Multi-Scale Altitudinal Patterns of Soil and Litter Invertebrate Communities in a Warm Temperate Deciduous Broadleaf Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites
2.2. Soil Invertebrate Collection
2.3. Statistical Analyses
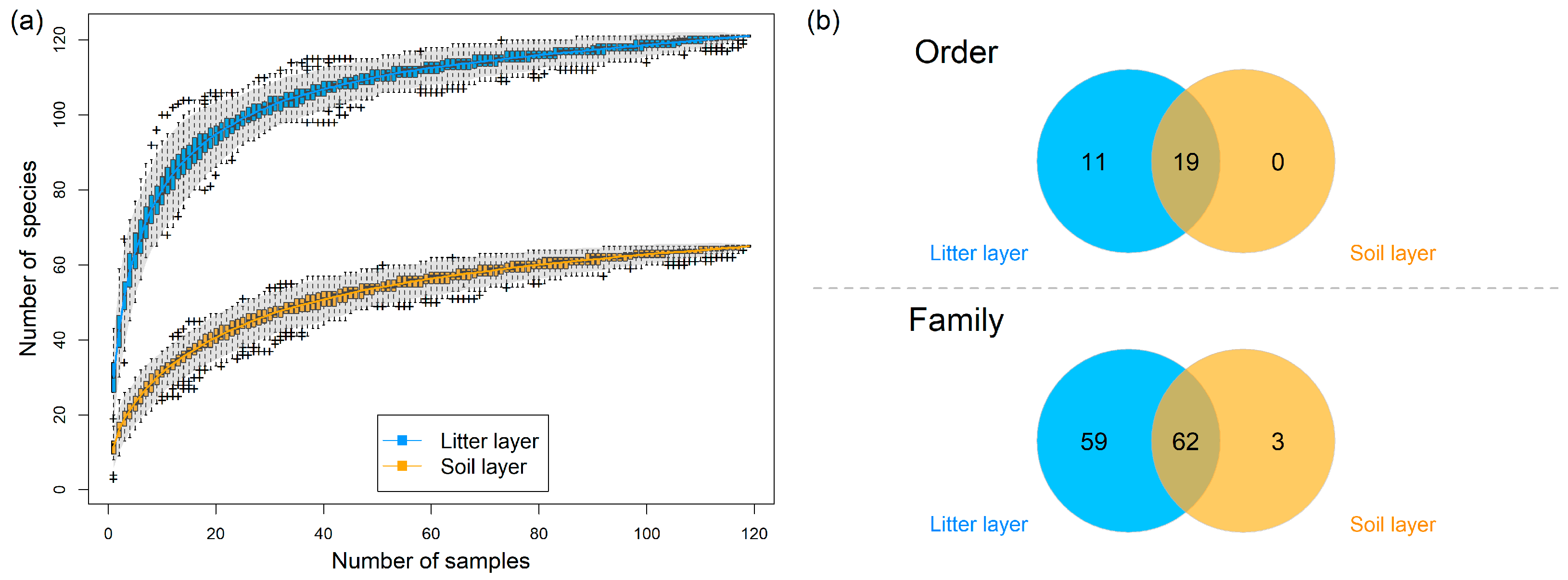
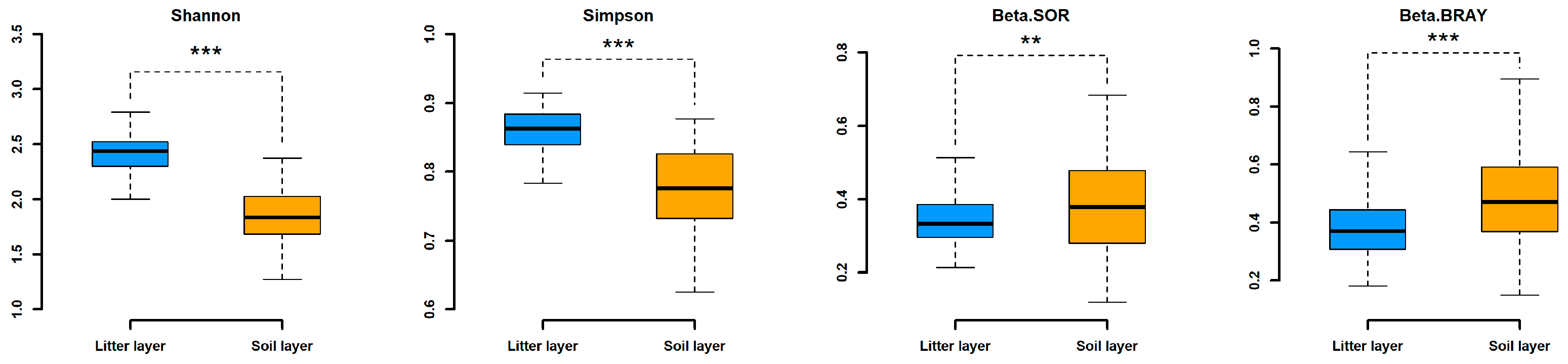
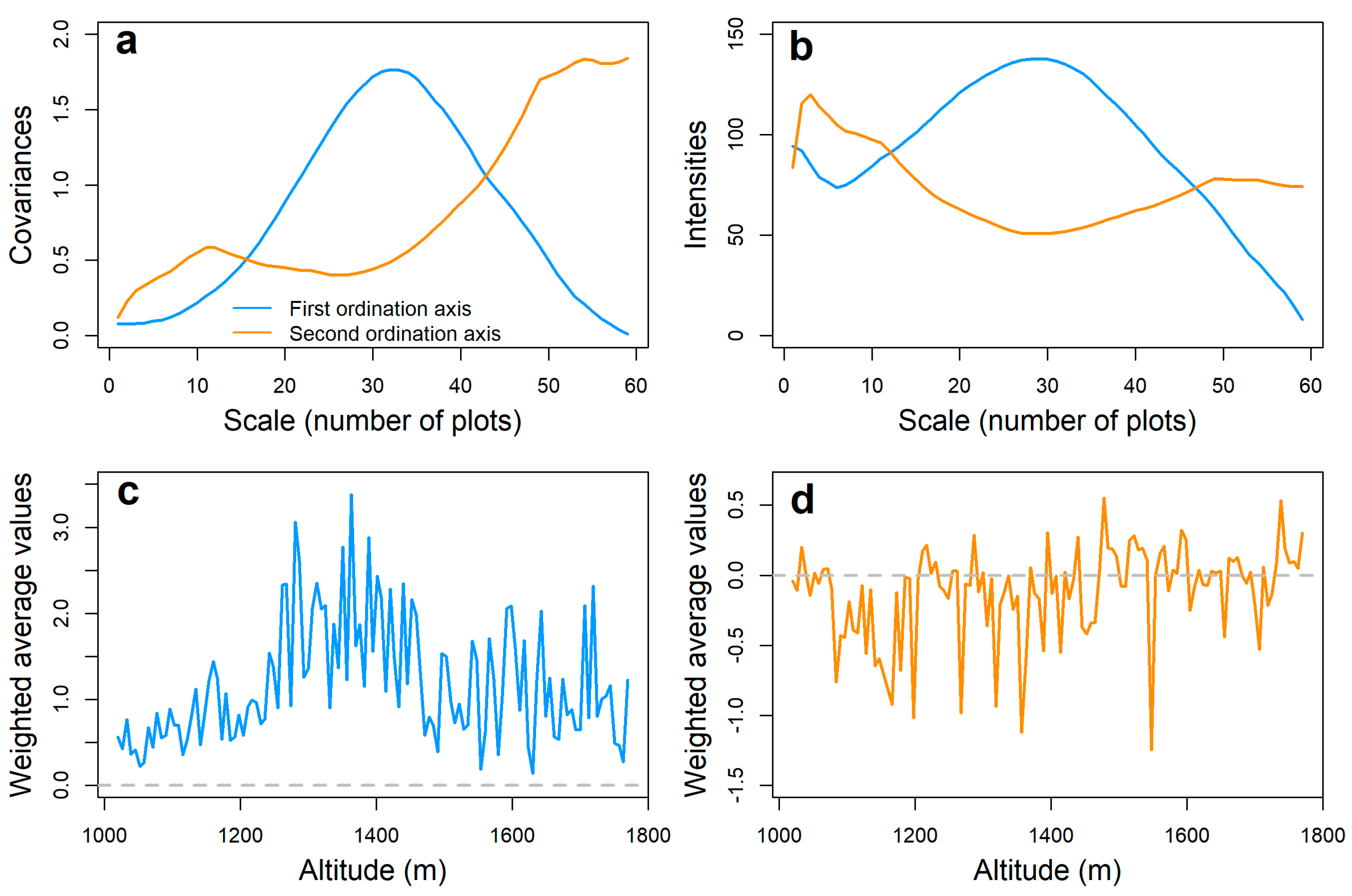
3. Results
3.1. Soil Invertebrate Diversity
3.2. Pattern Analysis of Soil Invertebrate Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, R.A. Effects of habitat complexity and composition on a diverse litter microarthropod assemblage. Ecology 2000, 81, 1120–1132. [Google Scholar] [CrossRef]
- García-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol. Lett. 2013, 16, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Osler, G.H.R.; Sommerkorn, M. Toward a complete soil C and N cycle: Incorporating the soil fauna. Ecology 2007, 88, 1611–1621. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.R.; Nagendra, U.J.; Taylor, M.K.; Campbell, J.H.; Cunard, C.E.; Joslin, A.H.; Mundepi, A.; Phillips, C.A.; Callaham, M.A. Soil fauna responses to natural disturbances, invasive species, and global climate change: Current state of the science and a call to action. Soil Biol. Biochem. 2017, 110, 116–133. [Google Scholar] [CrossRef]
- Landesman, W.J.; Treonis, A.M.; Dighton, J. Effects of a one-year rainfall manipulation on soil nematode abundances and community composition. Pedobiologia 2011, 54, 87–91. [Google Scholar] [CrossRef]
- Ding, J.; Eldridge, D.J. Drivers of soil biodiversity vary with organism type along an extensive aridity gradient. Appl. Soil Ecol. 2022, 170, 104271. [Google Scholar] [CrossRef]
- Levin, S.A. The Problem of Pattern and Scale in Ecology: The Robert H. MacArthur Award Lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Chave, J. The problem of pattern and scale in ecology: What have we learned in 20 years? Ecol. Lett. 2013, 16, 4–16. [Google Scholar] [CrossRef]
