Abstract
Soil microorganisms play crucial roles in maintaining material circulation and energy flow in desert ecosystems. However, the structure and function of soil microorganisms in different forestlands are currently unclear, restricting the use of sand-fixing plants and the understanding of forest ecosystem functions. In this study, Artemisia ordosica, Caragana korshinskii, and Salix psammophila, three types of sand-fixing forests widely distributed in the Mu Us Sandy Land, were used to explore the effects of sand-fixing forests on soil physicochemical properties, soil enzyme activity, soil microbial biomass, microbial community structure, and inter-microbial species relationships. Soils of forestlands showed higher soil organic carbon (SOC), total phosphorus (TP), and total nitrogen (TN) contents than bare sandy land. The SOC in bare sandy soil was only 0.84 g kg−1, while it remained 1.55–3.46 g kg−1 in forestland soils. The TN in bare sandy land soil was 0.07 g kg−1, which was significantly lower than that in forestland soils (0.35–0.51 g kg−1). The TP in bare sandy soil was 0.18 g kg−1, significantly lower than that in forestland soils (0.46–0.69 g kg−1). Afforestation of bare sandy land improved soil microbial carbon and nitrogen contents and increased microbial enzyme activities of acid phosphatase and N-acetyl-β-D-glucosaminidase. Significant differences were observed between the three forestlands and bare sandy land in terms of soil microorganisms and community composition. With the establishment of a sand-fixing forest, the alpha diversity of soil bacteria significantly improved, whereas that of soil fungi remained stable. The bacterial community comprised 33 phyla, 106 classes, 273 orders, 453 families, and 842 genera. While five fungal phyla were detected by OTUs at a similarity of 97%, bacterial and fungal community structures were affected by the organic carbon content, sand particle content, soil pH, total nitrogen, and total phosphorus contents of soils. This study is helpful for vegetation construction and protection on sandy lands from the perspective of plant-microbe interactions.
1. Introduction
Nowadays, land desertification in terrestrial ecosystems has always been a hot topic in ecological and environmental issues worldwide [1,2,3]. In 2014, the total desert area in China was 2.6 × 106 km2, occupying 27.2% of the total area [4]. Generally, ecological restoration and reconstruction of land desertification are very urgent [5,6,7]. However, using plant sand fixation as the main strategy of restoration is still at an unstable stage because of its single ecological functions, weak anti-interference ability, poor stability, and proneness to degradation [8,9,10]. Therefore, timely and accurate monitoring and evaluation of desertification processes are very important to prevent land desertification, consolidate the effects of artificial sand fixation, and stabilise the ecological restoration of sandy areas [11].
Among various biochemical processes in soil, the activities of soil microorganisms are one of the most important components [12,13]. Soil microorganisms are the main decomposers in desert ecosystems, participating in the exchange and flow of soil materials and responding quickly and effectively to changes in soil ecosystems [14]. Soil microbial indicators, such as soil microbial composition, community structure, microbial biomass, and soil enzyme activity, can sufficiently reflect soil quality, fertility, and health [15,16,17,18]. Generally speaking, soil microorganisms can directly participate in the processes of the carbon and nitrogen cycles, have a positive impact on the formation of soil humus and the transformation of soil physiochemical processes, and play an important role in soil formation and fertility restoration [8].
In desert areas, research has focused more on the impact of environmental gradient changes on microbial communities and individual functional genes but still lacks a comprehensive understanding of the plant-soil-microbial system [19,20]. The diversity of soil microorganisms, community structure composition, interspecific interactions, and differences in carbon and nitrogen functional genes in different sand fixation forests are currently unclear. Fully analysing the impact of sand-fixing vegetation on the structure, interspecific relationships, and functional characteristics of soil microbial communities is necessary to deeply understand the important role of soil microorganisms in the construction of desert plant communities, soil carbon and nitrogen cycling, and other processes.
As a typical desert ecosystem, the Mu Us Sandy Land, located in the agro-pastoral transition zone of northern China, is one of the four major sandy lands in China [21]. In this site, Artemisia ordosica, Caragana korshinskii, and Salix psammophila are the main established species and are important for maintaining regional ecological security [22]. However, the effects of these three shrubs on the soil microbial community structure and interspecific interactions are unclear. In summary, this study aimed to answer the questions that (1) how the activity, diversity, and composition of soil microbial communities in three typical sand-fixing forestlands and (2) what are the interspecific interactions and influencing factors of soil microorganisms in the three typical sand-fixing forestlands. Based on the previous studies, we hypothesised that forestland soils have superior soil biochemical properties than bare sand soils and have distinguished bacterial and fungal community properties in different forestland soils.
2. Materials and Methods
2.1. Sample Collection
Three forestlands (Artemisia ordosica, Caragana korshinskii, and Salix psammophila) and a bare sandy area were selected as the four experimental sites in the Mu Us Desert, China. The chosen study sites were located at Dabaodang Town (38°38′06″ N, 109°59′50″ E), Yulin City, on the southeastern edge of Maowusu Sandy Land and the northern edge of the Loess Plateau in northern Shaanxi Province. This study area has a typical temperate semi-arid continental monsoon climate, with a mean annual temperature of 8.4 °C and a mean annual precipitation of 2111 mm. The altitude of this study site is 1095–1225 m. Three experimental plots (100 m × 100 m) were randomly set at each forestland and bare sandy land. The selected plots were free of livestock grazing or human damage. The distance between each plot was more than 20 m. The nearby bare sandy land was chosen to be under the control of the three forestlands. Soil samples of 0–10 cm depth were collected from the 12 plots using a sterile soil drill with a diameter of 5 cm. Soil samples were collected from four directions: southeast, northwest, and northwest, to ensure the uniformity of the collected soil samples. The soil samples collected from each sampling site were mixed as a mixing sample to guarantee the representativeness of the samples. After discarding the plant residues, the soil samples were kept in sterilised sampling test tubes, placed in a vehicle refrigerator, and immediately brought to the laboratory for further analysis. Each sample was divided into three parts in the laboratory. The first part was stored at 4 °C to measure soil microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), soil water content (SWC), and enzyme activity. The second part was stored at −80 °C for high-throughput sequencing of 16S rRNA and ITS. The last part was air-dried for the determination of soil physicochemical properties.
2.2. Soil Physicochemical Properties, Enzyme Activity, and Biomass Determination
Freshly weighed soil was placed in an oven and dried at 105 °C to a constant weight, and the weight of the soil after drying was measured. The soil moisture content was measured based on the difference in mass before and after balance.
According to the water-soil ratio of 1:2.5 (g/v), distilled water was added to the air-dried soil, stirred for 1–2 min, and allowed to stand for 30 min before measuring with a pH meter.
The bulk density was measured using the ring-knife method [23]. The amount of organic carbon was measured using the potassium dichromate oxidation method [24]; total nitrogen was measured using a fully automatic Kjeldahl nitrogen analyser [25]; and total phosphorus was measured using the molybdenum antimony colorimetric method [26].
The activities of two enzymes (acid phosphatase and N-acetyl-β-D-glucosaminidase) were determined using the disodium phenylene phosphate colorimetric method [27,28]. The soil MBC and MBN were determined using the chloroform fumigation extraction method [29].
2.3. High-Throughput Sequencing and Bioinformatics Analysis
Total DNA was extracted from the soil microbiomes. The 16S rRNA (515F/806R) and ITS (ITS2/2043R) loci were sequenced for the identification of bacterial and fungal communities separately, using Illumina MiSeq at the Shanghai Invitrogen Biological Technology Company, Ltd. (Beijing, China). The original sequences were spliced, filtered, and clustered into operational taxonomic units (OTUs) with a consistency of ≥97%. After the library construction is completed, Qubit2.0 is used for preliminary quantification, the library is then diluted, and Agilent2100 is used to detect the insert size of the library [30]. After meeting expectations, Q-PCR was used to accurately quantify the effective concentration to ensure the quality of the library. Species annotation was performed to obtain species information on Bacteria and Fungi in each sample and analyse the differences in the composition and structure of microbial communities among different soil samples using cluster analysis, dimensionality reduction analysis, and statistical analysis using R version 3.1.1.
2.4. Microbial Community Structure Analysis
Alpha diversity generally includes species richness and community evenness. Richness was characterised by the number of OTUs and the Chao1 index of the sample, and evenness was characterised by the Shannon-Wiener index (H).
Non-parametric testing methods, including analysis of similarities (ANOSIM), multiple response permutation process analysis (MRPP), and permutation multivariate analysis of variance (PMAV), were used to compare microbial communities among different soil samples. Non-metric multidimensional scaling (NMDS), principal coordinates analysis (PCoA), and one-way analysis of variance (ANOVA) were used to analyse the differences in microbial community structures between the soil samples. Redundancy analysis (RDA), Spearman’s correlation analysis (SCA), and canonical correspondence analysis (CCA) were used to study the relationships between microbial community structure and soil properties.
3. Results
3.1. Soil Physicochemical Properties, Enzyme Activity, and Biomass Determination
Compared with that of the bare sandy land, the soils of Artemisia ordosica and Salix psammophila forestlands had higher soil organic carbon (SOC), total phosphorus (TP), and total nitrogen (TN) contents (Table 1). There were significant differences in the SWC, SOC, and TN among the three forestlands (Artemisia ordosica, Caragana korshinskii, and Salix psammophila). The SOC was highest in the Artemisia ordosica forestland and lowest in the Caragana korshinskii forestland. The TN was highest in the Caragana korshinskii and Salix psammophila forestlands and lowest in the Artemisia ordosica forestland. The SWC was highest in the Artemisia ordosica forestland. There were no significant differences in soil pH among the three forestlands. The plant diversity of the Artemisia ordosica forestland was significantly higher than that of the Salix psammophila and Caragana korshinskii forestlands.

Table 1.
Soil properties of the four experimental sites. Different lowercase letters behind the data indicate significant differences among the four experimental sites for each soil property.
The soil microbial carbon and nitrogen contents in the three forestlands were significantly higher than those in the bare sandy land (Table 1). The microbial carbon content in the Artemisia ordosica forestland was significantly higher than that in the Salix psammophila and Caragana korshinskii forestlands, and that in the Salix psammophila forestland was significantly higher than that in the Caragana korshinskii forestland. The microbial nitrogen content in the Artemisia ordosica and Salix psammophila forestlands was significantly higher than that in the Caragana korshinskii forestland, whereas the difference between the Artemisia ordosica and Salix psammophila forestlands was not significant.
The enzyme activities of acid phosphatase and N-acetyl-β-D-glucosaminidase in the three forestlands were significantly higher than those in the bare sandy land. Moreover, the enzyme activities in the three forestlands were significantly different in the following order: Artemisia ordosica > Salix psammophila > Caragana korshinskii (Table 1).
