Abstract
Monitoring water consumption dynamics across the geographic range of an ecosystem may indicate the possible variation and stress in a biome. Here, model output data based on remote sensing (1979–2022) were used to examine the water consumption dynamics and effects on cone production in three geographic margins in the longleaf pine’s range (i.e., Bladen Lake State Forest, Escambia Experimental Forest, and Kisatchie National Forest) under varying climatic conditions. Results indicated that the mean annual transpiration at Escambia was approximately 431 mm and that at Bladen and Kisatchie was 500 mm. Mean monthly transpiration peaked twice (June and October) at Escambia but only once (August) at Bladen and Kisatchie. The mean annual evapotranspiration ranged from approximately 900 mm at Kisatchie to about 791 mm at Escambia and Bladen. The mean annual transpiration/evapotranspiration ratio was about 0.65 at Bladen and 0.55 at Escambia and Kisatchie. A significant correlation existed between evapotranspiration and specific humidity across the sites on a monthly scale but not on a yearly scale. Significant negative relationships existed between precipitation and the ratios of transpiration/precipitation and evapotranspiration/precipitation on the yearly scale across the sites. Negative power relationships were observed between precipitation and the specific humidity/precipitation ratio on monthly and yearly scales. Cone production was generally highest in years with moderate water consumption. These results provide baseline information on how hydrological and ecological processes of longleaf pine forests interact with climate across broad spatial and temporal scales.
1. Introduction
Longleaf pine (Pinus palustris Mill.) forests are among the most economically, ecologically, and culturally valued ecosystems in the southeastern United States [1,2]. Before European settlement, longleaf pine forests occupied 37 million hectares, ranging from eastern Texas to southeastern Virginia [3]. Currently, the longleaf pine ecosystem can be found in approximately 1.9 million ha of fragmented forests after decades of timber exploitation, fire suppression, and forest land conversion [4,5]. While still economically valued, longleaf pine forests contribute more to biodiversity refugia because their discontinuous canopy and frequent surface fire regimes create valuable habitats for many threatened and endangered species [6]. Also, longleaf pine forests may provide long-term carbon sequestration through their long lifespan potential and below-ground carbon allocation [7,8,9]. Thus, restoring longleaf pine forests has recently become a management priority [10]. However, changes in longleaf pine and other forests can alter the region’s water cycling, affecting the drinking water supply for 17 million people throughout the southeastern United States [11]. The climate of the southeast region is generally warm and humid, but the weather is quite variable and is influenced by several factors (e.g., latitude, topography, and distance to the ocean). Extreme weather phenomena (e.g., floods, droughts, hurricanes, tornadoes, and winter storms) are frequent in this region [12]. Climate fluctuation, extreme events (e.g., hurricanes, heatwaves, and flooding), and human activities could even cause the collapse of the ecosystem or some important ecological processes due to complicated ecological interactions [13].
Forest management activities at a large scale can also play a role in surface water availability by altering hydrological processes [14,15]. Longleaf pine forest restoration usually involves clearcutting or removing undesirable woody vegetation, replanting longleaf pine seedlings, and maintaining a frequently prescribed fire regime [16]. Regional deforestation and reforestation can alter the water cycle (e.g., evapotranspiration, soil water, and precipitation) in multiple ways [17]. For example, under a sufficiently wet atmosphere, forest transpiration can lead to atmospheric moisture convergence such that increased transpiration enhances atmospheric moisture import and results in more precipitation and flooding [17]. Restoration of open longleaf pine woodland could reduce evapotranspiration and increase stream flow in watersheds [18]. The hydrological simulation was also used to evaluate stream water flow under longleaf pine restoration [19]. Increased atmospheric water vapor can also amplify the warming effect caused by other greenhouse gases. Humidity plays a vital role in several ecosystem functions [20]. Longleaf pine stands are typically managed in low density, which can reduce humidity. Decreased humidity can significantly impact forested ecosystems through susceptibility to insect attack, infection, tree mortality, and wildfire. Over the past century, the longleaf pine range has experienced severe droughts and flooding [21]. Forests play an important role in recycling water from precipitation to evapotranspiration, soil water, and atmospheric humidity [22,23,24]. It is necessary to evaluate the possible hydrological regime transition under ecological restoration.
Another largely underexamined area is the potential interaction between the moisture regime and cone production. The climate is considered the primary driver in longleaf pine cone production. However, the relationship between climate and cone production is complicated [25,26]. Cone production is generally highest under mild climatic conditions [27]. Theoretically, forests experiencing increased cone production should require elevated transpiration for photosynthetic production during reproduction. However, it is currently unknown whether water consumption and cone production are positively correlated at a regional or landscape scale. Characterizing the spatial and temporal dynamics of water consumption (e.g., transpiration and evapotranspiration) and their relationship with longleaf pine cone production will be helpful in better understanding important ecological processes.
Long-term observations over a range of environmental conditions are important for understanding the complex interactions of hydrological processes [28]. Usually, eddy covariance-based fluxes are used to monitor ecosystem flux dynamics (e.g., air temperature and humidity, soil moisture, and transpiration). However, due to its high cost and small monitoring areas, this method is limited in its application at the landscape or regional scale. The multiple satellite-associated model data from NASA EarthData provide one potential resource to overcome spatial limitations and provide important information at larger spatial scales [14]. This study aims to characterize the dynamics of water consumption in longleaf pine forests across the geographic extent of its range. We hypothesized that (a) longleaf pine forests with higher evapotranspiration or transpiration in space and time tend to have higher atmospheric moisture and precipitation; (b) some kind of regime may exist in evapotranspiration and atmospheric moisture dynamics in longleaf pine forests; and (c) high transpiration in longleaf pine forests may lead to high cone production in the next year. The objectives include (i) characterizing the annual and seasonal dynamics of transpiration, evapotranspiration, and atmospheric moisture; (ii) studying the relationships among transpiration, evapotranspiration, atmospheric moisture, and precipitation at three locations; (iii) indicating the possible regime shift in the regional hydrological processes; and (iv) examining whether there is a relationship between water consumption and cone production. The results of this study will provide an easy approach to describe complex interactions in longleaf pine eco-hydrological activity at the regional scale over time with limited cost.
2. Materials and Methods
2.1. Study Areas
In this study, we selected three sites with relatively high concentrations of longleaf pine habitat at the geographic margins of its historical range. These sites comprise Bladen Lake State Forest in southeastern North Carolina (hereafter Bladen, 34.71° N, 78.56° W), representative of the northern range of the longleaf pine; Escambia Experimental Forest in southern Alabama (Escambia, 31.13° N, 87.16° W), representative of the southern range; and Kisatchie National Forest in central Louisiana (Kisatchie, 31.34° N, 92.41° W), representative of the western range (Figure 1). The map distance from these study sites to the nearby ocean (the Atlantic Ocean or the Gulf of Mexico) is over 60 km. Tree ages are mixed at each site, and the oldest longleaf pine trees at the three sites are approximately 100 years old. Detailed information (e.g., climate and environment) on these three sites can be found in [14,21,27]. Data on the annual cone production per longleaf pine were obtained from the long-term ground monitoring effort by the scientists at the USDA Forest Service [29].
