Microtopographic Variation in Biomass and Diversity of Living and Dead Wood in a Forest in Dongling Mountains, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.3. Data Analysis
3. Results
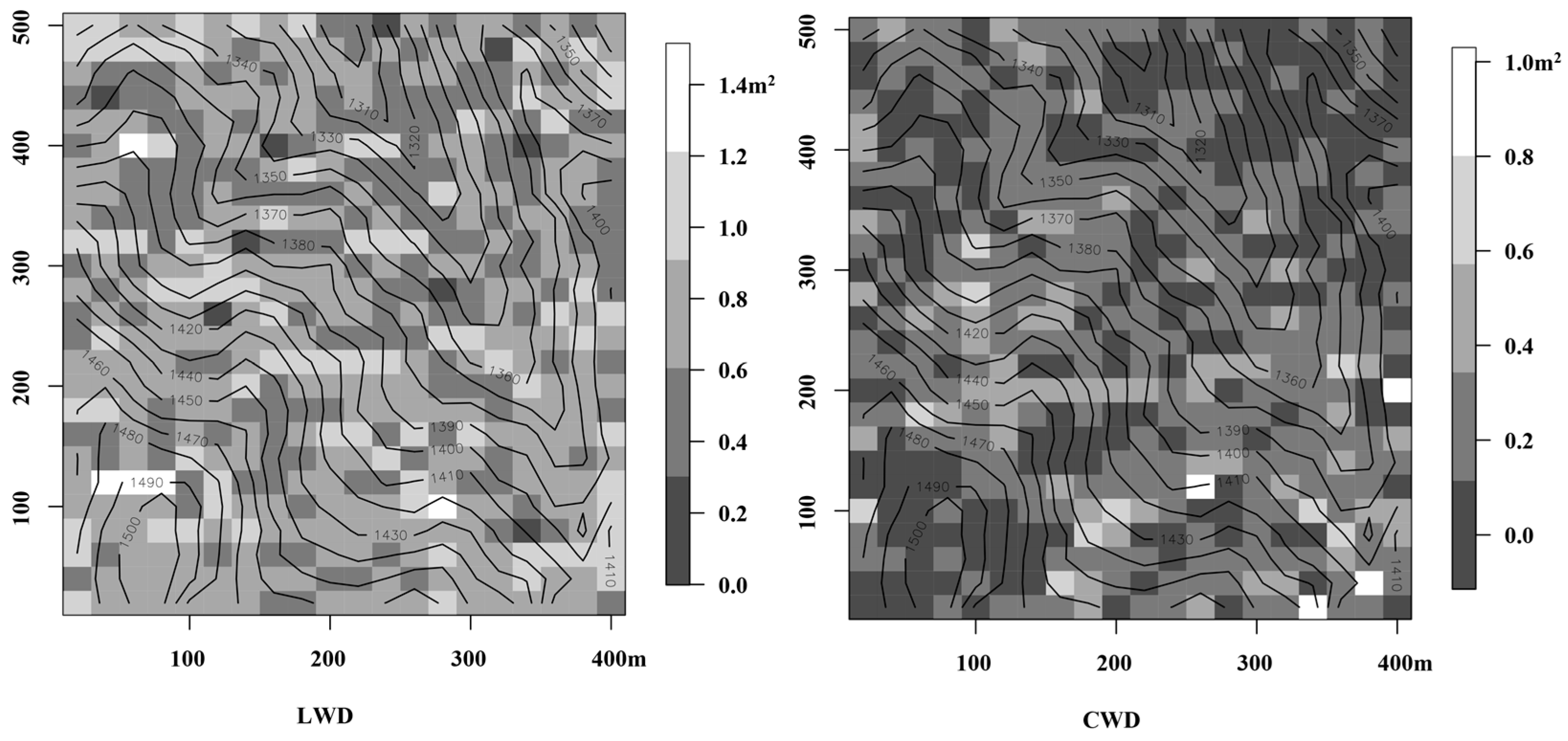
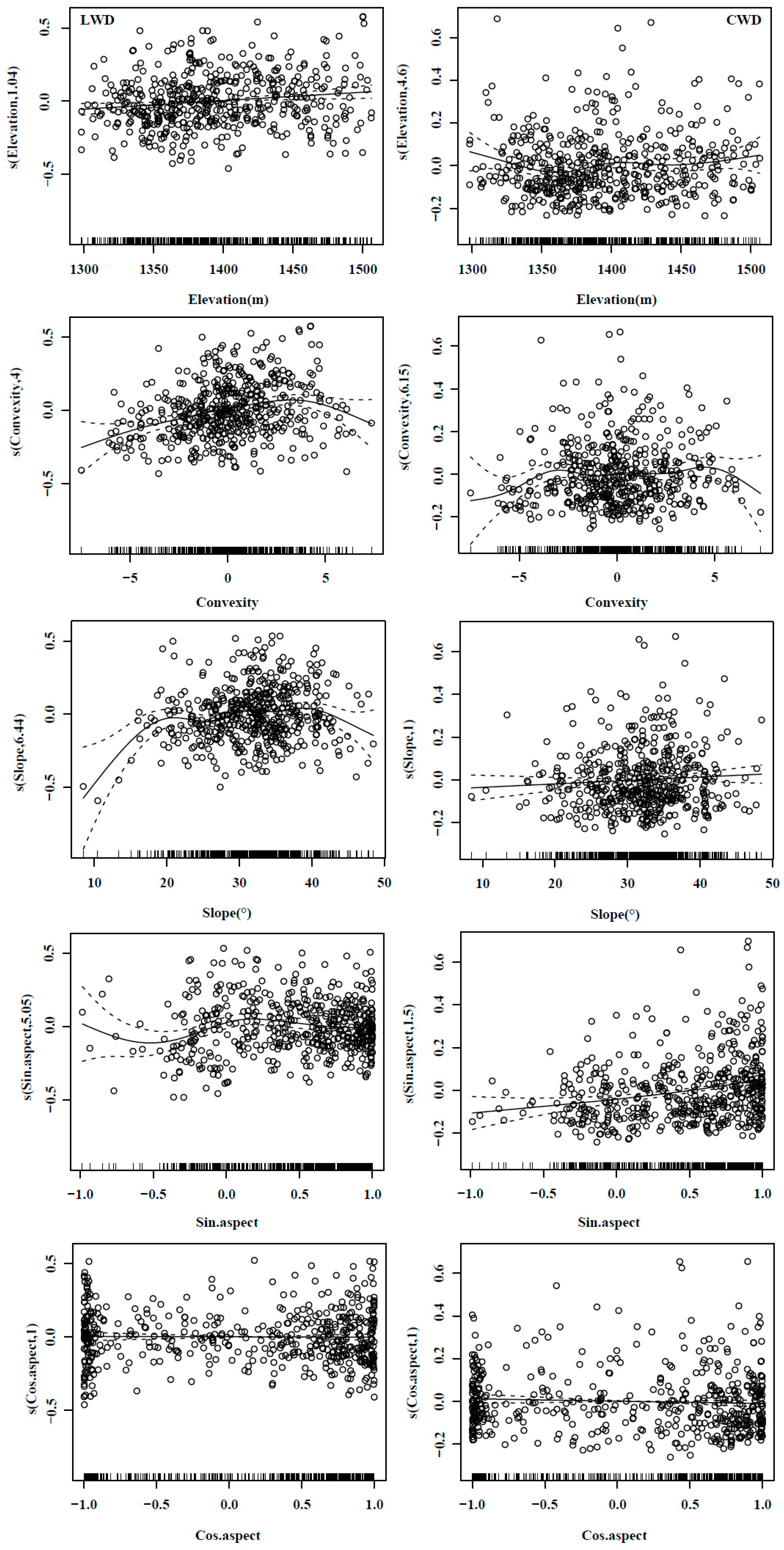
3.1. Effects of Microtopography on Total Basal Area
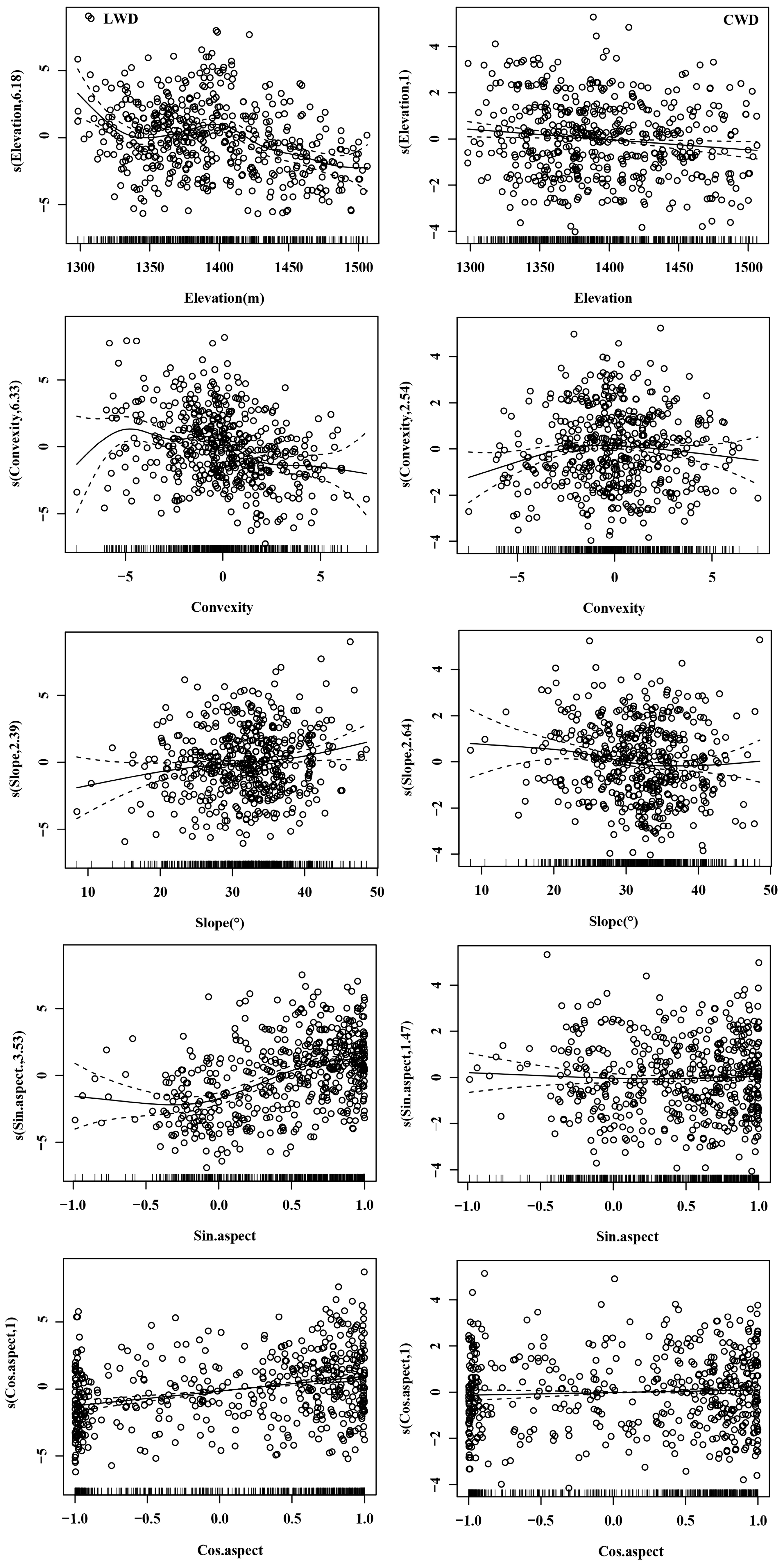
3.2. Effects of Microtopography on Species Richness
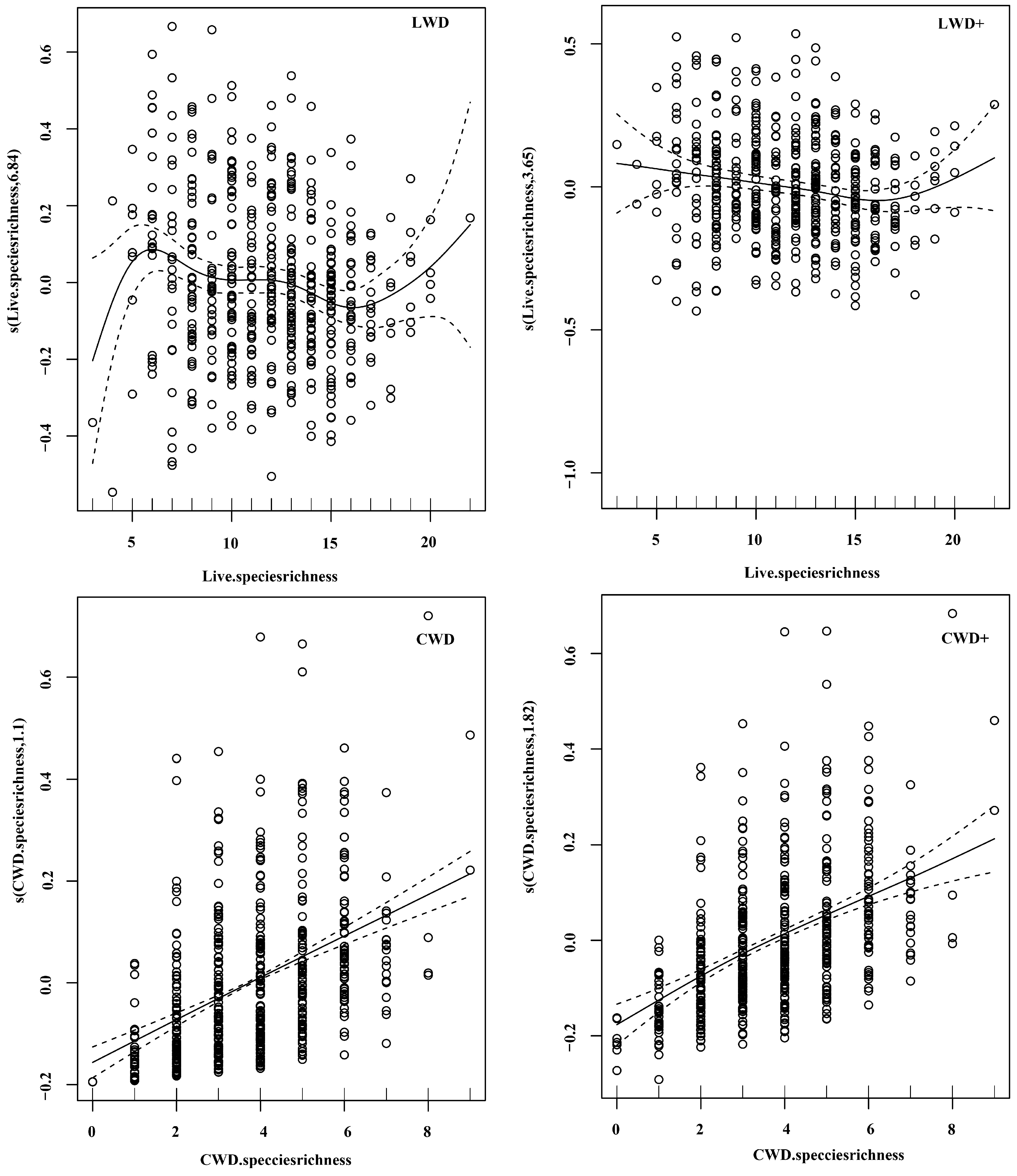
3.3. Effects of Microtopography on the Relationship of Species Richness to Total Basal Area
4. Discussion
4.1. The Response of Biomass (Total Basal Area) to Microtopography
4.2. Species Diversity (Species Richness) Response to Microtopography
4.3. The Response of the Species Diversity–Biomass Relationship to Microtopography
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murthy, I.K.; Khalid, M.A.; Sathyanarayan, V.; Beerappa, M.; Bhat, S.; Prashant, M.; Ravindranath, N.H. Biodiversity and biomass carbon dynamics: Insights from long-term monitoring in the western ghats. In Forest Dynamics and Conservation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 213–230. [Google Scholar]
- Houghton, R.A. Aboveground forest biomass and the global carbon balance. Global Change Biol. 2005, 11, 945–958. [Google Scholar] [CrossRef]
- Russell, M.B.; Fraver, S.; Aakala, T.; Gove, J.H.; Woodall, C.W.; D’Amato, A.W.; Ducey, M.J. Quantifying carbon stores and decomposition in dead wood: A review. Forest Ecol. Manag. 2015, 350, 107–128. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Global Change Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Fahey, T.J.; Woodbury, P.B.; Battles, J.J.; Goodale, C.L.; Hamburg, S.P.; Ollinger, S.V.; Woodall, C.W. Forest carbon storage: Ecology, management, and policy. Front Ecol. Environ. 2010, 8, 245–252. [Google Scholar] [CrossRef]
- McEwan, R.W.; Lin, Y.; Sun, I.; Hsieh, C.; Su, S.; Chang, L.; Song, G.-Z.M.; Wang, H.-H.; Hwong, J.-L.; Lin, K.-C.; et al. Topographic and biotic regulation of aboveground carbon storage in subtropical broad-leaved forests of taiwan. Forest Ecol. Manag. 2011, 262, 1817–1825. [Google Scholar] [CrossRef]
- Herrmann, S.; Kahl, T.; Bauhus, J. Decomposition dynamics of coarse woody debris of three important central european tree species. For. Ecosyst. 2015, 2, 27. [Google Scholar] [CrossRef]
