Populus trichocarpa PtHSFA4a Enhances Heat Tolerance by Regulating Expression of APX1 and HSPs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Stress Treatments
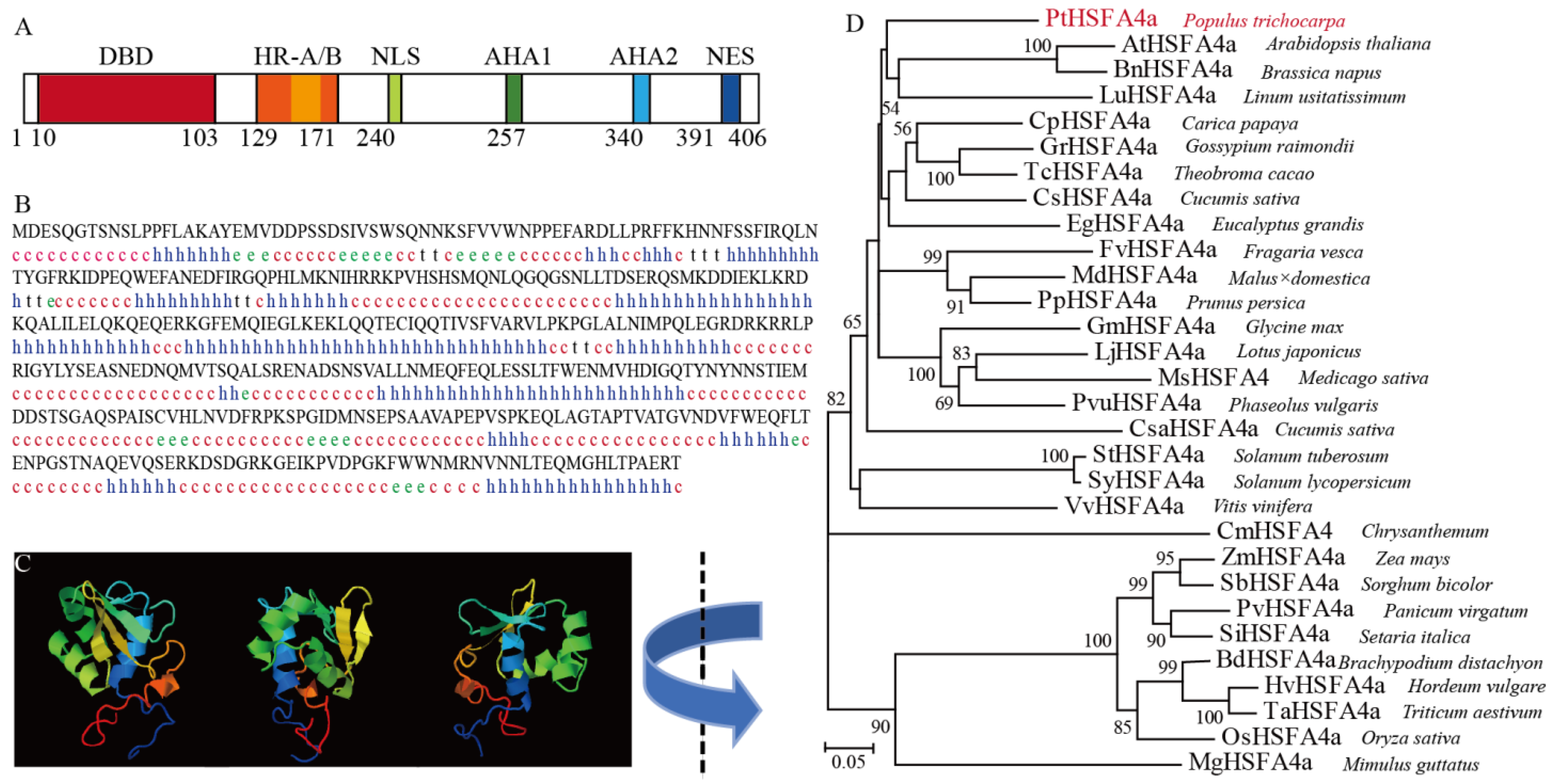
2.2. Secondary and Three-Dimensional (3D) Structural Analysis of the PtHSFA4a Protein
2.3. Phylogenetic Analysis of the HSFA4a Family
2.4. RNA Extraction and Reverse Transcription-Quantitative PCR
2.5. Generation of A. thaliana Transgenic Plants
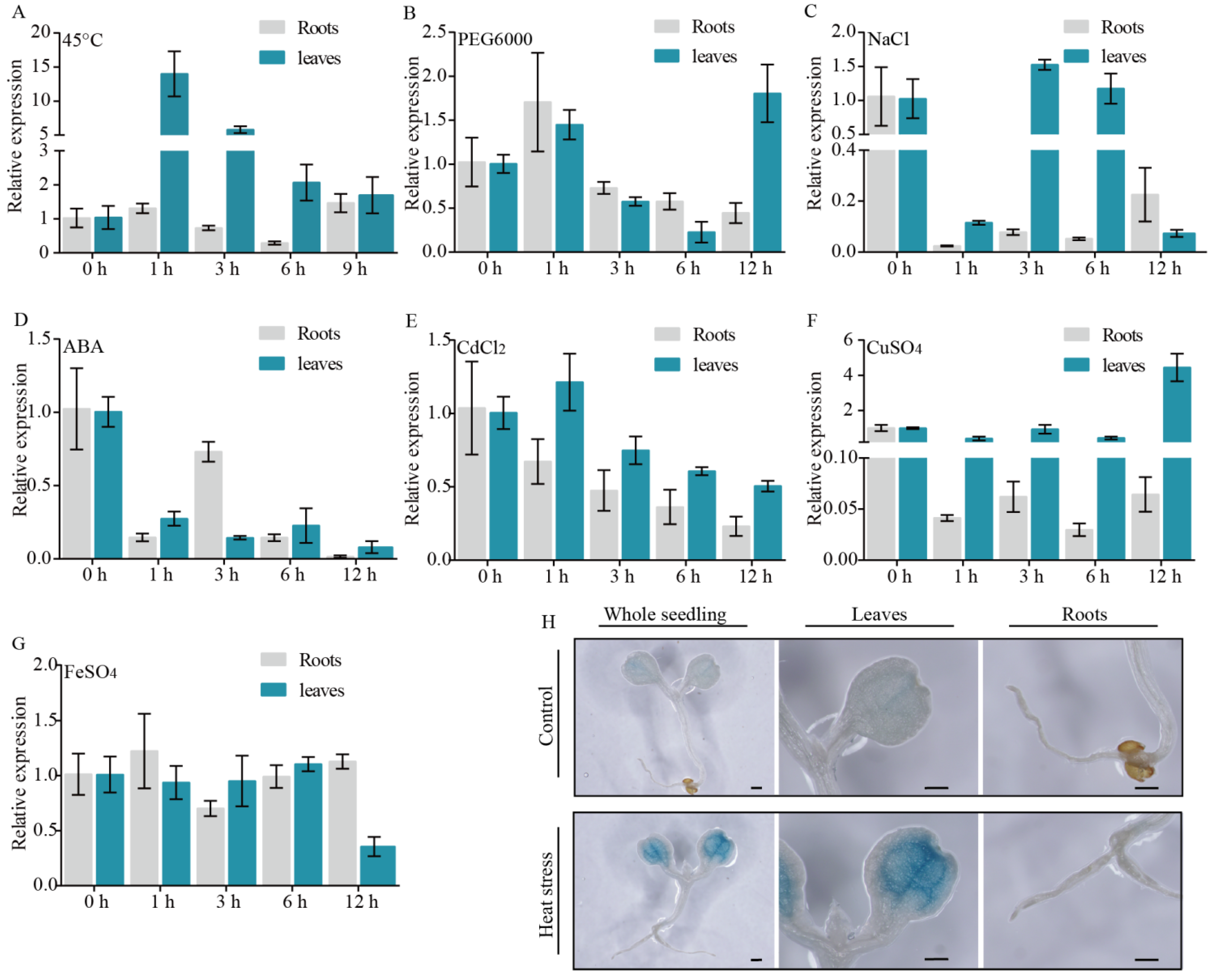
2.6. Analysis of PtHSFA4a Promoter Activity under Heat Stress
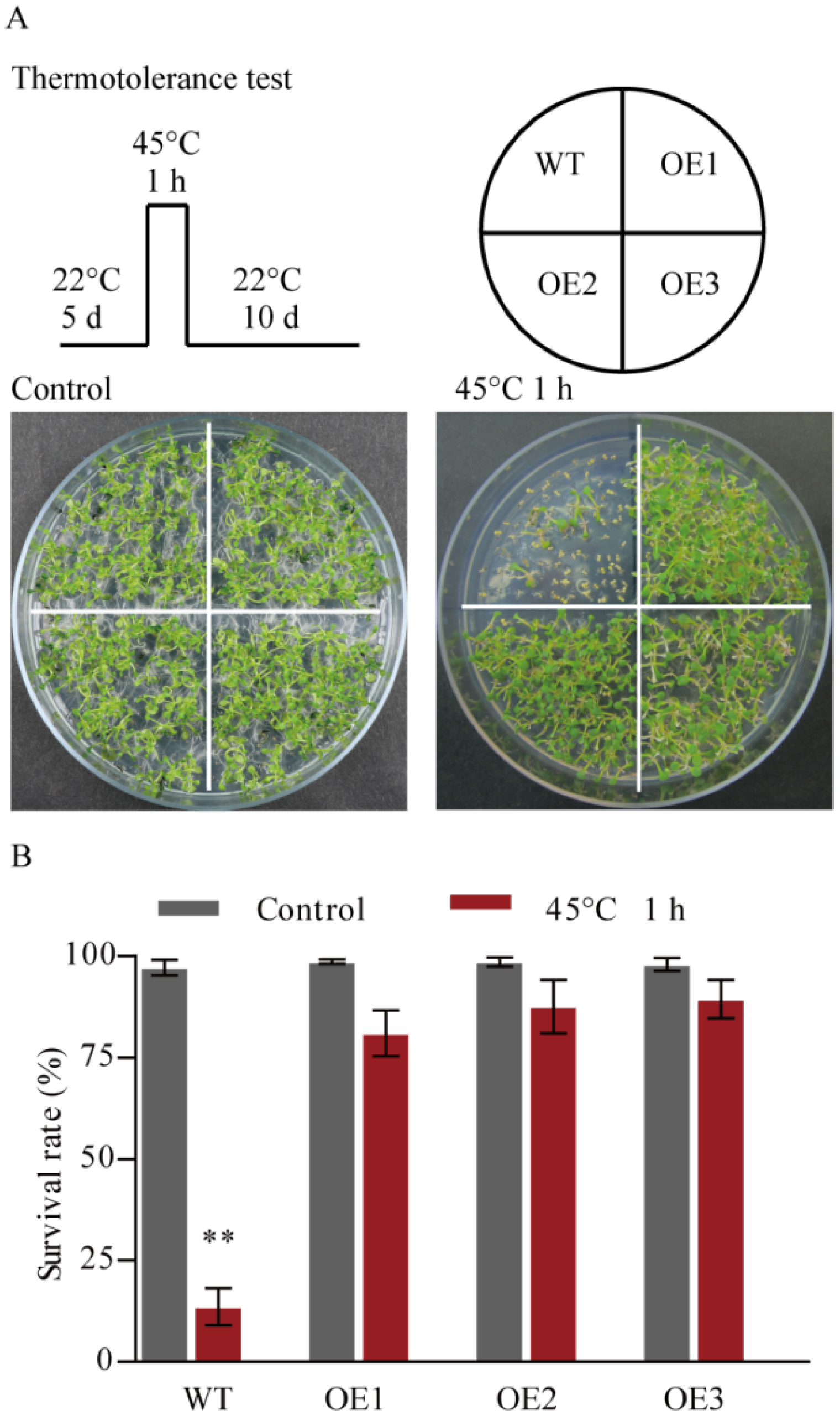
2.7. Thermotolerance Test
2.8. Measurement of Malondialdehyde, Electroleakage, and Reactive Oxygen Species Accumulation
2.9. Chromatin Immunoprecipitation-Quantitative PCR (ChIP-qPCR) Assays
2.10. Statistical Analysis
2.11. Accession Numbers
3. Results
3.1. Isolation and Characterization of PtHSFA4a
3.2. High Temperature Influences PtHSFA4a Expression in Leaf Tissues
3.3. PtHSFA4a Regulates Resistance to Heat Stress and High Temperature-Induced ROS Production in A. thaliana
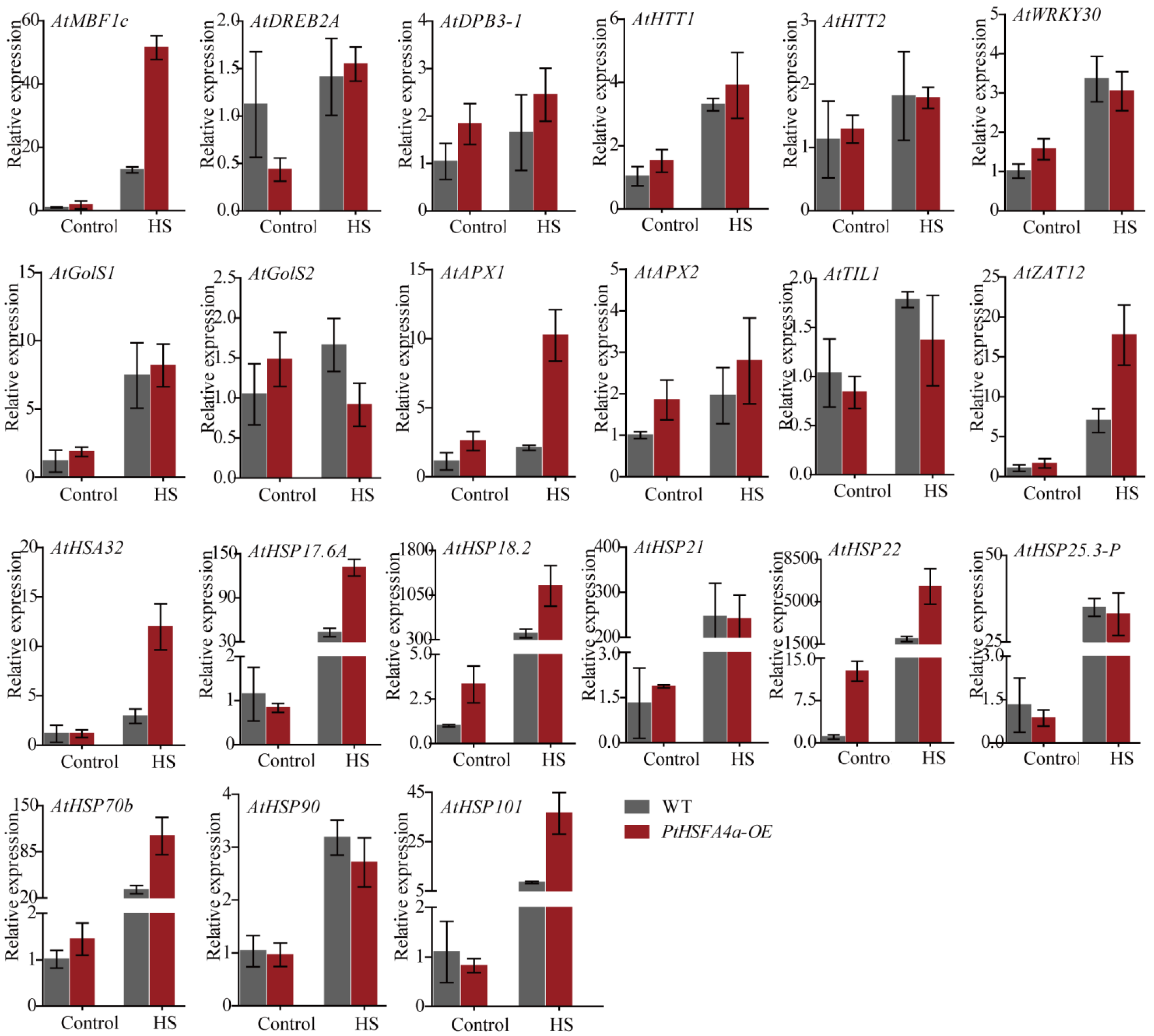
3.4. Overexpression of PtHSFA4a Upregulated Heat Stress-Responsive Genes in Transgenic A. thaliana Plants
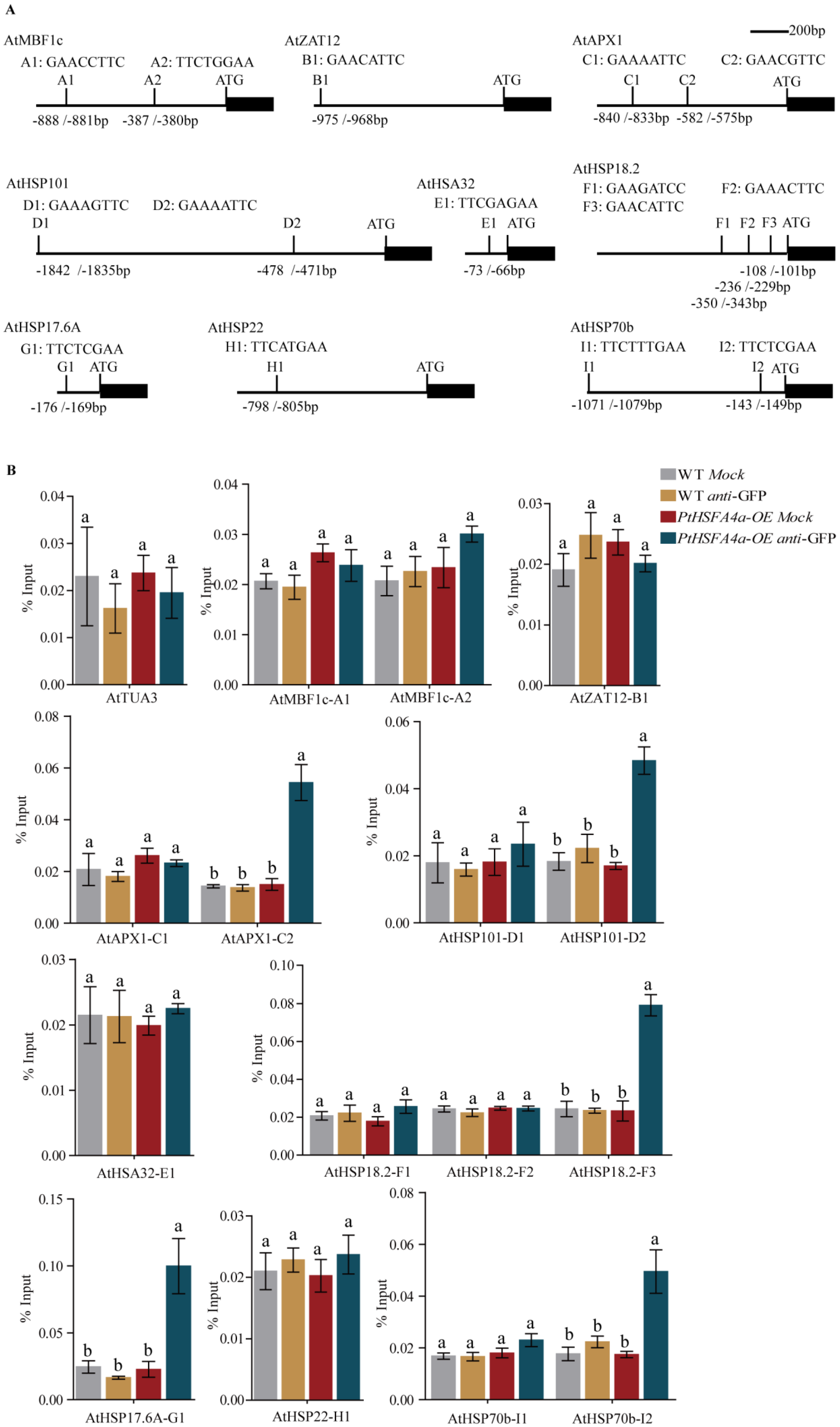
3.5. PtHSFA4a Directly Targets AtAPX1 and AtHSPs Promoters
4. Discussion
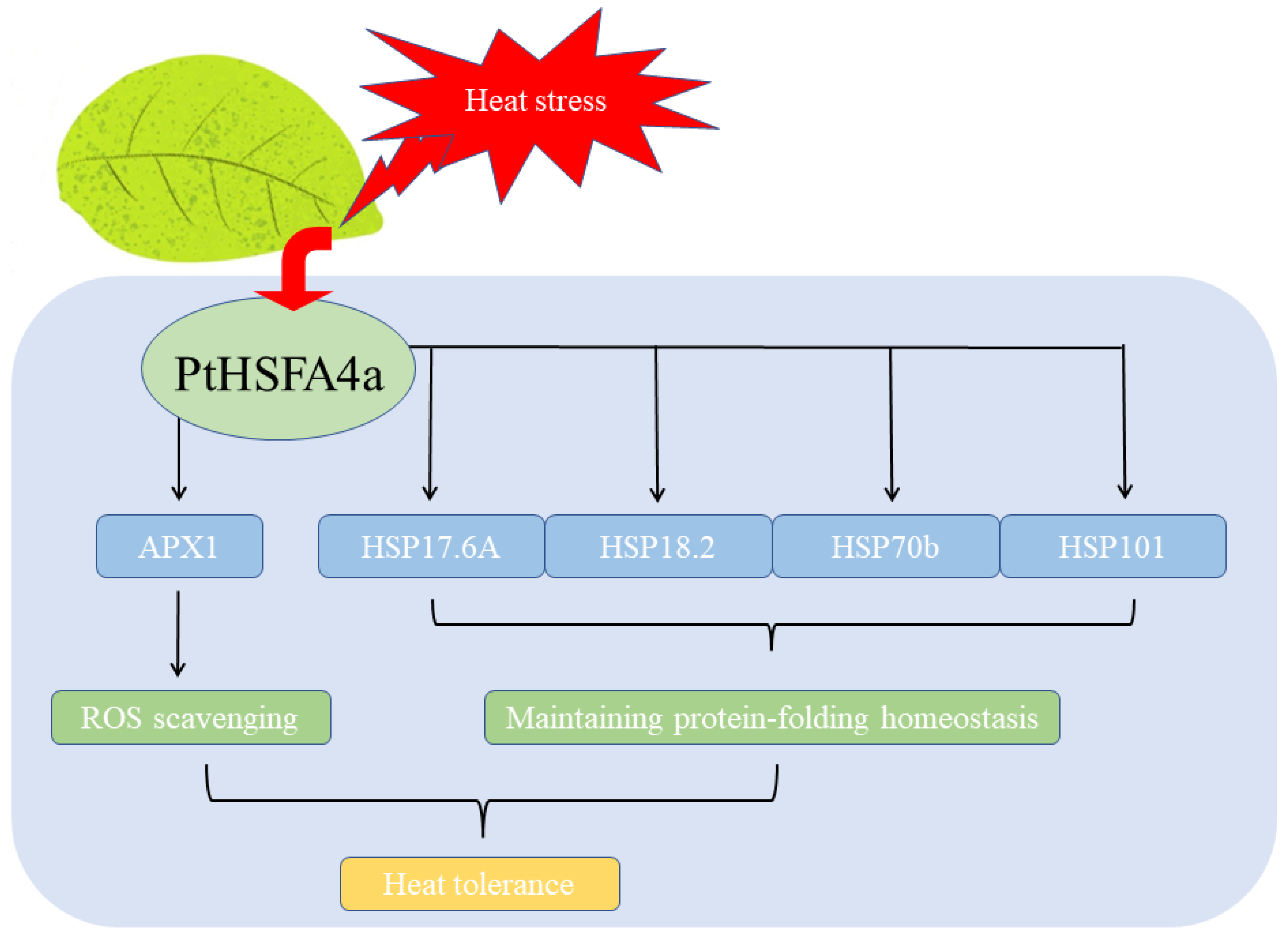
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chand, G.; Sinha, B.K.; Sharma, M.; Dogra, S. High temperature stress: Responses mechanism and management. In Abiotic & Biotic Stress Management in Plants, 1st ed.; Sinha, B.K., Reena, Eds.; E-Publishing Inc.: London, UK, 2022; Volume 1, pp. 51–93. [Google Scholar]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate change risks to global forest health: Emergence of unexpected events of elevated tree mortality worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.W.; Hao, G.Y. The synergistic effect of hydraulic and thermal impairments accounts for the severe crown damage in Fraxinus mandshurica seedlings following the combined drought-heatwave stress. Sci. Total Environ. 2023, 856, 159017. [Google Scholar] [PubMed]
