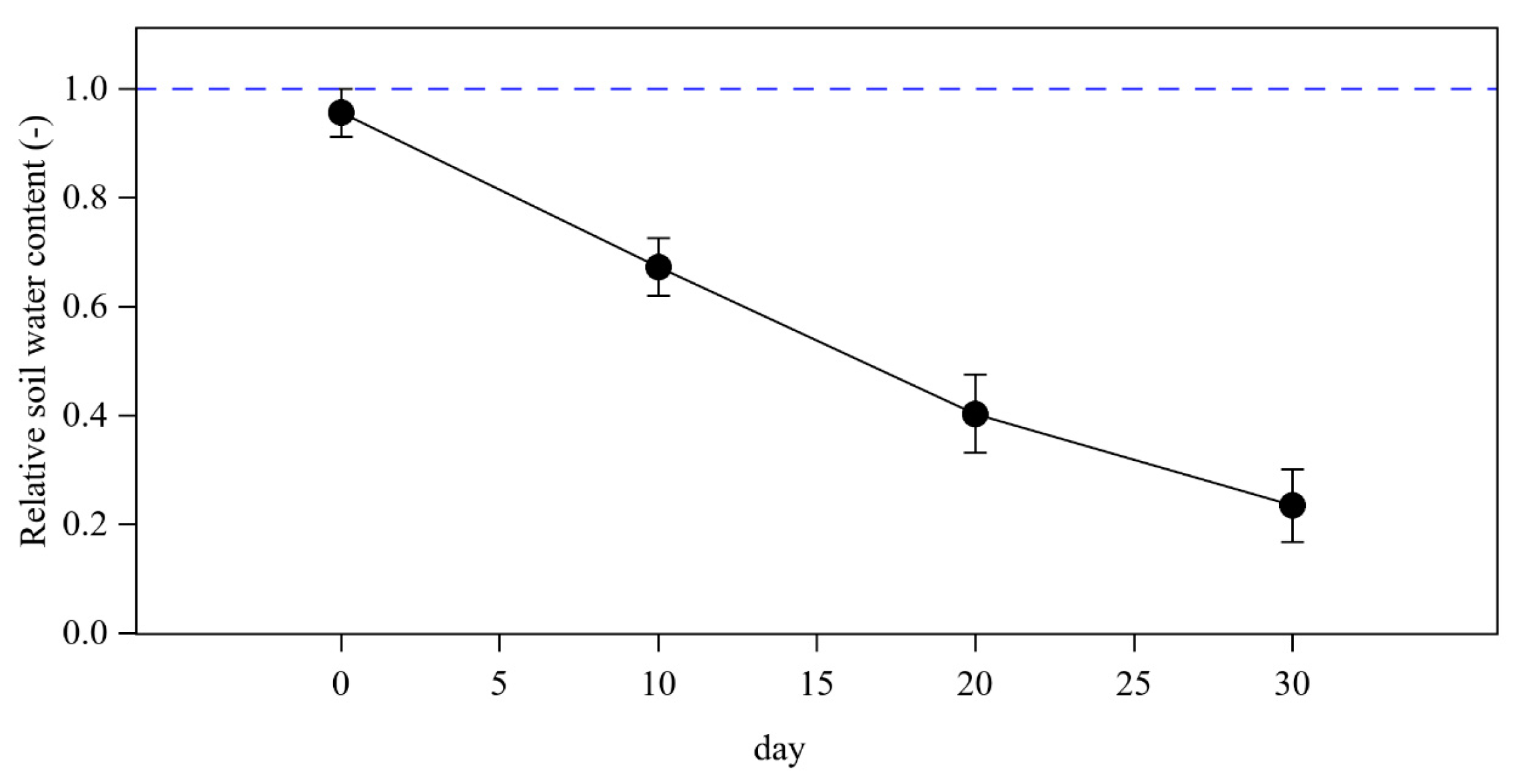
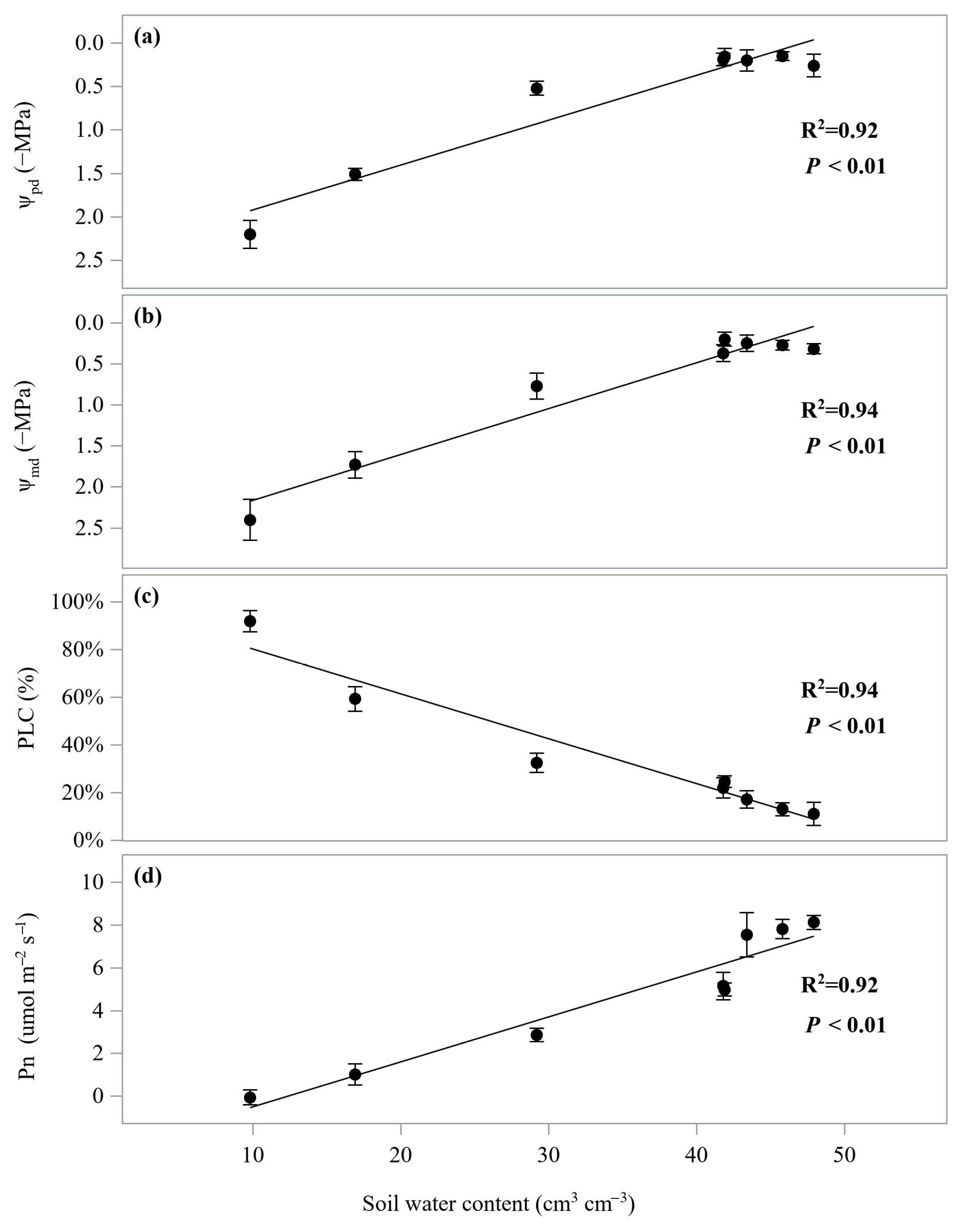
3.1. Hydraulic and Gas Exchange Dynamics
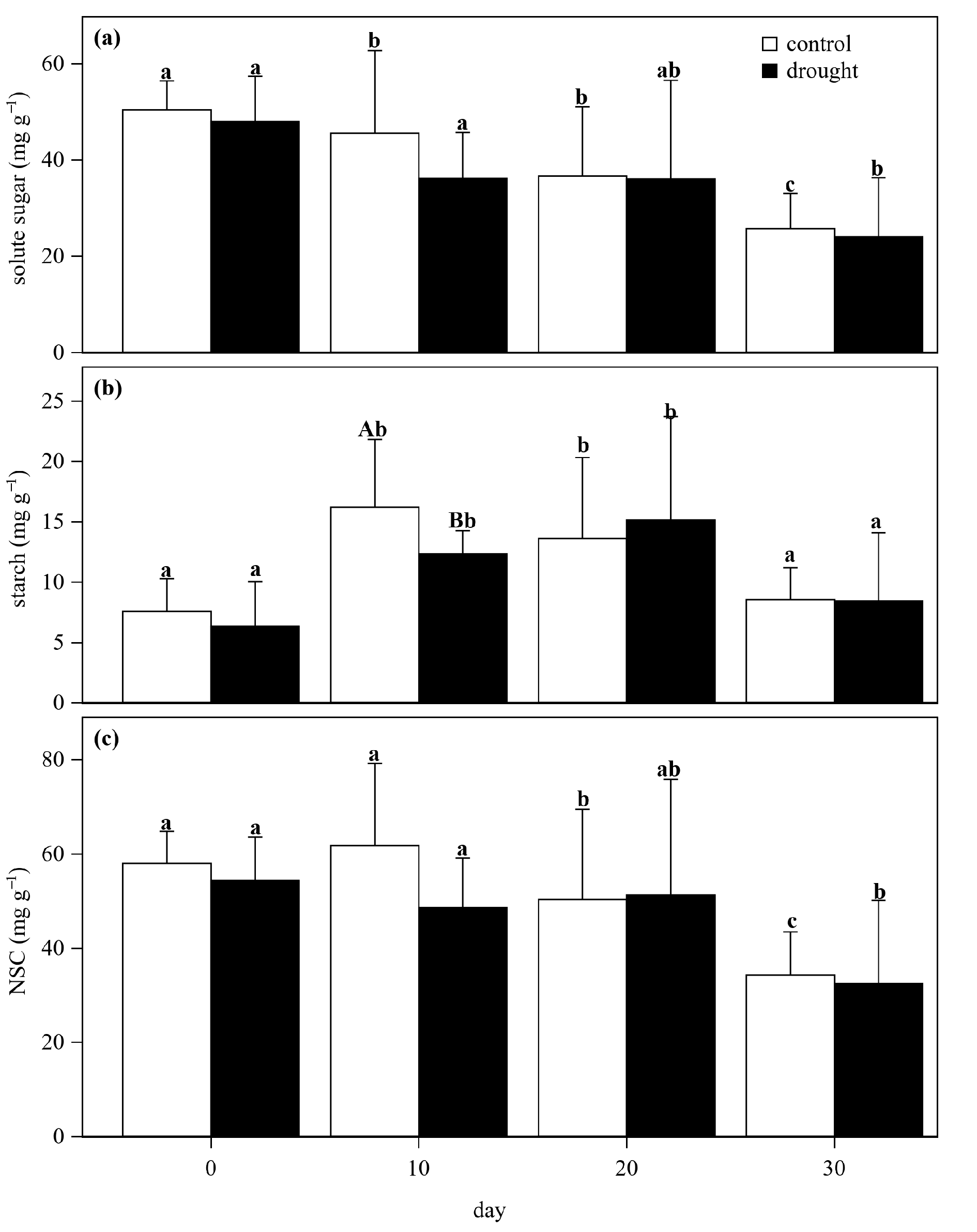
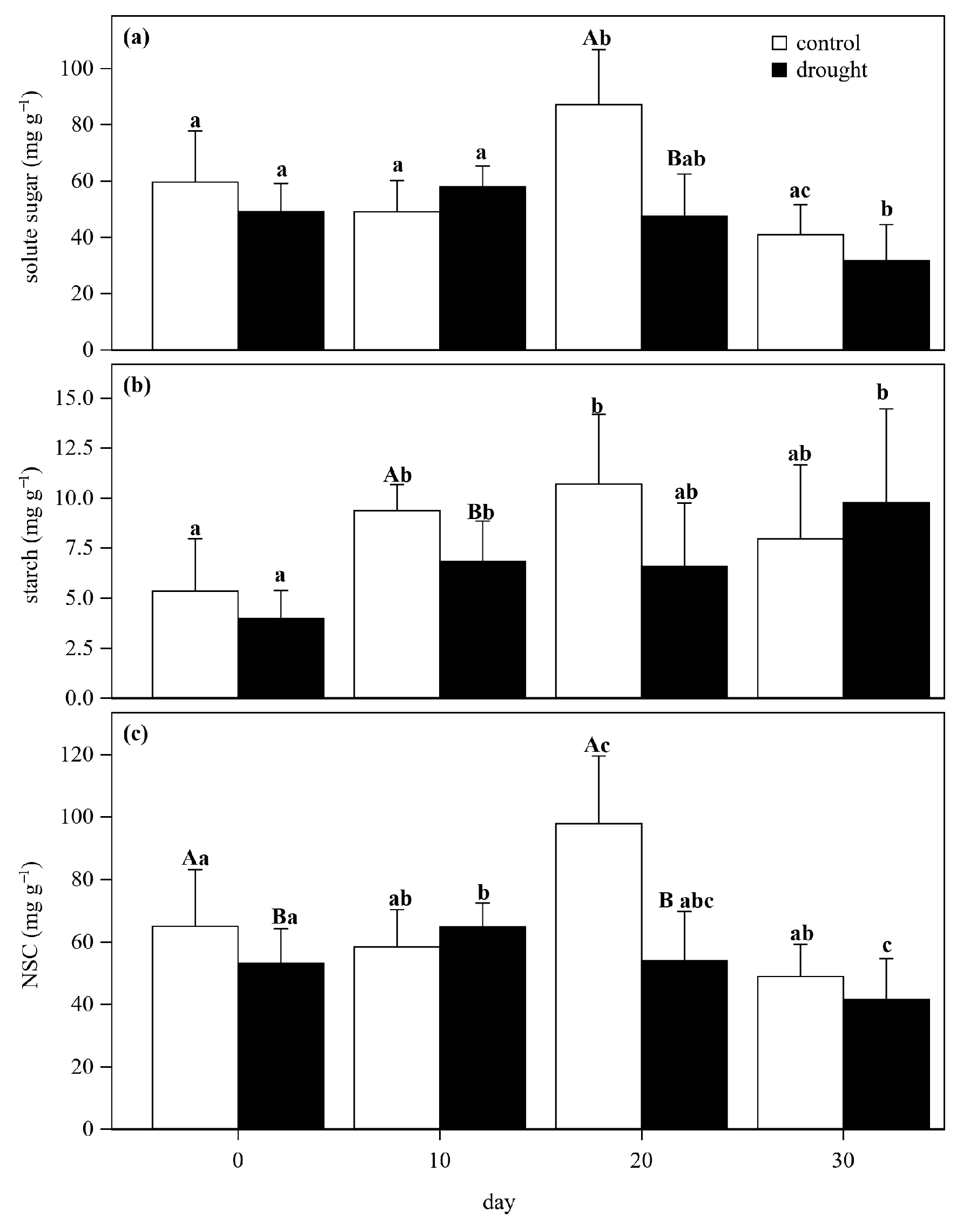
3.2. Nonstructural Carbon Dynamics
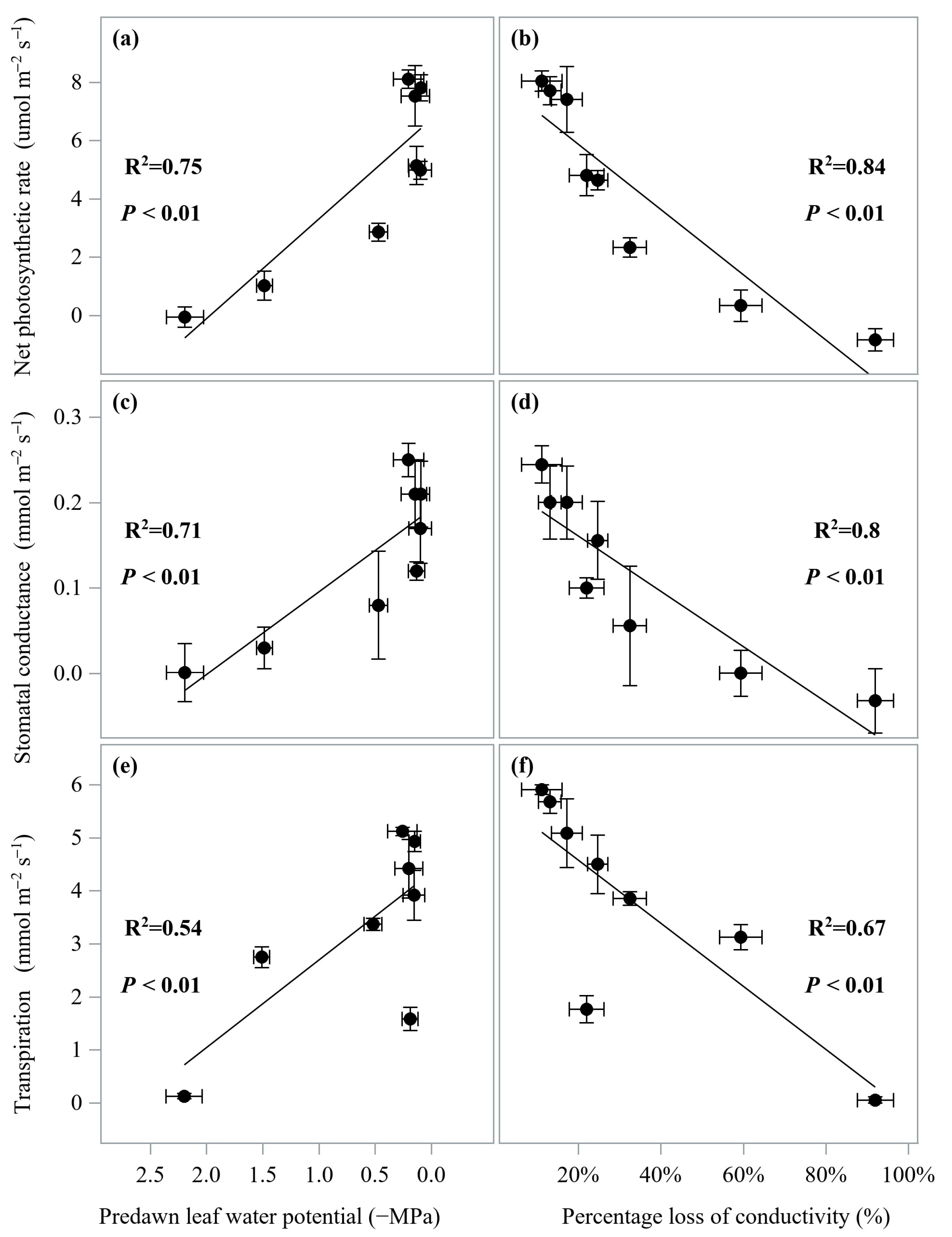
3.3. Correlation among Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adams, H.D.; Macalady, A.K.; Breshears, D.D.; Allen, C.D.; Stephenson, N.L.; Saleska, S.R.; Huxman, T.E.; McDowell, N.G. Climate-Induced Tree Mortality: Earth System Consequences. Eos Trans. Am. Geophys. Union 2010, 91, 153–154. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Klein, T.; Hartmann, H. Climate Change Drives Tree Mortality. Science 2018, 362, 758. [Google Scholar] [CrossRef]
- Powers, J.S.; Vargas, G.G.; Brodribb, T.J.; Schwartz, N.B.; Pérez-Aviles, D.; Smith-Martin, C.M.; Becknell, J.M.; Aureli, F.; Blanco, R.; Calderón-Morales, E.; et al. A Catastrophic Tropical Drought Kills Hydraulically Vulnerable Tree Species. Glob. Chang. Biol. 2020, 26, 3122–3133. [Google Scholar] [CrossRef]
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess Forest Mortality Is Consistently Linked to Drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- FAO. Proactive Approaches to Drought Preparedness—Where Are We Now and Where Do We Go from Here? FAO: Rome, Italy, 2019. [Google Scholar]