3.2. Bacterial Community Structure Characteristics
Based on the sequencing data, 5668 bacterial OTUs and 614,895 bacterial sequences were obtained. Subsequently, microbial diversity and community structure analyses were performed based on the minimum number of sample sequences. In the bare sandy land and sand-fixing forestlands, the total number of OTUs was 1150. The numbers of OTUs unique to the bare sandy land and Caragana korshinskii, Artemisia ordosica, and Salix psammophila forestlands were 447, 454, 628, and 695, respectively.
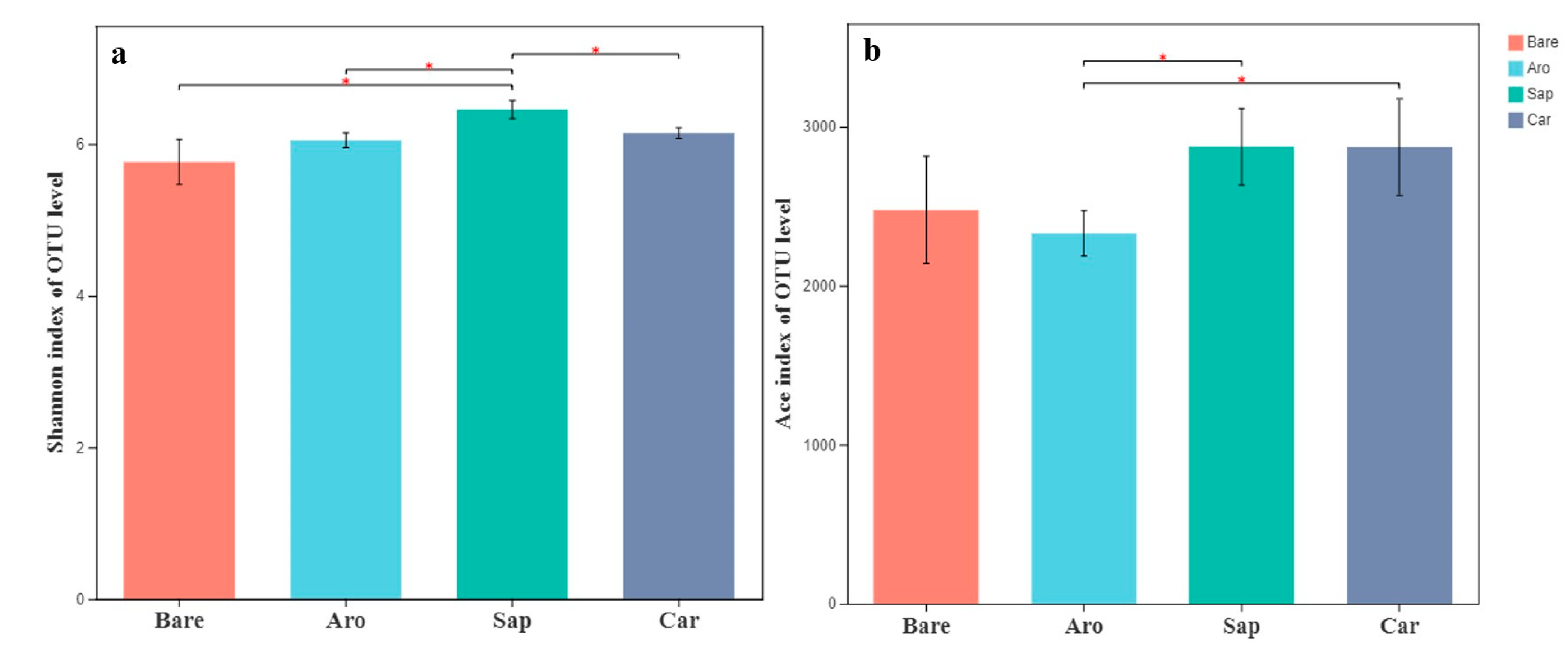
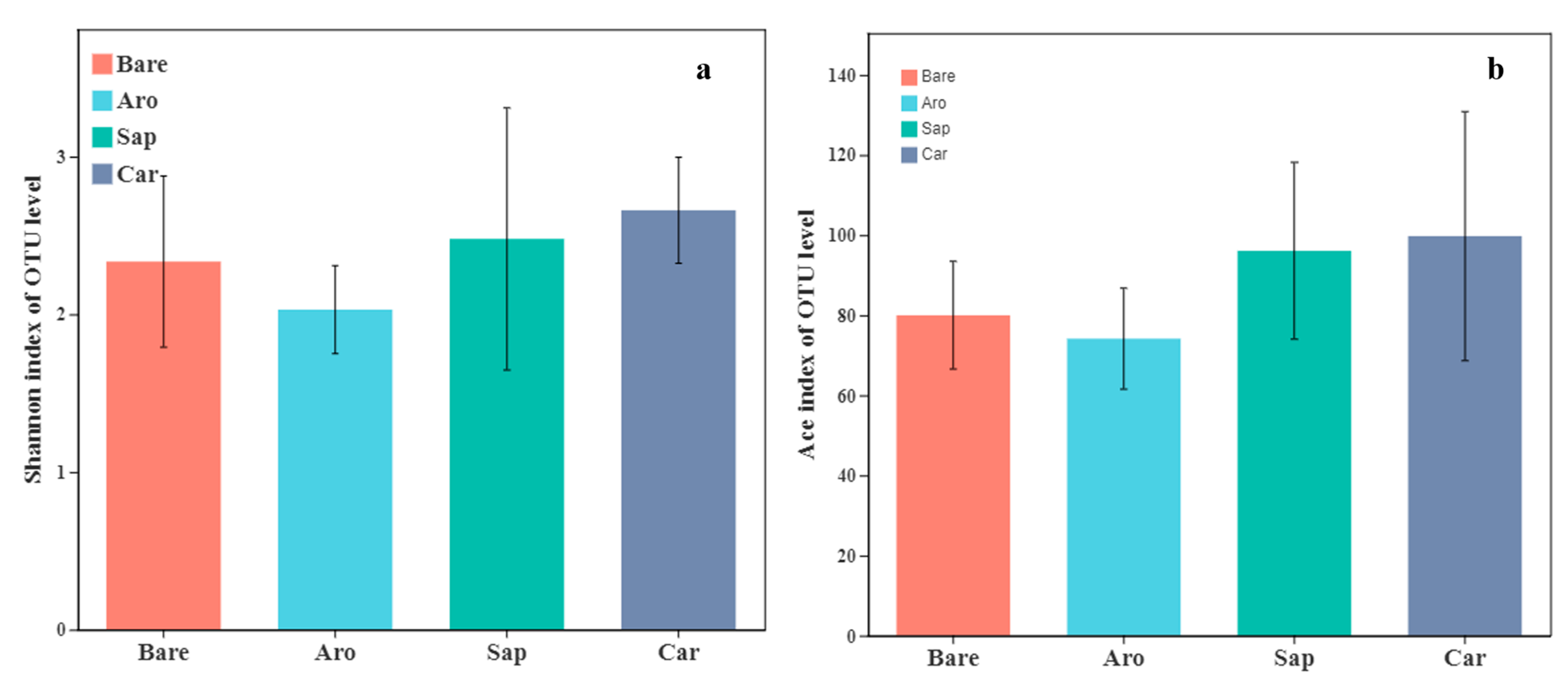
As shown in Figure 1, the Shannon and ACE indices of the bare sandy land were the lowest, whereas those of the forestlands were significantly higher. There were significant differences in microbial species diversity among the three forestlands. The order of the Shannon indices was as follows: Salix psammophila > Artemisia ordosica > Caragana korshinskii. The ACE indices in the Salix psammophila and Caragana korshinskii forestlands were significantly higher than those in the bare sandy land and Artemisia ordosica forestlands. These results showed that bacterial diversity increased significantly after the establishment of a sand-fixing forest.
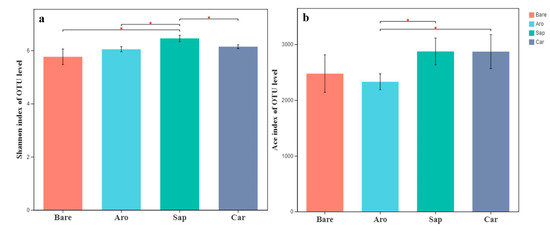
Figure 1.
Alpha diversity indices [Shannon index (a) and ACE index (b)] for the bacterial communities in the bare sandy land and different forestlands. * indicates significant results at p < 0.05.
