Changes in Soil Fungal Diversity and Composition along a Rural–Urban Gradient
Abstract
:1. Introduction
- (1)
- Spores are the main dispersal mode of fungi [24]. Forest patches in urban environments receive reduced numbers of migrating fungal spores, which could result in decreased fungal diversity and altered composition of the soil fungal community [18]. We therefore hypothesize that the species richness of fungi in the soil decreases with the increasing degree of urbanization and that increasing urbanization leads to shifts in the composition of fungi.
- (2)
- The different fungal phyla differ in their susceptibility to changes in biotic and abiotic characteristics of temperate forests [25]. Urbanization can change vegetation characteristics and soil properties [26]. We therefore hypothesize that urbanization-induced changes in forest characteristics will affect the different fungal phyla in different ways.
- (3)
- Symbiotrophic fungi are sensitive to disturbances [27]. This may result in a lower abundance and/or species richness of symbiotrophic soil fungi in urban than in rural habitats [28,29]. Based on these findings, we hypothesize that the abundance of symbiotrophic fungi decreases with the increasing degree of urbanization.
2. Materials and Methods
2.1. Study Area
2.2. Design of the Field Survey
2.3. Forest Vegetation Survey
2.4. Soil Sampling and Soil Properties
2.5. Soil Fungal Community
2.6. Data Analyses
3. Results
3.1. Vegetation Characteristics and Soil Properties
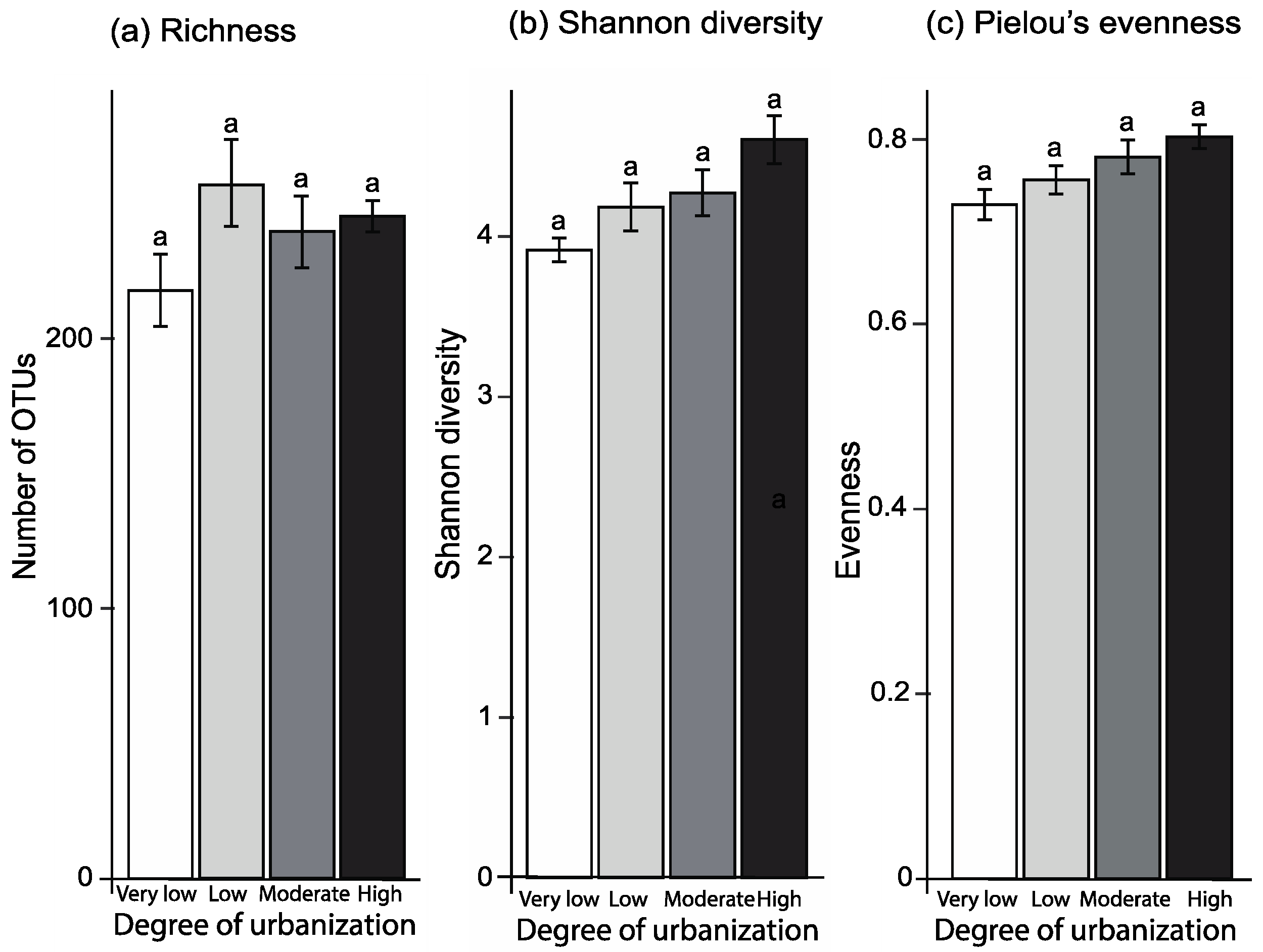
3.2. Diversity and Composition of Fungal Communities
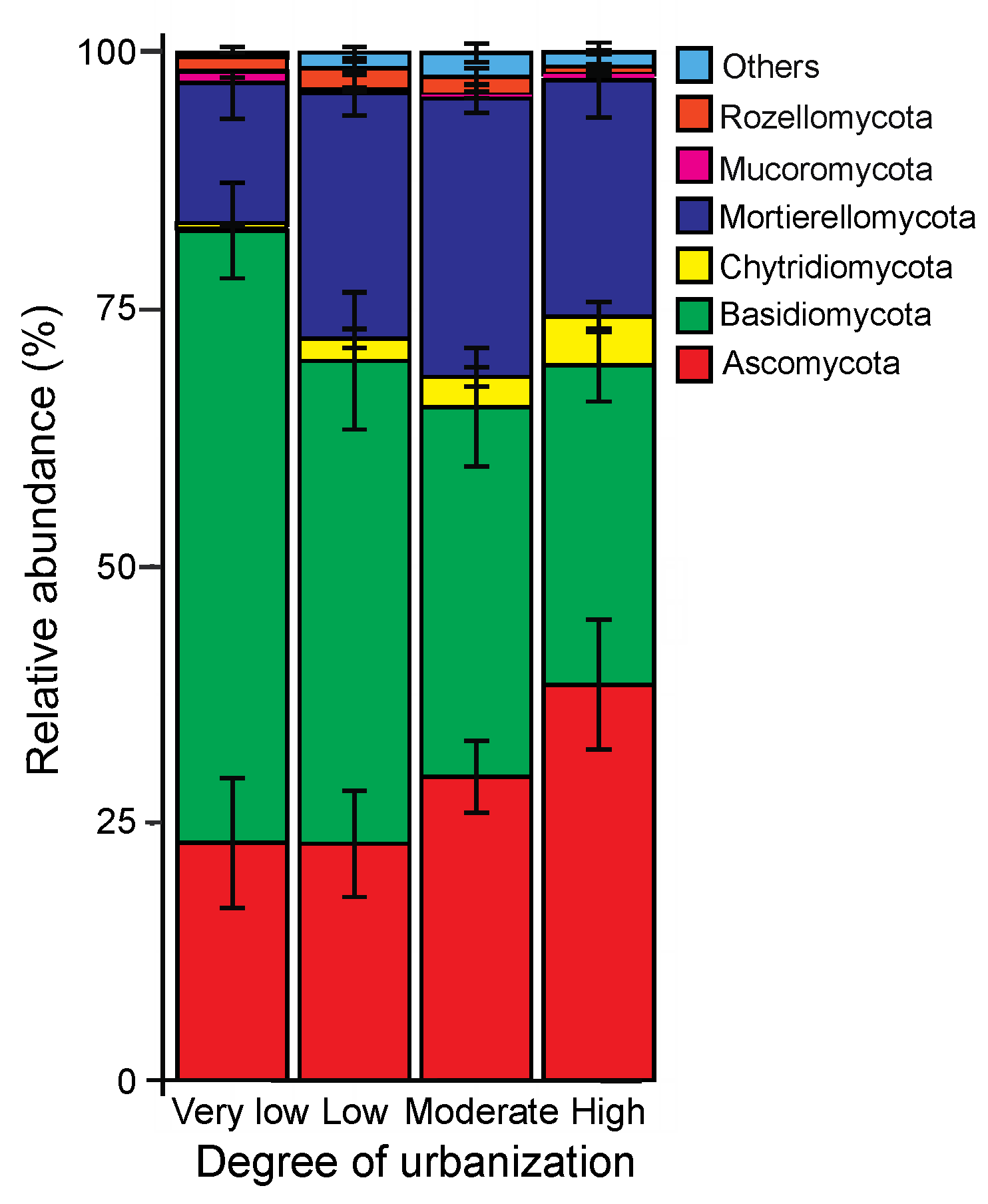
3.3. Taxonomic Composition of Soil Fungal Community
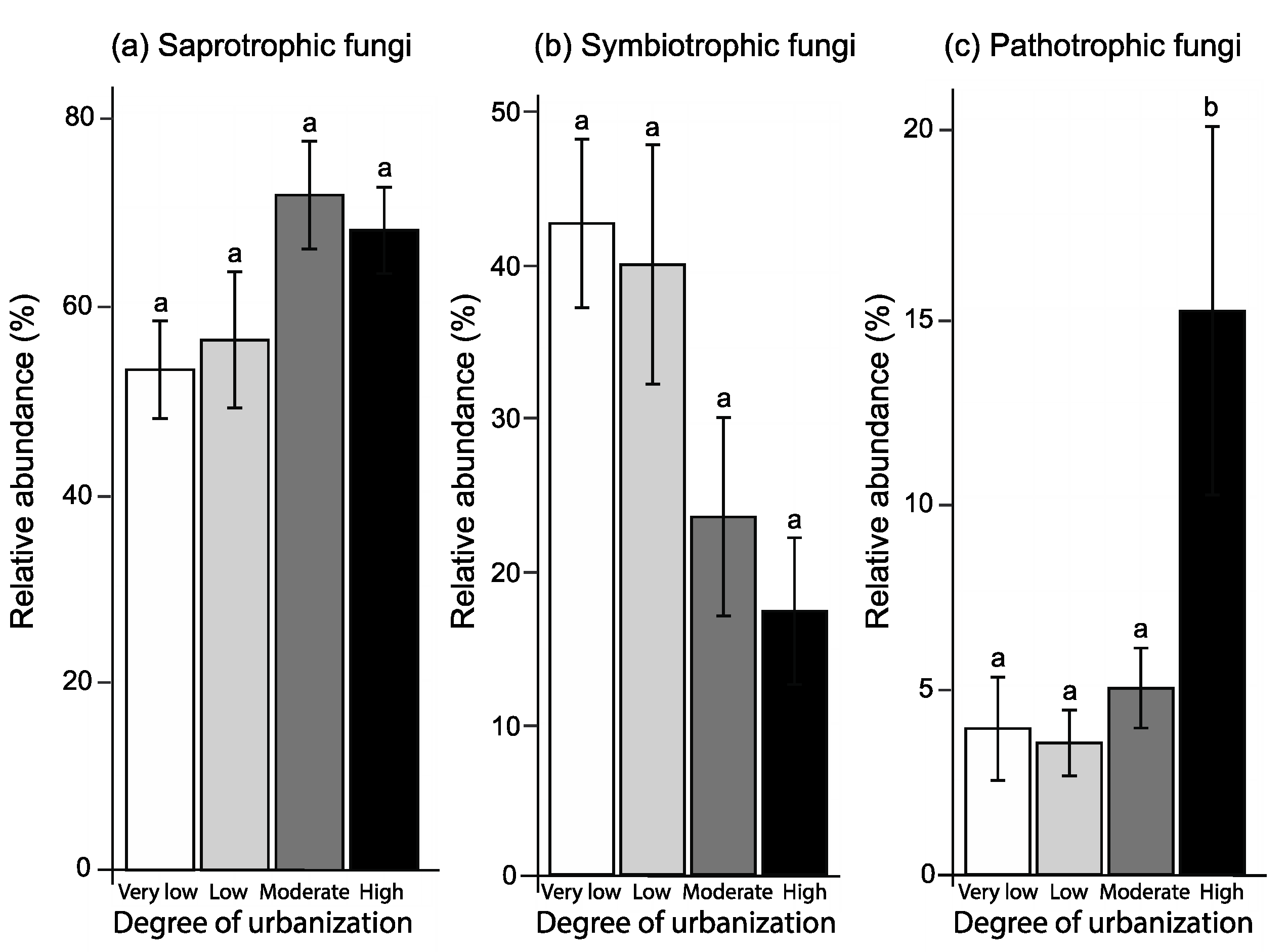
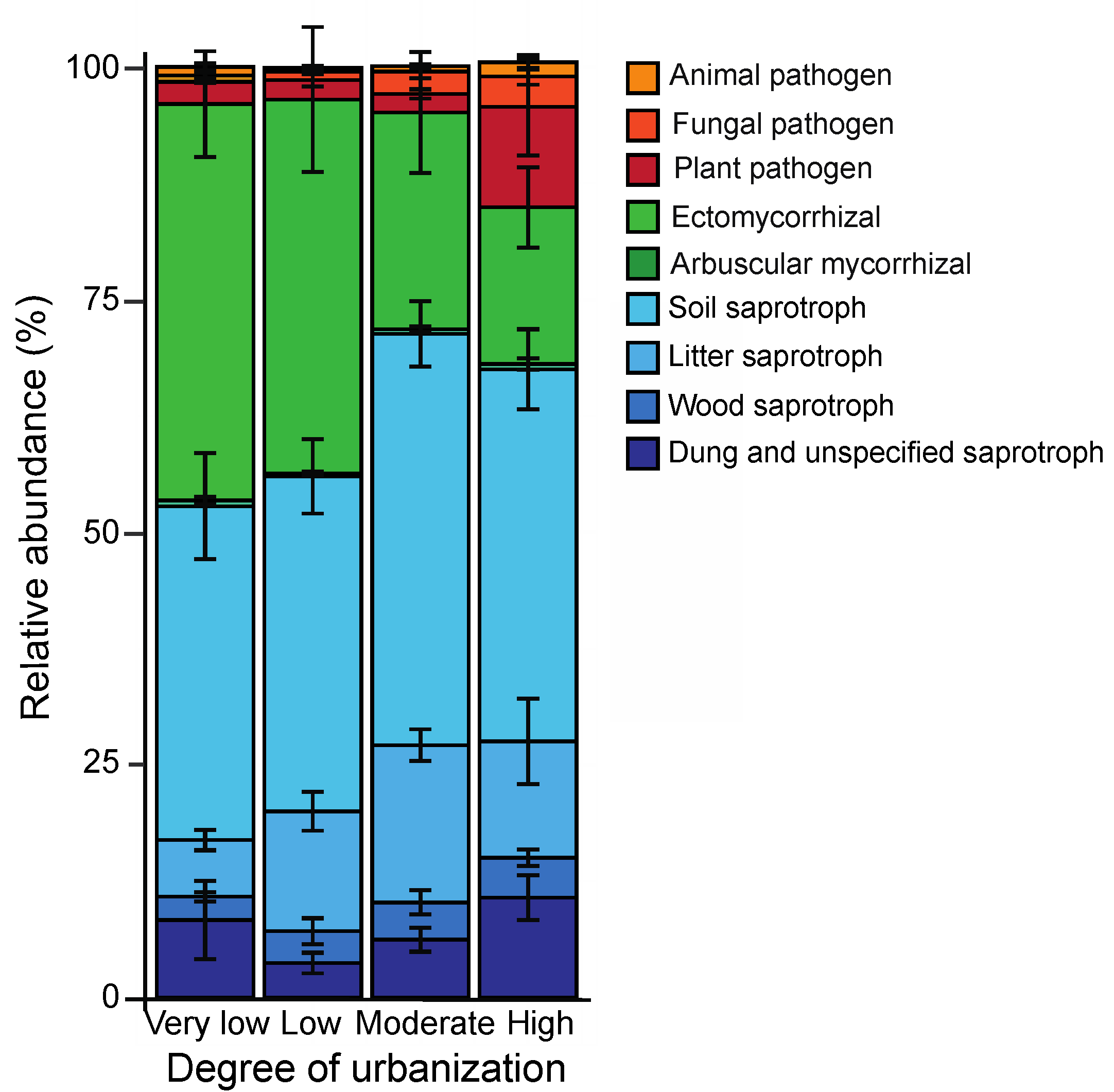
3.4. Soil Fungal Functional Composition
4. Discussion
4.1. Diversity and Composition of Fungal Communities
4.2. Composition of the Soil Fungal Communities at the Phylum Level
4.3. Soil Fungal Functional Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.G.; Bai, X.M.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Cvejić, R.; Eler, K.; Pintar, M.; Železnikar, Š.; Haase, D.; Kabisch, N.; Strohbach, M. A Typology of Urban Green Spaces, Ecosystem Services Provisioning Services and Demands; Report D3.1; European Union: Brüssel, Belgium, 2015. [Google Scholar]
