Abstract
We aimed to study the effects of mycorrhizal and extraradical hyphae on soil physical and chemical properties and enzyme activity characteristics in a subtropical plantation and to explore its indicative effect on the effectiveness of soil nutrients. In this study, three native afforestation tree species, Cunninghamia lanceolata, Schima superba, and Liquidambar formosana, with different biological characteristics, root functional traits, and nutrient acquisition strategies in subtropical regions were selected as the research objects. Based on the method of in-growth soil cores, the nylon mesh with different pore sizes was used to limited the root system and hypha into the soil column. The soil physical and chemical properties of five kinds of hydrolase related to the carbon (C), nitrogen (N), and phosphorus (P) cycles were determined in this study. The correlation of different tree species, roots, and mycelia with soil physicochemical properties, enzyme activity, and stoichiometric ratios was analyzed. The results revealed that mycorrhizal treatment significantly affected the soil total carbon (TC) and pH but had no significant effect on hydrolase activity and its stoichiometric ratio. Tree species significantly affected soil physical and chemical properties, soil β-1,4-N-acetylglucosaminidase (NAG), β-1,4-glucosidase (βG), and cellobiohydrolase (CB) activities and soil enzyme stoichiometric ratios. The soil enzyme activity and stoichiometric ratio of the Chinese fir forest had higher values than in monoculture broad-leaved stands of both Schima superba and Liquidambar formosana. There was no significant interaction effect of mycorrhizal treatments and tree species on all soil properties, enzyme activities, and stoichiometric ratios. In addition, the soil enzyme activity and stoichiometric characteristics were mainly affected by the pH. In this study, the soil enzyme activity ratios In(BG + CB):In(AP) and In(NAG + LAP):In(AP) were lower values than the global scale, while the ratios of In(βG + CB):In(NAG + LAP) were higher than the average, indicating that the soil microorganisms in this area were limited by C and P. Moreover, the soil enzyme activity and chemical metrology characteristics were mainly affected by the pH change. In conclusion, differences in litter quality and root functional traits of tree species affected the soil enzyme activity and its stoichiometric characteristics through the shaping of the forest environment by organic matter input, and the influence of pH was the main regulating factor.
1. Introduction
Soil enzymes are a class of catalytic proteins produced by soil microorganisms, plants, and animals that act as biocatalysts in ecochemical reactions and play an important role in biogeochemical processes [1]. Soil enzyme activities are closely linked to the efficiency of soil nutrients such as carbon (C), nitrogen (N), and phosphorus (P) [2]. During biogeochemical cycles, microorganisms secrete appropriate extracellular enzymes to convert soil organic matter into required elements, among which cellobiohydrolase (CB) and β-1,4-glucosidase (βG) are involved in processes related to the soil carbon cycle, Leucine aminopeptidase (LAP) and β-1,4-N-acetylglucosaminidase (NAG) are involved in processes related to the soil N cycle, and acid phosphatase (AP) is a phosphorus acquisition enzyme [3]. Changes in the soil enzyme activities are indicative of microbial nutrient demand characteristics and soil nutrient status, so soil enzyme activities are often used as an indicator for evaluating soil fertility and quality [4].
Soil enzyme stoichiometric ratios are the ratios of enzyme activities associated with C, N, and P nutrient cycling and may reflect biogeochemical balance patterns between plant, microbial community, and soil nutrients [5]. Currently, soil enzyme stoichiometric ratio research has become a focal point for nutrient cycling in ecosystems. It has been reported that the stoichiometric ratio of carbon, nitrogen, and phosphorus−Related hydrolases in soil stands at around 1:1:1 at a global scale [6]. However, due to variations in the surrounding environmental conditions and biological factors at different regional scales, the stoichiometric ratio will exhibit different patterns. Cui et al. [7] studied different vegetation species and soil types on the Loess Plateau of China and found that both plant species and soil types significantly affected the soil enzyme stoichiometry ratios, with forest stands responding more to enzyme activity and enzyme stoichiometry ratios than soil properties. And then Cui et al. [8] analyzed fir forests at high altitudes by a vector of enzyme stoichiometry and found that soil microorganisms were more limited in C and P with increasing altitude. Xu et al. [9] investigated forests in southern China and found that the soil enzyme activity ratios In (BG + CB):In(AP) and In(NAG + LAP):In(AP) were lower than the global scale, indicating that soil microorganisms in subtropical forest ecosystems in southern China were limited by P nutrient elements. In addition, under the interactive influence of altitude and location, soil nutrient restriction is also regulated by the pH, water content (WC), temperature, and nutrient metrology ratio [10]. Therefore, studying the soil nutrient limitation status by forest stand and environmental factors from the perspective of soil enzyme stoichiometric ratios can help to further understand the efficiency of nutrient utilization in different plantation forests in the subtropics.
