Impact of Fire Recurrence and Induced Water Stress on Seed Germination and Root Mitotic Cell Cycle of Pinus pinaster Aiton
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Dendrometric Evaluation
2.2. Plant Material
2.3. Water Stress Induction by PEG and Seed Germination
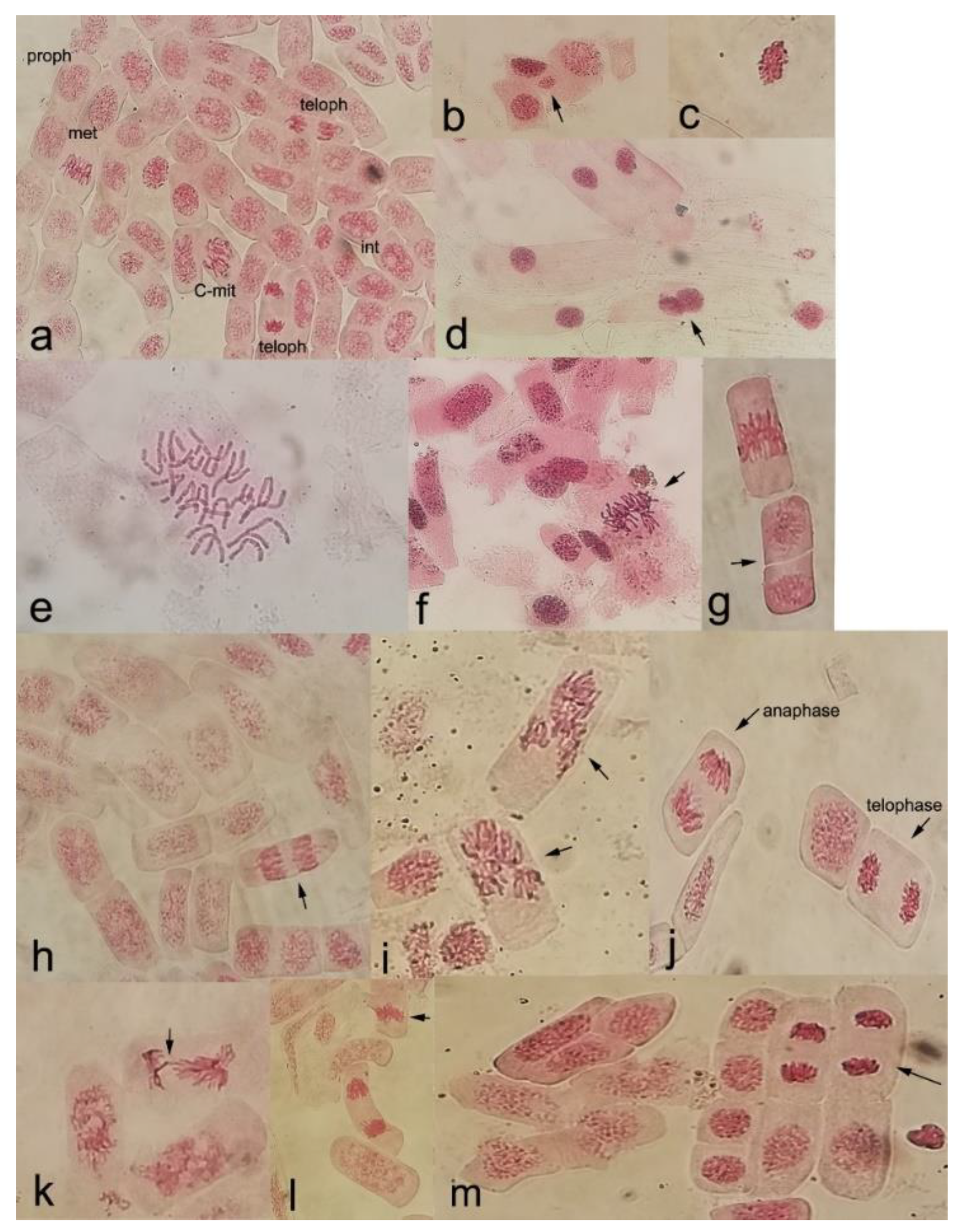
2.4. Cytogenetic Analysis of the Root Meristematic Cells
2.5. Statistical Analyses
3. Results
3.1. Germination Index and Mean Germination Time during Water Stress Induction and Recovery
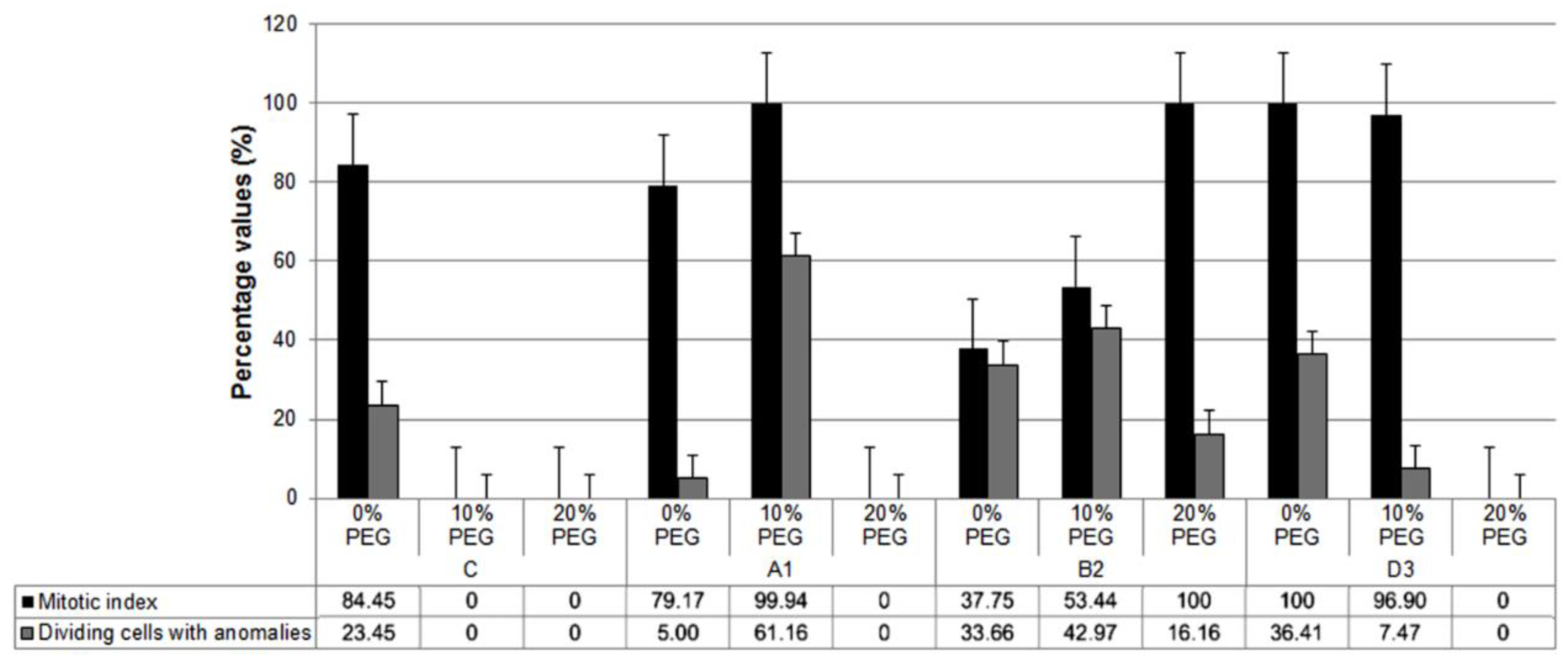
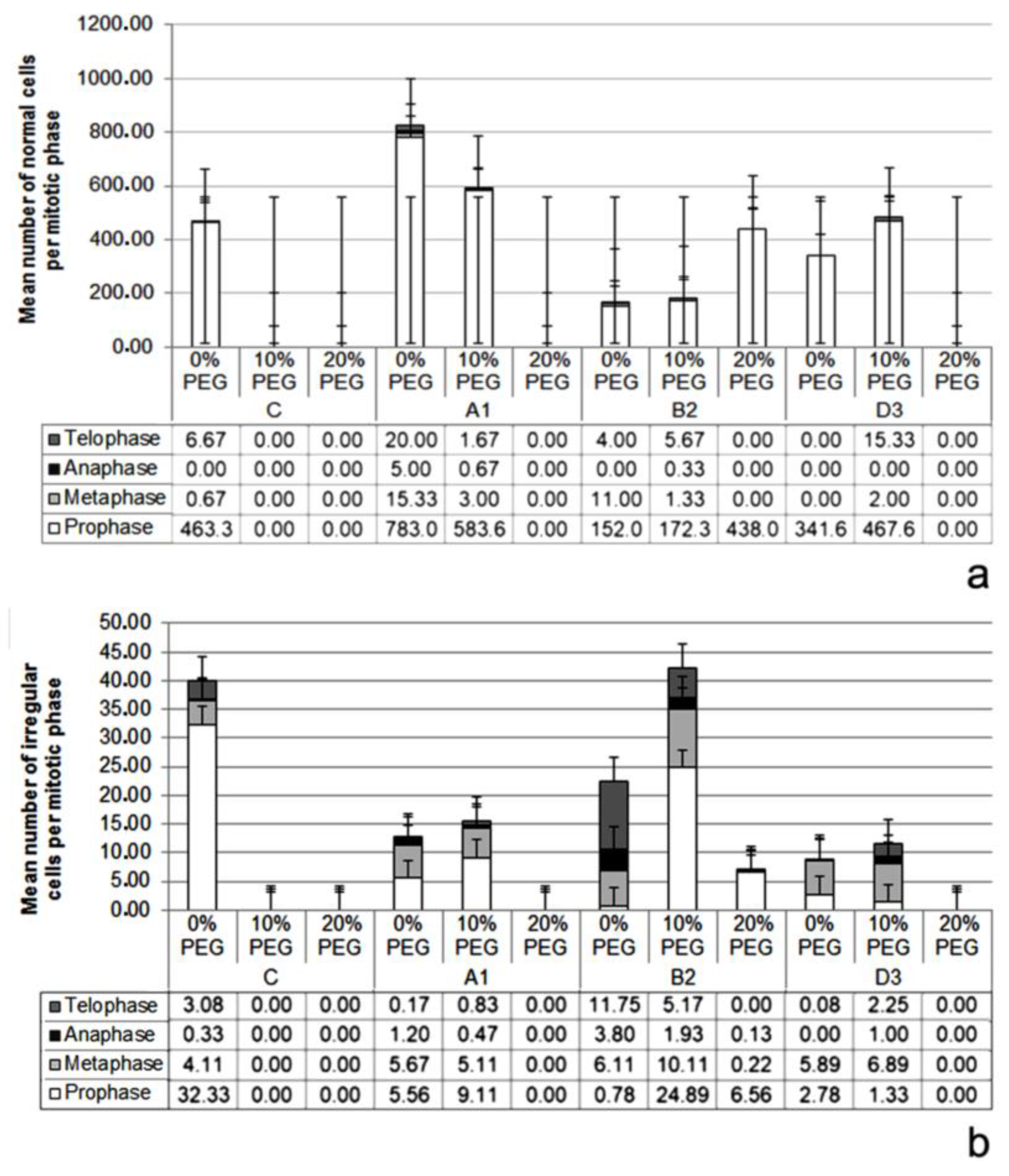
3.2. Root Mitotic Cell Cycle Analysis
4. Discussion
4.1. Impact of Fire Recurrence and/or Induced Water Stress on Seed Germination
4.2. Impact of Fire Recurrence and/or Induced Water Stress on Root Cell Division
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar]
- Ruano, I.; Manso, R.; Fortin, M.; Bravo, F. Extreme climate conditions limit seed availability to successfully attain natural regeneration of Pinus pinaster in sandy areas of central Spain. Can. J. For. Res. 2015, 45, 1795–1802. [Google Scholar] [CrossRef]
- Ribeiro, S.; Cerveira, A.; Soares, P.; Fonseca, T. Natural regeneration of maritime pine: A review of the influencing factors and proposals for management. Forests 2022, 13, 386. [Google Scholar] [CrossRef]
- Cunha, P.E.P.R. Fatores condicionantes da regeneração natural de Pinus pinaster Ait. após incêndio no Parque Nacional da Peneda Gerês. Relatório final de estágio Universidade de Trás-os-Montes e Alto Douro, Vila Real. 1993. [Google Scholar]
- Ruano, I.; Pando, V.; Bravo, F. How do light and water influence Pinus pinaster Ait. germination and early seedling development? For. Ecol. Manag. 2009, 258, 2647–2653. [Google Scholar] [CrossRef]
- Guignabert, A. Etude des Processus de Régénération Naturelle du pin Maritime en Contexte de Dune Forestière Gérée: Influence de la Sylviculture, du Climat et des Interactions Biotiques. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2018. [Google Scholar]
- Nuñez Paniagua, M.R.; Sierra de Grado, R.; Alía, R.; Bravo, F. Efecto del estrés hídrico y la oscilación de las temperaturas sobre la germinación de semillas de diversas procedencias de Pinus pinaster Ait. In Proceedings of the 6º Congreso Forestal Español: Servicios y Desarrollo Rural, Vitoria-Gasteiz, Spain, 10–14 June 2013. [Google Scholar]
- Lucas-Borja, M.E. Climate change and forest natural regeneration in mediterranean mountain areas. For. Res. 2014, 3, 1000e108. [Google Scholar] [CrossRef]