- Cui, B.S.; Zhai, H.J.; Dong, S.K.; Chen, B.; Liu, S.L. Multivariate analysis of the effects of edaphic and topographical factors on plant distribution in the yilong lake basin of yun-gui plateau, china. Can. J. Plant Sci. 2009, 89, 211–221. [Google Scholar] [CrossRef]
- Fortunel, C.; Lasky, J.R.; Uriarte, M.; Valencia, R.; Wright, S.J.; Garwood, N.C.; Kraft, N.J.B. Topography and neighborhood crowding can interact to shape species growth and distribution in a diverse amazonian forest. Ecology 2018, 99, 2272–2283. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F. Ecological relations between forest communities and environmental variables in the lishan mountain nature reserve, china. Afr. J. Agric. Res. 2011, 6, 248–259. [Google Scholar]
- Zhang, Z.; Hu, G.; Ni, J. Effects of topographical and edaphic factors on the distribution of plant communities in two subtropical karst forests, southwestern china. J. Mt. Sci. 2013, 10, 95–104. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.; Kursar, T.A.; Tyree, M.T. Drought effects on seedling survival in a tropical moist forest. Trees 2005, 19, 312–321. [Google Scholar] [CrossRef]
- Homeier, J.; Breckle, S.W.; Günter, S.; Rollenbeck, R.T.; Leuschner, C. Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich ecuadorian montane rain forest. Biotropica 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Girardin, C.A.J.; Malhi, Y.; Aragao, L.; Mamani, M.; Huaraca Huasco, W.; Durand, L.; Feeley, K.J.; Rapp, J.; Espejo, J.E.S.; Silman, M.; et al. Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the peruvian andes. Global Change Biol. 2010, 16, 3176–3192. [Google Scholar] [CrossRef]
- Tavili, A.; Jafari, M. Interrelations between plants and environmental variables. Int. J. Environ. Res. 2009, 2, 239–246. [Google Scholar]
- Unger, M.; Homeier, J.; Leuschner, C. Effects of soil chemistry on tropical forest biomass and productivity at different elevations in the equatorial andes. Oecologia 2012, 170, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Maza, B.; Rodes-Blanco, M.; Rojas, E. Aboveground biomass along an elevation gradient in an evergreen andean–amazonian forest in ecuador. Front. For. Glob. Change 2022, 5, 738585. [Google Scholar] [CrossRef]
- Woodall, C.W.; Westfall, J.A. Relationships between the stocking levels of live trees and dead tree attributes in forests of the united states. For. Ecol Manag. 2009, 258, 2602–2608. [Google Scholar] [CrossRef]
- Takeda, S.; Takahashi, K. Elevational variation in abundance of coarse woody debris in subalpine forests, central japan. For. Ecol Manag. 2020, 473, 118295. [Google Scholar] [CrossRef]
