Seed Dormancy Characteristics of Kadsura coccinea (Lem.) A. C. Smith, a Unique Medicinal Plant in Southeast Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Materials
2.2. Seed Germination Experiment
2.3. Observation of Seed Coat Structure
2.4. Seed Coat Permeability
2.5. Endogenous Seed Germination Inhibitor
2.6. Embryo Culture
2.7. Statistical Analysis
3. Results
3.1. Effect of K. coccinea Seed Coat on Germination
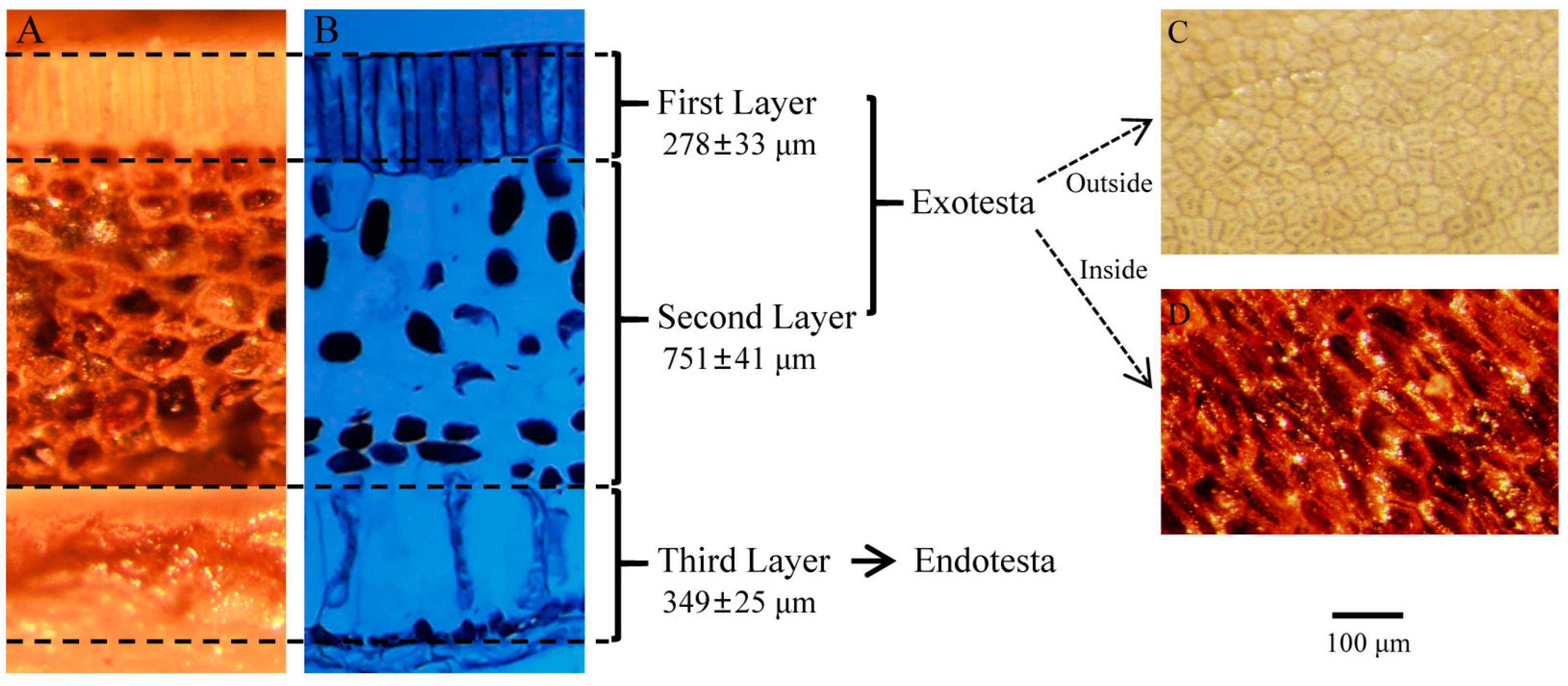
3.2. The Structure of K. coccinea Seed Coat
3.3. Permeability of K. coccinea Seed Coat
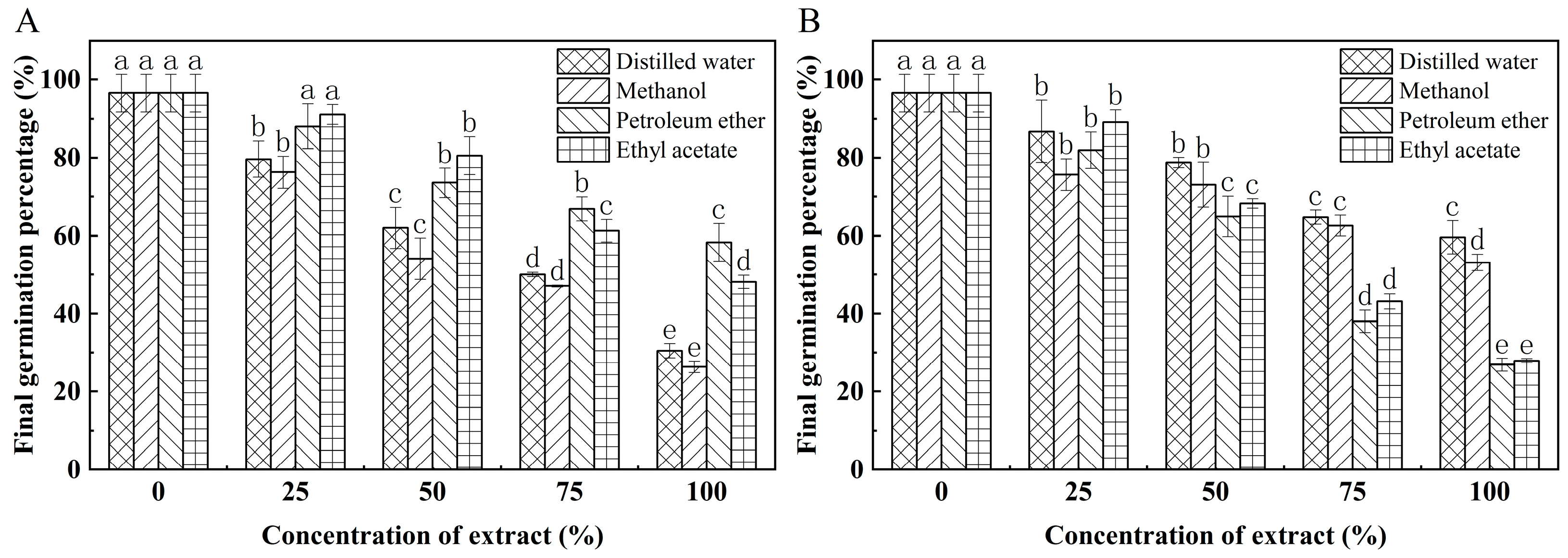
3.4. Effect of K. coccinea Seed Extract on Germination Percentage
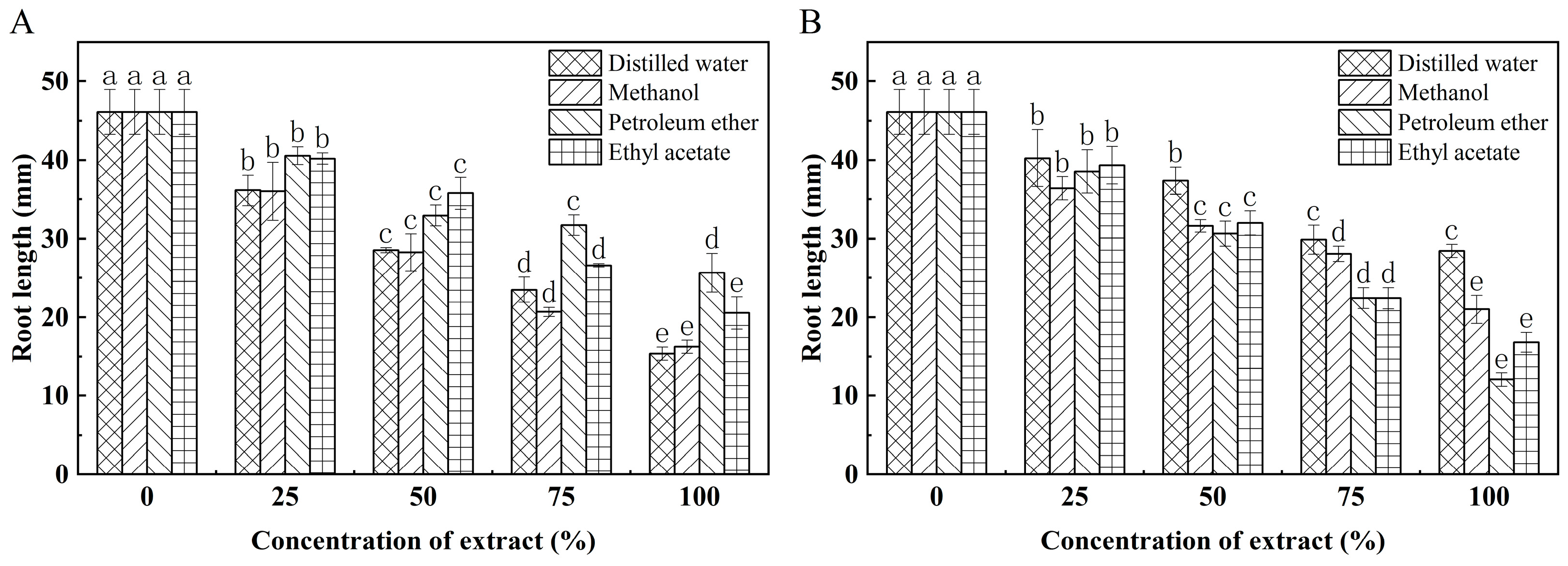
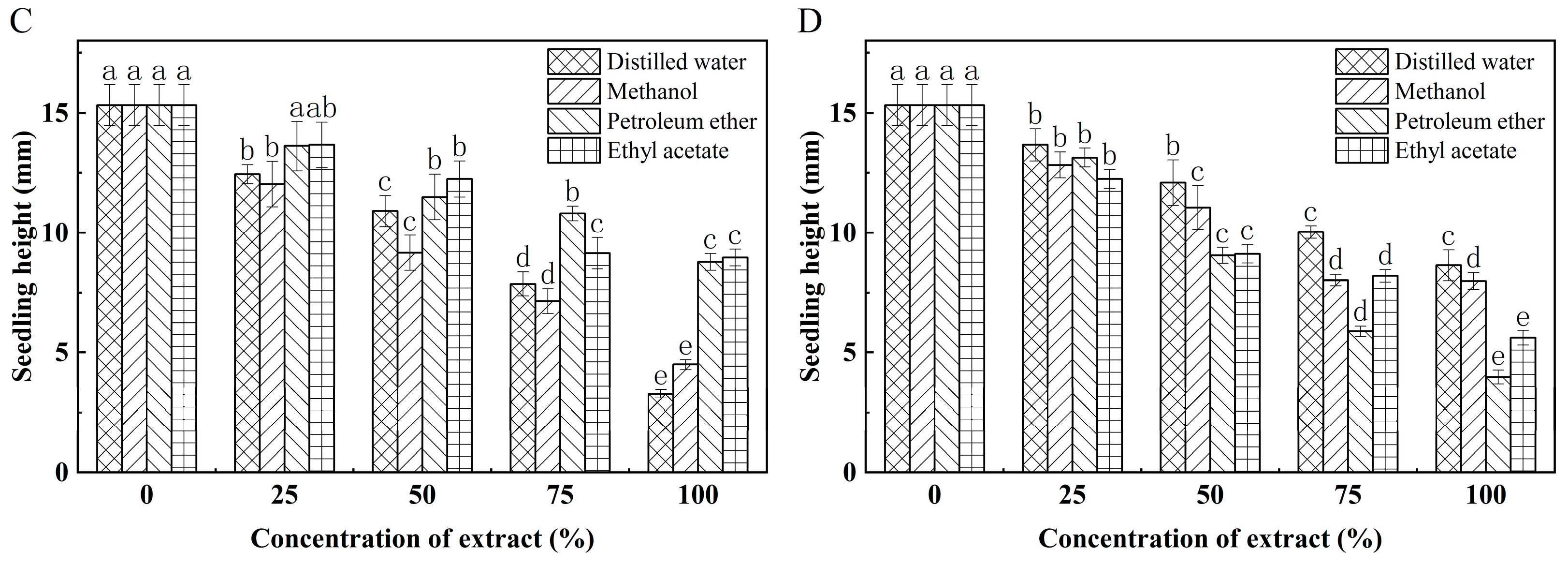
3.5. Effect of K. coccinea Seed Extract on Growth
3.6. Embryo of K. coccinea Culture In Vitro
4. Discussion
4.1. Physical Dormancy of K. coccinea Seeds
4.2. Physiological Dormancy of K. coccinea Seeds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sritalahareuthai, V.; Aursalung, A.; On-nom, N.; Temviriyanukul, P.; Charoenkiatkul, S.; Suttisansanee, U. Nutritional composition of conserved Kadsura spp. plants in northern Thailand. Heliyon 2020, 6, e04451. [Google Scholar] [CrossRef]
- Deng, B.L.; Xie, B.X.; Zhang, C. Germplasm resources of Kadsura and their utilization in China. J. Central South Univ. For. Technol. 2008, 28, 90–94. [Google Scholar] [CrossRef]
- Bi, H.Y.; Lin, Q.; Liu, C.J.; Zhao, J.C. Seed morphology of Kadsura Juss. (Schisandraceae) in relation to its taxonomic significance. Acta Phytotaxon. Sin. 2002, 40, 501–510. [Google Scholar]
