Effects of Different Nitrogen Application Rates on Root Growth and Distribution of Fine Root Length across Diameter Classes of Wolfberry (Lycium barbarum L.)
Abstract
:1. Introduction
2. Materials and Methods
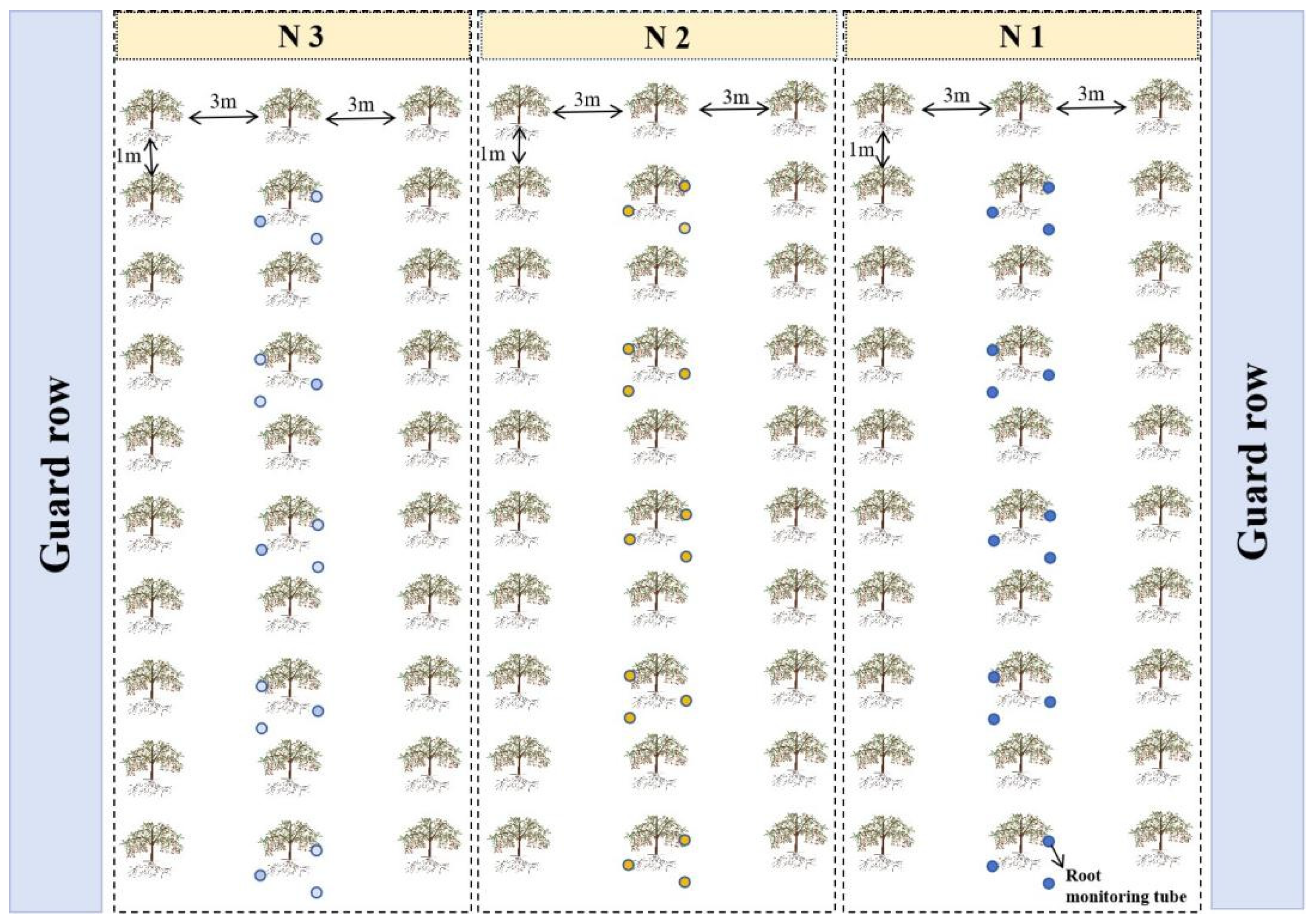
2.1. Basic Conditions of the Experimental Site and Experimental Design
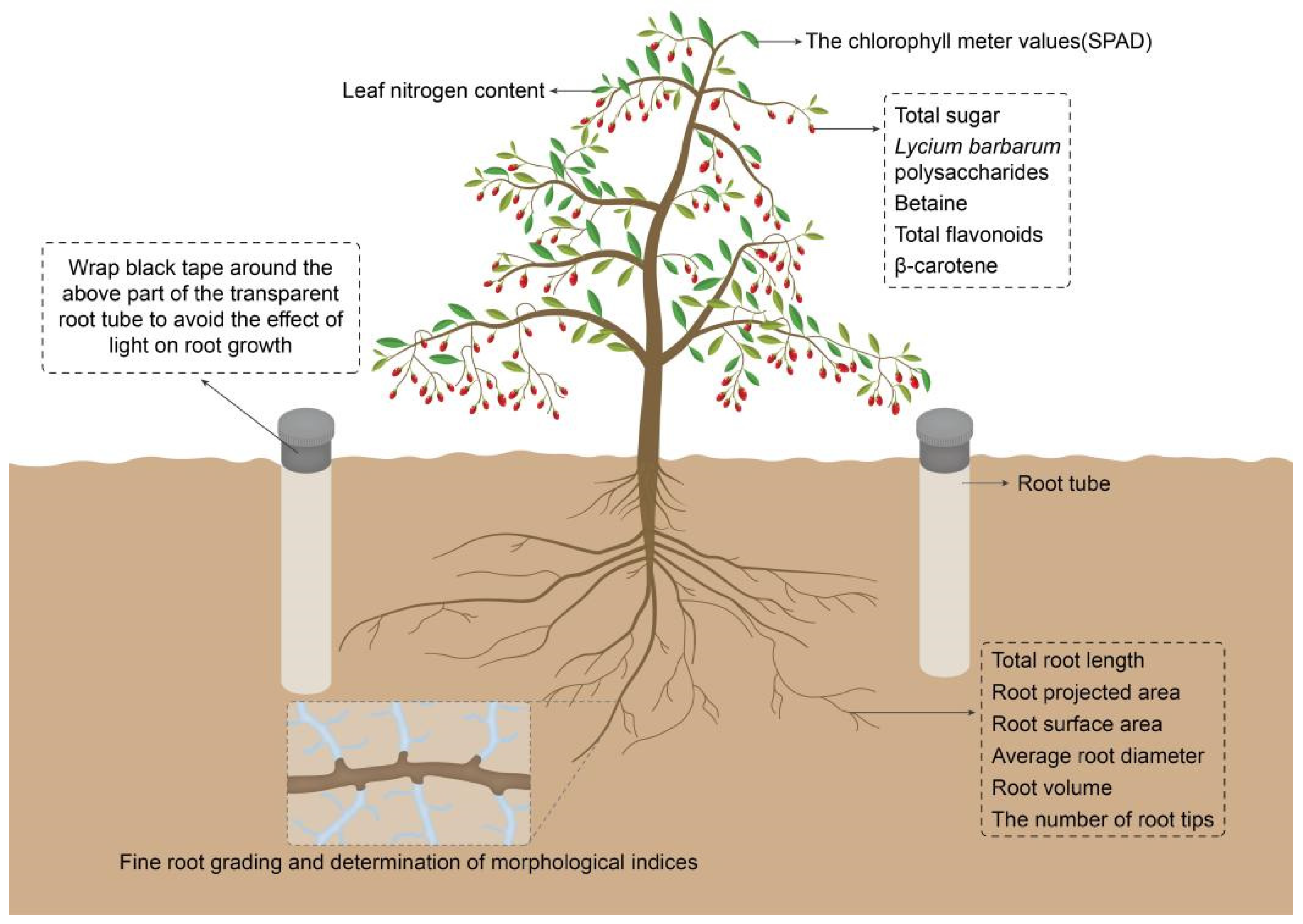
2.2. Investigated Parameters and Determination Methods
2.2.1. Root Morphological Indices
2.2.2. Fine Root Grading and Determination of Morphological Indices
2.2.3. Determination of Soil Chemical Properties
2.2.4. Determination of Leaf Nitrogen Content
2.2.5. Determination of Relative Chlorophyll Content
2.2.6. Determination of the Nutritional Composition of Wolfberry Fruits
2.2.7. Data Processing
3. Results
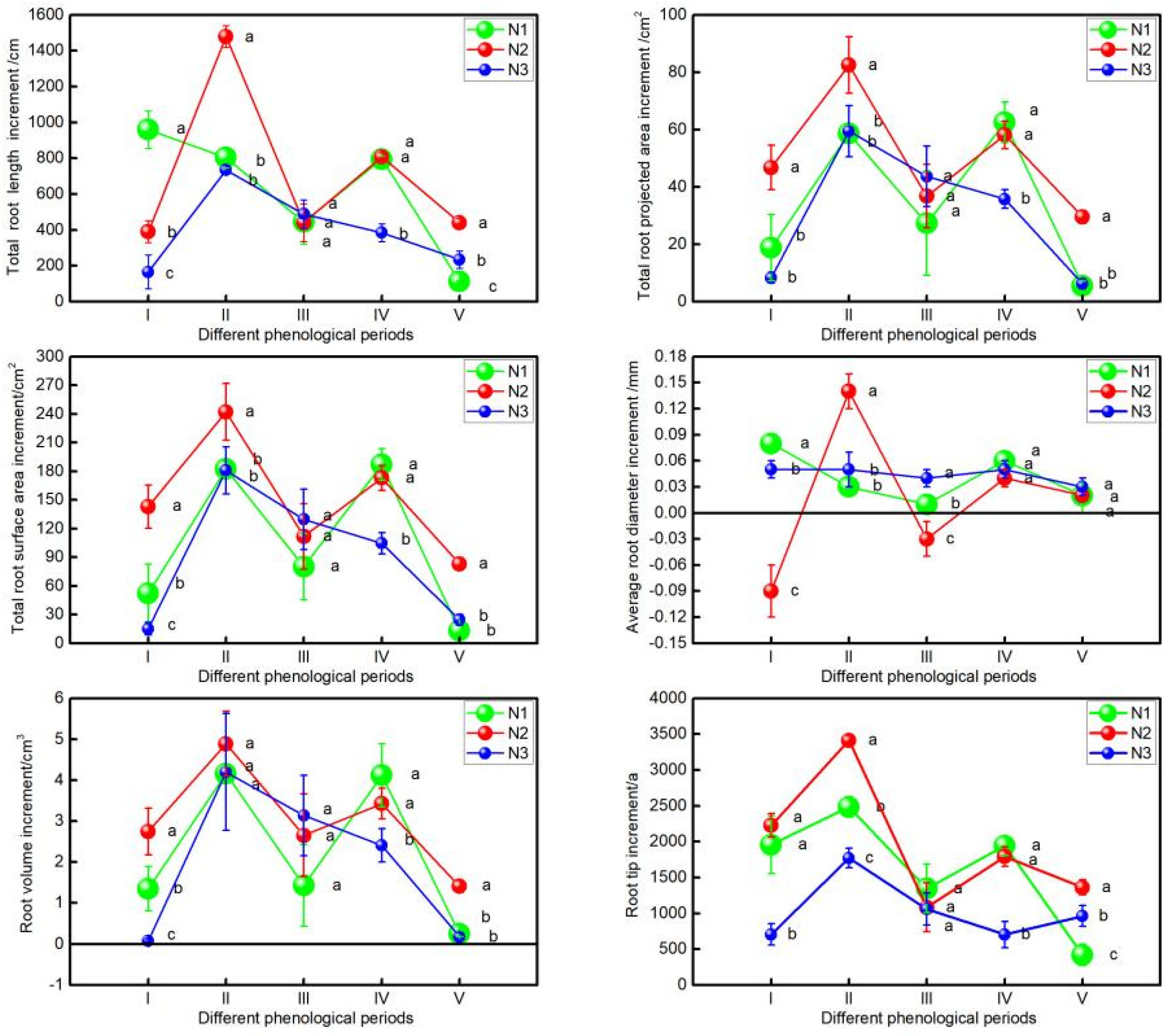
3.1. ANOVA of the Increment of Root Growth of Wolfberry under Different Nitrogen Application Treatments
3.2. ANOVA of the Increment of Root Growth of Wolfberry in Different Diameter Classes under Different Nitrogen Application Treatments
3.3. Monthly Growth Increment of Fine Roots in Different Diameter Classes at Different Soil Depths
3.4. ANOVA of Nitrogen Contents and SPAD Values of Wolfberry Leaves under Different Nitrogen Application Treatments
3.5. ANOVA of the Nutritional Composition of Wolfberry Fruits under Different Nitrogen Application Treatments
3.6. Comprehensive Evaluation of the Effects of Different Nitrogen Application Levels on the Growth and Development of Wolfberry Plants
4. Discussion
4.1. Effects of Different Nitrogen Application Rates on the Root Growth of Wolfberry
4.2. Effects of Different Nitrogen Application Rates on the Distribution of Wolfberry Fine Roots in Different Diameter Size Classes
4.3. Effects of Different Nitrogen Application Rates on Nitrogen Contents and SPAD Values of Wolfberry Leaves