- Holeksa, J.; Saniga, M.; Szwagrzyk, J.; Dziedzic, T.; Ferenc, S.; Wodka, M. Altitudinal variability of stand structure and regeneration in the subalpine spruce forests of the pol’ana biosphere reserve, central slovakia. Eur. J. Forest Res. 2007, 126, 303–313. [Google Scholar] [CrossRef]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Odor, P.; Standovár, T.; Rozenbergar, D.; Vrska, T. Dead wood in european beech (fagus sylvatica) forest reserves. Forest Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Katsumata, S.; Mori, A.S.; Osono, T.; Takeda, H. Accumulation and decay dynamics of coarse woody debris in a japanese old-growth subalpine coniferous forest. Ecol. Res. 2014, 29, 257–269. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, f8957. [Google Scholar] [CrossRef]
- Li, S.; Su, J.; Lang, X.; Liu, W.; Ou, G. Positive relationship between species richness and aboveground biomass across forest strata in a primary pinus kesiya forest. Sci. Rep. 2018, 8, 2227. [Google Scholar] [CrossRef]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; De Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Baert, J.M.; Janssen, C.R.; Sabbe, K.; De Laender, F. Per capita interactions and stress tolerance drive stress-induced changes in biodiversity effects on ecosystem functions. Nat. Commun. 2016, 7, 12486. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Ramírez, N.R.; Craven, D.; Reich, P.B.; Ewel, J.J.; Isbell, F.; Koricheva, J.; Eisenhauer, N. Diversity-dependent temporal divergence of ecosystem functioning in experimental ecosystems. Nat. Ecol. Evol. 2017, 1, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 2001, 412, 72–76. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Tang, Z.; Shen, Z.; Zheng, C.; Xia, X.; Fang, J. The relationship between species richness and biomass changes from boreal to subtropical forests in china. Ecography 2015, 38, 602–613. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.; Taylor, A.R. Aboveground biomass of understorey vegetation has a negligible or negative association with overstorey tree species diversity in natural forests. Global Ecol. Biogeogr. 2016, 25, 141–150. [Google Scholar] [CrossRef]
- Dai, W.; Fu, W.; Jiang, P.; Zhao, K.; Li, Y.; Tao, J. Spatial pattern of carbon stocks in forest ecosystems of a typical subtropical region of southeastern china. For. Ecol Manag. 2018, 409, 288–297. [Google Scholar] [CrossRef]