- Grammatikopoulou, I.; Vackarova, D. The value of forest ecosystem services: A meta-analysis at the European scale and application to national ecosystem accounting. Ecosyst. Serv. 2021, 48, 101262. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Boone, C.G.; Groffman, P.M.; Irwin, E.; Kaushal, S.S.; Marshall, V.; McGrath, B.P.; Nilon, C.H.; et al. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manag. 2011, 92, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Melliger, R.L.; Braschler, B.; Rusterholz, H.P.; Baur, B. Diverse effects of degree of urbanisation and forest size on species richness and functional diversity of plants, and ground surface-active ants and spiders. PLoS ONE 2018, 13, e0199245. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, M.S.; Rossetti, M.R.; Videla, M. Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1412–1429. [Google Scholar] [CrossRef]
- Meyer, S.; Rusterholz, H.P.; Baur, B. Saproxylic insects and fungi in deciduous forests along a rural–urban gradient. Ecol. Evol. 2021, 11, 1634–1652. [Google Scholar] [CrossRef] [PubMed]
- Chapin III, F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Decon, J.W. Fungal Biology, 4th ed.; John Willey & Sons: New York, NY, USA, 2005; p. 384. [Google Scholar]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on land. Microb. Molec. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Ecol. 2017, 41, 109–130. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Kadowaki, K.; Yamamoto, S.; Sato, H.; Tanabe, A.S.; Hidaka, A.; Toju, H. Mycorrhizal fungi mediate the direction and strength of plant-soil feedbacks differently between arbuscular mycorrhizal and ectomycorrhizal communities. Community Biol. 2018, 1, 196. [Google Scholar] [CrossRef] [PubMed]
- Gomdola, D.; Bhunjun, C.S.; Hyde, K.D.; Jeewon, R.; Pem, D.; Jayawardena, R.S. Ten important forest fungal pathogens: A review on their emergence and biology. Mycosphere 2022, 13, 612–671. [Google Scholar] [CrossRef]
- Terhonen, E.; Kovalchuk, A.; Zarsav, A.; Asiegbu, F.O. Biocontrol potential of forest tree endophytes. In Endophytes of Forest Trees: Biology and Applications, 2nd ed.; Pirttilä, M.A., Frank, A.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 283–318. [Google Scholar] [CrossRef]