- Liang, Z.H.; Li, X.; Li, P.; Deng, Y.; Zhong, Y.; Yang, H. Kadsura coccinea lignan metabolism based on metabolome and transcriptome analysis. J. Oncol. 2022, 2022, 3152155. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Cao, C.Y.; Jin, A.; Wu, W.H. Advances in chemical constituents and pharmacological activities of lignans from Kadsura coccinea. Nat. Prod. Res. Dev. 2022, 34, 491–504. [Google Scholar] [CrossRef]
- Zou, J.W.; Rao, H.X.; Luo, X.Q.; Zhang, M.; Liu, W. Characterization of the processing characteristics and nutritional components of Kadsura coccinea fruit. J. Hunan Agric. Univ. 2019, 45, 679–684. [Google Scholar] [CrossRef]
- Wang, L.J.; Liao, S.Q.; Long, H.R.; Xia, X.H.; Chen, Q.P.; Liang, J.; Wei, S.G. Analysis and evaluation of nutritional components of Kadsura coccinea fruit peel and pulp. Food Ferm. Ind. 2021, 47, 124–131. [Google Scholar] [CrossRef]
- Xu, L.; Su, K.D.; Wei, X.C.; Zhang, J.; Li, H.R. Chemical constituents of Kadsura coccinea. Chem. Nat. Compd. 2018, 54, 242–244. [Google Scholar] [CrossRef]
- Liu, Y.B.; Yang, Y.P.; Tasneem, S.; Hussain, N.; Daniyal, M.; Yuan, H.; Xie, Q.L.; Liu, B.; Sun, J.; Jian, Y.Q.; et al. Lignans from Tujia ethnomedicine Heilaohu: Chemical characterization and evaluation of their cytotoxicity and antioxidant activities. Molecules 2018, 23, 2147. [Google Scholar] [CrossRef]
- Zhao, Q.J.; Song, Y.; Chen, H.S. Cytotoxic dibenzocyclooctadiene lignans from Kadsura coccinea. Arch. Pharm. Res. 2014, 37, 1375–1379. [Google Scholar] [CrossRef]
- Ban, N.K.; Thanh, B.V.; Kiem, P.V.; Minh, C.V.; Cuong, N.X.; Nhiem, N.X.; Huong, H.T.; Anh, H.T.; Park, E.J.; Sohn, D.H.; et al. Dibenzocyclooctadiene lignans and lanostane derivatives from the roots of Kadsura coccinea and their protective effects on primary rat hepatocyte injury induced by t-butyl hydroperoxide. Planta Med. 2009, 75, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Xie, C.; Wang, H.; Jin, D.Q.; Xu, J.; Guo, Y.; Ma, Y. Lignans from the roots of Kadsura coccinea and their inhibitory activities on LPS-induced NO production. Phytochem. Lett. 2014, 9, 158–162. [Google Scholar] [CrossRef]