- Ma, F.; Zhuang, L.; Wang, S.; Sang, W. Coarse woody debris features of a warm temperate deciduous broad-leaved forest, northern China. J. For. Res. 2021, 32, 1105–1114. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Tree species distributions and local habitat variation in the amazon: Large forest plot in eastern ecuador. J. Ecol. 2004, 92, 214–229. [CrossRef]
- Lai, J.; Mi, X.; Ren, H.; Ma, K. Species-habitat associations change in a subtropical forest of china. J. Veg. Sci. 2009, 20, 415–423. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Wood, S.N. Low-rank scale-invariant tensor product smooths for generalized additive mixed models. Biometrics 2006, 62, 1025–1036. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Swartzman, G.; Huang, C.; Kaluzny, S. Spatial analysis of bering sea groundfish survey data using generalized additive models. Can. J. Fish Aquat. Sci. 1992, 49, 1366–1378. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Zhou, Y.; Wang, Z.; Gong, L.; Chen, A.; Liu, N.; Li, S.; Sun, H.; Yang, Z.; You, J. Functional characterization of three mate genes in relation to aluminum-induced citrate efflux from soybean root. Plant Soil. 2019, 443, 121–138. [Google Scholar] [CrossRef]
- Chun, J.; Ali, A.; Lee, C. Topography and forest diversity facets regulate overstory and understory aboveground biomass in a temperate forest of south korea. Sci. Total Environ. 2020, 744, 140783. [Google Scholar] [CrossRef]
- Punchi Manage, R.; Getzin, S.; Wiegand, T.; Kanagaraj, R.; Savitri Gunatilleke, C.V.; Nimal Gunatilleke, I.; Wiegand, K.; Huth, A. Effects of topography on structuring local species assemblages in a Sri Lankan mixed dipterocarp forest. J. Ecol. 2013, 101, 149–160. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Field, R.; Whittaker, R.J. Climatic gradients in woody plant (tree and shrub) diversity: Water-energy dynamics, residual variation, and topography. Oikos 2000, 89, 588–600. [Google Scholar] [CrossRef]
- Alves, L.F.; Vieira, S.A.; Scaranello, M.A.; Camargo, P.B.; Santos, F.A.; Joly, C.A.; Martinelli, L.A. Forest structure and live aboveground biomass variation along an elevational gradient of tropical atlantic moist forest (brazil). For. Ecol. Manag. 2010, 260, 679–691. [Google Scholar] [CrossRef]
- Fauzi, N.; Hambali, K.; Nawawi, S.A.; Busu, I.; Yew, S.K. Biomass and carbon stock estimation along different altitudinal gradients in tropical forest of gunung basor, kelantan, malaysia. Malay. Nat. J. 2017, 69, 57–62. [Google Scholar]