- Hoerling, M.; Eischeid, J.; Kumar, A.; Leung, R.; Mariotti, A.; Mo, K.; Schubert, S.; Seager, R. Causes and Predictability of the 2012 Great Plains Drought. Bull. Am. Meteorol. Soc. 2014, 95, 269–282. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, Z.; Pan, M.; Shi, C. Microwave Remote Sensing of Short-term Droughts during Crop Growing Seasons. Geophys. Res. Lett. 2015, 42, 4394–4401. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, L.; Wu, P.; Ji, P.; Sheffield, J.; Zhang, M. Anthropogenic Shift towards Higher Risk of Flash Drought over China. Nat. Commun. 2019, 10, 4661. [Google Scholar] [CrossRef]
- Jentsch, A.; Kreyling, J.; Beierkuhnlein, C. A New Generation of Climate-Change Experiments: Events, Not Trends. Front. Ecol. Environ. 2007, 5, 365–374. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Ong, R.; Reynolds, G. Intra-Annual Plasticity of Growth Mediates Drought Resilience over Multiple Years in Tropical Seedling Communities. Glob. Chang. Biol. 2017, 23, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global Convergence in the Vulnerability of Forests to Drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, N.; Zhang, X. A Comparison of Large-Scale Climate Signals and the North American Multi-Model Ensemble (NMME) for Drought Prediction in China. J. Hydrol. 