- Liang, C.Q.; Shi, Y.M.; Luo, R.H.; Li, X.Y.; Gao, Z.H.; Li, X.N.; Yang, L.M.; Shang, S.Z.; Li, Y.; Zheng, Y.T.; et al. Kadcoccitones A and B, two new 6/6/5/5-fused tetracyclic triterpenoids from Kadsura coccinea. Org. Lett. 2012, 14, 6362–6365. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Sun, J.; Feng, Y.; Zhuo, S.Y.; Le, N.; Yao, J.Y.; Long, X. An experimental animal investigation on toxicity and blood lipid modulating effect of Kadsura coccinea fruit. Food Sci. 2011, 32, 203–205. [Google Scholar]
- Sun, J.; Yao, J.Y.; Huang, S.X.; Long, X.; Wang, J.B.; Garcia-Garcia, E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) AC Smith. Food Chem. 2009, 117, 276–281. [Google Scholar] [CrossRef]
- Yan, Z.H.; Gao, Y.Q.; Kong, L.Y.; An, S.S.; Wang, X.; Li, L. Whitening effect of essence product containing Kadsura coccinea extract. Chin. J. Dermatovenereol. 2015, 29, 528–530. [Google Scholar] [CrossRef]
- He, T.D.; Shao, F.X.; Zhang, S.Y.; Chen, J.; Yang, C.H.; Wang, S. Fruit development and seed oil accumulation of Kadsura coccinea. Sci. Silv. Sin. 2023, 59, 75–85. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Dormancy and the control of germination. In Seeds; Springer: Boston, MA, USA, 1994; pp. 199–271. [Google Scholar]
- Sun, H.F. Some dormancy performance of angiosperm zygotes. Bull. Biol. 1990, 12. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Cao, Y.M.; Zhang, J.; Xiang, Y.; Zhao, Z.J.; Fei, Y.J. Study on the dormancy characteristics and dormancy breaking techniques of Paliurus hemsleyanus Rehd. Seed. Seed 2019, 38, 73–76. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.A.; Li, J.; Meng, M.; Feng, X.; Liu, J.M. A preliminary study on dormancy mechanism of Kadsura ananosma seeds. J. West Chin. For. Sci. 2020, 49, 43–47+56. [Google Scholar] [CrossRef]
- Ma, L.Q.H. Study on Dormancy Characteristics and Germination Physiology of Four Varieties sabina chinensis Seeds. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2019. [Google Scholar]
- Ke, B.Y.; Zhou, C.Y.; Li, J.Y.; Pan, J.; Zhou, P. Study on methods of seed dormancy and dormancy-release for rare species Acrocarpus fraxinifolius. Seed 2020, 39, 139–143. [Google Scholar] [CrossRef]
- Bhat, H.A.; Asif, M.; Mir, N.; Aijaz-Un-Nabi; Gatto, A.A.; Ahmad, F.; Hussain, N. Maturity indices and dormancy breaking methods of black locust (Rhobinia pseudoacacia) seeds under temperate Kashmir condition. Ecol. Environ. Cons. 2014, 20, 1769–1775. [Google Scholar]
- Pipinis, E.; Stampoulidis, A.; Milios, E.; Kitikidou, K.; Akritidou, S.; Theodoridou, S.; Radoglou, K. Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds. J. For. Res. 2020, 31, 743–749. [Google Scholar] [CrossRef]
- Chien, C.T.; Chen, S.Y.; Chang, S.H.; Chung, J.D. Dormancy and germination in seeds of the medicinal Asian tree species Phellodendron amurense var. Wilsonii (Rutaceae). Seed Sci. Technol. 2006, 34, 561–571. [Google Scholar] [CrossRef]
- Qi, Y.; Bilan, M.; Chin, K. New method for breaking Korean pine seed dormancy. J. Arbor. 1993, 19, 113–117. [Google Scholar] [CrossRef]
- Cao, D.C.; Xu, H.M.; Zhao, Y.Y.; Deng, X.; Liu, Y.X.; Soppe, W.J.J.; Lin, J.X. Transcriptome and degradome sequencing reveals dormancy mechanisms of Cunninghamia Lanceolata seeds. Plant Phys. 2016, 172, 2347–2362. [Google Scholar] [CrossRef]