- Caruso, T.; Taormina, M.; Migliorini, M. Relative role of deterministic and stochastic determinants of soil animal community: A spatially explicit analysis of oribatid mites. J. Anim. Ecol. 2012, 81, 214–221. [Google Scholar] [CrossRef]
- Magilton, M.; Maraun, M.; Emmerson, M.; Caruso, T. Oribatid mites reveal that competition for resources and trophic structure combine to regulate the assembly of diverse soil animal communities. Ecol. Evol. 2019, 9, 8320–8330. [Google Scholar] [CrossRef]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lin, Y.; Zhang, S.; Zhang, Y.; Li, G.; Ma, K. Shifting mechanisms of elevational diversity and biomass patterns in soil invertebrates at treeline. Soil Biol. Biochem. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and Ecosystem Responses to Elevational Gradients: Processes, Mechanisms, and Insights for Global Change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef]
- Wu, P.; Wang, C. Differences in spatiotemporal dynamics between soil macrofauna and mesofauna communities in forest ecosystems: The significance for soil fauna diversity monitoring. Geoderma 2019, 337, 266–272. [Google Scholar] [CrossRef]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef]
- Weiher, E.; Freund, D.; Bunton, T.; Stefanski, A.; Lee, T.; Bentivenga, S. Advances, challenges and a developing synthesis of ecological community assembly theory. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2403–2413. [Google Scholar] [CrossRef]
- Romdhane, S.; Spor, A.; Aubert, J.; Bru, D.; Breuil, M.-C.; Hallin, S.; Mounier, A.; Ouadah, S.; Tsiknia, M.; Philippot, L. Unraveling negative biotic interactions determining soil microbial community assembly and functioning. ISME J. 2022, 16, 296–306. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Zhang, D.; Zhang, J. The effects of gap size and litter species on colonization of soil fauna during litter decomposition in Pinus massoniana plantations. Appl. Soil Ecol. 2020, 155, 103611. [Google Scholar] [CrossRef]