- Seidel, H.; Schunk, C.; Matiu, M.; Menzel, A. Diverging drought resistance of Scots pine provenances revealed by infrared thermography. Front. Plant Sci. 2016, 7, 1247. [Google Scholar] [CrossRef] [PubMed]
- Seidel, H.; Menzel, A. Above-ground dimensions and acclimation explain variation in drought mortality of Scots pine seedlings from various provenances. Front. Plant Sci. 2016, 7, 1014. [Google Scholar] [CrossRef] [PubMed]
- Topacoglu, O.; Sevik, H.; Akkuzu, E. Effects of water stress on germination of Pinus nigra Arnold seeds. Pak. J. Bot. 2016, 48, 447–453. [Google Scholar]
- Lorenz, W.; Sun, F.; Liang, C.; Kolychev, D.; Wang, H.; Zhao, X.; Cordonnier-Pratt, M.-M.; Pratt, L.; Dean, J. Water stress-responsive genes in loblolly pine (Pinus taeda) roots identified by analyses of expressed sequence tag libraries. Tree physiol. 2005, 26, 1–16. [Google Scholar] [CrossRef]
- Juez, L.; González-Martínez, S.C.; Nanos, N.; de-Lucas, A.I.; Ordóñez, C.; del Peso, C.; Bravo, F. Can seed production and restricted dispersal limit recruitment in Pinus pinaster Aiton from the Spanish Northern Plateau? For. Ecol. Manag. 2014, 313, 329–339. [Google Scholar] [CrossRef]
- Pancou, J.-P.; Sin, F. Synthèse Bibliographique: Méthodologies et Problématiques de Régénérations Appliquées Aux Forêts Littorales Françaises; Interreg Sudoe ForManRisk: Bordeaux, France, 2020; p. 32. [Google Scholar]
- Ouallet, P. Quels peuvent-être les facteurs écologiques responsables des échecs de régénération naturelle du pin maritime sur les dunes littorales des forêts domaniales de Biscarrosse et de Sainte-Eulalie? Bordeaux, France. 2012. [Google Scholar]
- Carvalho, A.; Leal, F.; Matos, M.; Lima-Brito, J. Effects of heat stress in the leaf mitotic cell cycle and chromosomes of four wine-producing grapevine varieties. Protoplasma 2018, 255, 1725–1740. [Google Scholar] [CrossRef]
- Carvalho, A.; Leal, F.; Matos, M.; Lima-Brito, J. Heat stress tolerance assayed in four wine-producing grapevine varieties using a cytogenetic approach. Ciência Téc. Vitiv. 2019, 34, 61–70. [Google Scholar] [CrossRef]
- Carvalho, A.; Gaivão, I.; Lima-Brito, J. Seed osmopriming with PEG solutions in seeds of three infraspecific taxa of Pinus nigra: Impacts on germination, mitosis and nuclear DNA. For. Ecol. Manag. 2020, 456, 117739. [Google Scholar] [CrossRef]
