Physiological and Biochemical Effects of Exogenous Calcium on Camellia oleifera Abel under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Field
2.2. Experimental Materials
2.3. Experimental Design
2.4. Experimental Methods
2.5. Statistical Analysis
3. Results
3.1. Effect of Exogenous Calcium on Water Content in C. oleifera Plants under Drought Stress
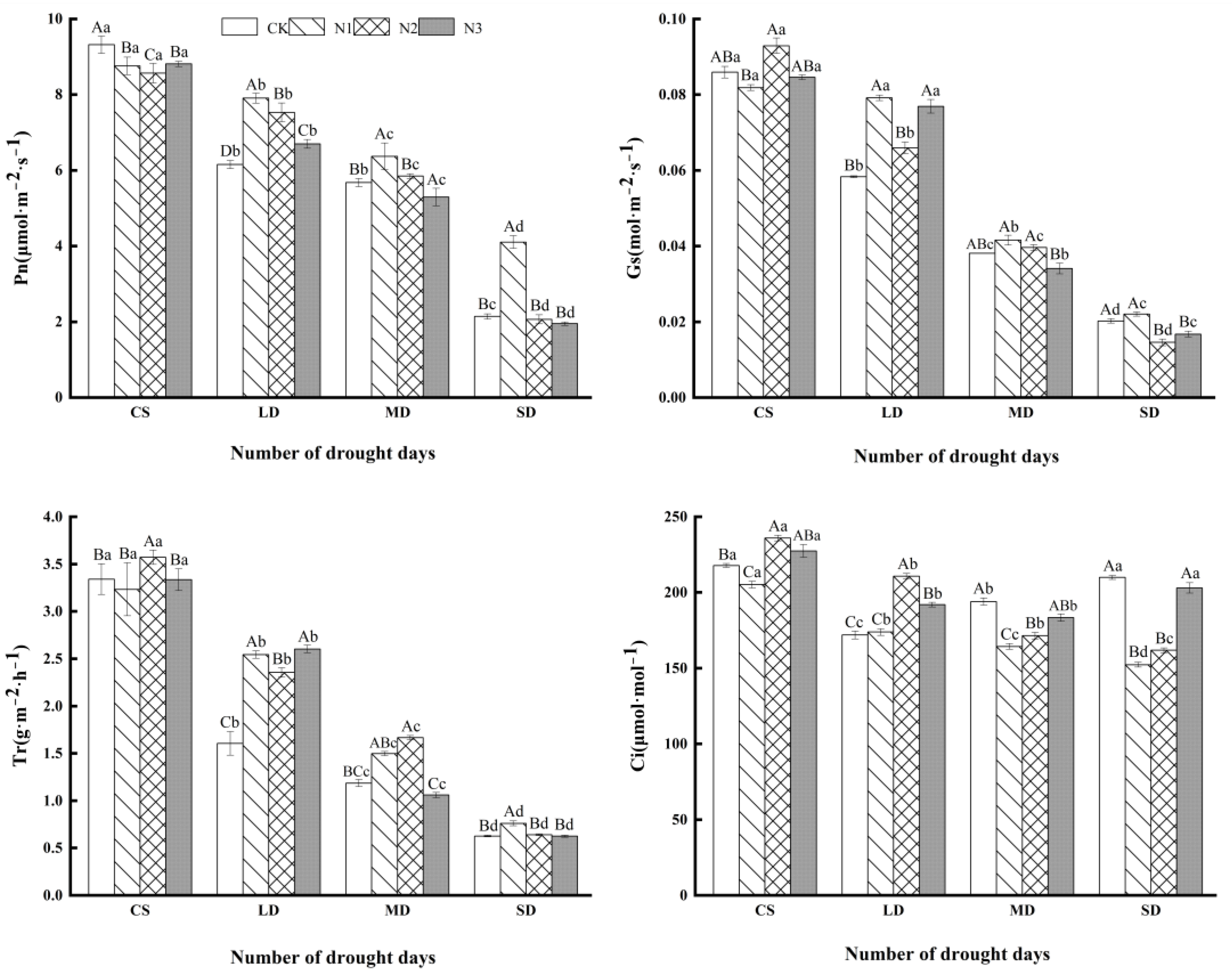
3.2. Effect of Exogenous Calcium on Photosynthetic Parameters of C. oleifera Plants under Drought Stress
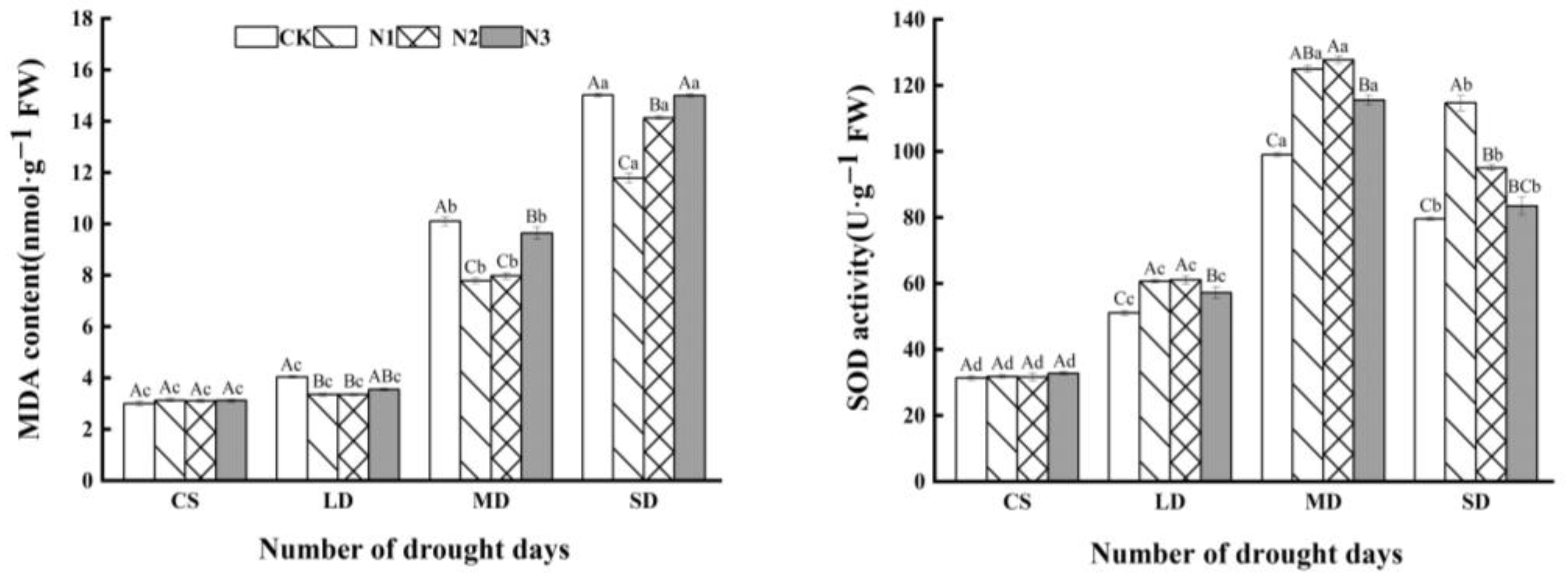
3.3. Effects of Exogenous Calcium on Biochemical Indicators in C. oleifera Plants under Drought Stress
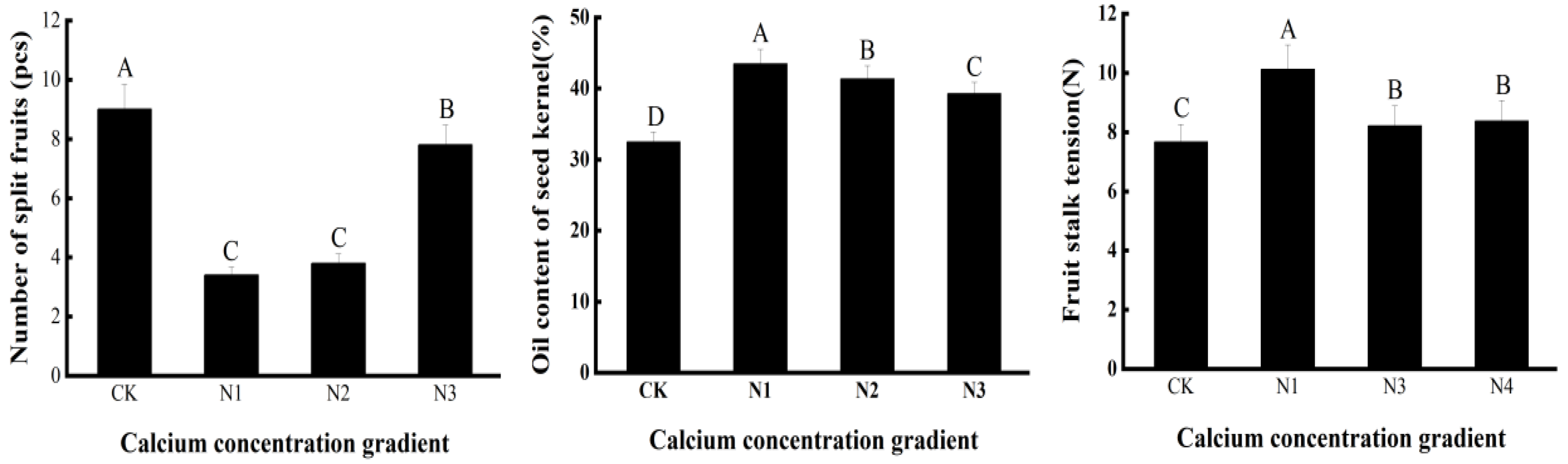
3.4. Effect of Exogenous Calcium on Fruits of C. oleifera Plants under Drought Stress
4. Discussion
4.1. Effect of Exogenous Calcium on Photosynthesis in C. oleifera under Drought Stress
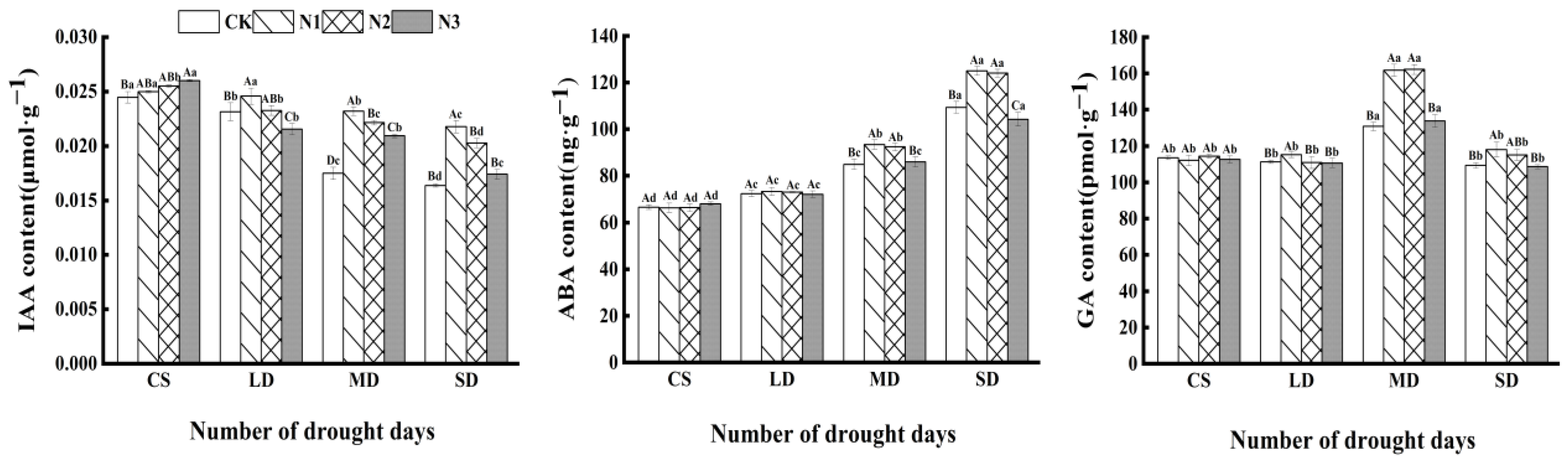
4.2. Effect of Exogenous Calcium on Endogenous Hormone Content in C. oleifera under Drought Stress
4.3. Effect of Exogenous Calcium on Biochemical Indicators in C. oleifera under Drought Stress
4.4. Effect of Exogenous Calcium on C. oleifera Fruits under Drought Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, R.; Zhi, X.F. Spatial-temporal characteristics of the drought and flood in Southern China. Sci. Meteorol. Sin. 2009, 29, 598–605. [Google Scholar]
- Bai, Y.Q.; Zhi, X.F.; Qi, H.X.; Zhang, L. Severe drought monitoring in South China based on the standardized precipitation index at different scales. Sci. Meteorol. Sin. 2010, 30, 292–300. [Google Scholar]
- Asgharipour, M.R.; Mosapour, H. A foliar application silicon enchances drought tolerance in fennel. J. Anim. Plant Sci. 2016, 26, 1056–1062. [Google Scholar]