Forest stands are the main factor influencing soil nutrients, with forest litter and root systems being the main input sources of soil nutrients [11]. Compared with litter, organic matter secreted by the root system not only provides rich sources of C and N for soil microorganisms but also alters the number and activity of microorganisms, thus affecting organic matter decomposition and nutrient metabolism processes to a greater extent [12]. At the same time, the microorganisms also coordinate the production of enzymes related to C, N, and P cycling to fulfill their nutrient requirements through “optimal resource allocation” [13]. In addition, roots also form mycorrhizal symbionts with mycorrhizal fungi and mycelium, as the epitaxy of roots, expanding the absorptive area of the plant’s root system, while the mycorrhizal fungi infestation increases the number of bacteria and actinomycetes in the rhizosphere soil. It has been found that plants allocate significantly higher levels of photosynthetic carbon to the mycelial pathway of mycorrhizal fungi than to the rhizosphere pathway [14]. Zhang et al. [15] found in vitro culture experiments that Arbuscular mycorrhiza fungi (AMF) promote phosphorus-solubilizing bacteria secretion and enhance intercellular phosphatase activity. AMF can also increase soil microbial levels and enhance the effectiveness of soil nutrients [16]. However, it was also found that cellulose hydrolase activity was lowest when mycorrhizal symbiosis was present, with no significant effect on phenol oxidase, and that the presence of mycorrhiza may have a negative effect on soil microorganisms [17]. It can be seen that the regulatory mechanism of the effect of root and mycelial growth on soil nutrients is not clear, and the effect of root and mycelial growth on soil enzyme activities and stoichiometric ratios may also be affected by tree species, mycorrhizal type, and the environment in which they are located; further research is needed.
Cunninghamia lanceolata (CL), Schima superba (SS), and Liquidambar formosana (LF) are important native silvicultural species in subtropical China, all of which are clumped arbuscular mycorrhizal (AM) species but with large differences in growth type and functional characteristics of the root system. SS has a developed fibrous root system and is usually used as a biological fireproof tree [18]. LF is a deciduous broad-leaved tree with obvious primary roots and slender and well-developed lateral roots, which can efficiently absorb nutrients [19]. It is an early tree species in subtropical secondary succession. CL is a coniferous tree species, preferring fertile soil, with weak root penetration, mainly distributed in the shallow soil layer, and the efficiency and rate of nutrient uptake is less than that of the other two broad-leaved forests [20]. Differences in stand type and nutrient acquisition strategies lead to differences in the quantity and quality of soil organic matter, which can alter the structure of the soil microbial community and nutrient utilization efficiency, affecting soil enzyme activities and stoichiometric ratios [21,22]. At present, there are fewer studies on AM mycorrhizal symbiosis and mycelium on soil enzyme activities and their stoichiometric ratios in these three species of plantation forests in the subtropics. This study investigated the differences and relationships between AM mycorrhizal symbiosis and mycelium on soil physicochemical properties and enzyme activities and their stoichiometric ratios of the three tree species based on the in-growth core method, to gain a deeper understanding of the characteristics of nutrient cycling of subtropical plantation forest ecosystems and to provide a theoretical basis for nutrient management in the major plantation forests in subtropical regions and for the sustainable operation of plantation forests.
2. Materials and Methods
2.1. Research Area
The study area is located in Guanshan Forest Farm, Yongfeng County (26°38′ N, 115°56′ E), Ji’an City, Jiangxi Province, which is located in the subtropical monsoon climate zone with abundant rainfall, four distinct seasons, abundant sunshine, an average annual temperature of 18 °C, average annual rainfall of 1627.3 mm, a frost-free period of 279 days, and a relative humidity of 75%–80%. The landform of this region is low mountains and hills, with an average elevation of 251.6 m. The soil types in this region are rich, including seven soil types, namely, paddy soil, tidal soil, purple soil, carbonaceous soil, red soil, mountain yellow soil, and mountain yellow brown soil. Among them, red soil is the soil type with the largest distribution area in the local area, and the average soil thickness is about 1 m [23].
2.2. Experimental Design
According to the principle of the same slope direction and soil development conditions, three artificial pure forests of Cunninghamia lanceolata, Schima superba, and Liquidambar formosana were selected as experimental plots. Fixed quadrats were set up in three pure forests for the installation of internal growth soil columns. Four quadrats were set up in each plantation, and the area of each quadrat was 20 m × 20 m, with a total of 12 sample plots. The basic conditions of the different plantation plots are shown in Table 1.

Table 1.
The basic situation of the three plantations plots in this study.
In mid-July 2021, an equal amount of soil was randomly taken from the 0–10 cm soil layer of the three plantations. After coarse roots and stones were removed, the soil of the three plantations was thoroughly mixed and evenly sifted through a 4 mm soil screen. After air drying, the soil was used as the test soil to ensure the uniformity of the initial soil matrix. The total carbon content of the soil matrix was 12.63 g·kg−1, and the pH was 4.73. The contents of gravel, silt, and clay were 32.3%, 44.6%, and 23.2%, respectively. According to the international soil classification method [24], the soil texture used was red loam.
Fine root and AMF treatment was performed using nylon mesh with different pore sizes. The pore sizes of 1000 μm, 50 μm, and 1 μm corresponded to tree absorbent roots and mycelia extending into soil column at the same time (+R + H), only mycelia extending into soil column (−R + H), and both mycelia and absorbent roots being restricted (−R − H) [25]. The nylon mesh was processed into a cylindrical mesh bag (height 20 cm/diameter 4.6 cm), and the mesh bag was filled with the soil matrix according to the soil bulk weight of 1.0 g·cm−3.