- Elzanati, O.; Mouzeyar, S.; Roche, J. Dynamics of the transcriptome response to heat in the moss, physcomitrella patens. Int. J. Mol. Sci. 2020, 21, 1512. [Google Scholar] [CrossRef] [PubMed]
- Kour, J.; Khanna, K.; Singh, A.D.; Dhiman, S.; Bhardwaj, T.; Devi, K.; Sharma, N.; Ohri, P.; Bhardwaj, R. Calcium’s multifaceted functions: From nutrient to secondary messenger during stress. S. Afr. J. Bot. 2023, 152, 247–263. [Google Scholar]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar]
- Chi, W.T.; Fung, R.W.; Liu, H.C.; Hsu, C.C.; Charng, Y.Y. Temperature-induced lipocalin is required for basal and acquired thermotolerance in Arabidopsis. Plant Cell Environ. 2009, 32, 917–927. [Google Scholar] [CrossRef]
- He, F.; Li, H.G.; Wang, J.J.; Su, Y.; Wang, H.L.; Feng, C.H.; Yang, Y.; Niu, M.X.; Liu, C.; Yin, W.; et al. PeSTZ1, a C2H2-type zinc finger transcription factor from Populus euphratica, enhances freezing tolerance through modulation of ROS scavenging by directly regulating PeAPX2. Plant Biotechnol. J. 2019, 17, 2169–2183. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Z.; Teng, R.; Xu, S.; Cao, X.; Yuan, G.; Zhang, D.; Teng, N. LlWRKY39 is involved in thermotolerance by activating LlMBF1c and interacting with LlCaM3 in lily (Lilium longiflorum). Hortic. Res. 2021, 8, 36. [Google Scholar]
- Fortunato, S.; Lasorella, C.; Dipierro, N.; Vita, F.; de Pinto, M.C. Redox signaling in plant heat stress response. Antioxidants 2023, 12, 605. [Google Scholar]
- Mao, L.; Deng, M.; Jiang, S.; Zhu, H.; Yang, Z.; Yue, Y.; Zhao, K. Characterization of the DREBA4-type transcription factor (SlDREBA4), which contributes to heat tolerance in tomatoes. Front. Plant Sci. 2020, 11, 554520. [Google Scholar] [CrossRef]