- Reis, S.; Pavia, I.; Carvalho, A.; Moutinho-Pereira, J.; Correia, C.; Lima-Brito, J. Seed priming with iron and zinc in bread wheat: Effects in germination, mitosis and grain yield. Protoplasma 2018, 255, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Carvalho, A.; Pavia, I.; Bacelar, E.; Lima-Brito, J. Development of grapevine plants under hydroponic copper-enriched solutions induced morpho-histological, biochemical and cytogenetic changes. Plant Physiol. Biochem. 2021, 166, 887–901. [Google Scholar] [CrossRef]
- Castro, C.; Carvalho, A.; Pavia, I.; Bacelar, E.; Lima-Brito, J. Grapevine varieties with differential tolerance to Zinc analysed by morpho-histological and cytogenetic approaches. Sci. Hortic. 2021, 288, 110386. [Google Scholar] [CrossRef]
- Santos, C.L.V.; Pourrut, B.; Ferreira de Oliveira, J.M. The use of comet assay in plant toxicology: Recent advances. Front. Genet. 2015, 6, 216. [Google Scholar] [CrossRef]
- Smertenko, A.; Dráber, P.; Viklický, V.; Opatrný, Z. Heat stress affects the organization of microtubules and cell division in Nicotiana tabacum cells. Plant Cell Environ. 1997, 20, 1534–1542. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Z.; Qin, R.; Zhang, H.; Zou, J.; Jiang, W.; Liu, D. Accumulation and cellular toxicity of aluminum in seedling of Pinus massoniana. BMC Plant Biol. 2014, 14, 264. [Google Scholar] [CrossRef]
- Khalil, S.K.; Mexal, J.G.; Ortiz, M. Osmotic priming hastens germination and improves seedling size of Pinus brutia var. eldarica. Tree Plant. Notes 1997, 48, 24–27. [Google Scholar]
- Ochatt, S.J.; Marconi, P.L.; Radice, S.; Arnozis, P.A.; Caso, O.H. In vitro recurrent selection of potato: Production and characterization of salt tolerant cell lines and plants. Plant Cell Tissue Organ Cult. 1999, 55, 1–8. [Google Scholar] [CrossRef]
- Guóth, A.; Benyó, D.; Csiszár, J.; Gallé, Á.; Horváth, F.; Cseuz, L.; Erdei, L.; Tari, I. Relationship between osmotic stress-induced abscisic acid accumulation, biomass production and plant growth in drought-tolerant and sensitive wheat cultivars. Acta Physiol. Plant. 2010, 32, 719–727. [Google Scholar] [CrossRef]
- Macovei, A.; Balestrazzi, A.; Confalonieri, M.; Carbonera, D. The tyrosyl-DNA phosphodiesterase gene family in Medicago truncatula Gaertn.: Bioinformatic investigation and expression profiles in response to copper- and PEG-mediated stress. Planta 2010, 232, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Balestrazzi, A.; Confalonieri, M.; Macovei, A.; Carbonera, D. Seed imbibition in Medicago truncatula Gaertn.: Expression profiles of DNA repair genes in relation to PEG-mediated stress. J. Plant Physiol. 2011, 168, 706–713. [Google Scholar] [CrossRef]