- Chen, Y.Z.; Xiao, Z.H.; Peng, S.F.; Yang, X.H.; Li, D.X.; Wang, X.N.; Duan, W. Study of fruit growing specialties and its oil content in oil-tea Camellia. For. Res. 2006, 19, 9–14. [Google Scholar]
- Ding, S.J.; Zhong, Q.P.; Yuan, T.T.; Cao, L.Q.; Yan, C.; Yuan, Y.Q.; Zhang, X.H.; Lin, J.P. Effects of drought stress on Camellia oleifera flower-bud growth and production. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2017, 41, 197–202. [Google Scholar]
- Chen, Y.Z.; Luo, J.; Chen, L.S.; Peng, S.F.; Liao, Y.F.; Peng, J.D.; Du, D.S.; Zhao, H. Drought injury and countermeasure of Camellia oleifera in Hunan Province. Non-Wood For. Res. 2014, 32, 22–29+53. [Google Scholar]
- Xie, B.C.; Guo, L.Y.; Du, D.S.; Tan, Y.; Wang, G.D. Responses of Camellia oleifera Yield to heat accumulation temperature and high temperature days in key growth period. Sci. Silvae Sin. 2021, 57, 34–42. [Google Scholar]
- Naeem, M.; Naeem, M.S.; Ahmad, R.; Ahmad, R. Foliar-applied calcium induces drought stress tolerance in maize by manipulating osmolyte accumulation and antioxidative responses. Pak. J. Bot 2017, 49, 427–434. [Google Scholar]
- Upadhyaya, H.; Panda, S.K.; Dutta, B.K. CaCl2 improves post-drought recovery potential in Camellia sinensis (L) O. Kuntze. Plant Cell Rep. 2011, 30, 495–503. [Google Scholar] [CrossRef]
- Li, B.; Liu, C.; Li, H.; Yang, Z. Analysis of the effect of exogenous calcium chloride on drought alleviation of ‘Longmu 807’ Alfalfa seedlings. Acta Agrestia Sin. 2020, 28, 990–997. [Google Scholar]
- Hu, J.Z.; Liu, L.Y.; Lu, S.G.; Wen, X.F. Effect and mechanism of supplementary application of exogenous calcium on growth of Hippohae rhamnoides under drought stress. Shanxi For. Sci. Technol. 2021, 50, 24–26+32. [Google Scholar]