- Nover, L.; Bharti, K.; Döring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K.D. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones 2001, 6, 177–189. [Google Scholar] [CrossRef]
- Shi, H.; Tan, D.X.; Reiter, R.J.; Ye, T.; Yang, F.; Chan, Z. Melatonin induces class A1 heat-shock factors (HSFA 1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, Y. Plant transcription factors and temperature stress. In Plant Transcription Factors, 1st ed.; Srivastava, V., Mishra, S., Eds.; E-Publishing Inc.: London, UK, 2023; pp. 287–300. [Google Scholar]
- Busch, W.; Wunderlich, M.; Schöffl, F. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J. 2005, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, S.; Liu, K.; Liu, Y.; Ni, L.; Zhang, K.; Zhang, L. Isolation of heat shock factor HSFA1a-binding sites in vivo revealed variations of heat shock elements in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Mesihovic, A.; Simm, S.; Paupière, M.J.; Hu, Y.; Paul, P.; Mishra, S.K.; Tschiersch, B.; Theres, K.; Bovy, A.; et al. HSFA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol. 2016, 170, 2461–2477. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Ye, C.J.; Lü, H.Y.; Xu, M.X.; Li, W.; Zhang, L.M.; Wang, C.; Luo, S.P.; Zhu, B.G. Cloning of GmHSFA1 gene and its overexpression leading to enhancement of heat tolerance in transgenic soybean. Yi Chuan Hered. 2006, 28, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HSFA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262. [Google Scholar]
- Cohen-Peer, R.; Schuster, S.; Meiri, D.; Breiman, A.; Avni, A. Arabidopsis heat shock factor A2 (HSFA2) modifies its activity during acquired thermotholerance. Plant Mol. Biol. 2010, 74, 33–45. [Google Scholar]
- Nishizawa-Yokoi, A.; Nosaka, R.; Hayashi, H.; Tainaka, H.; Maruta, T.; Tamoi, M.; Ikeda, M.; Ohme-Takagi, M.; Yoshimura, K.; Yabuta, Y.; et al. HSFA1d and HSFA1e involved in the transcriptional regulation of HSFA2 function as key regulators for the HSF signaling network in response to environmental stress. Plant Cell Physiol. 2011, 52, 933–945. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Xing, D.; Gao, C. Characterization of mitochondrial dynamics and subcellular localization of ROS reveal that HSFA2 alleviates oxidative damage caused by heat stress in Arabidopsis. J. Exp. Bot. 2009, 60, 2073–2091. [Google Scholar]