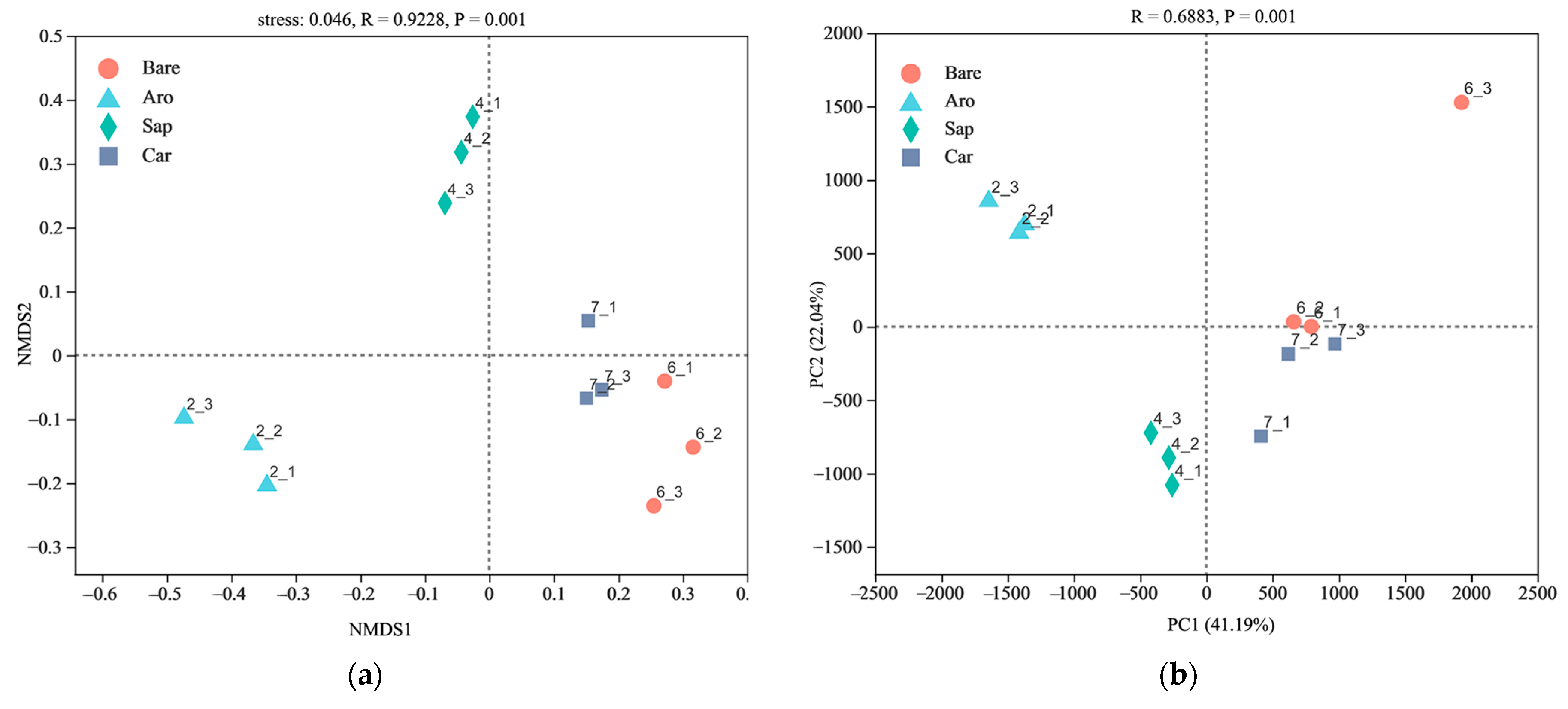
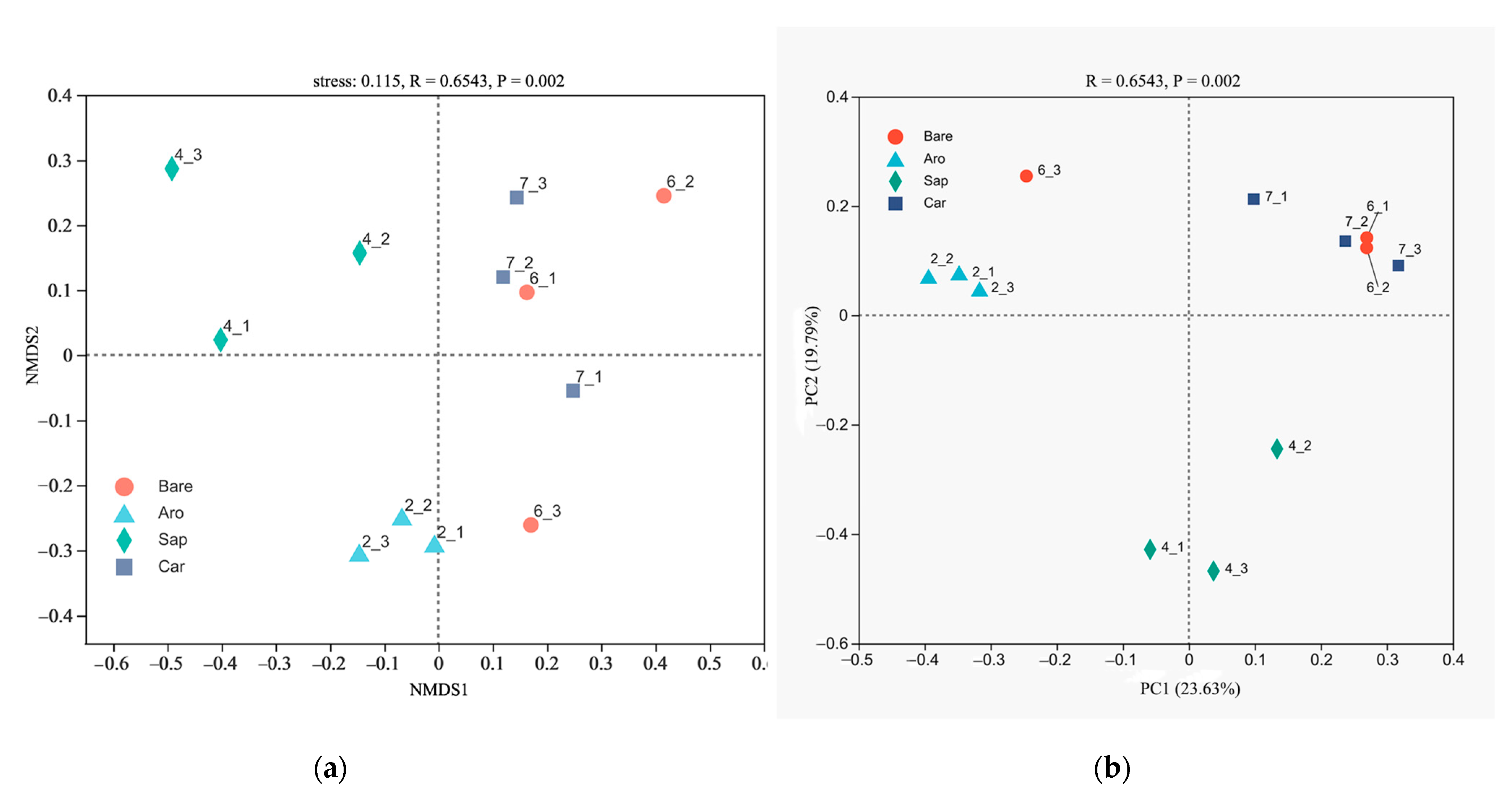
NMDS and PCoA were used to compare the bacterial community structures in the bare sandy land and three forestlands (Figure 2). The results showed that the cumulative explanations of the first- and second-ordering axes of the PCoA reached 26.63% and 36.95%, respectively. The bare sandy land and Artemisia ordosica, Salix psammophila, and Caragana korshinskii forestlands were distributed sequentially along the first sorting axis. They were separated from each other, indicating obvious differences in the bacterial communities between the bare sandy land and the three forestlands. The NMDS results further confirmed that the differences between the bacterial community structures in the bare sandy land and three forestlands were significant.
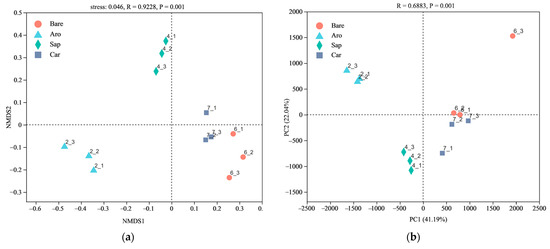
Figure 2.
Non-metric multidimensional scaling (NMDS, (a)) and principal coordinates analysis (PCoA, (b)) of the bacterial community structure in the bare sandy land and three forestlands. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land.
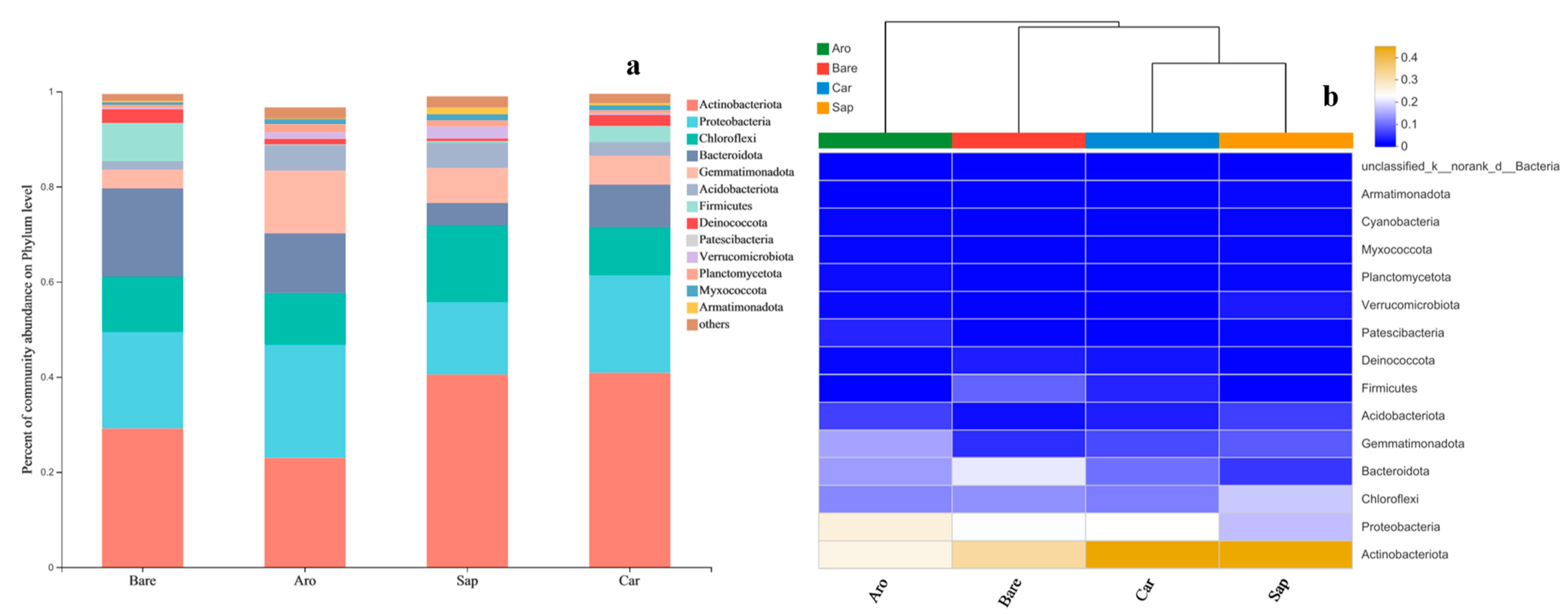
The bacterial community comprised 33 phyla, 106 classes, 273 orders, 453 families, and 842 genera. At a gene sequence similarity level of 97%, 13 phyla had relative abundances greater than 1%. The top 8 phyla were Actinobacteria (10.6%–19.4%), Proteobacteria (35.7%–64.5%), Chloroflexi (7.5%–10.2%), Bacteroidetes (5.1%–9.4%), Gemmatimonadetes (1.2%–2.5%), Verrucomicrobia (0.6%–11.9%), Planctomycetes (0.8%–3.0%), and Armatimonadetes. The relative abundance of bacteria in these categories accounted for more than 90% of the total bacterial abundance. In contrast, the relative abundance of bacteria in the other categories was less than 10% of the total abundance (Figure 3).