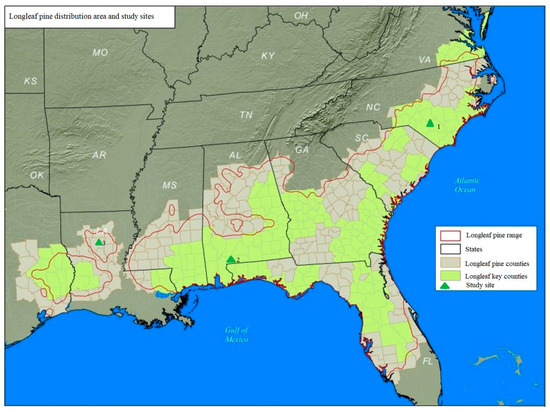
Figure 1.
The study sites and the historical range of longleaf pine (1: Bladen Lake State Forest, NC; 2: Escambia Experimental Forest, AL; 3: Kisatchie National Forest, LA) (map source is from the USDA NRCS).
2.2. Water Consumption and Related Data
Using multiple satellite data (e.g., moderate-resolution imaging spectroradiometer) and local meteorological data, land assimilation systems such as the North American land data assimilation system (NLDAS) produced land surface information, including heat and water fluxes based on the balance of water and energy distribution. These derived data were relatively accurate at a large scale [30,31]. The parameters used in this study are from these derived data from 1979 to 2022. Each physical index was calculated on an area-based average of the study sites and organized in monthly, yearly, and decadal values. The spatial resolution is approximately 0.1° in latitude and longitude and about 10 km × 10 km in the region. This spatial scale is to match the fragmented longleaf pine forests. All data in this study were downloaded from NASA EarthData (https://www.earthdata.nasa.gov, accessed on 17 October 2023). The framework of general practices is listed in Figure 2. The air temperature data from the derived data are correlated with the ground observation, such as at Escambia (Figure 3). There is no validation of transpiration, evapotranspiration, soil water content, and specific humidity because similar ground observation data are not available. Recent results from the longleaf pine watershed research indicate that the mean annual evapotranspiration ranged from 700 to 1100 mm [18], which is close to our results from 790 to 900 mm. Similar techniques were used to study the effects of forests on local environmental processes [32,33]. The detailed meaning of each physical index is listed here.
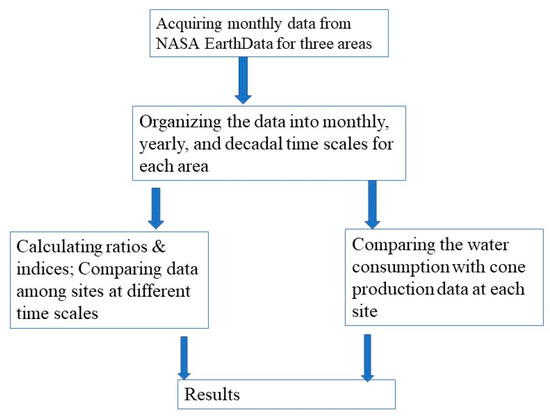
Figure 2.
The flowchart of general practices for this study.
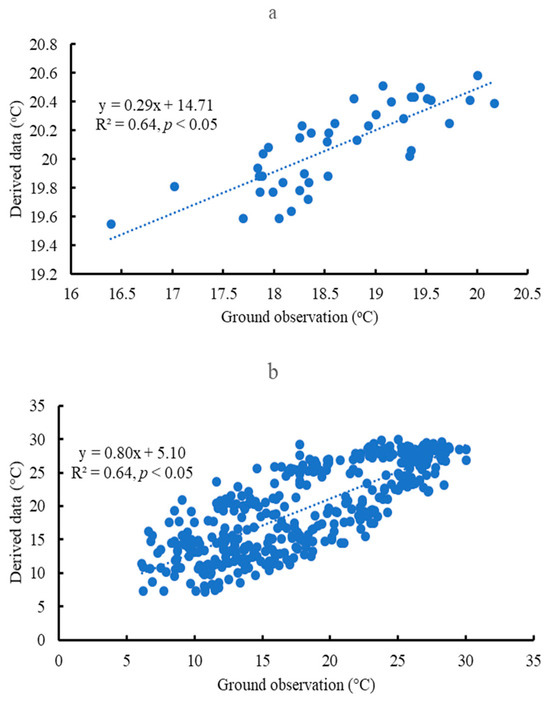
Figure 3.
The correlation of air temperature at Escambia through ground observation and derived data based on remote sensing at a yearly (a) and monthly (b) scale.
Transpiration: The loss of water as vapor through stomata in plant leaves. Evapotranspiration: The water is lost from a unit area of the surface, which is the sum of evaporation and plant transpiration. The unit of transpiration and evapotranspiration was W∙m−2 and was transformed to mm. Both data sources are the NLDAS model from 1979 to 2022. Soil surface water content (volume) (0–10 cm): Water in a specific soil layer beneath the surface is presented as the volume of water per unit volume of soil. Its unit is m3∙m−3. The data source is the FLDAS model from 1982 to 2022. Specific humidity: The ratio of the weight of water vapor in a specified volume to the weight of dry air in the same volume. The unit is kg∙kg−1. The data source is the FLDAS model from 1979 to 2022.
2.3. Regimes of Water Consumption
The monthly transpiration, evapotranspiration, precipitation, soil water content, and specific humidity at three sites were acquired from NASA EarthData. The dE/dP (E and P refer to evapotranspiration and precipitation, respectively) was used to characterize the water consumption regime in different ecosystems [17]. This study used the relationships between precipitation and transpiration/precipitation (T/P) and evapotranspiration/precipitation (E/P) in the yearly and monthly time scales to characterize the transpiration and evapotranspiration regimes in three areas. Similarly, the regimes of atmospheric moisture were indicated by the relationships between precipitation and atmospheric moisture/precipitation. Also, following [34], the transpiration/evapotranspiration (T/E) ratio was used to study the spatial and temporal characteristics of water consumption of longleaf pine forests.
2.4. Statistical Analysis
A two-way analysis of variance (ANOVA) (e.g., site and time) was used to examine statistical differences among the sites for each water consumption parameter from 1979 to 2022 at varying timescales (e.g., month and year). Monthly and yearly values were used because (i) most water-related indices have values at these timescales, and (ii) these timescales are suitable for distinguishing seasonal dynamics between sites. However, decadal data were also compared. Each index’s time series at the three sites were compared on monthly and yearly timescales in the past 43 years (516 months). The graphs presented long-term data according to the average monthly values across years for better visualization. Spearman’s correlation analysis and simple regression were conducted between selected parameters. Differences were considered significant at α < 0.05. All analyses were processed with SAS version 9.4 (The SAS Institute, Cary, NC, USA).