4.4. Effects of Different Nitrogen Application Rates on the Quality of Wolfberry Fruits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fukuda, T.; Yokoyama, J.; Ohashi, H. Phylogeny and biogeography of the genus Lycium (Solanaceae): Inferences from chloroplast DNA sequences. Mol. Phylogenet. Evol. 2001, 19, 246–258. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Meng, J.; Liu, Z.H.; Gou, C.H.; Rogers, K.; Yu, W.; Zhang, S.H.; Yuan, Y.; Zhang, L. Geographical origin of Chinese wolfberry (goji) determined by carbon isotope analysis of specific volatile compounds. J. Chromatogr. 2019, 1105, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, H.; Dong, X.; Yang, S.; Ma, S.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivities. Chin. Med. 2019, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Chen, C.C.; Ng, L.T. Nitrogen fertilization affects the growth performance, betaine and polysaccharide concentrations of Lycium barbarum. Ind. Crop Prod. 2010, 32, 650–655. [Google Scholar] [CrossRef]
- Wu, K.; Wang, S.; Song, W.; Zhang, J.; Wang, Y.; Liu, Q.; Yu, J.; Ye, Y.; Li, S.; Chen, J.; et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 367, eaaz3046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Zhang, W.; Cui, Z.; Ma, W.; Chen, X.; Jiang, R. Nutrient use efficiencies of major cereal crops in China and measures for improvement. Acta Pedol. Sin. 2008, 45, 915–924. [Google Scholar]
- Wu, D.L.; Xu, X.X.; Chen, Y.L.; Shao, H.; Sokolowski, E.; Mi, G.H. Effect of different drip fertigation methods on maize yield, nutrient and water productivity in two-soils in northeast China. Agric. Water Manag. 2019, 213, 200–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Zheng, G.; Wang, X.; Liu, G.; Zhang, Y.; Li, M. Effects of Different Fertilization Amounts on Growth, Yield and Appearance Quality of Lycium barbarum in Southern Xinjiang. Xinjiang Agric. Sci. 2018, 55, 2203. [Google Scholar]