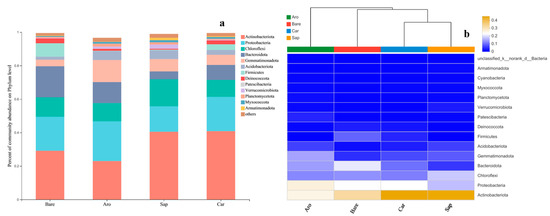
Figure 3.
Bacterial community composition in the bare sandy land and three forestlands. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land. (a) Bacterial community composition; (b) cluster analysis of bacterial community.
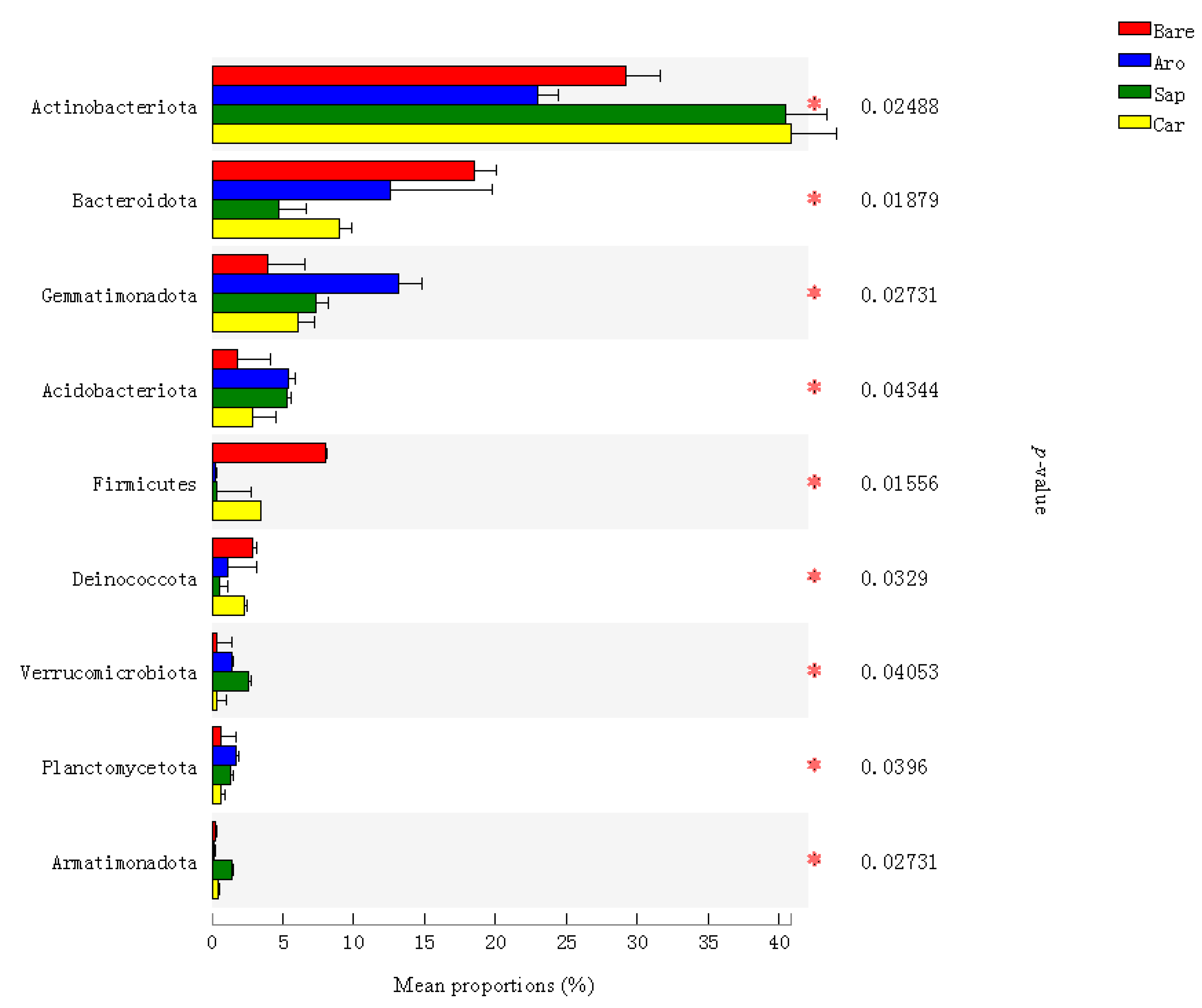
The relative abundance of bacteria at the phylum level was compared using a significance test analysis of the differences between the groups. The results (Figure 4) showed that there were significant differences in the relative abundance of bacteria at different phylum levels among the forestlands, mainly in Actinobacteria, Bacteroidetes, Gemmatimonadota, Acidobacteria, Firmicutes, Deinococcota, Verrucomicrobia, Planctomycetes, and bacterial species in the phylum Armorymycota. The abundance of Bacillus, Acidobacteria, and Plancomycetes was highest in Artemisia ordosica forestland soil, while the relative abundance of Actinobacteria was highest in Salix psammophila and Caragana korshinskii forestlands (Figure 4).
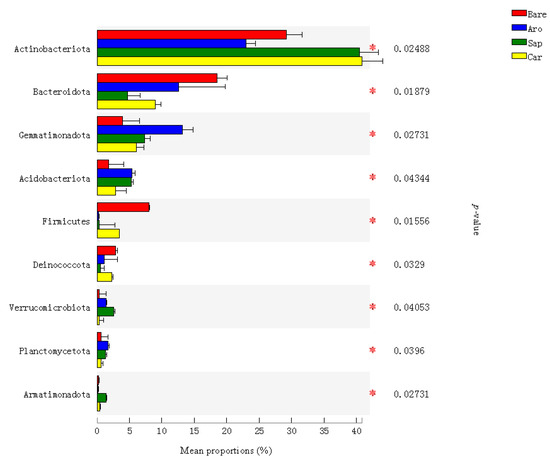
Figure 4.
Bacterial taxa with significant differences in the bare sandy land and three forestlands. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land. * indicates significant results at p < 0.05.
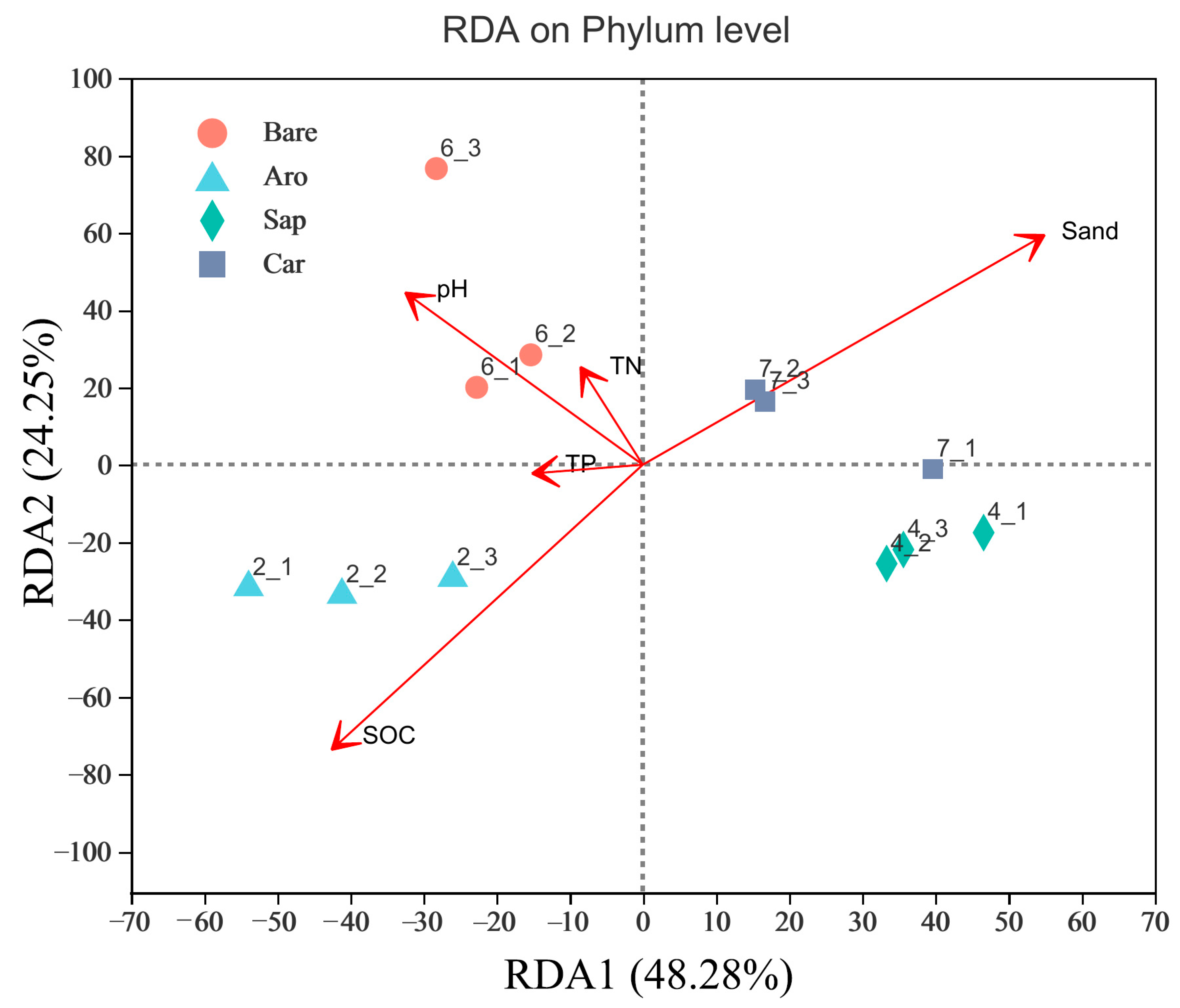
To explore the factors that affected the soil bacterial community structure in this study area, RDA was used to screen out the environmental variables that affect bacterial community changes (Figure 5). The Monte Carlo permutation test was used to analyse the correlation between environmental factors and the spatial distribution of the species (permutations = 999). The results showed that the environmental variables that affected the distribution of bacterial communities included SOC, TN, TP, pH, and sand content. Among them, the SOC had the greatest impact on community structure, followed by the sand content and pH. The explanation rates for the RDA1 and RDA2 axes were 24.2% and 48.28%, respectively. The composition of the bacterial community in Artemisia orchard woodland was closely related to the SOC. The bacterial community in the bare sandy land was most related to the pH, and the bacterial communities in Salix psammophila and Caragana korshinskii forestlands were most affected by the sand content.