2018, 557, 378–390. [Google Scholar] [CrossRef]
- Duan, H.; Resco de Dios, V.; Wang, D.; Zhao, N.; Huang, G.; Liu, W.; Wu, J.; Zhou, S.; Choat, B.; Tissue, D.T. Testing the Limits of Plant Drought Stress and Subsequent Recovery in Four Provenances of a Widely Distributed Subtropical Tree Species. Plant Cell Environ. 2022, 45, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Shao, C.; Luo, X.; Resco de Dios, V.; Tissue, D.T.; Ding, G. Root Relative Water Content Is a Potential Signal for Impending Mortality of a Subtropical Conifer during Extreme Drought Stress. Plant Cell Environ. 2023, 46, 2763–2777. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Duan, H.; Ding, G.; Luo, X.; Fu, Y.; Lou, Q. Physiological and Biochemical Dynamics of Pinus massoniana Lamb. Seedlings under Extreme Drought Stress and during Recovery. Forests 2022, 13, 65. [Google Scholar] [CrossRef]
- Ding, G.; Zhou, Z.; Wang, Z. Cultivation and Utilization of Pulpwood Stand for Pinus massoniana; China Forestry Publishing House: Beijing, China, 2006. [Google Scholar]
- Tong, C.; Zhang, X.; Xie, J.; Mei, T.; Fang, D.; Li, Y. Water Use Strategies of Different Aged Moso Bamboo Culms under Summer Drought. For. Ecol. Manag. 2021, 498, 119567. [Google Scholar] [CrossRef]
- Yu, F.; Xu, X.; Guy, R. Effects of Water Plus Heat Stress on Gas Exchange and Water Use Efficiency in Four Species of Coniferous Seedlings. Sci. Silvae Sin. 2004, 40, 38–44. [Google Scholar]
- Wang, Y.; ZHAN, C.; Ding, G.; LI, Z. Effect of Water Stress on Physiology and Photosynthesis Properties of Ectomycorrhizal Pinus massoniana Seedlings. J. Zhejiang For. Sci. Technol. 2019, 39, 471. [Google Scholar]
- Deng, X.; Shi, Z.; Xiao, W.; Zeng, L.; Lei, L. Effects of Drought and Shading on Growth and Photosynthetic Characteristics of Pinus massoniana Seedlings. Acta Ecol. Sin. 2020, 40, 2735–2742. [Google Scholar]
- Du, M.; Ding, G.; Zhao, X. Responses to Continuous Drought Stress and Drought Resistance Comprehensive Evaluation of Different Masson Pine Families. Sci. Silvae Sin. 2017, 53, 21–29. [Google Scholar]
- Li, M.; Wang, H.; Zhao, X.; Lu, Z.; Sun, X.; Ding, G. Role of Suillus placidus in Improving the Drought Tolerance of Masson Pine (Pinus massoniana Lamb.) Seedlings. Forests 2021, 12, 332. [Google Scholar] [CrossRef]