- Peña, M.A.; Feeley, K.J.; Duque, A. Effects of endogenous and exogenous processes on aboveground biomass stocks and dynamics in andean forests. Plant Ecol. 2018, 219, 1481–1492. [Google Scholar] [CrossRef]
- Kubota, Y.; Murata, H.; Kikuzawa, K. Effects of topographic heterogeneity on tree species richness and stand dynamics in a subtropical forest in okinawa island, southern japan. J. Ecol. 2004, 92, 230–240. [Google Scholar]
- Jia, X.; Zhu, Y.; Luo, Y. Soil moisture decline due to afforestation across the loess plateau, china. J. Hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Karahalil, U.; Başkent, E.Z.; Sivrikaya, F.; Kılıç, B. Analyzing deadwood volume of calabrian pine (pinus brutia ten.) In relation to stand and site parameters: A case study in köprülü canyon national park. Environ. Monit Assess. 2017, 189, 112. [Google Scholar] [CrossRef]
- Merganičová, K.; Merganič, J. Coarse woody debris carbon stocks in natural spruce forests of babia hora. J. For. Sci. 2010, 56, 397–405. [Google Scholar] [CrossRef]
- Scatena, F.N.; Lugo, A.E. Geomorphology, disturbance, and the soil and vegetation of two subtropical wet steepland watersheds of puerto rico. Geomorphology 1995, 13, 199–213. [Google Scholar] [CrossRef]
- Porder, S.; Paytan, A.; Vitousek, P.M. Erosion and landscape development affect plant nutrient status in the hawaiian islands. Oecologia 2005, 142, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, J.; Asner, G.P.; Muller-Landau, H.C.; van Breugel, M.; Hall, J.; Dahlin, K. Controls over aboveground forest carbon density on barro colorado island, panama. Biogeosciences 2011, 8, 1615–1629. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, H.; Guo, S. Soil organic carbon as a function of land use and topography on the loess plateau of china. Ecol. Eng. 2015, 83, 249–257. [Google Scholar] [CrossRef]
- Warren, R.J. Mechanisms driving understory evergreen herb distributions across slope aspects: As derived from landscape position. Plant Ecol. 2008, 198, 297–308. [Google Scholar] [CrossRef]
- Meng, Z.; Wei, L.; Yanyan, Q.; Jianjun, C.; Huiya, L.; Yu, Z. Distribution of soil organic carbon at hillslope scale in forest-steppe zone of qilian mountains. J. Desert Res. 2016, 36, 741. [Google Scholar]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Gairola, S.; Ghildiyal, S.K.; Sharma, C.M.; Suyal, S. Species richness and diversity along an altitudinal gradient in moist temperate forest of garhwal himalaya. Am. J. Sci. 2009, 5, 119–128. [Google Scholar]