- Leng, X.H. Effects of Exogenous Calcium on Physiological and Biochemical Characteristics of Handeliodendron bodinieri (Levl.) Rehd under Drought Stress. Master’s Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 2020. [Google Scholar]
- Zhang, X.R.; Xu, X.Y. Comparative study on drought and wind erosion resistance of Calligonum species. J. Arid. Land Resour. Environ. 1992, 4, 55–62. [Google Scholar]
- Wu, J.W.; Li, G.Q.; Guan, J.H.; Tang, X.Y.; Yang, S.H.; Qiu, M.; Li, Q.T.; Lu, S.Z.; Huang, X.Y. Effects of different treatments on oil body morphology and oil content of postharvest Camellia oleifera seeds. Food Res. Dev. 2020, 41, 73–78. [Google Scholar]
- Gu, Y.; Zhang, F.; Zeng, Y.; Tan, X.; Cao, H.; Li, Z. Physiological responses of tung tree (Vernicia fordii) saplings to different red, white and blue light-emitting diodes. Int. J. Agric. Biol. 2019, 22, 569–577. [Google Scholar]
- Deeba, F.; Pandey, A.K.; Ranjan, S.; Mishra, A.; Singh, R.; Sharma, Y.K.; Pandey, V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 2012, 53, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Schromm, M.J. Analysis of stomatal and nonstomatal components in theenviron mental control of CO2 exchanges in leaves of Welwitschia mirabilis. Plant Physiol. 1986, 82, 173–1781. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.H.; Wen, B.Q.; Fang, J.M. Progress about the relationship between calcium fertilizers and drought-resistance of plants. Subtrop. Agric. Res. 2005, 1, 19–25. [Google Scholar]
- Li, H.X.; Mi, Y.F.; Chen, S.C. Influences of exogenous calcium on PSII function and light energy allocation in peony with different resistance to drought stress. Jiangsu Agric. Sci. 2022, 50, 120–127. [Google Scholar]
- Ou, E.L.; Ban, S.; Tian, F.; Xu, Y.J.; Zhong, L.; Yang, C.Y. Effects of drought on the growth and endogenous hormones of Festuca arundinacea seedlings. Mol. Plant Breed. 2023, 21, 4085–4093. [Google Scholar]