- Song, C.; Chung, W.S.; Lim, C.O. Overexpression of heat shock factor gene HSFA3 increases galactinol levels and oxidative stress tolerance in Arabidopsis. Mol. Cells 2016, 39, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Schramm, F.; Larkindale, J.; Kiehlmann, E.; Ganguli, A.; Englich, G.; Vierling, E.; Von Koskull-Döring, P. A cascade of transcription factor DREB2A and heat stress transcription factor HSFA3 regulates the heat stress response of Arabidopsis. Plant J. 2008, 53, 264–274. [Google Scholar] [PubMed]
- Yoshida, T.; Ohama, N.; Nakajima, J.; Kidokoro, S.; Mizoi, J.; Nakashima, K.; Maruyama, K.; Kim, J.M.; Seki, M.; Todaka, D.; et al. Arabidopsis HSFA1 transcription factors function as the main positive regulators in heat shock responsive gene expression. Mol. Genet. Genom. 2011, 286, 321–332. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chang, K.Y.; Wu, S.J. High irradiance sensitive phenotype of Arabidopsis hit2/xpo1a mutant is caused in part by nuclear confinement of AtHSFA4a. Biol. Plant. 2018, 62, 69–79. [Google Scholar] [CrossRef]
- Andrási, N.; Rigó, G.; Zsigmond, L.; Pérez-Salamó, I.; Papdi, C.; Klement, E.; Pettkó-Szandtner, A.; Baba, A.I.; Ayaydin, F.; Dasari, R.; et al. The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses. J. Exp. Bot. 2019, 70, 4903–4918. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, U.; Yano, M.; Lin, H.; Ashikari, M.; Yamada, K. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc. Natl. Acad. Sci. USA 2002, 99, 7530–7535. [Google Scholar] [PubMed]