3. Results
3.1. Transpiration
Mean annual transpiration at Escambia (431.21 ± 24.50 mm) was significantly lower than at Bladen (513.54 ± 31.06 mm) and Kisatchie (497.12 ± 31.95 mm) (p < 0.05) (Figure 4a). There was no significant difference in mean annual transpiration between Bladen and Kisatchie. Similarly, there was no correlation in annual transpiration rates across the sites. However, there were some common transpiration peaks across the sites, such as in 1991 and 2013.
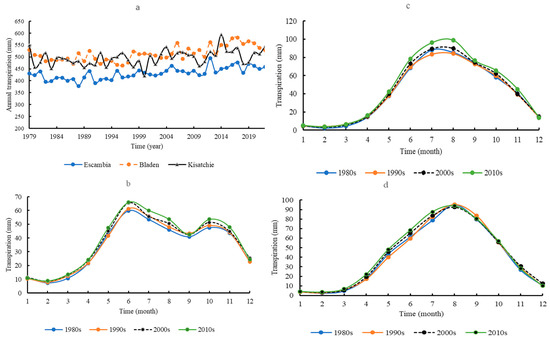
Figure 4.
Annual transpiration in the three areas (a); seasonal transpiration at Escambia (b), Bladen (c), and Kisatchie (d).
The seasonal transpiration dynamics at Escambia differed from those at Bladen and Kisatchie (Figure 4b–d). There were two peaks in transpiration (June and October) at Escambia, while only one peak (August) occurred at Bladen and Kisatchie, respectively. Seasonal changes in transpiration did not differ significantly over the past four decades.
3.2. Evapotranspiration
Mean annual evapotranspiration at Kisatchie was 900.67 ± 54.30 mm, which was significantly higher than at Escambia (791.05 ± 25.80 mm) and Bladen (795.88 ± 30.80 mm) (p < 0.05) (Figure 5a). Escambia and Bladen did not significantly differ in their mean annual evapotranspiration. Seasonal evapotranspiration dynamics were similar across the sites (Figure 5b–d). Mean monthly evapotranspiration gradually increased from January, peaked in August, and then declined. During the past four decades, there was no significant change in seasonal evapotranspiration across the sites. Evapotranspiration and transpiration were significantly correlated on a monthly scale across the sites (p < 0.05) (Figure 6a–c). However, on a yearly scale, evapotranspiration and transpiration were significantly correlated only at Kisatchie (Figure 6d).
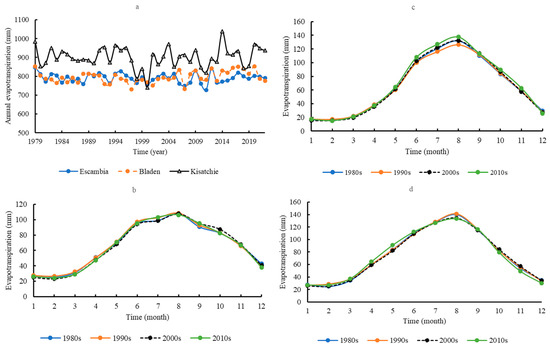
Figure 5.
Annual evapotranspiration in the three areas (a); seasonal evapotranspiration at Escambia (b), Bladen (c), and Kisatchie (d).
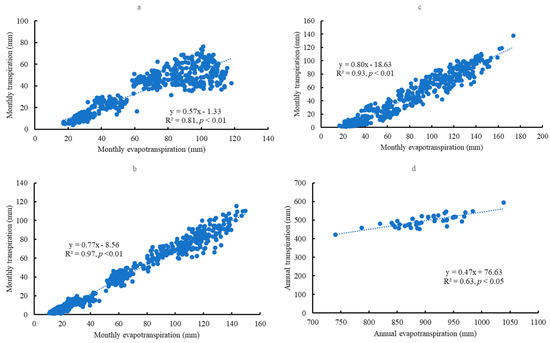
Figure 6.
The correlation between transpiration and evapotranspiration on the monthly scale at Escambia (a), Bladen (b), and Kisatchie (c). The correlation between transpiration and evapotranspiration on the yearly scale (d).
3.3. Transpiration/Evapotranspiration (T/E)
The mean T/E ratio was significantly higher at Bladen (0.6452 ± 0.03) than at Escambia (0.5455 ± 0.03) and Kisatchie (0.5523 ± 0.02) (p < 0.05) (Figure 7a). Kisatchie and Escambia did not significantly differ in mean T/E. Seasonal dynamics of T/E differed across the sites (Figure 7b–d). At Escambia, mean T/E peaked in June and November and experienced valleys in February and August. At Bladen, the seasonal dynamics of T/E were like Escambia, but neither the August nor September valley was as deep. However, T/E peaked in September or October and reached its nadir in February.
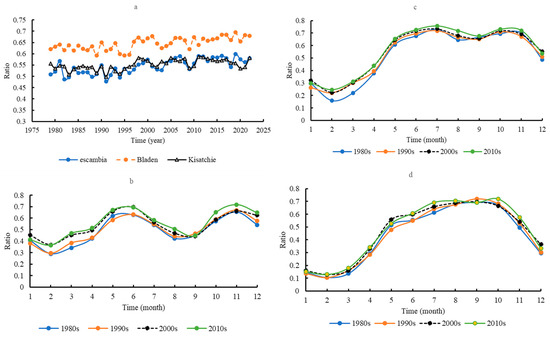
Figure 7.
The ratios of transpiration/evapotranspiration on the yearly scale in the three areas (a); the seasonal dynamics of the ratio of transpiration/evapotranspiration on the monthly scale in Escambia (b), Bladen (c), and Kisatchie (d).
3.4. Atmospheric Humidity
Mean annual specific humidity was significantly lower at Bladen (0.009 ± 0.0004 kg ∙ kg−1) than at Escambia (0.011 ± 0.0004 kg ∙ kg−1) and Kisatchie (0.010 ± 0.0004 kg ∙ kg−1) (p < 0.05) (Figure 8a). There was no significant trend of increasing specific humidity across sites. Seasonal dynamics in specific humidity were similar across the sites (Figure 8b–d). Specific humidity gradually increased from January, peaked in July or August, and then declined.