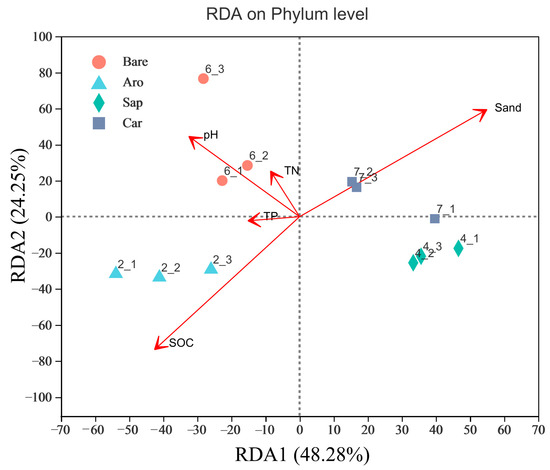
Figure 5.
Redundancy analysis (RDA) of the environmental factors that affected soil bacterial communities. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land.
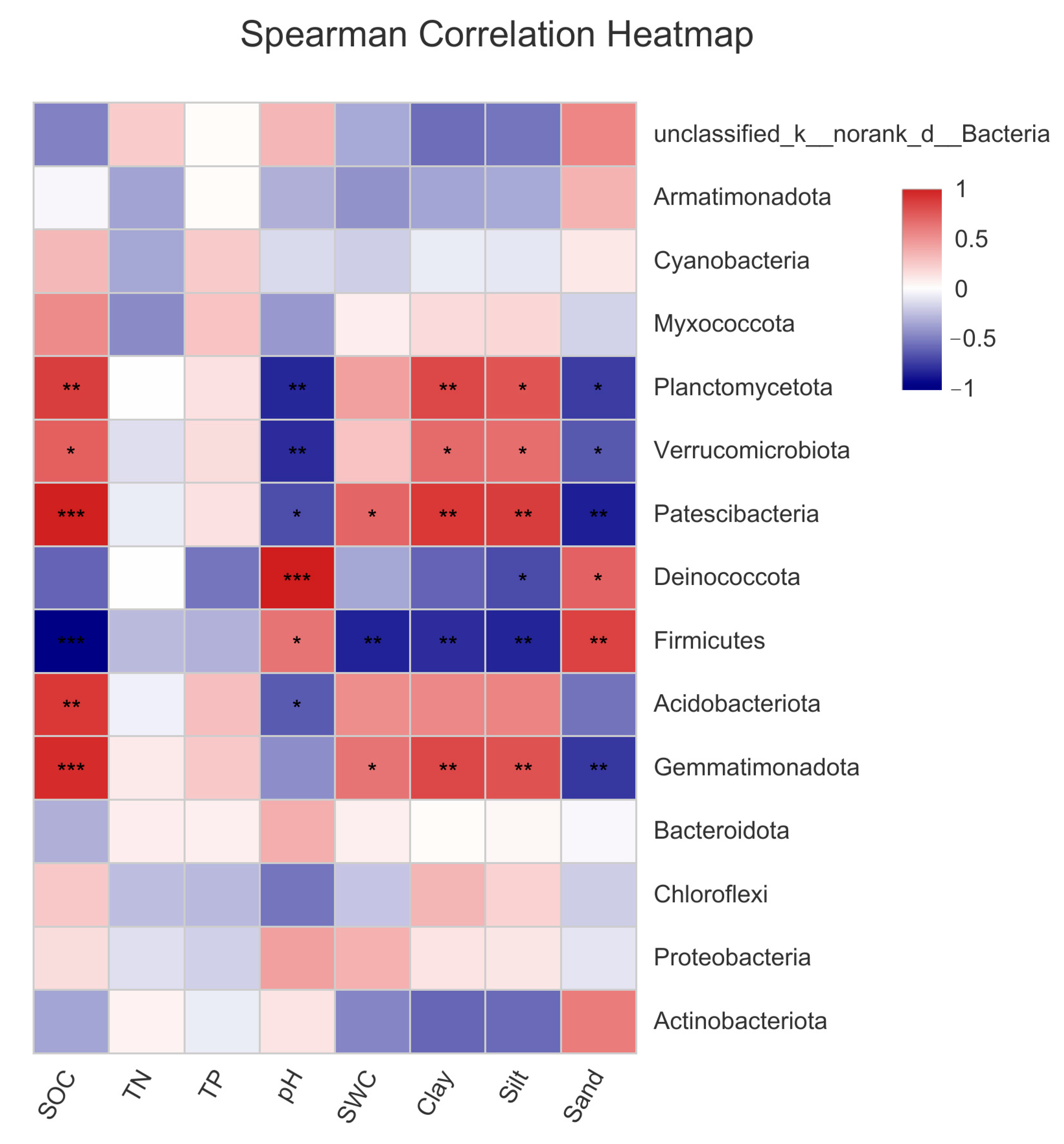
As shown in Figure 6, there was a significant correlation between SOC, pH, sand, silt, and clay content, and bacterial community composition. SCA showed that each environmental factor was closely related to the dominant bacterial phyla. The SOC, SWC, and silt and clay contents of the bare sandy land and three forestlands had significant or extremely significant positive correlations with Planctomycetota, Verrucomicrobiota, Bacteroidota, and Patescibacteria. A significant negative correlation was observed between Muricutes and Deinococccota. Sand content and pH showed a significant negative correlation with Planctomycetota, Verrucomicrobiota, Acidobacteriota, and Bacteroidota and a significant or extremely significant positive correlation with Deinococccota and Firmicutes. The above results further verified that the measured environmental factors had an important impact on the bacterial community composition.
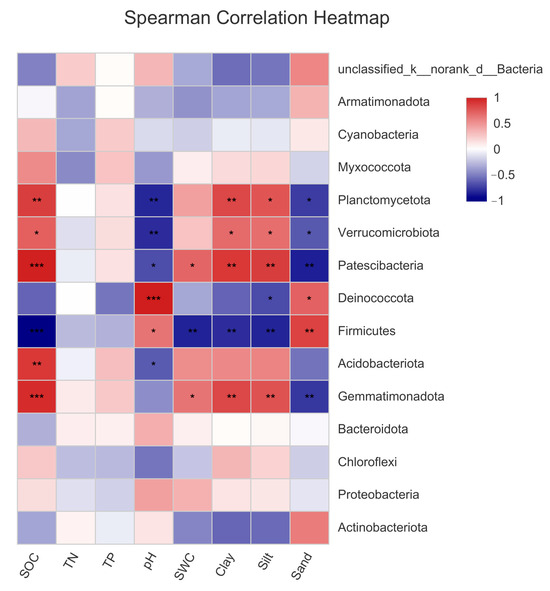
Figure 6.
Correlations between the dominant bacterial phyla and environmental factors in the bare sandy land and three forestlands. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land. * means significant results at p < 0.05, ** means significant results at p < 0.01, and *** means significant results at p < 0.001.
3.3. Fungal Community Structure Characteristics
Based on the sequencing data, 197 fungal OTUs and 782,024 fungal sequences were identified. Subsequently, microbial diversity and community structure analyses were performed based on the minimum number of sample sequences. As shown in Figure 7, there were no significant differences in the Shannon and ACE indices between the bare sandy land and Artemisia oleifera forestland. There was no significant difference between the bare sandy land and Artemisia oleracea, Caragana korshinskii, and Salix psammophila forestlands, indicating that the establishment of sand-fixing shrub forests had no significant impact on fungal diversity.
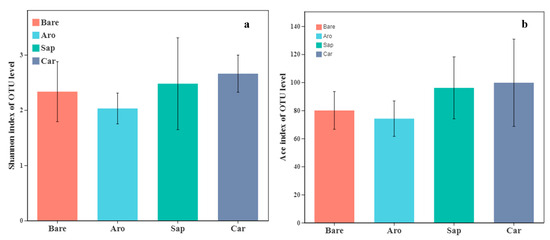
Figure 7.
Alpha diversity indices [Shannon index (a) and ACE index (b)] for the fungal communities in the bare sandy land and different forestlands. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land.
NMDS and PCoA were used to compare the fungal community structures in the bare sandy land and three forestlands (Figure 8). The results showed that the cumulative explanations of the first- and second-ordering axes of the PCoA reached 19.79% and 23.63%, respectively. The bare sandy land and Artemisia oleracea, Caragana korshinskii, and Salix psammophila forestlands were distributed sequentially along the first sorting axis. They were separated from each other, indicating that there were obvious differences in the fungal communities between the bare sandy land and the three forestlands. The NMDS results further confirmed that the differences between the fungal community structures in the bare sandy land and forestlands reached a significant level.
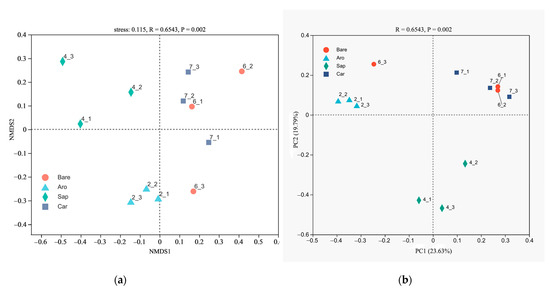
Figure 8.
Non-metric multidimensional scaling (NMDS, (a)) and principal coordinates analysis (PCoA, (b)) of the fungal community structure in the bare sandy land and three forestlands. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land.