- Li, Z.H.; Xu, R.H.; Ren, M.J.; Li, L.H. Advances in phytochrome regulating seed dormancy and germination by sensing light and temperature signals. Plant Phys. J. 2019, 55, 539–546. [Google Scholar] [CrossRef]
- Qin, A.L.; Guo, Q.S.; Ma, F.Q.; Jian, Z.J.; Zheng, X.K. Effects of temperature, light and water conditions on seed germination of Thuja sutchuenensis franch. Seed 2020, 39, 15–20. [Google Scholar] [CrossRef]
- Li, T.H.; Min, X.H. Dormancy characteristics and germination requirements of Phoebe bournei seed. Sci. Hortic. 2020, 260, 108903. [Google Scholar] [CrossRef]
- Lu, J.J.; Tan, D.Y.; Baskin, J.M.; Baskin, C.C. Trade-offs between seed dispersal and dormancy in an amphi-basicarpic cold desert annual. Ann. Bot. 2013, 112, 1815–1827. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.L.; Yao, L.J.; Zhang, Y.; Baskin, J.M.; Baskin, C.C.; Xiong, Z.M.; Tao, J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta 2019, 249, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhou, D.B.; Wang, W.Z.; Nie, S.Q. Preliminary study on the causes of seed dormancy of Schisandra chinensis (Turcz.) Baill. Terr. Nat. Res. Study 1993, 2, 59–61. [Google Scholar] [CrossRef]
- Yang, J.H. Dormancy and Germination Characteristics of Cercis canadensis ‘Forest Pansy’ Seeds. Master’s Thesis, Yangzhou University, Yangzhou, China, 2021. [Google Scholar]
- Lu, Q. Study on the Methods and Preliminary Mechanism of Seed Dormancy Release of Melicope pteleifolia. Ph.D. Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2019. [Google Scholar]
- Shang, X.L.; Xu, X.Z.; Fang, S.Z. Seed dormancy mechanism of Cyclocarya paliurus. Sci. Silv. Sin. 2011, 47, 68–74. [Google Scholar]