- Rai, M.K.; Kalia, R.K.; Singh, R.; Gangola, M.P.; Dhawan, A.K. Developing stress tolerant plants through in vitro selection—An overview of the recent progress. Environ. Exp. Bot. 2011, 71, 89–98. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Shen, H. Somatic embryogenesis and plant regeneration from immature zygotic embryo cultures of mountain ash (Sorbus pohuashanensis). Plant Cell Tissue Organ Cult. 2012, 109, 547–556. [Google Scholar] [CrossRef]
- Elmaghrabi, A.M.; Rogers, H.J.; Francis, D.; Ochatt, S.J. PEG induces high expression of the cell cycle checkpoint gene WEE1 in embryogenic callus of Medicago truncatula: Potential link between cell cycle checkpoint regulation and osmotic stress. Front. Plant Sci. 2017, 8, 1479. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Ahrazem, O.; Candel-Pérez, D.; Moya, D.; Fonseca, T.; Hernández Tecles, E.; De las Heras, J.; Gómez-Gómez, L. Evaluation of fire recurrence effect on genetic diversity in maritime pine (Pinus pinaster Ait.) stands using Inter-Simple Sequence Repeat profiles. Sci. Total Environ. 2016, 572, 1322–1328. [Google Scholar] [CrossRef]
- ForManRisk, I.S. ForManRisk—Forest Management and Natural Risks. Available online: https://formanrisk.eu/pt/accueil-portugues/ (accessed on 8 June 2022).
- Maia, P.; Pausas, J.G.; Vasques, A.; Keizer, J.J. Fire severity as a key factor in post-fire regeneration of Pinus pinaster (Ait.) in Central Portugal. Ann. For. Sci. 2012, 69, 489–498. [Google Scholar] [CrossRef]
- Maia, P.; Pausas, J.G.; Arcenegui, V.; Guerrero, C.; Pérez-Bejarano, A.; Mataix-Solera, J.; Varela, M.E.T.; Fernandes, I.; Pedrosa, E.T.; Keizer, J.J. Wildfire effects on the soil seed bank of a maritime pine stand — the importance of fire severity. Geoderma 2012, 191, 80–88. [Google Scholar] [CrossRef]
- Maia, P.A.A. Post-Fire Vegetation Regeneration in Portugal—Implications for Management. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 2014. [Google Scholar]
- Fernandes, P.M.; Rigolot, E. The fire ecology and management of maritime pine (Pinus pinaster Ait.). For. Ecol. Manag. 2007, 241, 1–13. [Google Scholar] [CrossRef]
- Tapias, R.; Climent, J.; Pardos, J.A.; Gil, L. Life histories of Mediterranean pines. Plant Ecol. 2004, 171, 53–68. [Google Scholar] [CrossRef]