- Ganault, P.; Nahmani, J.; Hättenschwiler, S.; Gillespie, L.M.; David, J.-F.; Henneron, L.; Iorio, E.; Mazzia, C.; Muys, B.; Pasquet, A.; et al. Relative importance of tree species richness, tree functional type, and microenvironment for soil macrofauna communities in European forests. Oecologia 2021, 196, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Princeton University Press: Princeton, NJ, USA, 1984; pp. 1–269. [Google Scholar]
- Ushio, M.; Hsieh, C.-H.; Masuda, R.; Deyle, E.R.; Ye, H.; Chang, C.-W.; Sugihara, G.; Kondoh, M. Fluctuating interaction network and time-varying stability of a natural fish community. Nature 2018, 554, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Ma, K. Identifying changing interspecific associations along gradients at multiple scales using wavelet correlation networks. Ecology 2021, 102, e03360. [Google Scholar] [CrossRef]
- Szabó, B.; Korányi, D.; Gallé, R.; Lövei, G.L.; Bakonyi, G.; Batáry, P. Urbanization decreases species richness, and increases abundance in dry climates whereas decreases in wet climates: A global meta-analysis. Sci. Total. Environ. 2023, 859, 160145. [Google Scholar] [CrossRef]
- Noy-Meir, I.; Anderson, D.J. Multiple Pattern Analysis or Multiscale Ordination: Pathway to a Vegetation Hologram. In Statistical Ecology III. Many Species Populations, Ecosystems and Systems Analysis; Patil, G.P., Pielou, E.C., Waters, W.E., Eds.; Pennsylvania State University Press: University Park, PA, USA, 1971; pp. 207–232. [Google Scholar]
- Dale, M.; Zbigniewicz, M. The evaluation of multi-species pattern. J. Veg. Sci. 1995, 6, 391–398. [Google Scholar] [CrossRef]
- R Core Team. R Version 3.5.3: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.r-project.org (accessed on 6 October 2023).