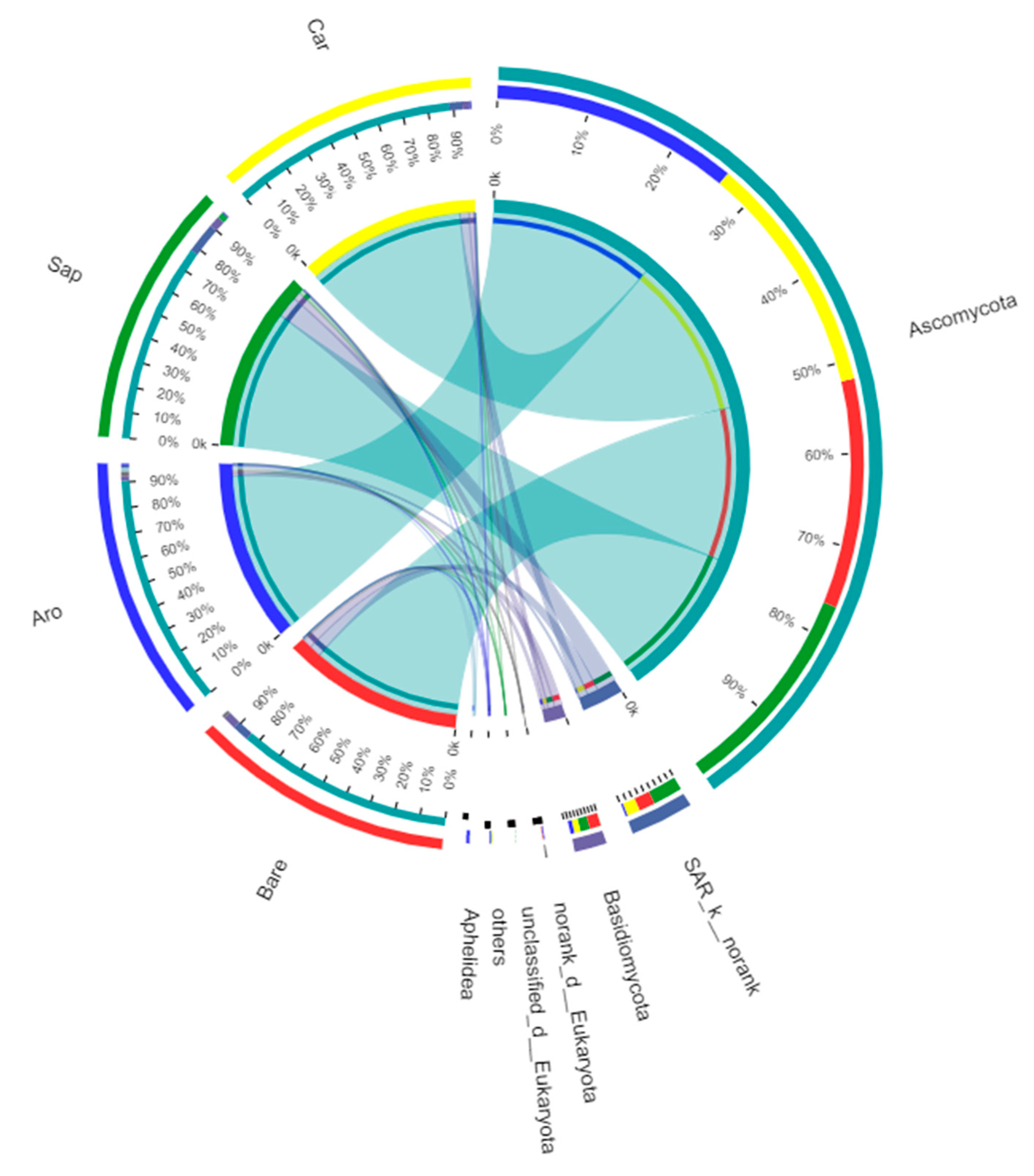
At a similarity level of 97%, all detected OTUs were divided into five fungal phyla. Among them, there were two phyla with relative abundances of >1% (Figure 9), namely Ascomycota (bare sandy land, 86.56%; Artemisia ordosica, 93.08%; Salix psammophila, 80.10%; Caragana korshinskii, 90.86%) and Basidiomycota (bare sandy land, 5.22%; Artemisia ordosica, 1.91%; Salix psammophila, 4.43%; Caragana korshinskii, 2.78%). Other phyla with relative abundances less than 1% included Blastocladiomycota (bare sandy land, 0.1%; Artemisia ordosica, 0%; Salix psammophila, 0%; Caragana korshinskii, 0.10%), Chytridiomycota (bare sandy land, 0%; Artemisia ordosica, 0.03%; Salix psammophila, 0%; Caragana korshinskii, 0%), Mucoromycota (bare sandy land, 0%; Artemisia ordosica, 0.68%; Salix psammophila, 0.20%; Caragana korshinskii, 0.03%), and unrecognised categories (bare sandy land, 8.21%; Artemisia ordosica, 4.31%; Salix psammophila, 15.26%; Caragana korshinskii, 6.32%).
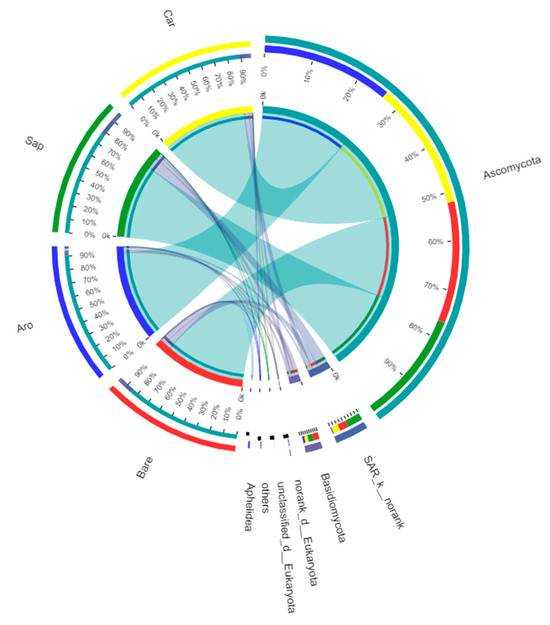
Figure 9.
Fungal community composition in the bare sandy land and three forestlands. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land.
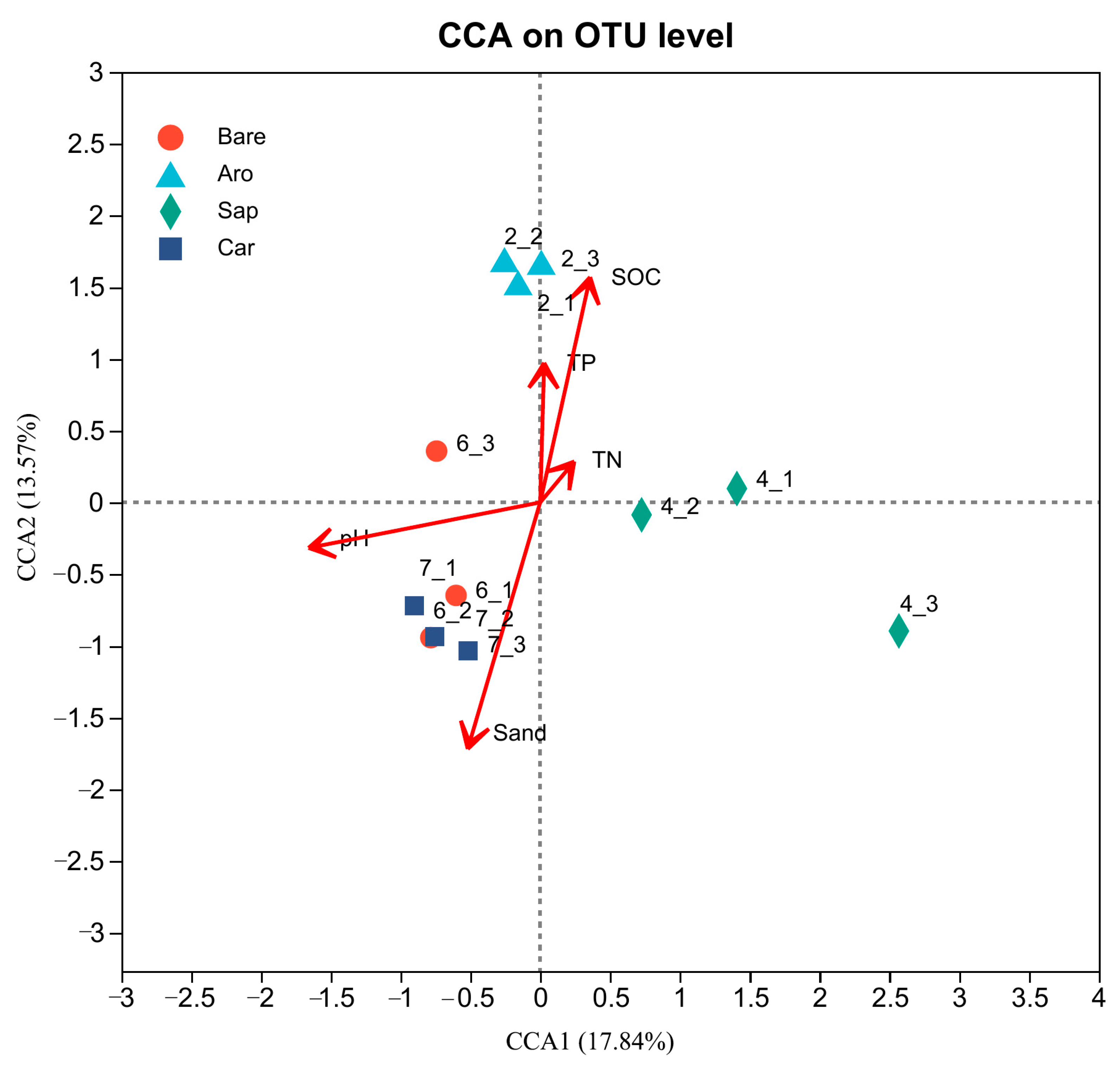
To explore the factors that affected the soil fungal community structure in this study area, CCA was used to screen out the environmental variables that affect fungal communities based on the measured environmental variables (Figure 10). The results showed that the environmental variables that affected the fungal community structure mainly included SOC, TN, TP, pH, and sand (sand content). Among them, the pH had the greatest impact on species distribution, followed by the SOC and sand content. The explanation rates for the CCA1 and CCA2 axes were 17.84% and 13.57%, respectively. The composition of the soil fungal community in the Artemisia ordosica forestland was most related to the SOC. In contrast, the fungal community in the bare sandy land and Caragana korshinskii forestland was more related to the sand content and pH.
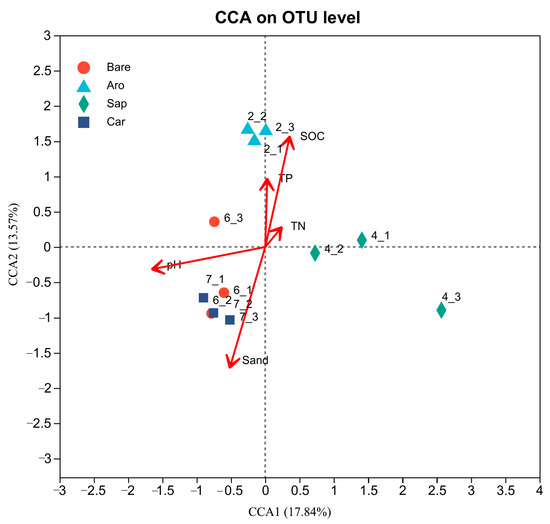
Figure 10.
Canonical correlation analysis (CCA) of the environmental factors that affected the soil fungal community. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land.