- Salvatore, R.; Moya, D.; Pulido, L.; Lovreglio, R.; López-Serrano, F.R.; De Las Heras, J.; Leone, V. Morphological and anatomical differences in Aleppo pine seeds from serotinous and non-serotinous cones. New For. 2010, 39, 329–341. [Google Scholar] [CrossRef]
- Calvo, L.; Hernández, V.; Valbuena, L.; Taboada, A. Provenance and seed mass determine seed tolerance to high temperatures associated to forest fires in Pinus pinaster. Ann. For. Sci. 2016, 73, 381–391. [Google Scholar] [CrossRef]
- Reineke, L.H. Perfecting a stand-density index for even-aged forests. Agric. Res. J. 1933, 46, 627–638. [Google Scholar]
- Luis, J.F.S.; Fonseca, T. The allometric model in the stand density management of Pinus pinaster Ait. in Portugal. Ann. For. Sci. 2004, 61, 807–814. [Google Scholar] [CrossRef]
- Marques, C.P. Evaluating site quality of even-aged maritime pine stands in northern Portugal using direct and indirect methods. For. Ecol. Manag. 1991, 41, 193–204. [Google Scholar] [CrossRef]
- Molina, C.; Tapias, R.; Gil, L. Influencia de la posición en la copa y del año de maduración en la germinación de las semillas de Pinus pinaster Ait. de la Sierra del Teleno (León, noroeste de España). Investig. Agrar. Sist. Recur. For. 1997, 6, 63–65. [Google Scholar] [CrossRef]
- Soares, P.; Calado, N.; Carneiro, S. Manual de Boas Práticas Para o Pinheiro-Bravo; Centro Pinus: Porto, Portugal, 2020; p. 32. [Google Scholar]
- Tapias, R.; Gil, L.; Fuentes-Utrilla, P.; Pardos, J.A. Canopy seed banks in Mediterranean pines of south-eastern Spain: A comparison between Pinus halepensis Mill., P. pinaster Ait., P. nigra Arn. and P. pinea L. J. Ecol. 2001, 89, 629–638. [Google Scholar] [CrossRef]
- Alvarez, R.; Valbuena, L.; Calvo, L. Influence of tree age on seed germination response to environmental factors and inhibitory substances in Pinus pinaster. IAWF 2005, 14, 277–284. [Google Scholar] [CrossRef]
- Correia, I.; Santos, L.; Faria, C.; Nóbrega, C.; Almeida, M.H.; David, T. Cone to seedling-variation between Pinus pinaster provenances from contrasting altitudes. For. Sci. 2014, 60, 724–732. [Google Scholar] [CrossRef]
- Freitas, T.M.D. Avaliação da Biomassa em Regeneração Natural de Pinheiro Bravo Pós-fogo. Relatório Final de Estágio. Master’s Thesis, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal, 2013. [Google Scholar]
- Santos, L.; Capelo, J.; Tavares, M. Germination patterns of soil seed banks in relation to fire in Portuguese littoral pine forest vegetation. Fire Ecol. 2010, 6, 1–15. [Google Scholar] [CrossRef]