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- González, G.; Lodge, D.J. Soil Biology Research across Latitude, Elevation and Disturbance Gradients: A Review of Forest Studies from Puerto Rico during the Past 25 Years. Forests 2017, 8, 178. [Google Scholar] [CrossRef]
- Birkhofer, K.; Henschel, J.R.; Scheu, S. Spatial-pattern analysis in a territorial spider: Evidence for multi-scale effects. Ecography 2006, 29, 641–648. [Google Scholar] [CrossRef]
- Davis, A.L.; Scholtz, C.H.; Deschodt, C. Multi-scale determinants of dung beetle assemblage structure across abiotic gradients of the Kalahari-Nama Karoo ecotone, South Africa. J. Biogeogr. 2008, 35, 1465–1480. [Google Scholar] [CrossRef]
- Reynolds, C.; Fletcher, R.J.; Carneiro, C.M.; Jennings, N.; Ke, A.; LaScaleia, M.C.; Lukhele, M.B.; Mamba, M.L.; Sibiya, M.D.; Austin, J.D.; et al. Inconsistent effects of landscape heterogeneity and land-use on animal diversity in an agricultural mosaic: A multi-scale and multi-taxon investigation. Landsc. Ecol. 2018, 33, 241–255. [Google Scholar] [CrossRef]
- Korboulewsky, N.; Perez, G.; Chauvat, M. How tree diversity affects soil fauna diversity: A review. Soil Biol. Biochem. 2016, 94, 94–106. [Google Scholar] [CrossRef]
- Sauvadet, M.; Chauvat, M.; Brunet, N.; Bertrand, I. Can changes in litter quality drive soil fauna structure and functions? Soil Biol. Biochem. 2017, 107, 94–103. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, K.; Liu, H.; Tang, Z. A trait-based approach reveals the importance of biotic filter for elevational herb richness pattern. J. Biogeogr. 2018, 45, 2288–2298. [Google Scholar] [CrossRef]
- Massaccesi, L.; Bardgett, R.D.; Agnelli, A.; Ostle, N.; Wilby, A.; Orwin, K.H. Impact of plant species evenness, dominant species identity and spatial arrangement on the structure and functioning of soil microbial communities in a model grassland. Oecologia 2015, 177, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, L.; Mazza, G.; D’Errico, G.; Fabiani, A.; Giuliani, C.; Inghilesi, A.F.; Lagomarsino, A.; Landi, S.; Lastrucci, L.; Pastorelli, R.; et al. How ecosystems change following invasion by Robinia pseudoacacia: Insights from soil chemical properties and soil microbial, nematode, microarthropod and plant communities. Sci. Total. Environ. 2018, 622, 1509–1518. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, Y.; Zhang, T.; Shi, Z.; Maimaitiaili, B.; Tian, C.; Feng, G. Elevated precipitation alters the community structure of spring ephemerals by changing dominant species density in Central Asia. Ecol. Evol. 2020, 10, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Morueta-Holme, N.; Blonder, B.; Sandel, B.; McGill, B.J.; Peet, R.K.; Ott, J.E.; Violle, C.; Enquist, B.J.; Jørgensen, P.M.; Svenning, J. A network approach for inferring species associations from co-occurrence data. Ecography 2016, 39, 1139–1150. [Google Scholar] [CrossRef]
- Uvarov, A.V.; Ilieva-Makulec, K.; Karaban, K.; Yakovenko, N.S.; Uchmański, J. Effects of Intra- and Interspecific Interactions in Earthworm Assemblages: A Comparative Study. Biol. Bull. 2019, 46, 475–482. [Google Scholar] [CrossRef]
- Chazot, N.; Willmott, K.R.; Endara, P.G.S.; Toporov, A.; Hill, R.I.; Jiggins, C.D.; Elias, M. Mutualistic Mimicry and Filtering by Altitude Shape the Structure of Andean Butterfly Communities. Am. Nat. 2014, 183, 26–39. [Google Scholar] [CrossRef]