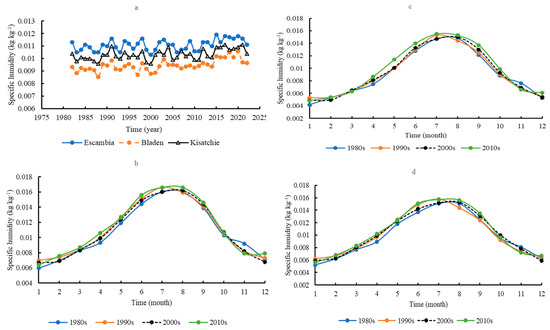
Figure 8.
Comparison of the annual specific humidity in the three areas (a); the seasonal dynamics of specific humidity in Escambia (b), Bladen (c), and Kisatchie (d).
3.5. Soil Water Content (0–10 cm)
Mean annual soil water content by volume was significantly higher at Escambia (0.3681 ± 0.022 m3 ∙ m−3) than at Bladen (0.3308 ± 0.018 m3 ∙ m−3) and Kisatchie (0.3243 ± 0.023 m3 ∙ m−3) (Figure 9a). Bladen and Kisatchie did not significantly differ in mean soil water content. While consistent trends in soil water content were absent, there were occasional troughs in soil water availability across the sites (e.g., 1990 and 2011). Seasonal dynamics in soil water content were similar between Escambia and Bladen (Figure 9b,c). Soil water content changed slowly; relatively low water content occurred in June and October. However, at Kisatchie, soil water content decreased continuously from February and reached its nadir in September or October before increasing (Figure 9d).
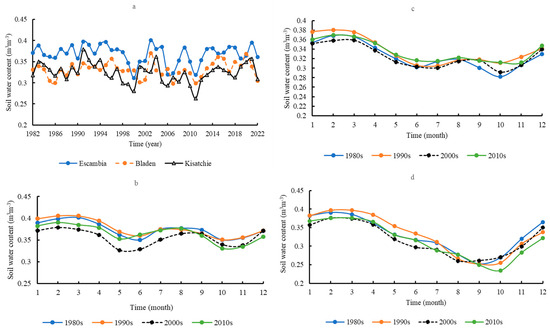
Figure 9.
Comparison of the annual soil surface water content (0–10 cm) by volume in the three areas (a); the seasonal dynamics of soil surface water content in Escambia (b), Bladen (c), and Kisatchie (d).
3.6. Relationships between Soil Water Content and Transpiration, Evapotranspiration, and Specific Humidity
Mean surface soil water content (0–10 cm) and transpiration were not significantly correlated at monthly and yearly scales. This trend was similar to the relationship between soil water content and evapotranspiration or specific humidity. A significant positive correlation existed between mean evapotranspiration and specific humidity at a monthly scale across the sites (Figure 10a–c). However, this correlation was not significant on a yearly scale.
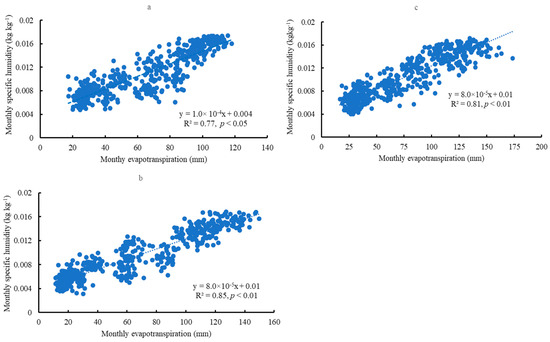
Figure 10.
The correlation between evapotranspiration and specific humidity at a monthly scale in Escambia (a), Bladen (b), and Kisatchie (c).
3.7. The Correlations with Precipitation
On either the yearly or monthly scale, there were no significant correlations between mean precipitation and mean transpiration, evapotranspiration, soil water content, or specific humidity across sites.
3.8. Regimes of Water Consumption
Significant and negative relationships existed between mean precipitation and the mean T/P ratio on the yearly scale across the sites (Figure 11a–c) (p < 0.05). However, this relationship was not significant on the monthly scale. The ratios of annual T/P decreased with increased annual precipitation. Like transpiration, significant and negative relationships existed between mean precipitation and mean evapotranspiration/precipitation across the sites on the yearly scale (Figure 11d–f) (p < 0.05) but not on the monthly scale. The annual E/P ratios decreased with increased annual precipitation.
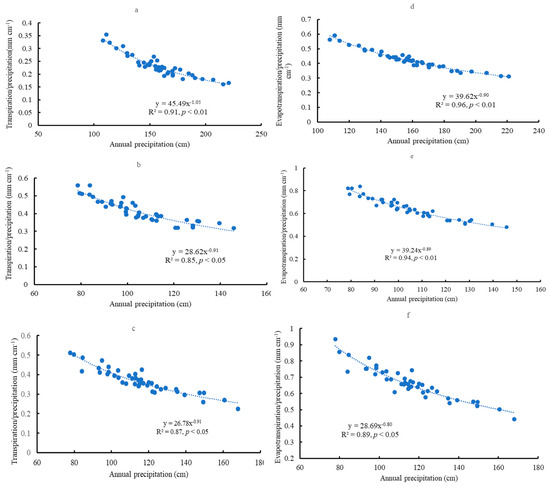
Figure 11.
Relationships between precipitation and transpiration/precipitation on the yearly scale in Escambia (a), Bladen (b), and Kisatchie (c); relationships between precipitation and evapotranspiration/precipitation on the yearly scale in Escambia (d), Bladen (e), and Kisatchie (f).
For specific humidity, significant and negative relationships existed between mean precipitation and mean specific humidity/precipitation (SH/P) on both the yearly and monthly scales across the sites (Figure 12a–f). The ratios of SH/P decreased with increasing precipitation. On the monthly scale, two domains and one transition zone existed. One domain is related to low precipitation or drought (around 20 cm), and another is under high precipitation (e.g., above 100 cm).
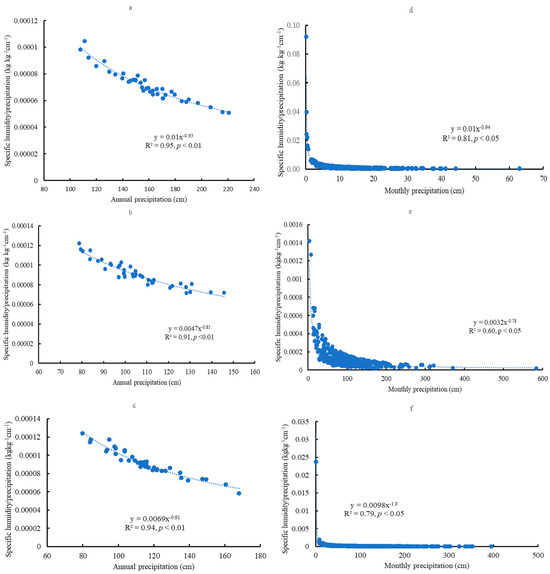
Figure 12.