- Dobson, A.; Crawley, W. Pathogens and the structure of plant-communities. Trends Ecol. Evol. 1994, 9, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Abrego, N.; Crosier, B.; Somervuo, P.; Ivanova, N.; Abrahamyan, A.; Abdi, A.; Hamalainen, K.; Junninen, K.; Maunula, M.; Purhonen, J.; et al. Fungal communities decline with urbanization—More in air than in soil. ISME J. 2020, 14, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Martinez, A.; Cleavenger, S.; Rudolph, J.; Barberan, A. Changes in soil microbial communities across an urbanization gradient: A local-scale temporal study in the arid Southwestern USA. Microorganisms 2021, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Barberan, A.; Gao, C.; Zhang, Z.C.; Wang, M.; Wurzburger, N.; Wang, X.; Zhang, R.; Li, J.X.; Zhang, J. Impact of urbanization on soil microbial diversity and composition in the megacity of Shanghai. Land Degrad. Dev. 2022, 33, 282–293. [Google Scholar] [CrossRef]
- Whitehead, J.; Roy, J.; Hempel, S.; Rillig, M.C. Soil microbial communities shift along an urban gradient in Berlin, Germany. Front. Microbiol. 2022, 13, 972052. [Google Scholar] [CrossRef]
- Tan, X.L.; Kan, L.; Su, Z.Y.; Liu, X.D.; Zhang, L. The composition and diversity of soil bacterial and fungal communities along an urban-to-rural gradient in South China. Forests 2019, 10, 797. [Google Scholar] [CrossRef]
- Wang, M.; Yu, S.Q.; Chen, X.H.; Liu, X.D.; Zeng, H.X.; Wu, W.K.; Liu, M.Y.; Su, C.H.; Xu, G.L. Soil microbial community changes in response to the environmental gradients of urbanization in Guangzhou City. Urban Ecosyst. 2022, 25, 1865–1874. [Google Scholar] [CrossRef]
- Chaudhary, V.B.; Aguilar-Trigueros, C.A.; Mansour, I.; Rillig, M.C. Fungal dispersal across spatial scales. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 69–85. [Google Scholar] [CrossRef]
- Osburn, E.D.; McBride, S.G.; Aylward, F.O.; Badgley, B.D.; Strahm, B.D.; Knoepp, J.D.; Barrett, J.E. Soil bacterial and fungal communities exhibit distinct long-term responses to disturbance in temperate forests. Front. Microbiol. 2019, 10, 2872. [Google Scholar] [CrossRef] [PubMed]
- Enescu, R.E.; Dinca, L.; Zup, M.; Davidescu, S.; Vasile, D. Assessment of soil physical and chemical properties among urban and peri-urban forests: A case study from metropolitan area of Brasov. Forests 2022, 13, 1070. [Google Scholar] [CrossRef]