In each sample plot, 12 average trees were selected, and three 20 cm deep boreholes were randomly drilled at 30–80 cm from the base of each tree with an earth auger, the distance between each borehole not exceeding 1 m. The mesh bag with soil was placed into the PVC pipe (70% of the PVC pipe opening area) and then together into the borehole, buried with in situ soil, and then covered with litter. A total of 432 soil columns were buried in the three plantation forests, with the intention of being sampled across three growing seasons. In this study, the samples were collected after the first growing season. As the study principally examined how the root system of the forest impacted soil enzyme activities along with their stoichiometric ratios, the understory vegetation surrounding buried soil columns was manually cut prior to the peak growing season (July) each year to diminish any impacts of the understory vegetation root system on the soil properties within the soil column.
2.3. Soil Sampling
After one growing season (July 2021 to November 2021), four test trees were randomly selected from each sample plot for destructive sampling in December 2021, the buried PVC pipe was drilled with an earth auger with a slightly larger hole size, and the nylon mesh-filled soil columns were removed and brought back indoors for processing to remove the root system within each soil column. Soil column soil with the same aperture nylon net in the same square was mixed, and the soil was divided into three parts; one part was kept fresh at low temperature at 4 °C for extracting soil available nitrogen and determining the mass water content, and the other part was preserved at −80 °C for determining the soil enzyme activity. The residual soil was dried and used to determine the soil pH, TC, TP, TN, and other nutrients.
2.4. Sampling Analyses
2.4.1. Soil Chemical Analyses
The soil water content (WC) was determined by the drying method [26], and the soil pH was determined potentiometrically (PHS-3C, INESA (Instruments and Electronics Associates, Shanghai, China) [27]. The total carbon (TC) was determined by a total organic carbon analyzer (Multi N/C 2100S, Analytic Jena, Germany). The soil was digested with H2SO4−HClO4, and the total nitrogen (TN) and total phosphorus (TP) were determined with a fully automated chemical analyzer (Smartchem 200, ALLIANCE, France). The soil samples were leached using 2 mol·L−1 KCl solution (soil–water ratio of 1:10), and the contents of ammonium nitrogen (AN) and nitrate nitrogen (NN) were determined by automatic discontinuous chemical analyzer (AMS SmartChem140, AMS-Alliance, Weston, FL, USA).
2.4.2. Soil Enzyme Activities
Five hydrolytic enzymes in soil were determined by the 96-microtiter plate fluorescence method [28], weighing about 1.0 g of soil samples in 250 mL conical flasks, adding 100 mL of sodium acetate buffer (adjusting pH to the average value of the soil), and stirring for 5 min with a magnetic stirrer to homogenize the soil under the condition of room temperature. The soil suspension, substrate, acetate buffer, and standard solution were added to a 96-microtiter plate in that order, and the hydrolase plate was incubated in an incubator at 25 °C for 3 h under light protection. The fluorescence intensity of the hydrolase was measured using a multifunctional enzyme marker (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA) at an excitation wavelength of 365 nm and an emission wavelength of 450 nm.
2.5. Statistical Analysis
The data were collated, statistically analyzed, and plotted using Microsoft Excel 365 and R (version R 4.1.3; R Core Team 2022) software. The aov() function of the R basic package was used to test the significance of differences between tree species and mycorrhizal treatments on soil physicochemical properties and enzyme activities, and the leveneTest() and shapiro.test() functions were used to test for the chi-squaredness of the variance and normal distribution of the data; the data that did not conform to the normal distribution and the chi-squaredness were subjected to log or square root transformation. Pearson correlation was used to analyze the correlation between the soil physicochemical properties and soil hydrolase activities (Hmisc’s rcorr() function). The rda() function of the vegan package was used to analyze the relationship between the physical and chemical properties of different soils and the stoichiometric ratio of enzymes, and the data on basic soil physicochemical properties were log transformed and then Hellinger normalized to the enzyme stoichiometric ratio matrix (decostand() function). In order to prevent collinearity among soil indicators, the degree of collinearity among the explanatory variables was determined by variance expansion factor (VIF). If the VIF was less than 10, the model was selected, and a Monte Carlo permutation test was used. The envfit function tested the significance of each explanatory variable and visualized the redundancy analysis (RDA) results using ggplot. The soil enzyme stoichiometric ratio was calculated by the ratio of hydrolase activity related to C, N, and P, as follows:
enzyme C:N activity ratio = In(βG + CB):In(NAG + LAP)
enzyme C:P activity ratio = In(βG + CB):In(AP)
enzyme N:P activity ratio = In(NAG + LAP):In(AP)
The energy and nutrient limitations of soil microorganisms were measured by creating a vector starting at the origin of the coordinates and ending at the enzyme stoichiometric ratios [29], calculated as follows:
X = (βG + CB):(βG + CB + AP)
Y = (βG + CB):(βG + CB + NAG + LAP)
VL = Sqrt (X2 + Y2)
VA = Degrees [Atan2(X, Y)]
The vector length (VL) indicates microbial C relative to N and P limitation, where the longer the vector, the more the microbe is C-limited; the vector angle (VA) indicates microbial P relative to N limitation, where VA > 45° indicates microbial P limitation and VA < 45° indicates microbial N limitation.