- Boydak, M.; Dirik, H.; Tilki, F.; Çalıkoğlu, M. Effects of water stress on germination in six provenances of Pinus brutia seeds from different bioclimatic zones in Turkey. Turk. J. Agric. For. 2003, 27, 91–97. [Google Scholar]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics. A Biometrical Approach, 2nd ed.; McGraw-Hill International Book Company: New York, NY, USA, 1981; p. 633. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry—The Principles and Practice of Statistics in Biological Research, 2nd ed.; Freeman and Company: New York, NY, USA, 1981; p. 859. [Google Scholar]
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Lima-Brito, J.; Guedes-Pinto, H.; Harrison, G.E.; Heslop-Harrison, J.S. Chromosome identification and nuclear architecture in triticale × tritordeum F1 hybrids. J. Exp. Bot. 1996, 47, 583–588. [Google Scholar] [CrossRef]
- Marôco, J. Análise Estatística Com o SPSS Statistics, 6th ed.; ReportNumber: Pêro Pinheiro, Portugal, 2014; p. 990. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson: London, UK, 2009; p. 816. [Google Scholar]
- Falusi, M.; Calamassi, R.; Tocci, A. Sensitivity of seed germination and seedling root growth to moisture stress in four provenances of Pinus halepensis Mill. Silvae Genet. 1983, 32, 4–9. [Google Scholar]
- Falleri, E. Effect of water stress on germination in six provenances of Pinus pinaster Ait. Seed Sci. Technol. 1994, 22, 591–599. [Google Scholar]
- Djavanshir, K.; Reid, C.P.P. Effect of moisture stress on germination and radicle development of Pinus eldarica Medw. and Pinus ponderosa Laws. Can. J. For. Res. 1975, 5, 80–83. [Google Scholar] [CrossRef]
- Reyes, O.; Casal, M. Germination behaviour of 3 species of the genus Pinus in relation to high temperatures suffered during forest fires. Ann. For. Sci. 1995, 52, 385–392. [Google Scholar] [CrossRef][Green Version]
- Ne’eman, G.; Goubitz, S.; Nathan, R. Reproductive traits of Pinus halepensis in the light of fire—A critical review. Plant Ecol. 2004, 171, 69–79. [Google Scholar] [CrossRef]
- Moya, D.; Espelta, J.M.; Verkaik, I.; López-Serrano, F.R.; De Las Heras, J. Tree density and site quality influence on Pinus halepensis Mill. reproductive characteristics after large fires. Ann. For. Sci. 2007, 64, 649–656. [Google Scholar] [CrossRef]
- Moya, D.; Saracino, A.; Salvatore, R.; Lovreglio, R.; De Las Heras, J.; Leone, V. Anatomic basis and insulation of serotinous cones in Pinus halepensis Mill. Trees 2008, 22, 511–519. [Google Scholar] [CrossRef]