- Shi, Z.; Wei, F.; Wan, R.; Li, Y.; Wang, Y.; An, W.; Qin, K.; Dai, G.; Cao, Y.; Feng, J. Impact of nitrogen fertilizer levels on metabolite profiling of the Lycium barbarum L. fruit. Molecules 2019, 24, 3879. [Google Scholar] [CrossRef]
- Lv, J.; Sheng, H.; Hua, M.; Nie, Y.; Gao, Y.; Xu, M.; Wei, J. Effects of Different Nitrogen Application Rates Combined with Nitrification Inhbibitor on Wolfberry Yield, Nitrogen Uptake and Utilization in Qaidam. Acta Agric. Boreali-Occident. Sin. 2023, 32, 1058–1067. [Google Scholar]
- Zeng, X.; Li, W.; Qiang, S.; Pan, J.; Zhang, Y.; Qi, G. Effects of straw mulch and irrigation on growth and water use efficiency of Lycium. Agric. Res. Arid. Areas 2013, 31, 61–65. [Google Scholar]
- Song, Y.; Chen, X.; Ren, X.; Gao, X. Effects of regulated deficit irrigation and reduced nitrogen fertilization on growth and yield of Lycium barbarum. Acta Agric. Boreali-Occident. Sin. 2019, 28, 1666–1673. [Google Scholar]
- Li, H.; Sun, Y.; Qu, J.; Wei, C.; Sun, G.; Zhao, Y.; Chai, Y. Influence of nitrogen levels on morphological and physiological characteristics of root system in japonica rice in northeast China. Chin. J. Rice Sci. 2012, 26, 723–730. [Google Scholar]
- Brinkman, M.; Rho, Y. Response of three oat cultivars to N fertilizer. Crop Sci. 1984, 24, 973–977. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, F.; Li, R.; Wang, X.; Xie, Y.; Wen, R.; Jia, Q. Optimal Resolution and Probability Threshold for the Semantic Segmentation of Spring Maize Root Image. J. Nucl. Agric. Sci. 2023, 37, 1690–1699. [Google Scholar]
- Jia, Q.; Xie, Y.; Zhao, Y.; Wang, R.; Liu, J.; Wen, R. Research on automatic recognition of plant root system image. J. Meteorol. Environ. 2022, 38, 105–111. [Google Scholar]