- Rodrigez-Ramos, J.C.; Cale, J.A.; Cahill, J.F., Jr.; Simard, S.W.; Karst, J.; Erbilgin, N. Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phyt. 2021, 229, 105–1117. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.J.E.; Pouyat, R.; Szlavecz, K.; Setälä, H.; Kotze, D.J.; Yesilonis, I.; Cilliers, S.; Hornung, E.; Dombos, M.; Yarwood, S.A. Urbanization erodes ectomycorrhizal fungal diversity and may cause microbial communities to converge. Nat. Ecol. Evol. 2017, 1, 0123. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.Y.; Yang, B.S.; Wang, H.; Sun, W.; Jiao, K.Q.; Qin, G.H. Changes in soil ectomycorrhizal fungi community in oak forests along the urban-rural gradient. Forests 2022, 13, 675. [Google Scholar] [CrossRef]
- Statistischer Atlas der Schweiz 2021. Available online: https://www.bfs.admin.ch/bfs/de/home/statistiken/regionalstatistik/atlanten/statistischer-atlas-schweiz.html (accessed on 10 May 2023).
- Burnand, J.; Hasspacher, B. Waldstandorte beider Basel. In Quellen und Forschungen zur Geschichte und Landeskunde des Kanton Basel-Landschaft, 2nd ed.; Verlag des Kantons Basel-Landschaft: Liestal, Switzerland, 1999; p. 266. [Google Scholar]
- Walthert, L.; Zimmermann, S.; Blaser, P.; Luster, J.; Lüscher, P. Waldböden der Schweiz 1. Grundlagen und Region Jura; Ott Verlag: Thun, Switzerland, 2004; p. 768. [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Blackburn Press: Caldwell, NJ, USA, 2002; p. 547. [Google Scholar]
- Allen, S.E. Chemical Analysis of Ecological Materials, 2nd ed.; Blackwell Scientific: Oxford, UK, 1989; p. 368. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfan, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 7, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2018, 47, D259–D264. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Version 3.6.3; Available online: https://www.R-project.org/ (accessed on 13 September 2023).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, R.; Stevens, M.H.H.; Wagner, H. Vegan Community Ecology Package. R Package Version 2.0–10. Available online: http://CRAN.R-project.org/package=vegan (accessed on 11 May 2022).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Goldmann, K.; Schröter, K.; Pena, R.; Schöning, I.; Schrumpf, M.; Buscot, F.; Polle, A.; Wubet, T. Divergent habitat filtering of root and soil fungal communities in temperate beech forests. Sci. Rep. 2016, 6, 31439. [Google Scholar] [CrossRef]