3. Results
3.1. Effects of Tree Species and Mycorrhizal Treatment on Soil Physicochemical Properties
The analysis of variance (ANOVA) showed that tree species significantly affected the soil pH, WC, TN, N:P, C:N, and AN. Different mycorrhizal treatments affected only the pH and TC values. The interaction between tree species and mycorrhizal treatments did not affect the soil physicochemical properties (Table 2).

Table 2.
Effects of tree species (TS) and mycorrhizal treatment (MT) on soil physical and chemical properties, enzyme activity, and stoichiometric ratio (F value).
The results of multiple comparative analyses showed that the soil pH value and the WC of CL were significantly higher than those of CL and SS (p < 0.05), and the difference between CL and SS was not significant. The TN content of LF was higher than that of the other two stands. The content of AN was the highest in SS, followed by LF, and the lowest in CL (Table 3). The soil C:N ratios were significantly greater in the SS forest than in the CL and LF forests, but the soil N:P ratios were smaller than in the other two species (Table 4). Only the soil pH and soil TC differed significantly between mycorrhizal limitation treatments (p < 0.05). The soil pH was highest under the −R − H mycorrhizal treatment, with the difference reaching the level of significance in both the LF and SS stands. The soil TC reached a significant difference level in SS, as +R + H > −R − H > −R + H (Table 3).

Table 3.
Soil physicochemical properties in different tree species and mycorrhizal treatments (mean ± SD, n = 4).

Table 4.
Effects of different tree species and mycorrhizal treatments on soil nutrient ratios and enzyme stoichiometry.
3.2. Effects of Tree Species and Mycorrhizal Treatment on Soil Enzyme Activity and Stoichiometric Ratio
The analysis of variance (ANOVA) showed that mycorrhizal restriction treatments did not affect the measured hydrolytic enzyme activities at a significant level (Table 2). The tree species had significant effects on NAG, βG, and CB activities (p < 0.05), and the activities of the three enzymes showed similar variation rules among different tree species, showing as CL > SS > LF (Figure 1b–d). The LAP and AP activities did not show significant changes among different artificial forests (Figure 1a,e).
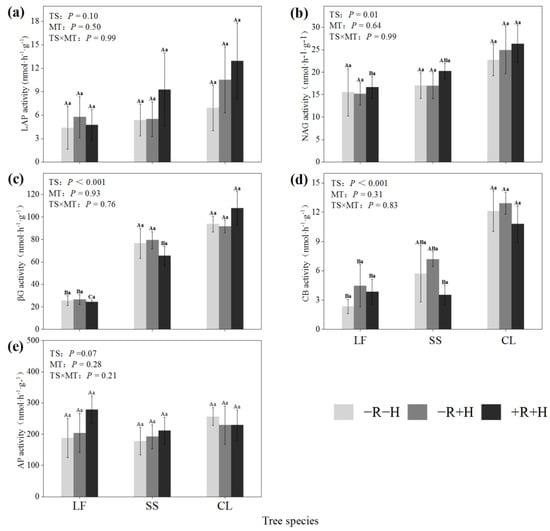
Figure 1.
Soil hydrolase activity under different tree species and mycorrhizal restriction treatments (a–e). CL: Cunninghamia lanceolata; LF: Liquidambar formosana; SS: Schima superba; Different lowercase letters indicate that there were significant differences in different mycorrhizal restriction treatments of the same tree species (p < 0.05). Different capital letters indicate that there were significant indigenous differences among different tree species under the same mycorrhizal restriction treatment (p < 0.05).
The mycorrhizal treatment had no significant effect on the soil enzyme stoichiometry, vector length, and angle (Table 4). The tree species significantly affected the soil enzyme stoichiometry, VL, and VA, generally showing that the enzyme stoichiometry and VL of LF were smaller than those of CL and SS, indicating that LF was less limited by C than the other two forest species. All three stand VAs were greater than 45° (Table 4), indicating that microorganisms in the area were limited by P.
3.3. Correlation Analysis of Soil Physicochemical Properties and Enzyme Activities with Their Stoichiometric Ratios
The correlation coefficients of soil physical and chemical properties, enzyme activities, and their stoichiometric ratios are shown in Table 5. NAG, βG, and CB activities showed significant negative correlations with pH (p < 0.05), while βG and AP activities showed significant negative and positive correlations with water content (p < 0.05), respectively. βG and CB activities were positively correlated with NN content (p < 0.05). The In(βG + CB):In(NAG + LAP) and In(βG + CB):In(AP) ratios were significantly negatively correlated with WC (p < 0.01), and the In(NAG + LAP):In(AP) ratio was significantly negatively correlated with pH and AN content (p < 0.05). There was no significant correlation between soil nutrients and their stoichiometry ratios and enzyme activities and their stoichiometry ratios.

Table 5.
Correlation between soil physical and chemical properties with soil enzyme activity and enzyme stoichiometric ratio.