- Liu, X. Studies on Seed Dormancy Mechanism of Celtis julianae Schneid. Seeds. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2019. [Google Scholar]
- Zhang, W.; Qu, L.W.; Zhao, J.; Xue, L.; Dai, H.P.; Xing, G.M.; Lei, J.J. Practical methods for breaking seed dormancy in a wild or-namental tulip species Tulipa thianschanica regel. Agronomy 2020, 10, 1765. [Google Scholar] [CrossRef]
- Liu, C.G.; Chen, L.; Li, Y.; Dai, S.J.; Chen, W.J.; Pan, L.; Xu, Y.J. Study on dormancy and germination technology of Taxus chinensis var. mairei seeds. J. Southwest For. Coll. 2015, 35, 25–29. [Google Scholar]
- Zheng, Q.L.; Hao, L.Z.; Yang, Z.R.; Zhang, F.L. Research progress of Pugionium gaertn plant seeds. Seed 2017, 36, 46–50. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Zhong, W.; Shao, F.; Guo, H.; Yan, C.; Ge, X.; Wang, J.; Wang, S. Seed Dormancy Characteristics of Kadsura coccinea (Lem.) A. C. Smith, a Unique Medicinal Plant in Southeast Asia. Forests 2023, 14, 1928. https://doi.org/10.3390/f14101928
He T, Zhong W, Shao F, Guo H, Yan C, Ge X, Wang J, Wang S. Seed Dormancy Characteristics of Kadsura coccinea (Lem.) A. C. Smith, a Unique Medicinal Plant in Southeast Asia. Forests. 2023; 14(10):1928. https://doi.org/10.3390/f14101928
Chicago/Turabian StyleHe, Tieding, Wenbin Zhong, Fengxia Shao, Hongyan Guo, Chao Yan, Xiaoning Ge, Jia Wang, and Sen Wang. 2023. "Seed Dormancy Characteristics of Kadsura coccinea (Lem.) A. C. Smith, a Unique Medicinal Plant in Southeast Asia" Forests 14, no. 10: 1928. https://doi.org/10.3390/f14101928
APA StyleHe, T., Zhong, W., Shao, F., Guo, H., Yan, C., Ge, X., Wang, J., & Wang, S. (2023). Seed Dormancy Characteristics of Kadsura coccinea (Lem.) A. C. Smith, a Unique Medicinal Plant in Southeast Asia. Forests, 14(10), 1928. https://doi.org/10.3390/f14101928