- Bahnmann, B.; Masinova, T.; Halvorsen, R.; Davey, M.L.; Sedlak, P.; Tomsovsky, M.; Baldrian, P. Effects of oak, beech and spruce on the distribution and community structure of fungi in litter and soils across a temperate forest. Soil Biol. Biochem. 2018, 119, 162–173. [Google Scholar] [CrossRef]
- Clausing, S.; Likulunga, L.E.; Janz, D.; Feng, H.Y.; Schneider, D.; Daniel, R.; Kruger, J.; Lang, F.; Polle, A. Impact of nitrogen and phosphorus addition on resident soil and root mycobiomes in beech forests. Biol. Fertil. Soils 2021, 57, 1031–1052. [Google Scholar] [CrossRef]
- Gorfer, M.; Mayer, M.; Berger, H.; Rewald, B.; Tallian, C.; Matthews, B.; Sanden, H.; Katzensteiner, K.; Godbold, D.L. High fungal diversity but low seasonal dynamics and ectomycorrhizal abundance in a mountain beech forest. Microb. Ecol. 2021, 82, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Scholier, T.; Lavrinienko, A.; Brila, I.; Tukalenko, E.; Hindstrom, R.; Vasylenko, A.; Cayol, C.; Ecke, F.; Singh, N.J.; Forsman, J.T.; et al. Urban forest soils harbour distinct and more diverse communities of bacteria and fungi compared to less disturbed forest soils. Mol. Ecol. 2023, 32, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Drenkhan, R.; Pritsch, K.; Buegger, F.; Padari, A.; Hagh-Doust, N.; Mikryukov, V.; Gohar, D.; et al. Regional-scale in-depth analysis of soil fungal diversity reveals strong pH and plant species effects in Northern Europe. Front. Microbiol. 2020, 11, 1953. [Google Scholar] [CrossRef] [PubMed]
- MacKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Christel, A.; Dequiedt, S.; Chemidlin-Prevost-Bouré, N.; Mercier, F.; Tripied, J.; Comment, G.; Djemiel, C.; Bargeot, L.; Matagne, E.; Fougeron, A.; et al. Urban land uses shape soil microbial abundance and diversity. Sci. Total Environ. 2023, 883, 163455. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Cajthaml, T.; Polme, S.; Hiiesalu, I.; Anslan, S.; Harend, H.; Buegger, F.; Pritsch, K.; Koricheva, J.; et al. Tree diversity and species identity effects on soil fungi, protists and animals are context dependent. ISME J. 2016, 10, 346–362. [Google Scholar] [CrossRef]