After removing collinear relationship variables, eight factors including the pH, WC, TC, TN, C:N, C:P, AN, and NN were retained. RDA was further conducted on the relationship between the retained environmental factors and soil enzyme activity and its stoichiometric characteristics. The first two axes (RDA1 and RDA2) explained 48.38% of the variation of soil enzyme activity and its stoichiometry characteristics (RDA1:42.76%; RDA:5.62%) (Figure 2). The results of the Monte Carlo displacement test showed (Table 6) that only the pH value had the largest explanatory power to soil enzyme activity and stoichiometry ratio after one growing season (R2 = 17%).
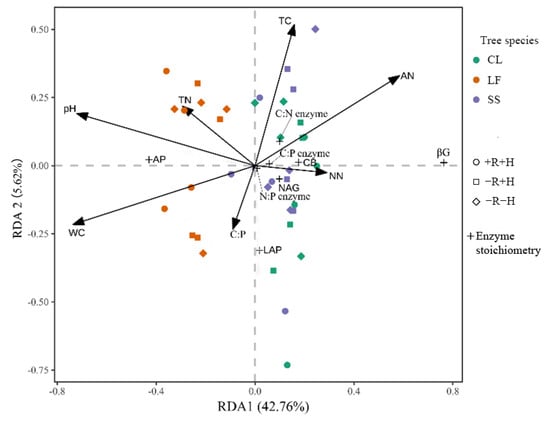
Figure 2.
Redundancy analysis (RDA) of soil physicochemical properties and enzyme activity and enzyme stoichiometry. CL: Cunninghamia Lanceolata; LF: Liquidambar formosana; SS: Schima superba; +R + H: treatment with roots and hyphae; −R + H: treatment with hyphae but without roots; −R−H: treatment without either; WC: water content; TC: total carbon; TN: total nitrogen; TP: total phosphorus; C:N: carbon–nitrogen ratio; C:P: carbon–phosphorus ratio; N:P: nitrogen–phosphorus ratio; AN: ammonium nitrogen; NN: nitrate nitrogen; LAP: leucine aminopeptidase; NAG: β-1,4-N- acetylglucosaminidase; AP: acid phosphatase; βG: β-1,4-glucosidase; CB: β-D-cellobiosidase; C:N enzyme: In(βG + CB):In(NAG + LAP); C:P enzyme: In(βG + CB):In(AP); C:P enzyme: In(NAG + LAP):In(AP).

Table 6.
Monte Carlo permutation test of explanatory variables for redundancy analysis (RDA).
4. Discussion
4.1. Effect of Tree Species on Soil Enzyme Activities and Their Stoichiometric Ratios
The soil enzyme activity varies with the different litter input and root functional traits in different forests. In this study, it was found that there was a highly significant correlation between different tree species on the activities of soil C cycle-related enzymes (βG and CB), and the βG and CB activities were significantly higher under CL than under the other two broad-leaved forests. The differences in enzyme activity within different stands were mainly in the microbial response to degrading organic matter from different sources. On one hand, the differences in root chemical properties and fine root turnover rate of the three tree species led to differences in the quantity and quality of the fine root residue input. Studies have found that the fine root yield of CL plantation is lower than that of natural forest, but the proportion of dead fine roots in the total fine root in the early growth stage of the stand is significantly higher than that of natural forest and some broad-leaved trees [30]. The fine root decomposition rate of low-quality CL is not always lower than that of high-quality broad-leaved trees. Soil microorganisms significantly increase the mineralization rate of soil organic matter by secreting more enzymes, so as to alleviate the demand for nutrients of CL and microorganisms [31]. On the other hand, CL belongs to coniferous forests, which are generally believed to have higher lignin and cellulose content in litters [9]. During the decomposition of soil organic matter, higher cellulose content will be preferentially decomposed by microorganisms, so cellulase activity will increase. Meanwhile, in the process of cellulose decomposition, glucose dimer and part of cellulose oligosaccharides will be decomposed into small-molecule glucose by β-1,4-glucosidase [32]. In addition, due to abundant rainfall in tropical and subtropical regions, soluble organic matter (DOM) produced by rainwater washing fresh leaves and apoplastic litter contributes significantly to the soil active carbon pool [30]. Some studies have found that the promotion of βG and CB activity of DOM produced by leaching from CL leaves after input into soil is higher than that of SS leaves [33]. This is similar to the results of the present study, in which the input of organic matter with high carbon content increased microbial biomass and changed the microbial community structure in the soil, which further increased soil enzyme activities.
It is generally accepted that the higher the soil enzyme activity ratios In(βG + CB):In(NAG + LAP) and In(βG + CB):In(AP), the higher the effective C source required by soil microorganisms. In this study, the ratio of enzyme activity to In(βG + CB):In(NAG + LAP) and In(βG + CB):In(AP) in LF soil was much lower than that of CL and SS, indicating that more C was available to microorganisms in LF soil. This may be due to the well-developed fibrous root system of LF, with more root branches and higher specific root length. LF belongs to the nutrient harvesting type of tree, which can intercept nutrients through well-developed roots and is less dependent on rhizosphere microorganisms [18]. At present, there are fewer reports on the effects of root functional traits on below-ground ecological processes, and the mechanisms by which differences in functional traits and nutrient acquisition strategies between stands affect soil nutrients remain to be further investigated.