- Tejedor-Cano, J.; Prieto-Dapena, P.; Almoguera, C.; Carranco, R.; Hiratsu, K.; Ohme-Takagi, M.; Jordano, J. Loss of function of the HSFA9 seed longevity program. Plant Cell Environ. 2010, 33, 1408–1417. [Google Scholar]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis HSFB1 and HSFB2b act as repressors of the expression of heat-inducible HSFs but positively regulate the acquired thermotolerance. Plant Physiol. 2011, 157, 1243–1254. [Google Scholar] [CrossRef]
- AlGhamdi, R.; Aziz, A.; Alshehri, M.; Pardasani, K.R.; Aziz, T. Deep learning model with ensemble techniques to compute the secondary structure of proteins. J. Supercomput. 2021, 77, 5104–5119. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; De Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef]
- Berz, J.; Simm, S.; Schuster, S.; Scharf, K.D.; Schleiff, E.; Ebersberger, I. Heatster: A database and web server for identification and classification of heat stress transcription factors in plants. Bioinform. Biol. Insights 2019, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gang, H.; Li, R.; Zhao, Y.; Liu, G.; Chen, S.; Jiang, J. Loss of GLK1 transcription factor function reveals new insights in chlorophyll biosynthesis and chloroplast development. J. Exp. Bot. 2019, 70, 3125–3138. [Google Scholar] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.; Li, W.; Chen, Y.; Lu, H.; Zhao, S.; Li, D.; Wei, M.; Li, C. PuHSFA4a enhances tolerance to excess zinc by regulating reactive oxygen species production and root development in Populus. Plant Physiol. 2019, 180, 2254–2271. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Meng, H.; Wen, H.; Fan, Y.; Zhao, J. Maize ABP9 enhances tolerance to multiple stresses in transgenic Arabidopsis by modulating ABA signaling and cellular levels of reactive oxygen species. Plant Mol. Biol. 2011, 75, 365–378. [Google Scholar]
- Baliardini, C.; Meyer, C.L.; Salis, P.; Saumitoulaprade, P.; Verbruggen, N. CATION EXCHANGER1 cosegregates with cadmium tolerance in the metal hyperaccumulator Arabidopsis halleri and plays a role in limiting oxidative stress in Arabidopsis spp. Plant Physiol. 2015, 169, 549–559. [Google Scholar] [CrossRef]