- Wubet, T.; Christ, S.; Schoning, I.; Boch, S.; Gawlich, M.; Schnabel, B.; Fischer, M.; Buscot, F. Differences in soil fungal communities between European Beech (Fagus sylvatica L.) dominated forests are related to soil and understory vegetation. PLoS ONE 2012, 7, e47500. [Google Scholar] [CrossRef]
- Kameshwar, A.K.S.; Qin, W.S. Systematic review of publicly available non-Dikarya fungal proteomes for understanding their plant biomass-degrading and bioremediation potentials. Bioresour. Bioprocess. 2019, 6, 30. [Google Scholar] [CrossRef]
- Hanrahan-Tan, D.G.; Lilje, O.; Henderson, L. Chytrids in soil environments: Unique adaptations and distributions. Encyclopedia 2023, 3, 642–664. [Google Scholar] [CrossRef]
- Blackwell, M. The Fungi: 1, 2, 3… 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kuang, J.L.; Wang, P.D.; Shu, W.S.; Barberan, A. Associations between human impacts and forest soil microbial communities. Elementa 2020, 8, 005. [Google Scholar] [CrossRef]
- Leake, J.R.; Johnson, D.; Donnelly, D.P.; Muckle, G.E.; Boddy, L.; Read, D.J. Networks of power and influence: The role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can. J. Bot. 2004, 82, 1016–1045. [Google Scholar] [CrossRef]
- Bödeker, I.T.M.; Lindahl, B.D.; Olson, Å.; Clemmensen, K.E. Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef]
- Marx, D.H. Ectomycorrhizae as biological deterrents to pathogenic root infections. Annu. Rev. Phytopathol. 1972, 10, 429–441. [Google Scholar] [CrossRef] [PubMed]
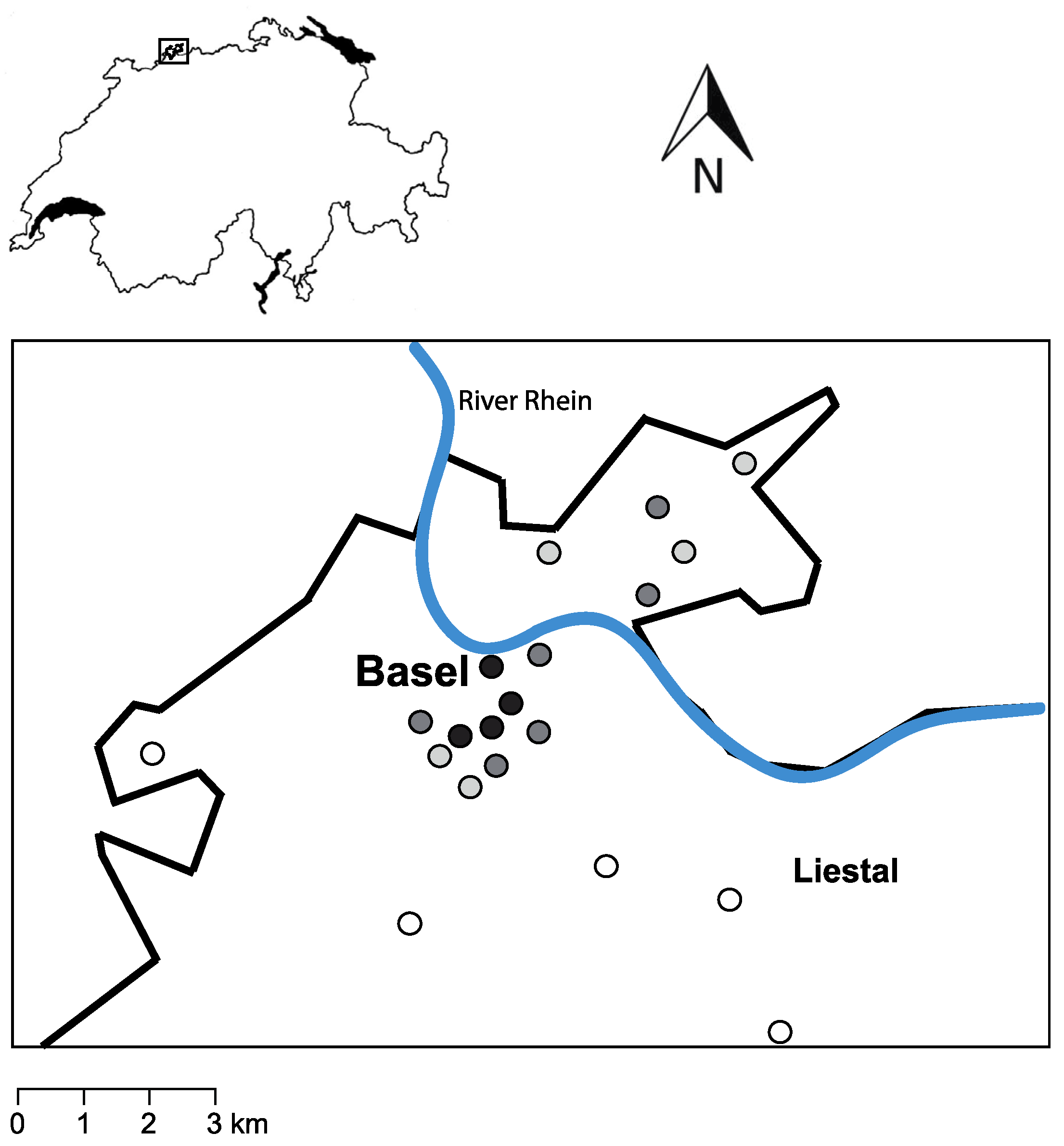

| Forest | Coordinates | Historical Development 1 | Forest Vegetation 2 | Elevation (m a.s.l.) | Exposure 3 | % Cover of Sealed Area (r = 500 m) | Degree of Urbanization 4 | Forest Area (ha) | % Cover of Forest (r = 500 m) |
|---|---|---|---|---|---|---|---|---|---|
| BS1 | 47°33′13″ N 7°36′17″ E | Planted | Galio-Fagetum Pulmonarietosum | 363 | WNW | 59 | 4 | 0.33 | 2 |
| BS2 | 47°33′14″ N 7°36′49″ E | Fragment | Galio Odorati-Fagetum Cornetosum | 262 | NE | 39 | 3 | 1.42 | 3 |
| BS3 | 47°33′55″ N 7°38′41″ E | Planted | Galio Odorati-Fagetum Pulmonarietosum | 319 | NNW | 30 | 3 | 0.41 | 56 |
| BS4 | 47°32′12″ N 7°36′6″ E | Fragment | Galio Odorati-Fagetum Cornetosum | 321 | NE | 54 | 4 | 1.16 | 13 |
| BS5 | 47°32′04.6″ N 7°31′16.2″ E | Forest | Galio-Fagetum Pulmonarietosum | 351 | – | 1 | 1 | 76.2 | 45 |