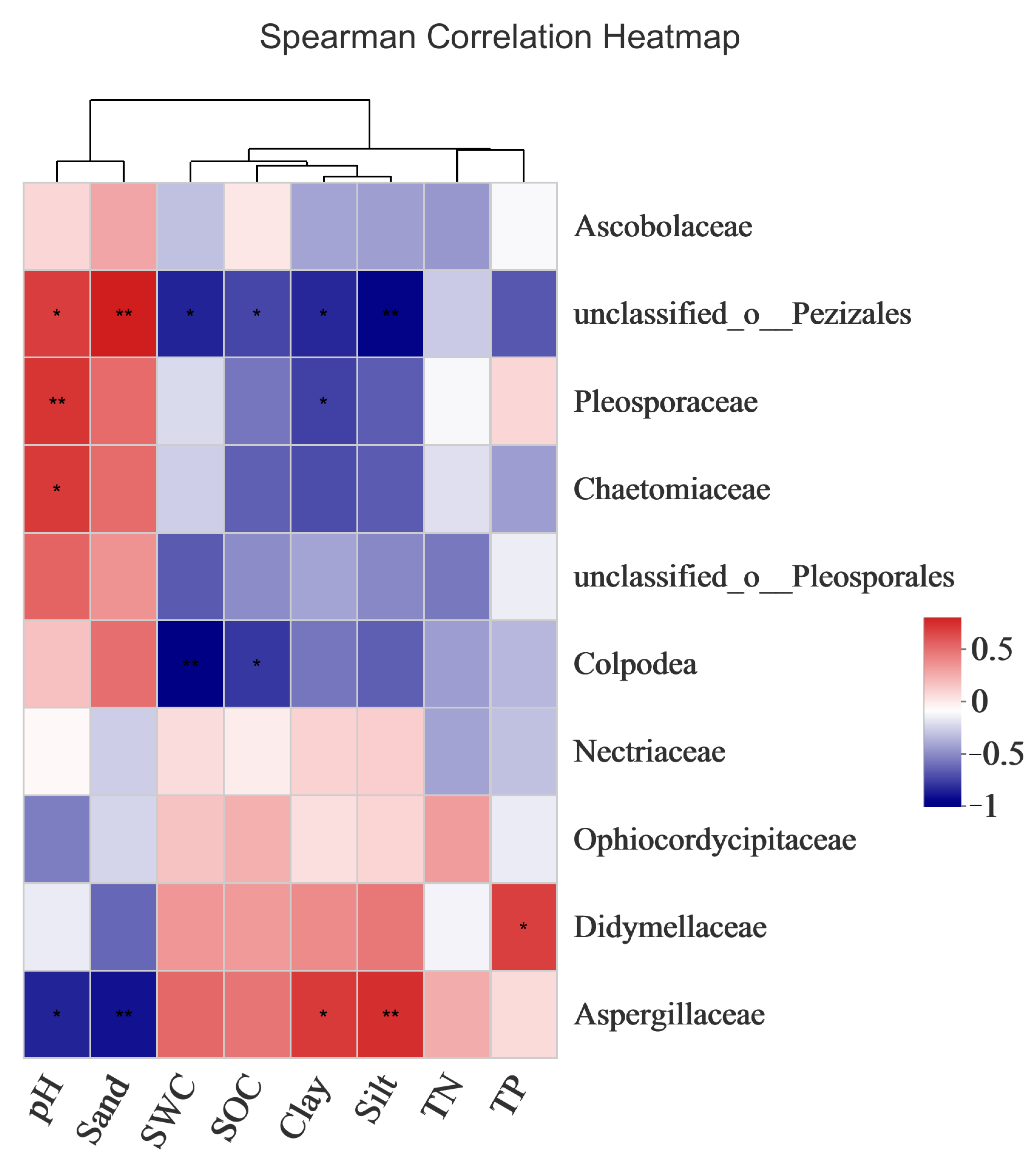
As shown in Figure 11, there was a significant correlation between pH, sand content, silt content, clay content, TP, SOC, and the fungal community composition; that is, these factors significantly affected the fungal community compositions of the bare sandy land and Artemisia ordosica, Salix psammophila, and Caragana korshinskii forestlands. SCA showed that each factor was closely related to the dominant fungal phyla. The pH of the bare sandy land and three forestlands showed a significant positive correlation with the families Discomycetaceae, Spyrozoaceae, and Chaetomaceae. The sand content was positively correlated with the family Discomycetaceae. The SWC, SOC, and powder content were positively correlated with Didymellaceae and were negatively correlated with the unclassified families of Pezizales and Colpodea. The silt content, cohesive content, and TP showed a significant positive correlation with Aspergillus and Didymellaceae. These results further verified that the measured environmental factors had an important impact on the fungal community composition.
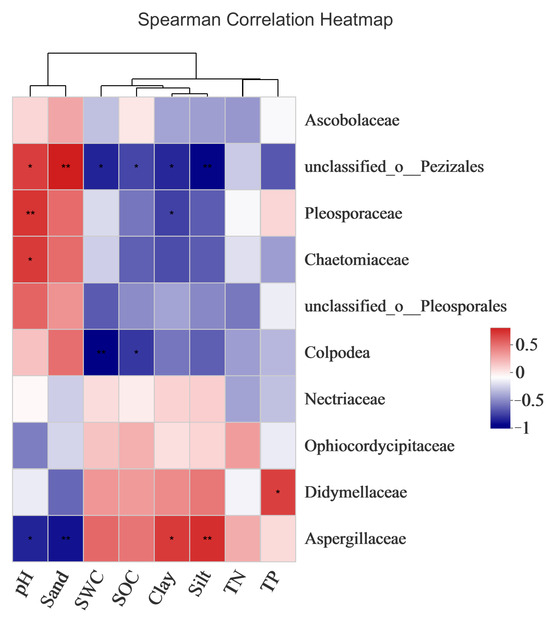
Figure 11.
Correlations between the dominant fungal phyla and environmental factors in the bare sandy land and three forestlands. Note: Aro, Artemisia ordosica; Sap, Salix psammophila; Car, Caragana korshinskii; Bare, bare sandy land. * means significant results at p < 0.05, and ** means significant results at p < 0.01.
4. Discussion
According to our hypothesis, our study demonstrated that the afforestation of bare sandy lands can significantly improve the physicochemical properties, microbial enzyme activity, and microbial biomass conditions in soil [31,32,33,34,35,36]. The soil moisture and nutrient conditions of the Artemisia ordosica forestland were much better than those in the soils of the Caragana korshinskii and Salix psammophila forestlands. Withered root secretions and biological knot skins are important sources of SOC, as well as the main energy and substrate source of soil microorganisms [37]. A higher coverage of vegetation in Artemisia ordosica forestland can reduce the evaporation of soil moisture, thereby promoting the growth of Artemisia ordosica. In the Artemisia ordosica forest, in addition to higher water and nutrient conditions, other factors can also lead to an improvement in the number and activity of soil microorganisms. Some studies have shown that some soil microorganisms use compounds released by plants as carbon and energy sources to promote their metabolism and growth [38], while the volatile chemicals from Artemisia ordosica stems and leaves may affect the metabolism of soil microorganisms, thereby promoting microbial activities [39]. On the other hand, the composition of carbon in the litter, which is provided by different plants, may vary, such as the types and proportions of active and inert carbon, which can affect the metabolic processes of soil microorganisms [40], thereby affecting enzyme activity and microbial biomass. Therefore, the input of litter and root exudates from different shrub species may also cause differences in soil microbial activity.
Importantly, we demonstrated that afforestation of bare sandy land can significantly improve the diversity of soil bacterial communities in this study. The diversity of above-ground plant species increased after afforestation, which further improved the nutrient conditions of the soil, creating a better growth and breeding environment for soil microorganisms and increasing the number and communities of bacteria. The diversity of soil bacteria was significantly different among the soils of the three forestlands, with the highest diversity found in Salix psammophila. The diversity of the bacterial community was closely related to the sand grain content and soil physicochemical properties. Recent research has also shown that the composition of soil microorganisms changes with the SOC content [41,42]. Particularly, the active SOC content is the main driving force affecting the diversity of bacterial communities [43]. Because of the soil texture of the research area, plant leaves and withering roots were decomposed by microorganisms and stored more active SOC [44]. Studies have shown that the relative abundance of certain soil bacteria is significantly positively or negatively correlated with SOC [45]. Similarly, the results of the RDA analysis also showed a close correlation between SOC content and bacterial community structure. Bacteroidetes are eutrophic bacteria [45], which have a higher relative abundance in Artemisia ordosica forests with better nutrient conditions. However, the higher relative abundance in the soil of Caragana korshinskii forests with poorer nutrient conditions indicates that the relative abundance of some groups of Bacteroidetes is not only affected by SOC content; it may also be influenced by other factors, such as soil texture [46]. This study found that sand content is also an important factor affecting microbial composition. In contrast, in drought and semi-arid areas, water availability is a key factor that limits plant diversity and productivity and indirectly affects the quantity and quality of proclaimed substances in the soil input, thereby affecting the diversity of soil microorganisms. In this study, the high water content in the shrub-covered soil may also have been a reason for the high diversity of the bacterial community.
Based on our results, Ascomycota and Basidiomycota were the most abundant fungal phyla, accounting for 90% of the fungi detected in the soils of the three forestry lands and bare sandy land. This result may be attributed to the proportion of approximately 98% of fungi belonging to Ascomycota and Basidiomycota [47,48,49]. Second, because of the desert ecosystem and lack of soil nutrients, Ascomycota has strong compression under harsh environmental conditions, owing to its faster evolutionary speed and rich species. Therefore, in dry sand and harsh survival conditions, Ascomycota fungi are more suited for survival than those from Basidiomycota. Our results showed that there are significant differences in the soil fungal community structure among the three types of forests. There is a significant correlation between SOC, fungal community structure, and soil physicochemical properties. Previous studies have also shown that SOC content determines changes in fungal community composition [50]. The research results showed that there are significant differences in SOC content among the three types of forests, which may lead to differences in fungal composition. In addition, unlike bacteria, which are more susceptible to soil properties, the composition of fungal communities is more closely related to plants. After vegetation restoration using different shrub species, the improvement of microclimate and soil nutrient conditions under shrubs will promote the growth of herbaceous plants, thereby affecting the composition of fungal communities. With the introduction of plants, the composition of soil fungal communities will change, and their changes often depend on the species of dominant plants.
5. Conclusions
There were significant differences among the three forestlands (Artemisia ordosica, Caragana korshinskii, and Salix psammophila) and bare sandy land regarding soil microorganisms and community compositions. Our results showed that the afforestation of bare sandy land can significantly improve its soil physicochemical properties and increase microbial enzyme activity and biomass. We found that the alpha diversity of the soil bacteria significantly improved; however, that of the soil fungi was unchanged. The bacterial and fungal community structures were affected by the SOC, sand particle content, soil pH, TN, and TP. This study’s results can aid in a better understanding of plant-microbe interactions and therefore aid in vegetation protection and management on sandy lands.