- Quan, W.X.; Ding, G.J. Dynamic of volatiles and endogenous hormones in Pinus massoniana needles under drought stress. Sci. Silvae Sin. 2017, 53, 49–55. [Google Scholar]
- Ao, H.; Wang, Y. Response of endogenous hormones and stomatal regulation of spruce to drought stress. Non-Wood For. Res. 2011, 29, 28–34. [Google Scholar]
- Han, R.H.; Zhang, Y.G.; Tian, H.; Lu, X.S. Study on changes of endogenous hormones in the leaves of Alfalfa under drought stress. Acta Agric. Boreali-Sin. 2008, 3, 81–84. [Google Scholar]
- Gusta, L.V.; Trischuk, R.; Weiser, C.J. Plant cold acclimation: The role of abscisic acid. J. Plant Growth Regul. 2005, 24, 308–318. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Liu, Y.C.; Li, Y.T.; Huang, S.R.; Zhang, J. Effects of drought stress on endogenous hormones in potted seedlings of Ulmus pumila ‘Jinye’. J. West China For. Sci. 2021, 50, 40–45. [Google Scholar]
- Zhou, L.; Xu, H.; Zhu, X.J.; Chen, X.; Wang, Y.H.; Fang, W.P.; Li, X.H. Effect of abscisic acid on physiological characteristics of tea plant under drought stress. J. Tea Sci. 2014, 34, 473–480. [Google Scholar]
- Wang, Y.; Li, Y.; Wang, S.; Xiang, Z.; Zhao, D.G. Changes of growth regulators of Camellia sinensis (L.) Kuntze var. niaowangensis Q.H.Chen under low temperature stress in Guizhou. Hubei Agric. Sci. 2020, 59, 99–102. [Google Scholar]
- Jinmin, F.; Bingru, H. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar]
- Abdel-Basset, R. Calcium channels and membrane disorders induced by drought stress in Vicia faba plants supplemented with calcium. Acta Physiol. Plant. 1998, 20, 149–153. [Google Scholar] [CrossRef]