4.2. Effect of Mycorrhizal Treatments on Soil Enzyme Activities and Their Stoichiometric Ratios
There were no significant differences in soil enzyme activities and stoichiometric ratios under different mycorrhizal treatments, which is different from the results of some previous studies. Zhang et al. [34], based on the in-growth soil column method and using subalpine coniferous forests in southwest China, found that mycelium inputs more new C into the soil, promotes intermycelial microbial activities, increases soil enzyme activities, and enhances SOM decomposition, compared with root C. Maillard used the same method to study coniferous forests in Finland [35], and the results showed that the presence of roots accelerated SOM decomposition and improved the soil enzyme activity in the short term, but the longer-term (3 years) test results showed that soil enzyme activity had no significant effect on mycorrhizal restriction treatment. Possible reasons why different mycorrhizal treatments did not affect soil enzyme activities and stoichiometric ratios in this study were as follows: On one hand, the soil columns in this study were installed for less than half a year, with a much shorter period than in the above studies, and it was difficult for the amount of input of root and mycelial secretions to cause significant changes in soil microorganisms and nutrients in a short period of time. On the other hand, the soil columns of the different mycorrhizal limitation treatments were not more than 1 m apart and were distributed around the trunks, and the soluble organic matter secreted by the root system and mycelium diffused into the 50 μm and 1 μm soil columns by the lateral flow of soil water; the leaching loss of soluble organic matter (DOM) from the soil columns of all treatments also occurred, leading to the homogenization of DOM in the soil of the different mycorrhizal limitation treatments [36]. In conclusion, nutrient input and substrate homogenization in different soil columns may be the reasons why enzyme activity and its stoichiometric ratio were not responsive to mycorrhizal treatment.
4.3. Drivers of Soil Enzyme Activities and Stoichiometric Ratios in Subtropical Plantation Forests
In this study, the correlation between soil enzyme activities and stoichiometry ratios and soil physical and chemical properties was explored by using correlation analysis and RDA. The results showed that soil enzyme activities and stoichiometry ratios were significantly affected by the soil moisture, pH, and available nitrogen. After eliminating the factors that had collinearity, the RDA analysis showed that the soil pH was the main factor influencing the soil enzyme activities and their stoichiometric ratios, with an explanatory value of 17%. The soil pH and C−Related enzyme activities and enzyme activity ratios In(βG + CB):In(NAG + LAP) and In(βG + CB):In(AP) showed a significant negative correlation, which was consistent with the research results of different tree species in the subtropical monsoon climate region by Wang et al. [37]. The pH can affect not only the structural composition of the enzyme itself but also the adsorption of soil mineral particles to the enzyme and the ionization of the substrate concentration and enzyme chemical reaction in solution. Fang et al. [38] also proved this conclusion that the increase in H+ can promote the aggregation of organic minerals in soil and effectively prevent the decomposition of soil organic matter by microorganisms, and soil pH affects enzyme activity and its stoichiometric ratio by changing the composition of organic matter and the availability of nutrients.
In this study, it was found that soil nutrients had no significant effect on enzyme activities. This finding was comparable to that in Xu et al.’s [39] research, which suggests that soil nutrient content indirectly influences soil enzymes by affecting the growth of plants and microorganisms. As soil nutrients increase or decrease, soil enzyme activities exhibit relatively complex alterations. Enzyme activities are co−Regulated by nutrient demand and supply, leading to an obscure correlation between the two factors. Previous studies have shown that soil enzyme stoichiometric ratios effectively respond to the ability of microorganisms to acquire C, N, and P nutrients [40]. In contrast, we found no significant correlation between enzyme stoichiometric ratios and both soil nutrients and their stoichiometric ratios. This may be due to the homogenization of the initial soil matrix of the soil columns in the three forests; the burial time is too short, and the difference in nutrient input and microbial structure is not significant. In addition, soil enzyme stoichiometric ratios are largely influenced by the soil microbial biomass. Therefore, over a short period of time (one growing season), soil environmental factors have a stronger effect on soil enzymes and their stoichiometric ratios than soil nutrient factors.
4.4. Soil Microbial Nutrient Limitation in Different Plantation Forests in the Subtropics
Soil enzyme stoichiometric ratios are related to nutrient limitation and can reflect soil microbial nutrient acquisition capacity and nutrient utilization, as well as being an important indicator of the soil fertility status [40]. In the present study, the enzyme activity ratios In(βG + CB):In(AP) were found to be 0.46, 0.14, and 0.41, and the In(NAG + LAP):In(AP) ratios were found to be 0.15, 0.10, and 0.13 for the soils of CL, SS, and LF, respectively, which were lower than the global average of 0.62 and 0.44 [6]. The VAs were all greater than 45°. This shows that the soil microorganisms in this study area were limited by P, which is similar to the previous results of P limitation in subtropical soil [41]. Due to abundant rainfall in subtropical regions, abundant precipitation leads to the loss of soil P. Also, phosphorus is adsorbed by soil iron and aluminum oxides to form a closed storage state of phosphorus in acidic red soil regions, leading to a decrease in the effectiveness of phosphorus in the soil [42]. Meanwhile, we found that the In(βG + CB):In(NAG + LAP) ratios of CL, LF, and SS in this region were 3.54, 3.65, and 1.62, respectively, which were all higher than the global average of 1.41 [6], indicating that soil microorganisms in this study area were also carbon limited, which may be a result of soil microorganisms being P-limited, which would have facilitated the release of soil C to alleviate nutrient deficiencies in soil [43].