Relationships between precipitation and specific humidity/precipitation on the yearly scale in Escambia (a), Bladen (b), and Kisatchie (c); Relationships between precipitation and specific humidity/precipitation on the monthly scale in Escambia (d), Bladen (e), and Kisatchie (f).
3.9. Relationships with Cone Production
Complex relationships existed between cone production per tree and the previous year’s mean transpiration, evapotranspiration, soil surface water content, and specific humidity (Figure 13). Cone production was maximized in the intermediate values of transpiration, evapotranspiration, soil surface water content, and specific humidity. The medians of monthly transpiration were 37, 40, and 42 mm in Escambia, Bladen, and Kisatchie, respectively. The medians of monthly evapotranspiration were 66, 67, and 73 mm in Escambia, Bladen, and Kisatchie, respectively. Likewise, the medians of soil surface water content were 0.38, 0.33, and 0.32 m3 ∙ m−3 for Escambia, Bladen, and Kisatchie, respectively. The median of specific humidity was about 0.01 kg ∙ kg−1 across the sites.
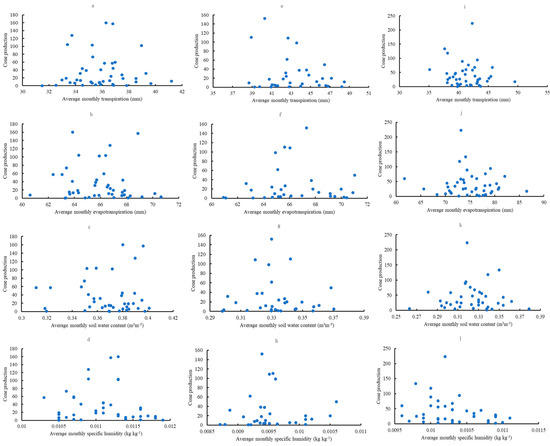
Figure 13.
Relationships between cone production (individuals per tree) and the previous year’s transpiration, evapotranspiration, soil surface water content, or specific humidity in Escambia (a–d), Bladen (e–h), and Kisatchie (i–l).
4. Discussion
4.1. Uncertainties
Uncertainties could exist in the remote sensing and derived data because these data were from different sources with varied resolutions. Also, some of these data cannot be validated because no corresponding ground observations cover the landscapes or small regions, such as transpiration and evapotranspiration. Eddy covariation can measure these parameters at a point or small area (homogeneous) level. However, the purpose of this study was to find patterns in water consumption across longleaf pine forests. The consistent patterns and the deviations we observed in this study provide helpful initial assessments of the effect of longleaf pine restoration on hydrological and ecological processes. The watershed approach can be used for water consumption in watersheds, but this approach also can be used for an area within or across watersheds. Further research to use some sites with detailed hydrological data (NEON or LTER) should be conducted for comparison.
4.2. Transpiration and Evapotranspiration
Transpiration and evapotranspiration are essential physiological processes of water loss from plants and landscapes. Transpiration is related to the plant’s stomatal aperture, photosynthesis, and cooling activities. A plant in a warm environment should transpire more than one in a cooler environment, although the southeastern region is generally warm and humid. Escambia is located south of Bladen and Kisatchie, and its summer air temperature is slightly higher than Kisatchie. However, it is surprising that the mean annual transpiration of longleaf pine forests reached about 500 mm at Bladen and Kisatchie but only about 430 mm at Escambia. Tree density, stand age, and site productivity might impact tree water use [34]. After comparing seasonal dynamics across the sites (Figure 4), two transpiration peaks existed at Escambia (June and October), while only one peak (August) occurred at Bladen and Kisatchie. The decline of transpiration from July to September at Escambia might be related to its local high air temperature or drought because plant water use can cease under extreme heat when transpiration becomes too severe [35,36]. This result suggests that spatial variation in the conservative nature of water use may exist across the range of the longleaf pine. Longleaf pine trees were reported to transpire more than four times more water on mesic sites than on xeric sites [37]. Thus, our result may indicate the physiological adaptation of longleaf pines to local hydrological conditions. Longleaf pines near the southern range boundary may experience stress. Understanding the transpiration dynamics may help to improve water management and minimize economic losses (e.g., tree mortality and early seed abortion) [38].
Landscape evapotranspiration includes vegetation transpiration and all evaporation from different ecosystems within the region. The mean annual evapotranspiration at Kisatchie (900 mm) was much higher than at Escambia and Bladen (791 mm). This trend may be related to the relatively high air temperature and precipitation at Kisatchie. The pattern of evapotranspiration across the sites mainly corresponds to the seasonal dynamics in air temperature. This pattern is reflected in the high correlation between evapotranspiration and transpiration across the sites on a monthly scale. However, the mean T/E ratio was higher at Bladen (0.65) than at Escambia and Kisatchie, although these ratios were in the general range of various ecosystems globally (0.45~0.77) [34]. Seasonal dynamics of the ratio varied across the sites (Figure 7). This pattern may be related to the differences in leaf area index and gross primary productivity, as the spatial variation in the ratio is considered to be driven mainly by vegetation and soil characteristics (e.g., leaf area index and soil properties) rather than by climate [34,39,40]. Thus, T/E may serve as an indicator of various ecosystem properties.
4.3. Specific Humidity, Soil Water Content, and Precipitation
Water on the land surface is sourced mainly from precipitation, but vegetation (e.g., plant species, types, and coverage) plays more or less of a role in water allocation and cycling in different regions. In this study, mean annual specific humidity was lower at Bladen than at Escambia and Kisatchie, although their seasonal dynamics were similar. Specific humidity is mainly related to evapotranspiration driven by air temperature. Because Bladen is located north of Escambia and Kisatchie, it has a lower air temperature than Escambia and Kisatchie. This pattern aligned with the result that evapotranspiration correlated with specific humidity across the sites at a monthly scale rather than yearly. At a yearly scale, wind, radiation, and other factors may affect this relationship [41].
Soil water content and its dynamics could be affected by the local climate, vegetation, and soil properties based on hydrological processes. Previous research revealed positive correlations between soil moisture and precipitation [42,43], which means wetter soil can provide abundant moisture to the atmosphere through evapotranspiration. The increased humidity and precipitation can also increase soil moisture and evapotranspiration. A negative relationship between soil water and precipitation was found in the eastern USA [44]. However, others have indicated that land cover and climate regime could alter this relationship because forest and dense vegetation can weaken this correlation [45]. In this study, no correlations existed between the surface soil water content and transpiration, evapotranspiration, or specific humidity. Although precipitation can affect water processes in longleaf pine forests, no correlations were found between precipitation and transpiration, evapotranspiration, soil water content, or specific humidity across the sites on monthly or yearly scales. Thus, the relationships between precipitation and transpiration, evapotranspiration, soil surface water content, and specific humidity are complicated. Part of the causes may be that air moisture or rain might be brought by the weather system. The regional high-pressure system (Bermuda High) can influence precipitation patterns depending on its location off the Atlantic Coast [13]. Longleaf pine forests could affect the water cycling processes across the landscape as their high canopy areas can intercept small amounts of rainfall without changing the soil water content. The result may indicate that further research should be conducted on whether forest management (e.g., longleaf pine restoration) could impact regional water availability [46].