- Swenson, N.G.; Anglada-Cordero, P.; Barone, J.A. Deterministic tropical tree community turnover: Evidence from patterns of functional beta diversity along an elevational gradient. Proc. R. Soc. B Biol. Sci. 2011, 278, 877–884. [Google Scholar] [CrossRef]
- Garten, C.T.; Taylor, G.E. Foliar δ 13 c within a temperate deciduous forest: Spatial, temporal, and species sources of variation. Oecologia 1992, 90, 1–7. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef]
- Jiang, L.; Wan, S.; Li, L. Species diversity and productivity: Why do results of diversity-manipulation experiments differ from natural patterns? J. Ecol. 2009, 97, 603–608. [Google Scholar] [CrossRef]
- Adler, P.B.; Seabloom, E.W.; Borer, E.T.; Hillebrand, H.; Hautier, Y.; Hector, A.; Harpole, W.S.; O’halloran, L.R.; Grace, J.B.; Anderson, T.M.; et al. Productivity is a poor predictor of plant species richness. Science 2011, 333, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.; Joshi, J.; Scherer-Lorenzen, M.; Schmid, B.; Spehn, E.M.; Wacker, L.; Weilenmann, M.; Bazeley-White, E.; Beierkuhnlein, C.; Caldeira, M.C.; et al. Biodiversity and ecosystem functioning: Reconciling the results of experimental and observational studies. Funct. Ecol. 2007, 998–1002. [Google Scholar] [CrossRef]
- Chisholm, R.A.; Muller Landau, H.C.; Abdul Rahman, K.; Bebber, D.P.; Bin, Y.; Bohlman, S.A.; Zimmerman, J.K. Scale-dependent relationships between tree species richness and ecosystem function in forests. J. Ecol. 2013, 101, 1214–1224. [Google Scholar] [CrossRef]
- Thompson, P.L.; Kéfi, S.; Zelnik, Y.R.; Dee, L.E.; Wang, S.; de Mazancourt, C.; Gonzalez, A. Scaling up biodiversity–ecosystem functioning relationships: The role of environmental heterogeneity in space and time. Proc. R. Soc. B 2021, 288, 20202779. [Google Scholar] [CrossRef]
- Mikoláš, M.; Svitok, M.; Bače, R.; Meigs, G.W.; Keeton, W.S.; Keith, H.; Buechling, A.; Trotsiuk, V.; Kozák, D.; Bollmann, K.; et al. Natural disturbance impacts on trade-offs and co-benefits of forest biodiversity and carbon. Proc. R. Soc. B 2021, 288, 20211631. [Google Scholar] [CrossRef]
- Deng, J.; Qin, B.; Wang, B. Quick implementing of generalized additive models using r and its application in blue-green algal bloom forecasting. Chin. J. Ecol. 2015, 34, 835. [Google Scholar]