- Ramel, F.; Sulmon, C.; Bogard, M.; Couée, I.; Gouesbet, G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009, 9, 28. [Google Scholar]
- Li, W.; Lin, Y.C.; Li, Q.; Shi, R.; Lin, C.Y.; Chen, H.; Chuang, L.; Qu, G.Z.; Sederoff, R.R.; Chiang, V.L. A robust chromatin immunoprecipitation protocol for studying transcription factor-DNA interactions and histone modifications in wood-forming tissue. Nat. Protoc. 2014, 9, 2180–2193. [Google Scholar] [CrossRef]
- Lämke, J.; Brzezinka, K.; Altmann, S.; Bäurle, I. A hit-and-run heat shock factor govern sustained histone methylation and transcriptional stress memory. EMBO J. 2016, 35, 162–175. [Google Scholar] [CrossRef]
- Rizhsky, L.; Davletova, S.; Liang, H.; Mittler, R. The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J. Biol. Chem. 2004, 279, 11736–11743. [Google Scholar] [CrossRef] [PubMed]
- Storozhenko, S.; De Pauw, P.; Van Montagu, M.; Inzé, D.; Kushnir, S. The heat-shock element is a functional component of the Arabidopsis APX1 gene promoter. Plant Physiol. 1998, 118, 1005–1014. [Google Scholar] [PubMed]
- Yamaguchi, N.; Winter, C.M.; Wu, M.F.; Kwon, C.S.; William, D.A.; Wagner, D. Protocols: Chromatin immunoprecipitation from Arabidopsis tissues. Arab. Book 2014, 12, e0170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.; Li, D.; Wei, M.; Li, C. PtHSFA4a gene play critical roles in the adaptation of Arabidopsis thaliana plants to high-Zinc stress. Plant Signal. Behav. 2019, 14, e1654353. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Sejima, H.; Tam, R.; Schlauch, K.; Mittler, R. Identification of the MBF1 heat-response regulon of Arabidopsis thaliana. Plant J. 2011, 66, 844–851. [Google Scholar]
- Sato, H.; Mizoi, J.; Tanaka, H.; Maruyama, K.; Qin, F.; Osakabe, Y.; Morimoto, K.; Ohori, T.; Kusakabe, K.; Nagata, M.; et al. Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with NF-Y subunits. Plant Cell 2014, 26, 4954–4973. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, Z.; Li, X.; Wu, F.; He, Y. Heat-induced TAS1 TARGET1 mediates thermotolerance via heat stress transcription factor A1a–directed pathways in Arabidopsis. Plant Cell 2014, 26, 1764–1780. [Google Scholar]