- Jia, Q.; Liu, X.; Xie, Y. A Root Image Recognition Method. CN114266903A, 1 April 2022. [Google Scholar]
- NY/T 1121.24–2012; Soil Testing—Part 24: Determination of Total Nitrogen in Soil—Automatic Kjeldahl Apparatus Method. Agricultural Industry Standards of People’s Republic of China: Beijing, China, 1 September 2012.
- NY/T 88–1988; Method for Determination of Soil Total Phosphorus. Agricultural Industry Standards of People’s Republic of China: Beijing, China, 1 March 1989.
- NY/T 87–1988; Method for Determination of Total Potassium in Soils. Agricultural Industry Standards of People’s Republic of China: Beijing, China, 1 March 1989.
- LY/T1228–2015; Nitrogen Determination Methods of Forest Soils. Forestry industry standard of the People’s Republic of China: Beijing, China, 1 January 2016.
- NY/T1121.7–2014; Soil Testing—Part 7: Method for Determination of Available Phosphorus in Soil. Agricultural Industry Standards of People’s Republic of China: Beijing, China, 1 January 2015.
- NY889–2004; Determination of Exchangeable Potassium and Non-Exchangeable Potassium Content in Soil. Agricultural Industry Standards of People’s Republic of China: Beijing, China, 1 February 2005.
- NY/T1121.6–2006; Soil Testing—Part 6: Method for Determination of Soil Organic Matter. Agricultural Industry Standards of People’s Republic of China: Beijing, China, 1 October 2006.
- HJ 802-2016; Soil Quality—Determination of Conductivity-Electrode Method. National Environmental Protection Standards of People’s Republic of China: Beijing, China, 1 August 2016.
- Trikilidou, E.; Samiotis, G.; Tsikritzis, L.; Amanatidou, E. Performance of Semi-Micro-Kjeldahl Nitrogen Method–uncertainty and nitrate interference. Int. J. Environ. Anal. Chem. 2022, 102, 6204–6214. [Google Scholar] [CrossRef]
- GB/T 18672-2014; Wolfberry. National Standards of People’s Republic of China: Beijing, China, 27 October 2014.