5. Conclusions
In conclusion, there was no significant effect of different mycorrhizae on the enzyme activities and their stoichiometric ratios, and the interaction between tree species and mycorrhizal treatments on soil enzyme activities and their stoichiometric ratios was not significant, suggesting that there was no tree species specificity in soil enzyme activities and their stoichiometric ratios for AM mycorrhizal symbiosis and mycelial growth in response to mycorrhizal growth in the short term. The soil enzyme activities and their stoichiometric ratios differed between three plantation forests in the subtropics, and the soil pH was the key factor driving the variation. Soil microorganisms of plantation forests in subtropical areas are severely limited by C and P. Therefore, during the management of subtropical plantation forests, attention should be paid to the management of the soil environment and the effectiveness of soil nutrients in order to maintain the productivity and sustainable development of the subtropical plantation forest ecosystem.
Author Contributions
Conceptualization, Y.C. and Y.Z.; methodology, Y.C., S.L., L.Z., B.A., T.X. and R.M.; software, Y.C.; validation, Y.C. and Y.Z.; formal analysis, Y.C.; investigation, Y.C.; resources, Y.C.; data curation, Y.C.; writing—original draft preparation, Y.C.; writing—review and editing, Y.C. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 31800524 and No. 32260378) and Natural Science Foundation of Jiangxi Province (20232BAB205043).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Qianguang Liu, Zhi Zheng, and Yong Zhu for participating in the field trial setup.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to resolve spelling and grammatical errors. This change does not affect the scientific content of the article.
References
- Sardans, J.; Peñuelas, J.; Estiarte, M. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; BeInap, J.; Findlay, S.G.; Shah, J.J.F.; Hill, B.H.; Kuehn, K.A.; Kuske, C.R.; Litvak, M.E.; Martinez, N.G.; Moorhead, D.L.; et al. Extracellular enzyme kinetics scale with resource availability. Biogeochemistry 2014, 121, 287–304. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Jicha, T.M.; Bolgrien, D.W.; Moffett, M.F. Sediment microbial enzyme activity as an indicator of nutrient limitation in the great rivers of the Upper Mississippi River basin. Biogeochemistry 2010, 97, 195–209. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.H.; Elonen, C.M.; Seifert, L.R.; May, A.A.; Tarquinio, E. Microbial enzyme stoichiometry and nutrient limitation in US streams and rivers. Ecol. Indic. 2012, 18, 540–551. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Zhang, Y.; Li, P.; Zhang, X. Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2021, 458, 7–20. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Hu, X.F.; Wan, S.; Wang, H.; Liang, C.; Chen, F.S. Litter manipulation effects on microbial communities and enzymatic activities vary with soil depth in a subtropical Chinese fir plantation. For. Ecol. Manag. 2021, 480, 118641. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; He, P.; Tang, S.; Yang, S.; Zhou, W.; Wang, X. The alleviation of acid soil stress in rice by inorganic or organic ameliorants is associated with changes in soil enzyme activity and microbial community composition. Biol. Fertil. Soils 2015, 51, 465–477. [Google Scholar] [CrossRef]
- Leff, J.W.; Wieder, W.R.; Taylor, P.G.; Townsend, A.R.; Nemergut, D.R.; Grandy, A.S.; Cleveland, C.C. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob. Chang. Biol. 2012, 18, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Kaiser, C.; Kilburn, M.R.; Clode, P.L.; Fuchslueger, L.; Koranda, M.; Cliff, J.B.; Solaiman, Z.M.; Murphy, D.V. Exploring the transfer of recent plant photosynthates to soil microbes: Mycorrhizal pathway vs direct root exudation. New Phytol. 2015, 205, 1537–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef]
- Xu, H.; Shao, H.; Lu, Y. Arbuscular mycorrhiza fungi and related soil microbial activity drive carbon mineralization in the maize rhizosphere. Ecotoxicol. Environ. Saf. 2019, 182, 109476. [Google Scholar] [CrossRef]
- Moore, J.A.; Jiang, J.; Patterson, C.M.; Mayes, M.A.; Wang, G.; Classen, A.T. Interactions among roots, mycorrhizas and free-living microbial communities differentially impact soil carbon processes. J. Ecol. 2015, 103, 1442–1453. [Google Scholar] [CrossRef]
- Yan, X.L.; Ma, X. Responses of root morphology and seedling growth in three tree species to heterogeneous supplies of ammonium and nitrate. For. Ecol. Manag. 2021, 479, 118538. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Mou, P.; Guo, J.; Li, S. Root order and initial moisture status influenced root decomposition in a subtropical tree species Liquidambar formosana. Plant Soil 2019, 443, 539–548. [Google Scholar] [CrossRef]