- Yer, E.N.; Baloglu, M.C.; Ayan, S. Identification and expression profiling of all HSP family member genes under salinity stress in different poplar clones. Gene 2018, 678, 324–336. [Google Scholar]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar]
- Medina, E.; Kim, S.H.; Yun, M.; Choi, W.G. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef]
- Naveen, J.; Hithamani, G.; Pushpalatha, H.G. Stress and Its Influence on the Generation of Reactive Oxygen Species and Oxidative Damage in Plants. In Plant Metabolites under Environmental Stress, 1st ed.; Desai, N.M., Pawar, U.R., Eds.; E-Publishing Inc.: New York, NY, USA, 2023; pp. 219–248. [Google Scholar]
- Mansoor, S.; Ali, W.O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2005, 17, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef] [PubMed]
- Panchuk, I.I.; Volkov, R.A.; Schöffl, F. Heat stress-and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 2002, 129, 838–853. [Google Scholar]
- Yadav, A.; Singh, J.; Ranjan, K.; Kumar, P.; Khanna, S.; Gupta, M.; Kumar, V.; Wani, S.H.; Sirohi, A. Heat shock proteins: Master players for heat-stress tolerance in plants during climate change. In Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives, 1st ed.; Wani, S.H., Kumar, V., Eds.; E-Publishing Inc.: New York, NY, USA, 2020; pp. 189–211. [Google Scholar]
- Wu, J.; Gao, T.; Hu, J.; Zhao, L.; Yu, C.; Ma, F. Research advances in function and regulation mechanisms of plant small heat shock proteins (sHSPs) under environmental stresses. Sci. Total Environ. 2022, 825, 154054. [Google Scholar]
- Osthoff, A.; Donà Dalle Rose, P.; Baldauf, J.A.; Piepho, H.P.; Hochholdinger, F. Transcriptomic reprogramming of barley seminal roots by combined water deficit and salt stress. BMC Genom. 2019, 20, 325. [Google Scholar]