| BS6 | 47°34′53″ N 7°38′52″ E | Planted | Galio Odorati-Fagetum | 283 | – | 33 | 3 | 0.33 | 1 |
| BS7 | 47°32′18″ N 7°35′39″ E | Planted | Aro-Fagetum | 325 | NE | 43 | 3 | 0.23 | 6 |
| BS8 | 47°31′49″ N 7°35′49″ E | Fragment | Galio Odorati-Fagetum Typicum | 370 | E | 23 | 2 | 2.70 | 11 |
| BS9 | 47°31′55″ N 7°36′6″ E | Fragment | Galio Odorati-Fagetum Typicum | 338 | NW | 44 | 3 | 2.10 | 19 |
| BS10 | 47°34′20″ N 7°37′6″ E | Forest | Galio-Carpinetum Corydalidetosum | 269 | – | 25 | 2 | 2.53 | 35 |
| BS11 | 47°29′11″ N 7°40′43″ E | Forest | Galio-Fagetum Pulmonarietosum | 565 | – | 3 | 1 | 186.4 | 92 |
| BS12 | 47°34′29″ N 7°39′58″ E | Forest | Galio Odorati-Fagetum Cornetosum | 450 | NW | 10 | 2 | 5.15 | 37 |
| BS13 | 47°35′18″ N 7°40′20″ E | Forest | Galio Odorati-Fagetum Cornetosum | 473 | SW | 13 | 2 | 3.42 | 54 |
| BS14 | 47°32′31″ N 7°35′2″ E | Fragment | Galio Odorati-Fagetum Cornetosum | 299 | NNE | 35 | 3 | 1.95 | 6 |
| BS15 | 47°30′53″ N 7°38′11″ E | Forest | Galio-Fagetum Pulmonarietosum | 418 | – | 2 | 1 | 79.0 | 43 |
| BS16 | 47°30′31″ N 7°40′04″ E | Forest | Aro-Fagetum | 454 | – | 1 | 1 | 337.0 | 66 |
| BS17 | 47°32′43″ N 7°36′27″ E | Planted | Aro-Fagetum | 276 | – | 69 | 4 | 0.37 | 2 |
| BS18 | 47°30′18″ N 7°34′46″ E | Forest | Galio Odorati-Fagetum Typicum | 380 | – | 2 | 1 | 237.7 | 59 |
| BS19 | 47°34′23″ N 7°39′16″ E | Planted | Galio Odorati-Fagetum Typicum | 380 | – | 9 | 2 | 1.28 | 38 |
| BS20 | 47°32′14″ N 7°35′26″ E | Fragment | Galio Odorati-Fagetum Cornetosum | 326 | E | 56 | 4 | 0.89 | 5 |
| Degree of Urbanization | |||||
|---|---|---|---|---|---|
| Very Low (Rural) | Low | Moderate | High | p | |
| (n = 5) | (n = 5) | (n = 6) | (n = 4) | ||
| Forest vegetation characteristics | |||||
| Ground vegetation cover (%) | 67.2 ± 9.5 | 56.2 ± 11.7 | 78.0 ± 18.1 | 73.9 ± 15.9 | N.S. |
| Herbaceous plant species richness 1 | 9.5 ± 1.4 a | 6.8 ± 0.6 a | 5.6 ± 0.4 b | 5.2 ± 0.2 b | 0.005 |
| Shrub species richness 2 | 4.0 ± 0.7 a | 4.0 ± 0.9 a | 6.0 ± 0.8 b | 7.5 ± 0.3 b | 0.034 |
| Tree species richness 2 | 3.8 ± 0.6 | 3.8 ± 0.5 | 3.8 ± 0.6 | 2.5 ± 0.8 | N.S. |
| Soil properties | |||||
| Moisture (%) | 31.4 ± 2.3 | 29.0 ± 1.5 | 28.8 ± 1.6 | 25.7 ± 2.1 | N.S. |
| pH | 5.6 ± 0.4 a | 5.7 ± 0.4 a | 6.6 ± 0.2 b | 7.2 ± 0.1 b | 0.004 |