4.4. Regime of Water Consumption
Regimes existed in transpiration, evapotranspiration, and specific humidity. There were negative power law relationships between precipitation and T/P or E/P on the yearly scale across the sites, although these relationships were not significant on the monthly scale. With an increase in precipitation, the ratios of T/P or E/P decreased on a yearly scale. This result suggests relatively less transpiration or evapotranspiration with increasing precipitation. However, with less precipitation, there was relatively more transpiration or evapotranspiration, which might lead to decreased photosynthesis based on previous observations [47]. This result may indicate that longleaf pine forests have a distinct zone of moisture availability where physiological activity is maximized. The annual precipitation of 80–120 cm may be suitable to maintain a high transpiration/precipitation or evapotranspiration/precipitation ratio. In fact, too much water or flooding may kill longleaf pine trees [48].
Significant and negative relationships existed between precipitation and SH/P on yearly and monthly scales across the sites (Figure 12). On the monthly scale, there were two domains (wet and drought) and one transition zone. The regional biosphere of the longleaf pine can maintain a similar regime through self-adjusting between the wet and drought domains. The transition zone may offer a unique opportunity to understand how longleaf pine forests respond to hydrological changes [49]. This result is aligned with the longleaf pine belowground carbon allocation [7,9]. The self-adjusting processes include returning water to the atmosphere through transpiration and evapotranspiration. Some of the water vapor in the atmosphere may condense and become precipitation again. A nonlinear relationship was found in the Amazon basin between hourly precipitation and atmospheric water content [50]. Even though these regimes on the monthly and yearly scales were relatively stable, they can be used to monitor the regional hydrological responses (through mediation from longleaf pine forests) to climate change [17,51]. Such a switch could occur quickly (e.g., on a monthly scale), where the transient dynamics between the wet and dry states have general implications for further study [52].
4.5. Relationships with Cone Production
The relationships between cone production per tree and the previous year’s mean transpiration, evapotranspiration, soil surface water content, or specific humidity were complicated. However, high cone production always occurred consistently in the intermediate values of transpiration, evapotranspiration, soil surface water content, and specific humidity across the sites. This is similar to the results of air temperature and precipitation on longleaf pine cone production [27]. One plausible explanation for this pattern relates to the extended reproduction process of the longleaf pine. During three years, any abnormal climatic event during this period may alter cone production. The optimal environmental conditions may be around the mid-values of each parameter (e.g., transpiration, evapotranspiration, soil moisture, and atmospheric humidity). Similar phenomena were observed in the relationship between precipitation and annual radial increment in Chinese Torreya (Torreya grandis) trees [53]. This pattern may be related to the middle domain hypothesis, which posits that if species’ ranges are distributed randomly within a bounded domain, more ranges will overlap in the middle of the domain than at the edges [54]. The middle domain effect can also exist in the non-spatial domain, and many hump-shaped relationships emerge across various gradients [55]. The middle domain hypothesis may provide a reasonable explanation for the climate effect on the cone production of longleaf pines. Suppose the climate or environmental factors related to cone production during three consecutive years are around the average conditions. In that case, it may lead to high cone production; otherwise, if one abnormal factor occurs, it may result in poor cone production. Evolutionarily, this mechanism might protect the offspring of the longleaf pine by avoiding abnormal climate conditions. Further research should be conducted to determine whether this middle domain hypothesis can distinguish from the stochasticity in the cone production of longleaf pines. Also, given the high annual variation in cone production of individual trees [56], it is unknown whether a tremendous sampling effort is needed to represent longleaf pine cone production more precisely at the regional scale.
5. Conclusions
Based on the above results and discussion, longleaf forest restoration in the southeastern United States may affect the regional eco-hydrological processes and services. It is critical to quantify water consumption (transpiration and evapotranspiration) and characterize the patterns in restoring the longleaf pine ecosystem within its historical range. Despite no data validation, multi-satellite derived information (e.g., NLDAS) could provide valuable information to monitor this ecological process at different time scales. The investigation of transpiration, evapotranspiration, and related soil water and air humidity at three geographically distinct locations of restored longleaf pine forest near its historical range boundary revealed some general patterns and spatial variations in transpiration, evapotranspiration, and related processes on yearly and monthly scales. Longleaf pines in the southern range (Escambia area) might experience some stress in the summertime. However, the regimes of water consumption were still relatively stable. The findings from this study may provide an understanding of the hydrological adaptation of longleaf pine forests to climatic variability. When these hydrological data are used to examine the annual cone production, possible mid-domain phenomena emerge. This study provides exciting results of some hydrological responses of longleaf pine forests over the past four decades. The approach can provide important eco-hydrological data to any land (minimum 0.1° × 0.1°) in North America with limited cost. The insights into the hydrological function of longleaf pine forests may have significant implications for restoration and conservation. Further detailed research should be conducted on the individual tree level and at the sites (e.g., NEON and LTER) with long-term ground monitoring data for comparison.
Author Contributions
X.C.: conceptualization; data analysis, writing; J.L.W. and Q.G.: investigation; methodology; visualization; writing; reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
USDA National Institute of Food and Agriculture Capacity Building Program (2021-38821-34596), McIntire Stennis project (7001918), and 1890COE (2022-38427-37307).
Data Availability Statement
Most data are free from the earthdata.nasa.gov website. Cone data can be requested from J.L.W.
Acknowledgments
This paper was written and prepared in part by a U.S. Government employee on official time, and therefore, it is in the public domain and not subject to copyright. The findings and conclusions in this publication are those of the author(s) and should not be construed to represent an official USDA, Forest Service, or United States Government determination or policy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hodges, A.W. The naval stores industry. In The Longleaf Pine Ecosystem: Ecology, Silviculture, and Restoration; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 43–48. [Google Scholar]
- Jose, S.; Jokela, E.J.; Miller, D.L. The Longleaf Pine Ecosystem: An overview. In The Longleaf Pine Ecosystem: Ecology, Silviculture, and Restoration; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 3–8. [Google Scholar]
- Frost, C.C. History and future of the longleaf pine ecosystem. In The Longleaf Pine Ecosystem: Ecology, Silviculture, and Restoration; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 9–42. [Google Scholar]
- Outcalt, K.W.; Sheffield, R.M. The Longleaf Pine Forest: Trends and Current Conditions; Resource Bulletin SRS-9; USDA Forest Service, Southern Research Station: Asheville, NC, USA, 1996.
- Oswalt, C.; Guldin, J.M. Status of Longleaf Pine in the South: An FIA Update; US Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2021.