- Fang, L.; Zhu, M.; Zheng, C.H. Determination of Betaine in Lycium chinense by HPLC. Drug Stand. China 2011, 12, 288–291. [Google Scholar]
- Zhang, Y.; Zhang, L.; Zhou, H. Determination of Flavonoids in Lycium barbarum from Different Places. Chin. J. Tradit. Med. Sci. Technol. 2004, 2, 102–103. [Google Scholar]
- Mi, J.; Lv, L.; Dai, G.; He, X.; Li, X.; Yan, Y.; Qin, K. Correlations between Skin Color and Carotenoid Contents in Wolfberry. Food Sci. 2018, 39, 81–86. [Google Scholar]
- Tian, Z.H.; Fan, Y.; Yin, M.; Wang, F.; Cai, J.; Jiang, D.; Dai, T. Genetic improvement of root growth and its relationship with grain yield of wheat cultivars in the middle-lower Yangtze River. Acta Agron. Sin. 2015, 41, 613–622. [Google Scholar] [CrossRef]
- Joseph, M. Competition for nutrients and optimal root allocation. Plant Soil 2006, 285, 171–185. [Google Scholar]
- Li, X.; Lin, L.; Wang, K.; Cai, M.; Cao, C.; Jiang, Y. Root characteristics of Cry2A* transgenic rice under different nitrogen fertilizer conditions. J. Huazhong Agric. Univ. 2023, 42, 125–131. [Google Scholar] [CrossRef]
- Qiao, H.; Yang, H.; Shen, W.; Jiang, Q.; You, S.H.; Zhang, L.; Ran, K.; Zhang, X. Effect of nitrogen-deficient and iron-deficient on root architecture of young seedlings of Malus hupehensis (Pamp) Rehd. Acta Hortic. Sin. 2009, 36, 321–326. [Google Scholar]
- Kang, S.H.; Shi, W.; Zhang, J. An improved water-use efficiency for maize grown under regulated deficit irrigation. Field Crops Res. 2000, 67, 207–214. [Google Scholar] [CrossRef]
- Ju, X.; Pan, J.; Liu, X. Study on the fate of nitrogen fertilizer in winter wheat/summer maize rotation system in Beijing suburban. Plant Nutr. Fertil. Sci. 2003, 9, 264–270. [Google Scholar]
- Walch, L.; Ivanov, I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J. Plant Physiol. 2008, 165, 942–951. [Google Scholar] [CrossRef]
- Tian, Q.; Sun, P.; Zhang, W. Ethylene is involved in nitrate-dependent root growth and branching in Arabidopsis thaliana. New Phytol. 2009, 184, 918–931. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, J.; Gong, L. The morphological and chemical properties of fine roots respond to nitrogen addition in a temperate Schrenk’s spruce (Picea schrenkiana) forest. Sci. Rep. 2021, 11, 3839. [Google Scholar] [CrossRef] [PubMed]
- Sierra Cornejo, N.; Hertel, D.; Becker, J.N.; Hemp, A.; Leuschner, C. Biomass, Morphology, and Dynamics of the Fine Root System Across a 3000-M Elevation Gradient on Mt. Kilimanjaro. Front. Plant Sci. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tu, L.; Peng, Y.; Hu, H.; Hu, T.; Xu, Z.H.; Liu, L.; Tang, Y. Effect of nitrogen additions on root morphology and chemistry in a subtropical bamboo forest. Plant Soil 2017, 412, 441–451. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Zeng, H.; Liu, M.; Yuan, M.; Wu, H.; Kardol, P. The nutrient absorption-transportation hypothesis: Optimizing structural traits in absorptive roots. New Phytol. 2016, 213, 1569–1572. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Wang, H.; Wang, Z.H.; Gu, J. Root tip morphology, anatomy, chemistry and potential hydraulic conductivity vary with soil depth in three temperate hardwood species. Tree Physiol. 2016, 36, 99–108. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Hoch, G.; Wang, Z.H.; Gu, J. Linkage of root morphology to anatomy with increasing nitrogen availability in six temperate tree species. Plant Soil 2018, 425, 189–200. [Google Scholar] [CrossRef]
- Wang, G.; Fahey, T.; Xue, S.; Liu, F. Root morphology and architecture respond to N addition in Pinus tabuliformis, west China. Oecologia 2013, 171, 583–590. [Google Scholar] [CrossRef]
- Noguchi, K.; Nagakura, J.; Kaneko, S. Biomass and morphology of fine roots of sugi (Cryptomeria japonica) after 3 years of nirogen fertilization. Front. Plant Sci. 2013, 4, 347. [Google Scholar] [CrossRef]
- Geng, P.; Jin, G. Fine root morphology and chemical responses to N addition depend on root function and soil depth in a Korean pine plantation in Northeast China. For. Ecol. Manag. 2022, 520, 120407. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Song, S. Relationships between SPAD Readings and the Contents of Chlorophy II and Nitrogen in Chinese Cabbage Leaves. North. Hortic. 2010, 23, 15–17. [Google Scholar]
- Song, Y. Study on absolute SPAD value and relative SPAD value of maize leaves in different treatments. China Seed Ind. 2021, 7, 66–69. [Google Scholar]
- Zhang, J.; Li, G.; Yuan, J.; Yan, L.; Wei, X.; Liu, S.H. Effects of water and nitrogen regulation on soil and leaf stoichiomertic characteristics of spring wheat in dry farming. Arid. Zone Res. 2021, 38, 1750–1759. Available online: http://kns.cnki.net/kcms/detail/65.1095.X.20210928.1840.004.html (accessed on 26 May 2023).