| SOM (%) | 18.3 ± 6.9 | 12.7 ± 1.9 | 16.6 ± 2.2 | 22.2 ± 3.4 | N.S. |
| Total organic nitrogen (%) | 0.298 ± 0.071 | 0.282 ± 0.034 | 0.313 ± 0.033 | 0.381 ± 0.047 | N.S. |
| Plant-available phosphorus (µg PO43−/g) | 26.9 ± 5.0 a | 19.4 ± 3.9 a | 35.8 ± 8.5 b | 46.4 ± 5.3 b | 0.07 |
| Richness | Shannon Diversity Index | Pielou’s Evenness | |
|---|---|---|---|
| Degree of urbanization | Chi23,16 = 16.79, p < 0.001 | F3,14 = 4.22, p = 0.026 | F3,11 = 5.14, p = 0.018 |
| Forest area (ha) 1 | Chi21,15 = 1.47, p = 0.226 | – | F1,11 = 3.51, p = 0.088 |
| % forest within 500 m 1 | Chi21,14 = 5.90, p = 0.015 | F1,14 = 1.82, p = 0.197 | F1,11 = 1.08, p = 0.322 |
| Plant species richness 1 | – | – | F1,11 = 2.82 p = 0.123 |
| Tree species richness 1 | Chi21,13 = 1.82, p = 0.177 | – | – |
| Soil moisture | Chi21,12 = 8.20, p = 0.004 | F1,14 = 1.68, p = 0.216 | F1,11 = 5.05, p = 0.046 |
| Soil pH | – | – | F1,11 = 1.23, p = 0.290 |
| Relative Abundance of | |||
|---|---|---|---|
| Saprotrophic Fungi | Symbiotrophic Fungi | Pathotrophic Fungi | |
| Degree of urbanization | F3,13 = 3.16, p = 0.061 | F3,12 = 4.37, p = 0.026 | F3,13 = 5.24, p = 0.014 |
| Ground vegetation cover (%) | F1,13 = 1.47, p = 0.247 | F1,12 = 2.08, p = 0.175 | F1,13 = 1.25, p = 0.284 |
| Plant species richness 1 | F1,13 = 2.55, p = 0.141 | F1,12 = 1.22, p = 0.290 | – |
| Tree species richness 1 | – | F1,12 = 1.85, p = 0.198 | – |
| Soil moisture (%) | F1,13 = 4.10, p = 0.046 | – | F1,13 = 3.46, p = 0.084 |
| Soil pH | – | F1,12 = 3.17, p = 0.100 | F1,13 = 2.20, p = 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusterholz, H.-P.; Baur, B. Changes in Soil Fungal Diversity and Composition along a Rural–Urban Gradient. Forests 2023, 14, 2226. https://doi.org/10.3390/f14112226
Rusterholz H-P, Baur B. Changes in Soil Fungal Diversity and Composition along a Rural–Urban Gradient. Forests. 2023; 14(11):2226. https://doi.org/10.3390/f14112226
Chicago/Turabian StyleRusterholz, Hans-Peter, and Bruno Baur. 2023. "Changes in Soil Fungal Diversity and Composition along a Rural–Urban Gradient" Forests 14, no. 11: 2226. https://doi.org/10.3390/f14112226
APA StyleRusterholz, H.-P., & Baur, B. (2023). Changes in Soil Fungal Diversity and Composition along a Rural–Urban Gradient. Forests, 14(11), 2226. https://doi.org/10.3390/f14112226