- Natural Resource Conservation Service (NRCS). Longleaf Pine Ecosystem Restoration; FY20-24 Implementation Strategy; USDA: Washington, DC, USA, 2020.
- Platt, W.J.; Evans, G.W.; Rathbun, S.L. The population dynamics of a long-lived conifer (Pinus palustris). Am. Nat. 1988, 131, 491–525. [Google Scholar] [CrossRef]
- Boyer, W.D. Pinus palustris Mill. Longleaf pine. In Silvics of North America; Conifers. Agriculture Handbook; Burns, R.M., Honkala, B.H., Eds.; USDA, Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 405–412. [Google Scholar]
- Samuelson, L.J.; Stokes, T.A.; Butnor, J.R.; Johnsen, K.H.; Gonzalez-Benecke, C.A.; Martin, T.A.; Cropper, W.P., Jr.; Anderson, P.H.; Ramirez, M.R.; Lewis, J.C. Ecosystem carbon density and allocation across a chronosequence of longleaf pine forests. Ecol. Appl. 2017, 27, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Guldin, J.M. Restoration of native fire-adapted southern pine-dominated forest ecosystems: Diversifying the tools in the silvicultural toolbox. For. Sci. 2019, 65, 508–518. [Google Scholar] [CrossRef]
- Caldwell, P.; Muldoon, C.; Ford-Miniat, C.; Cohen, E.; Krieger, S.; Sun, G.; McNulty, S.; Bosltad, P. Quantifying the Role of National Forests System Lands in Providing Drinking Water Supply for the Southern United States; General Technical Report SRS-197; U.S. Department of Agriculture Forest Service: Asheville, NC, USA, 2014; 135p.
- Ingram, K.; Dow, K.; Carter, L.; Anderson, J. Climate of the Southeast United States: Variability, Change, Impact, and Vulnerability; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Willcock, S.; Cooper, G.S.; Addy, J.; Dearing, J.A. Earlier collapse of Anthropocene ecosystems driven by multiple faster and noisier drivers. Nat. Sust. 2023. [Google Scholar] [CrossRef]
- Chen, X.; Willis, J.L. Interactions of biosphere and atmosphere within longleaf pine restoration areas. Atmosphere 2022, 13, 1733. [Google Scholar] [CrossRef]
- Boggs, J.; Sun, G.; McNulty, S. Converting naturally regenerated mixed pine-hardwood to loblolly pine plantation forests reduces streamflow in the Piedmont of North Carolina. In Enhancing Landscapes for Sustainable Intensification and Watershed Resiliency, Proceedings of the 7th Interagency Conference on Research in the Watersheds, Virtual, 16–19 November 2020; Latimer, J.S., Bosch, D.D., Faustini, J., Lane, C.R., Trettin, C.C., Eds.; U.S. Department of Agriculture Forest Service, Southern Research Station: Ashville, NC, USA, 2022; 217p. [Google Scholar]
- Johnson, R.; Gjerstad, D. Restoring the overstory of longleaf pine ecosystems. In The Longleaf Pine Ecosystem: Ecology, Silviculture, and Restoration; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 271–295. [Google Scholar]
- Makarieva, A.M.; Nefiodov, A.V.; Nobre, A.D.; Baudena, M.; Bardi, U.; Sheil, D.; Saleska, S.R.; Molina, R.D.; Rammig, A. The role of ecosystem transpiration in creating alternate moisture regimes by influencing atmospheric moisture convergence. Glob. Change Biol. 2023, 29, 2536–2556. [Google Scholar] [CrossRef]
- Younger, S.E.; Cannon, J.B.; Brantley, S.T. Impacts of longleaf pine (Pinus palustris Mill.) on long-term hydrology at the watershed scale. Sci. Total Environ. 2023, 902, 165999. [Google Scholar] [CrossRef]
- Qi, J.; Brantley, S.T.; Golladay, S.W. Simulated longleaf pine (Pinus palustris mill.) restoration increased streamflow—A case study in the lower Flint River basin. Ecohydrology 2022, 15, e2365. [Google Scholar] [CrossRef]
- Brown, J.J.; Pascual, M.; Wimberly, M.C.; Johnson, L.R.; Murdock, C.C. Humidity—The overlooked variable in the thermal biology of mosquito-borne Disease. Ecol. Lett. 2023, 26, 1029–1049. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Q.; Bowman, K.A. Climate variation within the range of longleaf pine forests during the past century. Atmosphere 2022, 13, 465. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, B.; Chen, S.; Wang, X.; Ma, X.; Pan, B. Large-scale afforestation enhances precipitation by intensifying the atmospheric water cycle over the Chinese Loess Plateau. JGR Atmos. 2022, 127, e2022JD036738. [Google Scholar] [CrossRef]
- Makarieva, A.M.; Gorshkov, V.G.; Li, B.-L. Revisiting forest impact on atmospheric water vapor transport and precipitation. Theor. Appl. Climatol. 2012, 111, 79–96. [Google Scholar] [CrossRef]
- Makarieva, A.M.; Gorshkov, V.G.; Sheil, D.; Nobre, A.D.; Bunyard, P.; Li, B.-L. Why does air passage over forest yield more rain? Examining the coupling between rainfall, pressure, and atmospheric moisture content. J. Hydrometeorol. 2014, 15, 411–426. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Q.; Brockway, D.G. Analyzing the complexity of cone production in longleaf pine by multiscale entropy. J. Sust. For. 2016, 35, 172–182. [Google Scholar] [CrossRef]
- Chen, X.; Willis, J.L.; Bowman, K.A. Assessing the influence of climate on cone production of longleaf pine forests. Trees For. People 2022, 9, 100297. [Google Scholar] [CrossRef]
- Guo, Q.; Zarnoch, S.J.; Chen, X.; Brockway, D.G. Life cycle and masting of a recovering keystone indicator species under climate change. Ecosystem Health Sust. 2016, 2, e01226. [Google Scholar] [CrossRef]
- Whelan, A.; Starr, G.; Staudhammer, C.L.; Loescher, H.W.; Mitchell, R.J. Effects of drought and prescribed fire on energy exchange in longleaf pine ecosystems. Ecosphere 2015, 6, 128. [Google Scholar] [CrossRef]
- Willis, J.L.; Brockway, D.G. Longleaf Pine Cone Prospects for 2023; Southern Research Station, USDA Forest Service: Asheville, NC, USA, 2023.