- Zhu, Q.; Mo, B.; Zh, E. Different nitrogen rate on growth and flowering of pepper. Guizhou Agric. Sci. 2008, 36, 438–442. [Google Scholar]
- Li, Z.; Li, A.; Zh, L.; Cao, C.H.; Tian, S. Effect of nitrogen fertilizer application on nitrogen content of different position leaves and yield of Jindan 84. J. Shanxi Agric. Sci. 2015, 43, 1285–1289. [Google Scholar]
- Maiti, D.; Das, D.; Karak, T.; Banerjee, M. Management of nitrogen through the use of leaf color chart (LCC) and soil plant analysis development (SPAD) or chlorophyll meter in rice under irrigated ecosystem. Sci. World J. 2004, 4, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, H.; Wang, Z.H. Evaluation of SPAD and Dualex for in-season corn nitrogen status estimation. Acta Agron. Sin. 2010, 36, 840–847. [Google Scholar]
- Sandhu, O.; Gupta, R.; Thind, H.; Jat, M.; Sidhu, H.; Singh, Y. Drip irrigation and nitrogen management for improving crop yields, nitrogen use efficiency and water productivity of maize-wheat system on permanent beds in northwest India. Agric. Water Manag. 2019, 219, 19–26. [Google Scholar] [CrossRef]
- Liu, X.; Ma, L.; Li, J.; Yang, R.; Liu, Y.; Zi, H. Effect of drip fertigation on lycium yield and quality in sandy land in northern shaanxi. J. Irri. Drain. 2020, 39, 13–16. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Han, H.; Yang, Q.; Liu, X. Coupling scheme optimization of panax notoginseng considering yield, quality and water-fertilizer use efficiency. Trans. Chin. Soc. Agric. Eng. 2021, 37, 139–146. [Google Scholar] [CrossRef]
- Ma, X.; Guo, Y.; Li, M.; Ma, X.; Zhang, Z.H.; Zhu, W.D. Leaf CO2 response curve and fruit medicinal components of Lycium ruthenicum affected by nitrogen application in the arid area. Acta Bot. Boreali-Occident. Sin. 2020, 40, 1209–1218. [Google Scholar]
- Ma, B.; Tian, J. Advance in research on water and fertilizer effect on yield and quality of Lycium Barbarum L. Water Sav. Irrig. 2020, 11, 6–11. [Google Scholar]
- Ma, Z.H.; Yin, J.; Yang, Y.; Sun, F.; Yang, Z.H. Effect of water and nitrogen coupling regulation on the growth, physiology, yield, and quality attributes and comprehensive evaluation of wolfberry (Lycium barbarum L.). Front. Plant Sci. 2023, 14, 1130109. [Google Scholar] [CrossRef] [PubMed]





| Different Treatment | Budding and Unfolding Stage | Leaf Growth Period and Flowering Period | Summer Fruit Period | Autumn Branch Budding Period and Autumn Flowering Period | Autumn Fruiting Period | Cumulative Increment |
|---|---|---|---|---|---|---|
| N1 | 959.58 ± 104.98 a | 803.20 ± 57.04 b | 443.69 ± 123.66 a | 795.19 ± 14.72 a | 111.99 ± 5.37 b | 3113.65 |
| N2 | 389.81 ± 61.66 b | 1479.95 ± 61.83 a | 440.25 ± 106.10 a | 806.94 ± 24.63 a | 440.55 ± 22.57 a | 3557.5 |
| N3 | 165.21 ± 94.08 b | 734.15 ± 24.49 b | 489.26 ± 79.42 a | 384.08 ± 48.84 b | 234.30 ± 48.57 b | 2007 |
| Phenological Period | Time | Increment of Different-Class Fine root Growth/(cm) | |||||
|---|---|---|---|---|---|---|---|
| Diameter 0–0.4 mm | Diameter 0.41–0.8 mm | Diameter 0.81–1.2 mm | Diameter 1.21–1.6 mm | Diameter 1.61–2.0 mm | Diameter >2.0 mm | ||
| Budding and unfolding stage | 4.10–5.10 | 211.49 ± 23.51 b | 547.44 ± 183.85 a | 159.51 ± 20.26 bc | 30.40 ± 8.22c | 7.64 ± 0.42 c | 3.09 ± 1.37 c |