- Guo, P.R.; Wu, L.L.; Wang, Y.; Liu, D.; Li, J.A. Effects of drought stress on the morphological structure and flower organ physiological characteristics of Camellia oleifera flower buds. Plants 2023, 12, 2585. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Yang, Y.R.; Zheng, Q.H. Effects of exogenous nitric oxide on antioxidase and chlorophyll fluorescence of seedling of alfalfa under drought stress. Agric. Res. Arid. Areas 2008, 2, 65–68. [Google Scholar]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Lopez, A.; Jeon, S.; de Freitas, S.T.; Yu, Q.; Wu, Z.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. 2019, 6, 17. [Google Scholar] [CrossRef]
- Wang, R.H.; Zhong, F.X.; Liao, W.T.; Li, T. Effects of soilmoisture on fruit growth of Camellia oleifera. Sci. Silvae Sin. 2014, 50, 40–46. [Google Scholar]
- Jiang, Y.H.; Liao, Y.F. Research summary on meteorological influence indicators of oil tea Camellia. Chin. Agric. Sci. Bull. 2015, 31, 179–183. [Google Scholar]
- Li, Z.J.; Hua, J.Q.; Zeng, Y.R. Oil content of Camellia oleifera fruit trees. J. Zhejiang For. Coll. 2010, 27, 935–940. [Google Scholar]
- Xu, T. Study on Regulating Mechanism of Calciumon the Abscission Oftomato Pedicel. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2008. [Google Scholar]


| Specimen | Treatment | CK | N1 | N2 | N3 |
|---|---|---|---|---|---|
| Leaf | CS | 62.32% ± 0.62 Aa | 62.17% ± 0.34 Aa | 62.21% ± 0.76 Aa | 62.35% ± 0.62 Aa |
| LD | 61.99% ± 0.35 Aa | 62.10% ± 0.96 Aa | 61.43% ± 0.29 Ba | 62.00% ± 0.51 Aa | |
| MD | 60.12% ± 0.39 Bb | 61.36% ± 0.12 Ab | 60.76% ± 0.63 ABb | 60.54% ± 0.45 ABb | |
| SD | 58.15% ± 0.45 Bc | 59.88% ± 0.14 Ab | 58.90% ± 0.56 Bc | 57.76% ± 0.61 Cc | |
| Pericarp | CS | 77.29% ± 0.25 Aa | 77.24% ± 0.19 Aa | 76.92% ± 0.32 Aa | 77.18% ± 0.10 Aa |
| LD | 77.03% ± 0.13 Aa | 77.43% ± 0.17 Aa | 77.04% ± 0.12 Aa | 77.05% ± 0.08 Aa | |
| MD | 70.33% ± 0.46 Bb | 71.33% ± 0.34 Ab | 70.84% ± 0.29 ABb | 70.34% ± 0.30 Bb | |