- Pekol, S.; Baloğlu, M.C.; Altunoğlu, Y.Ç. Evaluation of genotoxic and cytologic effects of environmental stressin wheat species with different ploidy levels. Turk. J. Biol. 2016, 40, 580–588. [Google Scholar] [CrossRef]
- Carvalho, A.; Pavia, I.; Fernandes, C.; Pires, J.; Correia, C.; Bacelar, E.; Moutinho-Pereira, J.; Gaspar, M.J.; Bento, J.; Silva, M.E.; et al. Differential physiological and genetic responses of five European Scots pine provenances to induced water stress. J. Plant Physiol. 2017, 215, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Grossnickle, S. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
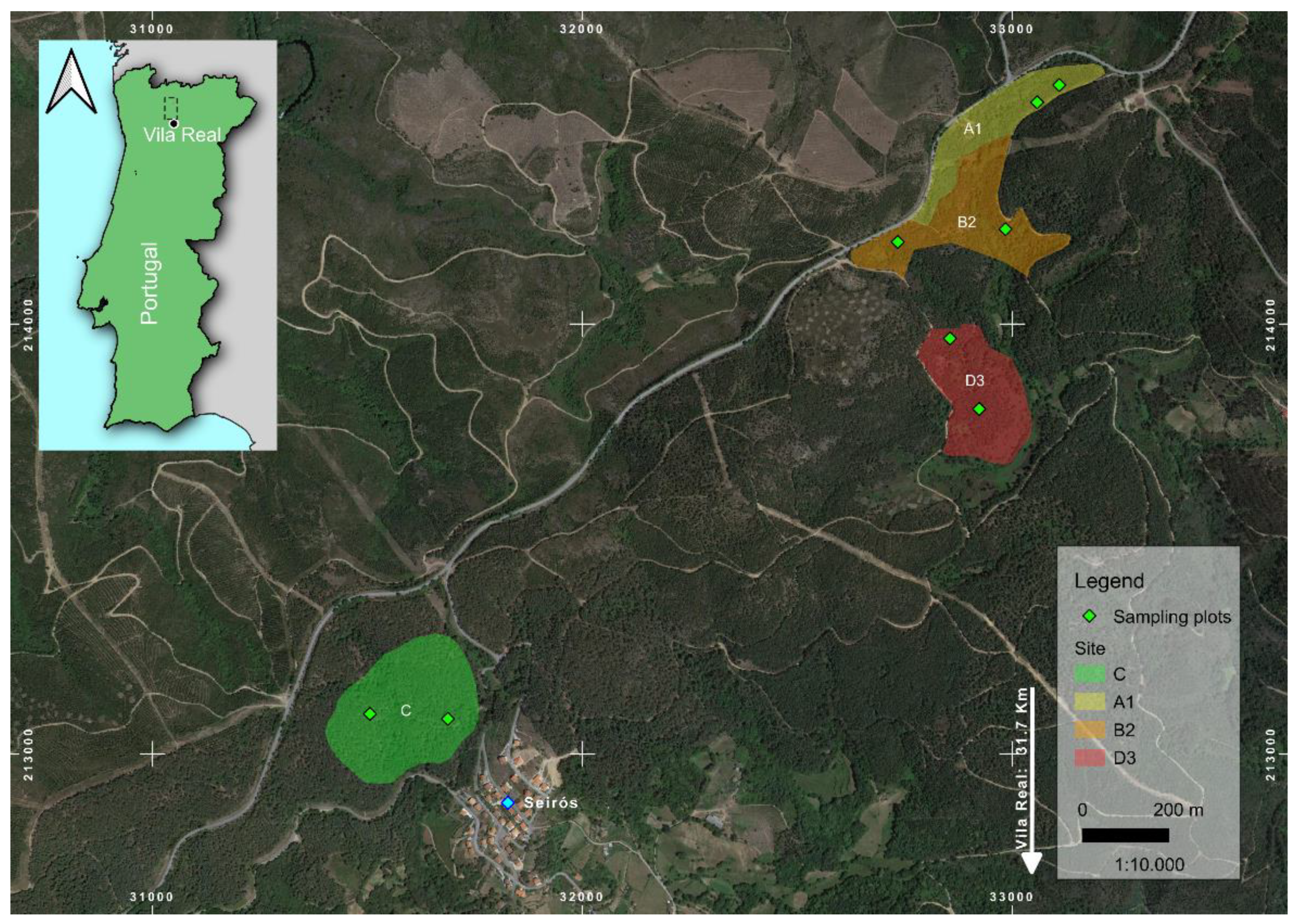
| Site | Area of the Stand (ha) | Fire Years |
|---|---|---|
| C | 8.6 | – |
| A1 | 4.0 | 1987 |
| B2 | 6.0 | 1987, 1998 |
| D3 | 5.7 | 1989, 1990, 1998 |
| Stand | Age (Years) | N (ha−1) | dg (cm) | SDI (%) | SI35 (m) | d ± S.D. (cm) | h ± S.D. (m) |
|---|---|---|---|---|---|---|---|
| C | 58 | 550 | 24.2 | 28 | 16.8 | 24.0 ± 3.7 | 16.6 ± 2.0 |
| A1 | 36 | 1000 | 22.4 | 44 | 17.3 | 21.3 ± 5.6 | 16.1 ± 2.7 |
| B2 | 22 | 1075 | 16.1 | 25 | 17.9 | 15.1 ± 4.8 | 10.4 ± 2.6 |
| D3 | 24 | 1800 | 13.4 | 30 | 16.6 | 13.0 ± 4.9 | 10.5 ± 3.5 |
| Germination Index (Stress) | Germination Index (Recovery) | MGT (Days) (Stress) | MGT (Days) (Recovery) | ||
|---|---|---|---|---|---|
| Stand (S) | C | 8.61 ± 5.77 a | 11.26 ± 8.15 a | 25.50 ± 3.50 a | 28.88 ± 6.88 a |
| A1 | 9.22 ± 4.12 a | 13.21 ± 6.17 a | 21.67 ± 5.90 a | 12.00 ± 8.54 a | |
| B2 | 8.61 ± 5.77 a | 13.82 ± 4.54 a | 23.84 ± 1.17 a | 16.75 ± 8.01 a | |
| D3 | 20.54 ± 4.70 a | 7.76 ± 5.06 a | 25.53 ± 0.99 a | 11.75 ± 10.75 a | |
| Treatment (T) | 0% PEG | 15. 22 ± 4.95 a | 12.09 ± 6.56 a | 20.53 ± 2.68 a | 21.08 ± 8.90 a |
| 10% PEG | 9.78 ± 4.97 a | 12.21 ± 4.88 a | 25.22 ± 0.40 ab | 15.25 ± 7.65 a | |
| 20% PEG | 10.23 ± 3.98 a | 10.23 ± 3.98 a | 28.25 ± 0.75 b | 15.00 ± 7.58 a | |
| ANOVA p-value | S | 0.336 | 0.863 | 0.030 | 0.706 |
| T | 0.654 | 0.948 | 0.008 | 0.780 | |
| S × T | 0.445 | 0.127 | 0.061 | 0.701 |
| MI (%) (Mean ± S.E.) | %DCA (Mean ± S.E.) | ||
|---|---|---|---|
| Stand (S) | C | 28.15 ± 14.14 a | 7.82 ± 4.89 a |
| A1 | 59.70 ± 15.28 b | 22.05 ± 13.13 a | |
| B2 | 63.06 ± 9.72 b,c | 30.03 ± 5.88 a | |
| D3 | 65.63 ± 16.42 c | 14.63 ± 10.73 a | |
| Treatment (T) | 0% PEG | 74.84 ± 7.34 c | 24.63 ± 8.09 b |