- Quan, W.; Ding, G. Root Tip Structure and Volatile Organic Compound Responses to Drought Stress in Masson Pine (Pinus massoniana Lamb.). Acta Physiol. Plant. 2017, 39, 258. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, G. Physiological Responses of Mycorrhizal Pinus massoniana Seedlings to Drought Stress and Drought Resistance Evaluation. Chin. J. Appl. Ecol. 2013, 24, 639–645. [Google Scholar]
- Shi, Z.; Deng, X.; Zeng, L.; Shi, S.; Lei, L.; Xiao, W. Acclimation Strategy of Masson Pine (Pinus massoniana) by Limiting Flavonoid and Terpenoid Production under Low Light and Drought. Int. J. Mol. Sci. 2022, 23, 8441. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Anderegg, L.D.L.; Kerr, K.L.; Trugman, A.T. Widespread Drought-Induced Tree Mortality at Dry Range Edges Indicates That Climate Stress Exceeds Species’ Compensating Mechanisms. Glob. Chang. Biol. 2019, 25, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How Do Trees Die? A Test of the Hydraulic Failure and Carbon Starvation Hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Tomasella, M.; Casolo, V.; Aichner, N.; Petruzzellis, F.; Savi, T.; Trifilò, P.; Nardini, A. Non-Structural Carbohydrate and Hydraulic Dynamics during Drought and Recovery in Fraxinus Ornus and Ostrya Carpinifolia Saplings. Plant Physiol. Biochem. 2019, 145, 1–9. [Google Scholar] [CrossRef]