Author Contributions
Conceptualization, L.W., and X.L.; methodology, L.W., and X.L.; software, L.W., and X.L.; validation, L.W., and X.L.; formal analysis, L.W., and X.L.; investigation, L.W., and X.L.; resources, L.W., and X.L.; data curation, L.W., and X.L.; writing—original draft preparation, L.W.; writing—review and editing, L.W., and X.L.; visualization, L.W., and X.L.; supervision, L.W., and X.L.; project administration, L.W.; funding acquisition, L.W. All authors have read and agreed to the published version of this manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities (2018ZY40).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bassett, T.J.; Zuéli, K.B. Environmental discourses and the Ivorian savanna. Ann. Am. Assoc. Geogr. 2000, 90, 67–95. [Google Scholar] [CrossRef]
- Wen, X.R.; Cheng, Y.P.; Zhang, J.K.; Dong, H. Ecological function zoning and protection of groundwater in Asia. J. Groundw. Sci. Eng. 2021, 9, 359–368. [Google Scholar]
- Oraon, P.R.; Sagar, V.; Beauty, K. Ecological restoration of degraded land through afforestation activities. Land. Environ. Manag. For. 2023, 1, 201–216. [Google Scholar] [CrossRef]
- Bulletin on Desertification and Desertification Status in China: State Forestry and Grassland Administration. 2015. Available online: http://www.forestry.gov.cn/uploadfile/main/2011-1/file/2011-1-5-59315b03587b4d7793d5d9c3aae7ca86.pdf (accessed on 13 November 2022).
- Liu, Q.; Zhang, Q.; Yan, Y.; Zhang, X.; Niu, J.; Svenning, J.C. Ecological restoration is the dominant driver of the recent reversal of desertification in the Mu Us Desert (China). J. Clean. Prod. 2020, 268, 122241. [Google Scholar] [CrossRef]
- Wei, Y.; Zhen, L.; Du, B. The evolution of desertification control and restoration technology in typical ecologically vulnerable regions. J. Resour. Ecol. 2022, 13, 775–785. [Google Scholar]
- Raj, A.; Jhariya, M.K.; Banerjee, A.; Nema, S.; Bargali, K. Land degradation and restoration: Implication and management perspective. Land. Environ. Manag. For. 2023, 1, 1–21. [Google Scholar] [CrossRef]
- Gao, S.; Wu, J.; Ma, L.; Gong, X.; Zhang, Q. Introduction to sand-restoration technology and model in China. Sustainability 2022, 15, 98. [Google Scholar] [CrossRef]
- Hagemann, M.; Henneberg, M.; Felde, V.J.; Drahorad, S.L.; Berkowicz, S.M.; Felix-Henningsen, P.; Kaplan, A. Cyanobacterial diversity in biological soil crusts along a precipitation gradient, Northwest Negev Desert, Israel. Microb. Ecol. 2015, 70, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Gao, G.L.; Qin, S.G.; Yu, M.H.; Ding, G.D. Desertification detection and the evaluation indicators: A review. J. Arid. Land. Resour. Environ. 2019, 33, 81–87. [Google Scholar]
- Yu, Z.L. Ecology: Current Knowledge and Future Challenges; High Education Press: Beijing, China, 2016. [Google Scholar]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Pett-Ridge, J. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Wanek, W. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil. Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L.; Holmes, A.J.; Westoby, M.; Oliver, I.; Briscoe, D.; Dangerfield, M.; Beattie, A.J. Spatial scaling of microbial eukaryote diversity. Nature 2004, 432, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, F.; Cui, G.; Wang, Y.; Yang, J.; Cheng, H.; Zhang, L. Effects of bio-organic fertilizer on soil fertility, microbial community composition, and potato growth. Sci. Asia 2021, 47, 347. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Ellert, B.H.; Bremer, E.; Smith, E.G.; Petri, R.M.; Neilson, J.A.; Janzen, H.H. Ramifications of crop residue loading for soil microbial community composition, activity and nutrient supply. Soil. Use Manag. 2023, 39, 402–414. [Google Scholar] [CrossRef]
- Weldmichael, T.G.; Szegi, T.; Denish, L.; Gangwar, R.K.; Michéli, E.; Simon, B. The patterns of soil microbial respiration and earthworm communities as influenced by soil and land-use type in selected soils of Hungary. Soil. Sci. Annu. 2020, 71, 43–52. [Google Scholar] [CrossRef]
- Song, H.K.; Shi, Y.; Yang, T.; Chu, H.; He, J.S.; Kim, H.; Adams, J.M. Environmental filtering of bacterial functional diversity along an aridity gradient. Sci. Rep. 2019, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Z.; Zhang, C.X.; Liu, J.L.; Zeng, X.W.; Li, F.R.; Wu, Y.C.; Jia, Z.J. Microbial community changes along a land-use gradient of desert soil origin. Pedosphere 2012, 22, 593–603. [Google Scholar] [CrossRef]
- Wu, B.; Ci, L.J. Landscape change and desertification development in the Mu Us Sandland, Northern China. J. Arid. Environ. 2002, 50, 429–444. [Google Scholar] [CrossRef]
- Yan, J.; Lou, L.; Bai, W.; Zhang, S.; Zhang, N. Phosphorus deficiency is the main limiting factor for re-vegetation and soil microorganisms in Mu Us Sandy Land, Northwest China. Sci. Total. Environ. 2023, 900, 165770. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, F.; Ren, X.; Meng, Z.; Dang, X.; Wu, X. Soil infiltration properties are affected by typical plant communities in a semi-arid desert grassland in China. Water 2022, 14, 3301. [Google Scholar] [CrossRef]
- Gerenfes, D.; Giorgis, A.; Negasa, G. Comparison of organic matter determination methods in soil by loss on ignition and potassium dichromate method. Int. J. Hortic. Food Sci. 2022, 4, 49–53. [Google Scholar] [CrossRef]
- Hicks, T.D.; Kuns, C.M.; Raman, C.; Bates, Z.T.; Nagarajan, S. Simplified Method for the Determination of Total Kjeldahl Nitrogen in Wastewater. Environments 2022, 9, 55. [Google Scholar] [CrossRef]
- Yan, S.; Liu, C.; Li, J.; Li, J.; Cui, C.; Fan, J.; Yan, C. Changes in the soil phosphorus supply with rice straw return in cold region. Agronomy 2023, 13, 2214. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total. Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Asiku, J.; Dvir, H.; Shtenberg, G. N-acetyl-β-d-glucosaminidase activity assay for monitoring insulin-dependent diabetes using Ag-porous Si SERS platform. Talanta 2022, 239, 123087. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Wang, S.; Wang, C.; Mo, J.; Zhang, W. Is microbial biomass measurement by the chloroform fumigation extraction method biased by experimental addition of N and P? iForest 2021, 14, 408. [Google Scholar] [CrossRef]
- Ren, X.; Hao, S.; Yang, C.; Yuan, L.; Zhou, X.; Zhao, H.; Yao, J. Alterations of intestinal microbiota in liver cirrhosis with muscle wasting. Nutrition 2021, 83, 111081. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, Z.; Liu, Y.; Wu, G.L. Carbon accumulation by Pinus sylvestris forest plantations after different periods of afforestation in a semiarid sandy ecosystem. Land. Degrad. Dev. 2021, 32, 2094–2104. [Google Scholar] [CrossRef]
- Cao, H.; Gao, G.; Zhang, Y.; Guo, M.; Ren, Y.; Ding, G. Soil bacterial approach to assessing afforestation in the desertfied Northern China. J. Clean. Prod. 2021, 292, 125935. [Google Scholar] [CrossRef]
- Güner, Ş.T.; Erkan, N.; Karataş, R. Effects of afforestation with different species on carbon pools and soil and forest floor properties. Catena 2021, 196, 104871. [Google Scholar] [CrossRef]
- Chen, W.; Yu, T.; Zhao, C.; Li, B.; Qin, Y.; Li, H.; Zhang, X. Development and determinants of topsoil bacterial and fungal communities of afforestation by aerial sowing in Tengger Desert, China. J. Fungi 2023, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shao, M.; Fu, X.; Wang, X.; Wei, X. Effect of grassland afforestation on soil N mineralization and its response to soil texture and slope position. Agric. Ecosyst. Environ. 2019, 276, 64–72. [Google Scholar] [CrossRef]
- Mongil-Manso, J.; Navarro-Hevia, J.; San Martín, R. Impact of land use change and afforestation on soil properties in a Mediterranean mountain area of central Spain. Land 2022, 11, 1043. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, M.M.M.; Malik, A.; Vashisth, M.; Singh, D.; Kumar, R.; Pandey, V. Rhizosphere, rhizosphere biology, and rhizospheric engineering. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer International Publishing: Cham, Switzerland, 2021; pp. 577–624. [Google Scholar]
- Vokou, D.; Chalkos, D.; Karamanlidou, G.; Yiangou, M. Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J. Chem. Ecol. 2002, 28, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deng, S.; De Philippis, R.; Chen, L.; Hu, C.; Zhang, W. Chemical composition of volatile oil from Artemisia ordosica and its allelopathic effects on desert soil microalgae, Palmellococcus miniatus. Plant. Physiol. Biochem. 2012, 51, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The M icrobial Efficiency-M atrix S tabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.P.; Zhang, L.M.; Guo, J.F.; Ray, J.L.; He, J.Z. Impact of long-term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China. Appl. Soil. Ecol. 2010, 46, 119–124. [Google Scholar] [CrossRef]
- Santonja, M.; Rancon, A.; Fromin, N.; Baldy, V.; Hättenschwiler, S.; Fernandez, C.; Mirleau, P. Plant litter diversity increases microbial abundance, fungal diversity, and carbon and nitrogen cycling in a Mediterranean shrubland. Soil. Biol. Biochem. 2017, 111, 124–134. [Google Scholar] [CrossRef]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil. Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil. Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Girvan, M.S.; Bullimore, J.; Pretty, J.N.; Osborn, A.M.; Ball, A.S. Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl. Environ. Microb. 2003, 69, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, S.; Huang, M.; Thorsten, L.H.; Wei, J. Ascomycota has a faster evolutionary rate and higher species diversity than Basidiomycota. Sci. China Life Sci. 2010, 53, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Berbee, M.L. Dating divergences in the Fungal Tree of Life: Review and new analyses. Mycologia 2006, 98, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.S.; Blackwell, M.; James, T.Y.; Spatafora, J.W.; Taylor, J.W.; Vilgalys, R. Phylogenetic taxon definitions for fungi, dikarya, ascomycota and basidiomycota. IMA Fungus 2018, 9, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).