- Luo, L.; Robock, A.; Mitchell, K.E.; Houser, P.R.; Wood, E.F.; Schaake, J.C.; Lohmann, D.; Cosgrove, B.; Wen, F.; Sheffield, J.; et al. Validation of the North American Land Data Assimilation System (NLDAS) retrospective forcing over the southern Great Plains. J. Geophys. Res. 2003, 108, 8843. [Google Scholar] [CrossRef]
- Mitchell, K.E.; Lohmann, D.; Houser, P.R.; Wood, E.F.; Schaake, J.C.; Robock, A.; Cosgrove, B.A.; Sheffield, J.; Duan, Q.; Luo, L.; et al. The multi-institution North American Land Data Assimilation System (NLDAS): Utilizing multiple GCIP products and partners in a continental distributed hydrological modeling system. J. Geophys. Res. 2004, 109, D07S90. [Google Scholar] [CrossRef]
- Chen, X. A case study of using remote sensing data to compare biophysical properties of a forest and an urban area in northern Alabama, USA. J. Sust. For. 2016, 35, 261–279. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H. Comparing environmental impacts of Chinese Torreya plantations and regular forests using remote sensing. Envir. Develop. Sust. 2021, 23, 133–150. [Google Scholar] [CrossRef]
- Nelson, J.A.; Pérez-Priego, O.; Zhou, S.; Royatos, R.; Zhang, Y.; Blanken, P.D.; Gimeno, T.E.; Wohlfahrt, G.; Gioli, B.; Limousin, J.M. Ecosystem transpiration and evaporation: Insights from three water flux partitioning methods across FLUXNET sites. Glob. Change Biol. 2020, 26, 6916–6930. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Amatya, D.M.; McNulty, S.G.; Skaggs, R.W.; Hughes, J.H. Climate change impacts on the hydrology and productivity of a pine plantation. J. Am. Water Res. Assoc. 2000, 36, 367–374. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogee, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Ford, C.R.; Mitchell, R.J.; Teskey, R.O. Water table depth affects productivity, water use, and the response to nitrogen addition in a savanna system. Can. J. For. Res. 2008, 38, 2118–2127. [Google Scholar] [CrossRef]
- Fisher, J.B.; Melton, F.; Middleton, E.; Hain, C.; Anderson, M.; Allen, R.; McCabe, M.C.; Hook, S.; Baldocchi, D.; Townsend, P.A.; et al. The future of evapotranspiration: Global requirements for ecosystem functioning, carbon and climate feedbacks, agricultural management, and water resources: The future of evapotranspiration. Water Resour. Res. 2017, 53, 2618–2626. [Google Scholar] [CrossRef]
- Berkelhammer, M.; Noone, D.C.; Wong, T.E.; Burns, S.P.; Knowles, J.F.; Kaushik, A.; Blanken, P.D.; Williams, M.W. Convergent approaches to determine an ecosystem’s transpiration fraction: Transpiration fraction of two forests. Glob. Biogeochem. Cycles 2016, 30, 933–951. [Google Scholar] [CrossRef]
- Fatichi, S.; Pappas, C. Constrained variability of modeled T:ET ratio across biomes: Transpiration: Evapotranspiration Ratio. Geophys. Res. Lett. 2017, 44, 6795–6803. [Google Scholar] [CrossRef]
- Jiang, K.; Pan, Z.; Pan, F.; Teuling, A.J.; Han, G.; An, P.; Chen, X.; Wang, J.; Song, Y.; Cheng, L.; et al. Combined influence of soil moisture and atmospheric humidity on land surface temperature under different climatic background. iScience 2023, 26, 106937. [Google Scholar] [CrossRef]
- Eltahir, E.A.B. A soil moisture–rainfall feedback mechanism: 1. Theory and observations. Water Resour. Res. 1998, 34, 765–776. [Google Scholar] [CrossRef]
- Sehler, R.; Li, J.; Reager, J.T.; Ye, H. Investigating relationship between soil moisture and precipitation globally using remote sensing observations. J. Contemp. Water Res. Edu. 2019, 168, 106–118. [Google Scholar] [CrossRef]
- Tuttle, S.; Salvucci, G. Empirical evidence of contrasting soil moisture–precipitation feed-backs across the United States. Science 2016, 352, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.W.; Rapp, A.D.; Quiring, S.M.; Blake, J. Soil moisture–precipitation coupling: Observations from the Oklahoma Mesonet and underlying physical mechanisms. Hydrol. Earth Syst. Sci. 2015, 19, 3617–3631. [Google Scholar] [CrossRef]
- Rascón-Ramos, A.E.; Martínez-Salvador, M.; Sosa-Pérez, G.; Villarreal-Guerrero, F.; Pinedo-Alvarez, A.; Santellano-Estrada, E.; Corrales-Lerma, R. Soil moisture dynamics in response to precipitation and thinning in a semi-dry forest in northern Mexico. Water 2021, 13, 105. [Google Scholar] [CrossRef]
- Powell, T.L.; Gholz, H.L.; Clark, K.L.; Starr, G.; Cropper, W.P., Jr.; Martin, T.A. Carbon exchange of a mature, naturally regenerated pine forest in north Florida. Glob. Change Biol. 2008, 14, 2523–2538. [Google Scholar] [CrossRef]
- MacRoberts, M.H.; MacRoberts, B.R. Longleaf pine (Pinus palustris Mill.) growth in bogs. Phytologia 1996, 81, 28–34. [Google Scholar]
- Foster, T.E.; Brooks, J.R. Long-term trends in growth of Pinus palustris and Pinus elliottii along a hydrological gradient in central Florida. Can. J. For. Res. 2001, 31, 1661–1670. [Google Scholar] [CrossRef]
- Baudena, M.; Tuinenburg, O.A.; Ferdinand, P.A.; Staal, A. Effects of land-use change in the Amazon on precipitation are likely underestimated. Glob. Change Biol. 2021, 27, 5580–5587. [Google Scholar] [CrossRef]
- Sheil, D. Forests, atmospheric water and an uncertain future: The new biology of the global water cycle. For. Ecosys. 2018, 5, 19. [Google Scholar] [CrossRef]
- Hasting, A.; Annott, K.C.; Cuddington, K.; Francis, T.; Gellner, G.; Lai, Y.-C.; Morozov, A.; Petrovskii, S.; Scranton, K.; Zeeman, M.L. Transient phenomena in ecology. Science 2018, 361, eaat6412. [Google Scholar] [CrossRef]
- Chen, X. Historical radial growth of Chinese Torreya trees and adaptation to climate change. Atmosphere 2020, 11, 691. [Google Scholar] [CrossRef]
- Colwell, R.K.; Hurtt, G.C. Nonbiological gradients in species richness and a spurious Rapoport effect. Am. Nat. 1994, 144, 570–595. [Google Scholar] [CrossRef]
- Letten, A.D.; Lyons, S.K.; Moles, A.T. The mid-domain effect: It’s not just about space. J. Biogeogr. 2013, 40, 2017–2019. [Google Scholar] [CrossRef]
- Chen, X.; Willis, J.L. Individuals’ behaviors of cone production in longleaf pine trees. Forests 2023, 14, 494. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).