- Yan, X.L.; Wang, C.; Ma, X.; Wu, P. Root morphology and seedling growth of three tree species in southern China in response to homogeneous and heterogeneous phosphorus supplies. Trees 2019, 33, 1283–1297. [Google Scholar] [CrossRef]
- Cheng, F.; Peng, X.; Zhao, P.; Yuan, J.; Zhong, C.; Cheng, Y.; Cui, C.; Zhang, S. Soil microbial biomass, basal respiration and enzyme activity of main forest types in the Qinling Mountains. PLoS ONE 2013, 8, e67353. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, J.; Liang, H.; Zhang, Y.; Yang, J.; Chen, F.; Wang, Y.; Yan, W. Changes in Soil Properties, Microbial Quantity and Enzyme Activities in Four Castanopsis hystrix Forest Types in Subtropical China. Plants 2023, 12, 2411. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guo, H.; Li, W.; Wang, J.; Huang, Z.; Zhang, J.; Liu, G.; Wang, Y.; Jiang, J. Study on the characteristics of mycorrhizal colonization in Chinese fir plantations at different ages. Acta Ecol. Sin. 2019, 39, 1926–1934. [Google Scholar]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sietiö, O.M.; Straková, P.; Prommer, J.; Wild, B.; Hagner, M.; Pihlatie, M.; Fritze, H.; Richter, A.; Heinonsalo, J. Plant roots increase both decomposition and stable organic matter formation in boreal forest soil. Nat. Commun. 2019, 10, 3982. [Google Scholar] [CrossRef]
- National Forestry Administration. Forestry Industry Standard of the People‘s Republic of China: Forest Soil Analysis Method; Standards Press of China: Beijing, China, 1999. [Google Scholar]
- Lu, Y. Soil Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 228–246. [Google Scholar]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Hu, M.; Zou, B.; Huang, Z.; Wang, S.; Su, X.; Ding, X.; Zheng, G.; Chen, H.Y. Fine root biomass and necromass dynamics of Chinese fir plantations and natural secondary forests in subtropical China. For. Ecol. Manag. 2021, 496, 119413. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Luo, Y.; Liu, S.; Xu, X.; Tong, C.; Shibistova, O.; Guggenberger, G.; Wu, J. Microbial stoichiometric flexibility regulates rice straw mineralization and its priming effect in paddy soil. Soil Biol. Biochem. 2018, 121, 67–76. [Google Scholar] [CrossRef]
- Šnajdr, J.; Valášková, V.; Merhautová, V.; Herinková, J.; Cajthaml, T.; Baldrian, P. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol. Biochem. 2008, 40, 2068–2075. [Google Scholar] [CrossRef]
- Mei, K.; Cheng, L.; Zhang, Q.; Lin, K.; Zhou, J.; Zeng, Q.; Wu, Y.; Xu, J.; Zhou, J.; Chen, Y. Effects of dissolved organic matter from different plant sources on soil enzyme activities in subtropical forests. Chin. J. Plant Ecol. 2020, 44, 1273–1284. [Google Scholar] [CrossRef]
- Zhang, Z.; Phillips, R.P.; Zhao, W.; Yuan, Y.; Liu, Q.; Yin, H. Mycelia-derived C contributes more to nitrogen cycling than root-derived C in ectomycorrhizal alpine forests. Funct. Ecol. 2019, 33, 346–359. [Google Scholar] [CrossRef]
- Maillard, F.; Kennedy, P.G.; Adamczyk, B.; Heinonsalo, J.; Buée, M. Root presence modifies the long-term decomposition dynamics of fungal necromass and the associated microbial communities in a boreal forest. Mol. Ecol. 2021, 30, 1921–1935. [Google Scholar] [CrossRef]
- Kaiser, K.; Kalbitz, K. Cycling downwards-dissolved organic matter in soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- Wang, Y.S.; Cheng, S.L.; Yu, G.R.; Fang, H.J.; Mo, J.M.; Xu, M.J.; Gao, W.L. Response of carbon utilization and enzymatic activities to nitrogen deposition in three forests of subtropical China. Can. J. For. Res. 2015, 45, 394–401. [Google Scholar] [CrossRef]
- Fang, H.; Cheng, S.; Yu, G.; Xu, M.; Wang, Y.; Li, L.; Dang, X.; Wang, L.; Li, Y. Experimental nitrogen deposition alters the quantity and quality of soil dissolved organic carbon in an alpine meadow on the Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2014, 81, 1–11. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, C. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Li, J.; Shangguan, Z.; Deng, L. Dynamics of soil microbial metabolic activity during grassland succession after farmland abandonment. Geoderma 2020, 363, 114167. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Farrington, H. Nutrient limitation and soil development: Experimental test of a biogeochemical theory. Biogeochemistry 1994, 37, 63–75. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, Z.J.; Zong, Y.Y.; Li, W.Q.; Chen, F.S.; Wang, G.G.; Li, J.J.; Fang, X.M. Mixing with coniferous tree species alleviates rhizosphere soil phosphorus limitation of broad-leaved trees in subtropical plantations. Soil Biol. Biochem. 2022, 175, 108853. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).