| Leaf growth period and flowering period | 5.10–6.10 | 186.68 ± 42.75 b | 303.55 ± 99.97 a | 237.46 ± 52.30 ab | 51.94 ± 3.71 c | 15.16 ± 3.25 c | 8.40 ± 2.51 c |
| Summer fruit period | 6.10–7.10 | 139.64 ± 53.15 ab | 234.52 ± 119.65 a | 52.45 ± 44.94 b | 13.87 ± 32.09 b | 2.76 ± 12.60 b | 0.47 ± 8.88 b |
| Autumn branch budding period and autumn flowering period | 7.10–8.10 | 109.57 ± 22.97 c | 389.85 ± 39.43 a | 235.89 ± 31.38 b | 41.29 ± 23.30 d | 13.20 ± 12.95 d | 5.39 ± 9.92 d |
| Autumn fruiting period | 8.10–9.10 | −36.68 ± 103.88 a | 65.34 ± 38.78 a | 14.50 ± 18.47 a | 0.69 ± 2.53 a | −0.66 ± 0.97 a | 1.22 ± 1.81 a |
| Cumulative root growth | 610.70 | 1540.70 | 699.81 | 138.19 | 38.10 | 18.57 | |
| The proportion of the total increment of the annual root length | 20.0% | 50.6% | 23.0% | 4.5% | 1.3% | 0.6% | |
| Phenological Period | Time | Increment of Different-Class Fine Root Growth/(cm) | |||||
|---|---|---|---|---|---|---|---|
| Diameter 0–0.4 mm | Diameter 0.41–0.8 mm | Diameter 0.81–1.2 mm | Diameter 1.21–1.6 mm | Diameter 1.61–2.0 mm | Diameter >2.0 mm | ||
| Budding and unfolding stage | 4.10–5.10 | 121.85 ±18.11b | 326.30 ± 62.33 a | −51.61 ± 21.32 c | −6.91 ± 3.70 c | 0.77 ± 0.35 c | −0.59 ± 2.91 c |
| Leaf growth period and flowering period | 5.10–6.10 | 306.19 ± 37.39 c | 601.35 ± 33.75 a | 460.92 ± 134.65 b | 87.98 ± 30.65 d | 17.26 ± 9.96 d | 6.24 ± 2.38 d |
| Summer fruit period | 6.10–7.10 | 59.74 ± 27.30 a | 162.95 ± 142.64 a | 166.50 ± 165.23 a | 38.11 ± 30.80 a | 9.51 ± 7.40 a | 3.44 ± 3.85 a |
| Autumn branch budding period and autumn flowering period | 7.10–8.10 | 135.10 ± 28.36 b | 438.28 ± 65.68 a | 194.79 ± 57.76 b | 31.18 ± 10.15 c | 6.00 ± 1.87 c | 1.59 ± 0.99 c |
| Autumn fruiting period | 8.10–9.10 | 94.06 ± 56.63 b | 282.44 ± 50.95 a | 55.25 ± 20.41 bc | 8.19 ± 4.67 c | 0.47 ± 0.99 c | 0.15 ± 0.26 c |
| Cumulative root growth | 716.94 | 1811.32 | 825.85 | 158.55 | 34.01 | 10.83 | |
| The proportion of the total increment of the annual root length | 20.15% | 50.92% | 23.21% | 4.46% | 0.96% | 0.30% | |
| Phenological Period | Time | Increment of Different-Class Fine Root Growth/(cm) | |||||
|---|---|---|---|---|---|---|---|
| Diameter 0–0.4 mm | Diameter 0.41–0.8 mm | Diameter 0.81–1.2 mm | Diameter 1.21–1.6 mm | Diameter 1.61–2.0 mm | Diameter >2.0 mm | ||
| Budding and unfolding stage | 4.10–5.10 | 4.39 ± 0.25 b | 138.47 ± 8.25 a | 5.61 ± 0.05 b | 9.52 ± 0.52 b | 5.43 ± 0.58 b | 1.79 ± 0.76 b |
| Leaf growth period and flowering period | 5.10–6.10 | 101.96 ± 70.45 b | 290.77 ± 40.54 a | 263.78 ± 110.95 a | 58.89 ± 34.27 b | 12.46 ± 6.41 b | 6.29 ± 2.42 b |
| Summer fruit period | 6.10–7.10 | 46.85 ± 2.44 b | 193.43 ± 40.00 a | 183.52 ± 112.72 a | 49.36 ± 35.85 b | 11.54 ± 9.54 b | 4.56 ± 5.06 b |
| Autumn branch budding period and autumn flowering period | 7.10–8.10 | −37.02 ± 75.36 b | 228.53 ± 62.77 a | 154.85 ± 58.04 a | 26.43 ± 19.63 b | 6.00 ± 3.95 b | 5.28 ± 7.02 b |
| Autumn fruiting period | 8.10–9.10 | 135.75 ± 43.14 a | 106.45 ± 61.55 a | −6.29 ± 15.68 b | −1.49 ± 3.70 b | −0.11 ± 1.33 b | −0.02 ± 0.33 b |