- Yoshida, K.; Kasai, T.; Garcia, M.R.; Sawada, S.; Shoji, T.; Shimizu, S.; Yamazaki, K.; Komeda, Y.; Shinmyo, A. Heat-inducible expression system for a foreign gene in cultured tobacco cells using the HSP18.2 promoter of Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 1995, 44, 466–472. [Google Scholar] [CrossRef]
- Sung, D.Y.; Vierling, E.; Guy, C.L. Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol. 2001, 126, 789–800. [Google Scholar]
- Babbar, R.; Tiwari, L.D.; Mishra, R.C.; Shimphrui, R.; Singh, A.A.; Goyal, I.; Rana, S.; Kumar, R.; Sharma, V.; Tripathi, G.; et al. Arabidopsis plants overexpressing additional copies of heat shock protein HSP101 showed high heat tolerance and endo-gene silencing. Plant Sci. 2023, 330, 111639. [Google Scholar]
- Kumar, R.; Khungar, L.; Shimphrui, R.; Tiwari, L.D.; Tripathi, G.; Sarkar, N.K.; Agarwal, S.K.; Agarwal, M.; Grover, A. AtHSP101 research sets course of action for the genetic improvement of crops against heat stress. J. Plant Biochem. Biotechnol. 2020, 29, 715–732. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, X.; Meng, M.; Di, H.; Wang, J. Populus trichocarpa PtHSFA4a Enhances Heat Tolerance by Regulating Expression of APX1 and HSPs. Forests 2023, 14, 2028. https://doi.org/10.3390/f14102028
Zhang H, Zhang X, Meng M, Di H, Wang J. Populus trichocarpa PtHSFA4a Enhances Heat Tolerance by Regulating Expression of APX1 and HSPs. Forests. 2023; 14(10):2028. https://doi.org/10.3390/f14102028
Chicago/Turabian StyleZhang, Haizhen, Xuetong Zhang, Meng Meng, Haoyang Di, and Jingang Wang. 2023. "Populus trichocarpa PtHSFA4a Enhances Heat Tolerance by Regulating Expression of APX1 and HSPs" Forests 14, no. 10: 2028. https://doi.org/10.3390/f14102028
APA StyleZhang, H., Zhang, X., Meng, M., Di, H., & Wang, J. (2023). Populus trichocarpa PtHSFA4a Enhances Heat Tolerance by Regulating Expression of APX1 and HSPs. Forests, 14(10), 2028. https://doi.org/10.3390/f14102028