- Tomasella, M.; Nardini, A.; Hesse, B.D.; Machlet, A.; Matyssek, R.; Häberle, K.-H. Close to the Edge: Effects of Repeated Severe Drought on Stem Hydraulics and Non-Structural Carbohydrates in European Beech Saplings. Tree Physiol. 2019, 39, 717–728. [Google Scholar] [CrossRef]
- Rowland, L.; Ramírez-Valiente, J.-A.; Hartley, I.P.; Mencuccini, M. How Woody Plants Adjust Above- and below-Ground Traits in Response to Sustained Drought. New Phytol. 2023, 239, 1173–1189. [Google Scholar] [CrossRef]
- Sperry, J.S.; Donnelly, J.R.; Tyree, M.T. A Method for Measuring Hydraulic Conductivity and Embolism in Xylem. Plant Cell Environ. 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Hernández, E.I.; Vilagrosa, A.; Pausas, J.G.; Bellot, J. Morphological Traits and Water Use Strategies in Seedlings of Mediterranean Coexisting Species. Plant Ecol. 2010, 207, 233–244. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The Physiology of Plant Responses to Drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined Effects of Drought and Shading on Growth and Non-Structural Carbohydrates in Pinus massoniana Lamb. Seedlings. Forests 2019, 11, 18. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, D.; Yao, H.; Huang, Y.; Li, W.; Mei, T.; Jin, S. The Composite Physiological Response of Hydraulic and Photosynthetic Traits and Nonstructural Carbon in Masson Pine Seedlings to Drought Associated with High Temperature. Forests 2023, 14, 2320. https://doi.org/10.3390/f14122320
Fang D, Yao H, Huang Y, Li W, Mei T, Jin S. The Composite Physiological Response of Hydraulic and Photosynthetic Traits and Nonstructural Carbon in Masson Pine Seedlings to Drought Associated with High Temperature. Forests. 2023; 14(12):2320. https://doi.org/10.3390/f14122320
Chicago/Turabian StyleFang, Dongming, Heting Yao, Yuelai Huang, Weijiao Li, Tingting Mei, and Songheng Jin. 2023. "The Composite Physiological Response of Hydraulic and Photosynthetic Traits and Nonstructural Carbon in Masson Pine Seedlings to Drought Associated with High Temperature" Forests 14, no. 12: 2320. https://doi.org/10.3390/f14122320
APA StyleFang, D., Yao, H., Huang, Y., Li, W., Mei, T., & Jin, S. (2023). The Composite Physiological Response of Hydraulic and Photosynthetic Traits and Nonstructural Carbon in Masson Pine Seedlings to Drought Associated with High Temperature. Forests, 14(12), 2320. https://doi.org/10.3390/f14122320