| Cumulative root growth | 251.93 | 957.65 | 601.47 | 142.71 | 35.32 | 17.9 | |
| The proportion of the total increment of the annual root length | 12.55% | 47.72% | 29.97% | 7.11% | 1.76% | 0.89% | |
| Leaf Blade Part | Nitrogen Content | SPAD | ||||
|---|---|---|---|---|---|---|
| N1 | N2 | N3 | N1 | N2 | N3 | |
| The tender tip leaf | 9.42 ± 1.93 b | 9.64 ± 2.03 a | 10.54 ± 1.04 a | 21.47 ± 6.40 b | 22.13 ± 6.22 a | 24.93 ± 3.21 a |
| Middle leaf | 11.48 ± 0.32 c | 12.02 ± 0.98 a | 11.74 ± 1.51 b | 27.88 ± 5.46 c | 29.62 ± 3.10 a | 28.74 ± 4.76 b |
| Basal leaf | 12.30 ± 1.36 c | 12.52 ± 1.36 a | 12.50 ± 1.30 b | 21.22 ± 4.30 b | 31.20 ± 4.92 a | 31.14 ± 4.12 b |
| Different Nitrogen Treatment | Total Sugar/(g·100 g−1) | Lycium Barbarum Polysaccharide/(g·100 g−1) | Total Flavonoids/(g·100 g−1) | Betaine/(g·100 g−1) | β-Carotene/(g·100 g−1) |
|---|---|---|---|---|---|
| N1 | 34.90 ± 0.00 c | 5.62 ± 0.07 a | 0.057 ± 0.000 a | 1.22 ± 0.01b | 9.66 ± 0.07 b |
| N2 | 35.63 ± 0.15 b | 4.96 ± 0.13 b | 0.049 ± 0.000 b | 1.22 ± 0.03b | 10.43 ± 0.19 a |
| N3 | 39.60 ± 0.00 a | 4.75 ± 0.19 b | 0.048 ± 0.000 c | 1.54 ± 0.03a | 10.61 ± 0.38 a |
| Project | PC1 | PC2 |
|---|---|---|
| Total root length increment | 0.346 | 0.120 |
| Total root projection area increment | 0.235 | 0.330 |
| Total root surface area increments | 0.237 | 0.327 |
| Root mean diameter increment | −0.221 | −0.344 |
| Root volume increment | 0.252 | 0.311 |
| The number of root tips increment | 0.336 | 0.158 |
| Mean leaf nitrogen content | −0.285 | 0.266 |
| Mean leaf SPAD | −0.216 | 0.348 |
| Total sugar | −0.356 | 0.064 |
| Lycium barbarum polysaccharide | 0.247 | −0.317 |
| Total flavonoids | 0.210 | −0.353 |
| Betaine | −0.360 | 0.001 |
| β-carotene | −0.232 | 0.332 |
| Eigenvalue | 7.698 | 5.302 |
| Contribution rate of variance | 59.212 | 40.788 |
| Cumulative variance contribution rate | 59.212 | 100.000 |
| Different Treatments | F1 | F2 | F | Rank |
|---|---|---|---|---|
| N1 | 1.594 | −2.308 | 0.002 | 2 |
| N2 | 1.609 | 2.301 | 1.891 | 1 |
| N3 | −3.203 | 0.006 | −1.894 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; An, W.; Li, Y.; Wang, Y.; Su, S. Effects of Different Nitrogen Application Rates on Root Growth and Distribution of Fine Root Length across Diameter Classes of Wolfberry (Lycium barbarum L.). Forests 2023, 14, 2317. https://doi.org/10.3390/f14122317
Liang X, An W, Li Y, Wang Y, Su S. Effects of Different Nitrogen Application Rates on Root Growth and Distribution of Fine Root Length across Diameter Classes of Wolfberry (Lycium barbarum L.). Forests. 2023; 14(12):2317. https://doi.org/10.3390/f14122317
Chicago/Turabian StyleLiang, Xiaojie, Wei An, Yuekun Li, Yajun Wang, and Shuchai Su. 2023. "Effects of Different Nitrogen Application Rates on Root Growth and Distribution of Fine Root Length across Diameter Classes of Wolfberry (Lycium barbarum L.)" Forests 14, no. 12: 2317. https://doi.org/10.3390/f14122317
APA StyleLiang, X., An, W., Li, Y., Wang, Y., & Su, S. (2023). Effects of Different Nitrogen Application Rates on Root Growth and Distribution of Fine Root Length across Diameter Classes of Wolfberry (Lycium barbarum L.). Forests, 14(12), 2317. https://doi.org/10.3390/f14122317