- Querner, P.; Bruckner, A. Combining pitfall traps and soil samples to collect Collembola for site scale biodiversity assessments. Appl. Soil Ecol. 2010, 45, 293–297. [Google Scholar] [CrossRef]
- Marsden, C.; Martin-Chave, A.; Cortet, J.; Hedde, M.; Capowiez, Y. How agroforestry systems influence soil fauna and their functions—A review. Plant Soil 2020, 453, 29–44. [Google Scholar] [CrossRef]
- Bae, S.; Heidrich, L.; Levick, S.R.; Gossner, M.M.; Seibold, S.; Weisser, W.W.; Magdon, P.; Serebryanyk, A.; Bässler, C.; Schäfer, D.; et al. Dispersal ability, trophic position and body size mediate species turnover processes: Insights from a multi-taxa and multi-scale approach. Divers. Distrib. 2021, 27, 439–453. [Google Scholar] [CrossRef]
- Potapov, A.M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M.; Goncharov, A.A.; Gongalsky, K.B.; Klarner, B.; Korobushkin, D.I.; et al. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biol. Rev. 2022, 97, 1057–1117. [Google Scholar] [CrossRef] [PubMed]

| First Ordination Axis | Second Ordination Axis | ||||
|---|---|---|---|---|---|
| Taxa | Eigenvector | Contribution | Taxa | Eigenvector | Contribution |
| Mesostigmata | 0.6951 | 0.4831 | Onychiuridae | −0.942 | 0.8873 |
| Apterogasterine oribatid mites | 0.5914 | 0.3497 | Isotomidae | 0.2439 | 0.0595 |
| Entomobryidae | 0.2901 | 0.0841 | Mesostigmata | 0.1644 | 0.027 |
| Macropylina oribatid mites | 0.1684 | 0.0283 | Macropylina oribatid mites | −0.0889 | 0.0079 |
| Tomoceridae | 0.1211 | 0.0147 | Tomoceridae | −0.0791 | 0.0063 |
| Formicidae | 0.0974 | 0.0095 | Pterogasterine oribatid mites | −0.0645 | 0.0042 |
| Neanuridae | 0.0935 | 0.0087 | Entomobryidae | −0.0495 | 0.0024 |
| First Ordination Axis | Second Ordination Axis | ||||
|---|---|---|---|---|---|
| Taxa | Eigenvector | Contribution | Taxa | Eigenvector | Contribution |
| Mesostigmata | 0.7754 | 0.6013 | Diadocidiidae | −0.6342 | 0.4023 |
| Apterogasterine oribatid mites | 0.5426 | 0.2944 | Apterogasterine oribatid mites | 0.5097 | 0.2598 |
| Isotomidae | 0.288 | 0.0829 | Mesostigmata | −0.4293 | 0.1843 |
| Macropylina oribatid mites | 0.1205 | 0.0145 | Isotomidae | 0.2426 | 0.0588 |
| Enchytraeidae | 0.051 | 0.0026 | Enchytraeidae | −0.1746 | 0.0305 |
| Formicidae | 0.0293 | 0.0009 | Macropylina oribatid mites | 0.1504 | 0.0226 |
| Empididae | 0.0279 | 0.0008 | Onychiuridae | −0.1207 | 0.0146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Xu, G.; Zhang, Y.; Zhang, S.; Ma, K. Multi-Scale Altitudinal Patterns of Soil and Litter Invertebrate Communities in a Warm Temperate Deciduous Broadleaf Forest. Forests 2023, 14, 2009. https://doi.org/10.3390/f14102009
Ding Z, Xu G, Zhang Y, Zhang S, Ma K. Multi-Scale Altitudinal Patterns of Soil and Litter Invertebrate Communities in a Warm Temperate Deciduous Broadleaf Forest. Forests. 2023; 14(10):2009. https://doi.org/10.3390/f14102009
Chicago/Turabian StyleDing, Zhangqi, Guorui Xu, Yuxin Zhang, Shuang Zhang, and Keming Ma. 2023. "Multi-Scale Altitudinal Patterns of Soil and Litter Invertebrate Communities in a Warm Temperate Deciduous Broadleaf Forest" Forests 14, no. 10: 2009. https://doi.org/10.3390/f14102009
APA StyleDing, Z., Xu, G., Zhang, Y., Zhang, S., & Ma, K. (2023). Multi-Scale Altitudinal Patterns of Soil and Litter Invertebrate Communities in a Warm Temperate Deciduous Broadleaf Forest. Forests, 14(10), 2009. https://doi.org/10.3390/f14102009