| 10% PEG | 62.57 ± 12.28 b | 27.90 ± 9.99 b | |
| 20% PEG | 25.00 ± 13.06 a | 4.04 ± 3.62 a | |
| ANOVA p-value | S | <0.0001 | 0.2222 |
| T | <0.0001 | 0.0444 | |
| S × T | <0.0001 | 0.0826 |
| Variables Influenced by the Stand Factor (Unburned or Burned One, Two or Three Times) | Variables Influenced by the PEG Treatment Factor (0% PEG, 10% PEG, and 20% PEG) |
|---|---|
| – | MGT (water stress period) |
| MI (%) | MI (%) |
| Mean number of normal metaphase and anaphase cells | Mean number of normal metaphase cells |
| – | DCA (%) |
| Mean number of irregular metaphase, anaphase, and telophase cells | Mean number of irregular metaphase, anaphase, and telophase cells |
| Stickiness of chromatin (metaphase, anaphase, telophase) | Stickiness of chromatin (metaphase, telophase) |
| Laggard chromosomes (anaphase, telophase) | Laggard chromosomes (anaphase, telophase) |
| Binucleate prophase cell | – |
| – | C-mitosis (metaphase) |
| Chromatin bridges in anaphase | Chromatin bridges in anaphase |
| Disturbed chromosomal orientation in anaphase and telophase | Disturbed chromosomal orientation in anaphase and telophase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, S.; Gaspar, M.J.; Lima-Brito, J.; Fonseca, T.; Soares, P.; Cerveira, A.; Fernandes, P.M.; Louzada, J.; Carvalho, A. Impact of Fire Recurrence and Induced Water Stress on Seed Germination and Root Mitotic Cell Cycle of Pinus pinaster Aiton. Forests 2023, 14, 78. https://doi.org/10.3390/f14010078
Ribeiro S, Gaspar MJ, Lima-Brito J, Fonseca T, Soares P, Cerveira A, Fernandes PM, Louzada J, Carvalho A. Impact of Fire Recurrence and Induced Water Stress on Seed Germination and Root Mitotic Cell Cycle of Pinus pinaster Aiton. Forests. 2023; 14(1):78. https://doi.org/10.3390/f14010078
Chicago/Turabian StyleRibeiro, Stéphanie, Maria João Gaspar, José Lima-Brito, Teresa Fonseca, Paula Soares, Adelaide Cerveira, Paulo M. Fernandes, José Louzada, and Ana Carvalho. 2023. "Impact of Fire Recurrence and Induced Water Stress on Seed Germination and Root Mitotic Cell Cycle of Pinus pinaster Aiton" Forests 14, no. 1: 78. https://doi.org/10.3390/f14010078
APA StyleRibeiro, S., Gaspar, M. J., Lima-Brito, J., Fonseca, T., Soares, P., Cerveira, A., Fernandes, P. M., Louzada, J., & Carvalho, A. (2023). Impact of Fire Recurrence and Induced Water Stress on Seed Germination and Root Mitotic Cell Cycle of Pinus pinaster Aiton. Forests, 14(1), 78. https://doi.org/10.3390/f14010078