- Guo, F.; Hu, H.; Jin, S.; Ma, Z.; Zhang, Y. Relationship between forest lighting fire occurrence and weather factors in daxing’an mountains based on negative binomial model and zero-inflated negative binomial models. J. Plant Ecol. 2010, 34, 571–577. [Google Scholar]
- Dong, X.; Bennion, H.; Maberly, S.C.; Sayer, C.D.; Simpson, G.L.; Battarbee, R.W. Nutrients exert a stronger control than climate on recent diatom communities in esthwaite water: Evidence from monitoring and palaeolimnological records. Freshw. Biol. 2012, 57, 2044–2056. [Google Scholar] [CrossRef]


| All Individuals | Environmental Parameters | Cumulative R2adj | Cumulative Explained Deviation (%) | Akaike Information Criterion (AIC) |
|---|---|---|---|---|
| LWD | s(Elevation) | 0.053 | 5.65% (5.65%) | −170.066 |
| s(Convexity) | 0.133 | 14.50% (12.70%) | −209.363 | |
| s(Slope) | 0.179 | 19.70% (6.40%) | −232.681 | |
| s(sin.Aspect) | 0.202 | 22.80% (4.58%) | −241.434 | |
| s(cos.Aspect) | 0.201 | 22.90% (1.55%) | −239.578 | |
| CWD | s(Elevation) | 0.017 | 2.49% (2.49%) | −442.809 |
| s(Convexity) | 0.034 | 5.51% (2.96%) | −444.669 | |
| s(Slope) | 0.033 | 5.63% (0.02%) | −443.262 | |
| s(sin.Aspect) | 0.067 | 8.24% (4.83%) | −464.660 | |
| s(cos.Aspect) | 0.076 | 10.20% (4.19%) | −463.908 |
| All Individuals | Smooth Terms | Edf | Ref.df | F | p-Value |
|---|---|---|---|---|---|
| LWD | s(Elevation) | 1.043 | 1.084 | 7.834 | 0.004 ** |
| s(Convexity) | 4.002 | 5.008 | 7.908 | 8.12 × 10−7 *** | |
| s(Slope) | 6.438 | 7.590 | 3.325 | 0.001 ** | |
| s(sin.Aspect) | 5.047 | 6.126 | 2.504 | 0.021 * | |
| s(cos.Aspect) | 1.000 | 1.000 | 0.023 | 0.879 | |
| CWD | s(Elevation) | 4.601 | 5.681 | 2.015 | 0.061 |
| s(Convexity) | 6.145 | 7.352 | 1.307 | 0.265 | |
| s(Slope) | 1.000 | 1.000 | 1.568 | 0.211 | |
| s(sin.Aspect) | 1.504 | 1.859 | 12.33 | 1.29 × 10−5 *** | |
| s(cos.Aspect) | 1.000 | 1.000 | 1.301 | 0.255 |
| N | Adjusted R2 | D2 | GCV | ||
|---|---|---|---|---|---|
| Model Performance | LWD | 500 | 0.201 | 0.229 | 0.036 |
| CWD | 500 | 0.075 | 0.102 | 0.023 |
| All Individuals | Environmental Parameters | Cumulative R2adj | Cumulative Explained Deviation (%) | Akaike Information Criterion (AIC) |
|---|---|---|---|---|
| LWD | s(Elevation) | 0.194 | 20.10% (20.10%) | 2538.12 |
| s(Convexity) | 0.224 | 23.20% (10.20%) | 2519.92 | |
| s(Slope) | 0.223 | 23.40% (2.78%) | 2521.53 | |
| s(sin.Aspect) | 0.394 | 41.10% (19.5%) | 2403.77 | |
| s(cos.Aspect) | 0.440 | 46.20% (22.4%) | 2369.90 | |