| SD | 68.10% ± 0.77 Bc | 68.81% ± 0.24 Ac | 68.34% ± 0.22 Ac | 67.32% ± 0.48 Cc | |
| Kernel | CS | 72.35% ± 0.50 Aa | 71.58% ± 0.46 Aa | 71.79% ± 0.27 Aa | 72.09% ± 0.54 Aa |
| LD | 72.05% ± 0.38 Aa | 71.79% ± 0.54 Aa | 71.95% ± 0.59 Aa | 71.74% ± 0.46 Aa | |
| MD | 68.11% ± 0.37 ABb | 68.49% ± 0.42 Ab | 67.85% ± 0.79 Bb | 67.49% ± 0.57 Bb | |
| SD | 64.66% ± 1.11 Bc | 65.64% ± 0.56 Ac | 65.25% ± 0.36 ABc | 63.94% ± 0.79 Cc | |
| Fruiting stem | CS | 77.29% ± 0.30 Aa | 77.24% ± 0.68 Aa | 76.92% ± 0.13 Aa | 77.18% ± 0.57 Aa |
| LD | 77.03% ± 0.16 Aa | 77.43% ± 0.32 Aa | 77.04% ± 0.40 Aa | 77.05% ± 0.19 Aa | |
| MD | 70.33% ± 0.19 Bb | 71.33% ± 1.00 Ab | 70.84% ± 0.41 ABb | 70.34% ± 0.49 Bb | |
| SD | 67.43% ± 0.21 Bc | 68.81% ± 1.07 Ac | 68.34% ± 0.58 Ac | 67.32% ± 0.75 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Liu, C.; Chen, Y.; Xu, Y.; Tang, W.; Chen, L.; Li, Z. Physiological and Biochemical Effects of Exogenous Calcium on Camellia oleifera Abel under Drought Stress. Forests 2023, 14, 2082. https://doi.org/10.3390/f14102082
Zhang T, Liu C, Chen Y, Xu Y, Tang W, Chen L, Li Z. Physiological and Biochemical Effects of Exogenous Calcium on Camellia oleifera Abel under Drought Stress. Forests. 2023; 14(10):2082. https://doi.org/10.3390/f14102082
Chicago/Turabian StyleZhang, Tao, Caixia Liu, Yongzhong Chen, Yanming Xu, Wei Tang, Longsheng Chen, and Ze Li. 2023. "Physiological and Biochemical Effects of Exogenous Calcium on Camellia oleifera Abel under Drought Stress" Forests 14, no. 10: 2082. https://doi.org/10.3390/f14102082
APA StyleZhang, T., Liu, C., Chen, Y., Xu, Y., Tang, W., Chen, L., & Li, Z. (2023). Physiological and Biochemical Effects of Exogenous Calcium on Camellia oleifera Abel under Drought Stress. Forests, 14(10), 2082. https://doi.org/10.3390/f14102082