| CWD | s(Elevation) | 0.006 | 0.75% (0.75%) | 1944.03 |
| s(Convexity) | 0.014 | 2.14% (1.19%) | 1942.14 | |
| s(Slope) | 0.033 | 4.56% (1.77%) | 1935.09 | |
| s(sin.Aspect) | 0.032 | 4.61% (0.87%) | 1937.69 | |
| s(cos.Aspect) | 0.033 | 4.95% (0.63%) | 1936.91 |
| All Individuals | Smooth Terms | Edf | Ref.df | F | p-Value |
|---|---|---|---|---|---|
| LWD | s(Elevation) | 6.184 | 7.354 | 9.693 | <2 × 10−16 *** |
| S(Convexity) | 6.334 | 7.527 | 7.201 | <2 × 10−16 *** | |
| s(Slope) | 2.389 | 3.045 | 2.807 | 0.036 * | |
| s(sin.Aspect) | 3.529 | 4.390 | 21.60 | <2 × 10−16 *** | |
| s(cos.Aspect) | 1.000 | 1.000 | 40.01 | <2 × 10−16 *** | |
| CWD | s(Elevation) | 1.000 | 1.000 | 6.815 | 0.001 ** |
| s(Convexity) | 2.535 | 3.226 | 2.701 | 0.044 * | |
| s(Slope) | 2.643 | 3.380 | 2.585 | 0.053 | |
| s(sin.Aspect) | 1.469 | 1.805 | 0.323 | 0.703 | |
| s(cos.Aspect) | 1.000 | 1.000 | 1.111 | 0.292 |
| N | Adjusted R2 | D2 | GCV | ||
|---|---|---|---|---|---|
| Model Performance | LWD | 500 | 0.440 | 0.462 | 6.684 |
| CWD | 500 | 0.033 | 0.049 | 2.812 |
| Model Performance | Deviance Explained (D2) | Adjusted R2 | GCV | |
|---|---|---|---|---|
| LWD | − topography | 0.046 | 0.033 | 0.043 |
| + topography | 0.277 | 0.233 | 0.035 | |
| CWD | − topography | 0.200 | 0.198 | 0.019 |
| + topography | 0.287 | 0.271 | 0.018 |
| Convexity | Slope | Aspect | |
|---|---|---|---|
| Elevation | 0.35 *** | −0.17 *** | 0.04 * |
| Convexity | 0 | 0.04 * | |
| Slope | 0.14 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Wang, S.; Sang, W.; Zhang, S.; Ma, K. Microtopographic Variation in Biomass and Diversity of Living and Dead Wood in a Forest in Dongling Mountains, China. Forests 2023, 14, 2111. https://doi.org/10.3390/f14102111
Ma F, Wang S, Sang W, Zhang S, Ma K. Microtopographic Variation in Biomass and Diversity of Living and Dead Wood in a Forest in Dongling Mountains, China. Forests. 2023; 14(10):2111. https://doi.org/10.3390/f14102111
Chicago/Turabian StyleMa, Fang, Shunzhong Wang, Weiguo Sang, Shuang Zhang, and Keming Ma. 2023. "Microtopographic Variation in Biomass and Diversity of Living and Dead Wood in a Forest in Dongling Mountains, China" Forests 14, no. 10: 2111. https://doi.org/10.3390/f14102111
APA StyleMa, F., Wang, S., Sang, W., Zhang, S., & Ma, K. (2023). Microtopographic Variation in Biomass and Diversity of Living and Dead Wood in a Forest in Dongling Mountains, China. Forests, 14(10), 2111. https://doi.org/10.3390/f14102111







