Abstract
Soil organic carbon (SOC) decomposition, a key process controlling the carbon (C) loss from terrestrial soils to the atmosphere, varies with soil aggregate size and is influenced by increasing nitrogen (N) and phosphorus (P) inputs from anthropogenic activities. However, how increasing N and P affects SOC decomposition and its temperature sensitivity (Q10) in soil aggregates remains unclear. Thus, we collected soils from a subtropical Cunninghamia lanceolata forest receiving N and P addition for 8 years to explore the interactive effects of N and P fertilization on SOC decomposition and its Q10 in mega-aggregates (>2 mm, MeA), macroaggregates (0.25–2.0 mm, MaA), and microaggregates (<0.25 mm, MiA). Results showed that aggregate size has a huge influence on SOC decomposition and its Q10. Specifically, SOC decomposition in MiA is 49.2% and 26.0% higher than MeA and MaA, respectively. Moreover, the averaged Q10 values were 2.29, 2.26 and 1.83 in MeA, MaA and MiA. SOC decomposition significantly increased by 39.4% in MaA and 23.7% in MiA with N fertilization, but P fertilization had less impact. However, P fertilization increased Q10 by 46.7% in MeA and 46.6% in MaA. Furthermore, we found P fertilization changed the influences of N fertilization on SOC decomposition in MaA and MiA but had no effect on responses of Q10 to N fertilization. Overall, our findings suggested that there were differences in SOC decomposition and Q10 among aggregates, and fertilization treatment had an impact on them. Our results highlighted the significance of considering differences in SOC decomposition and its response to climate warming and nutrient input among different aggregates in the prediction of SOC dynamics and its feedback to environmental changes in terrestrial ecosystems under climate warming scenarios.
1. Introduction
Soil organic carbon (SOC) decomposition is the key process of carbon (C) release from terrestrial soil to the atmosphere [1], and a small change in its rate will have profound effects on SOC dynamics and atmospheric CO2 concentration. The decomposition rate of SOC is strongly affected by temperature and usually increases with the exponential function of temperature [2,3]. The response of SOC decomposition rate to temperature change is called temperature sensitivity (Q10) that is defined as the relative proportional change in SOC decomposition with a 10 °C rise in temperature [4]. Q10 is a key parameter predicting SOC dynamics and climate change in Earth system models. Given that the annual CO2 emission through SOC decomposition is more than five times that through fossil fuel combustion [1], a small deviation in Q10 values would result in a significant bias in the estimation of CO2 emission in Earth system models.
Soil comprises different size aggregates with varying soil pore structures and provides different physical protection of SOC [5]. Different aggregates also have different SOC and nutrient availability for microbes [6,7,8] and different microbial community and enzyme activities [9,10] that control SOC decomposition and its Q10. Macroaggregates contain more active and labile plant-derived carbon and microaggregates contain recalcitrant microbial-reduced carbon [11] which lead to the differences of SOC decomposition among aggregates. On the one hand, total and labile SOC concentrations generally increase with increasing aggregate size [6,12], suggesting that SOC decomposition increases with increasing aggregate size. On the other hand, microaggregates increased contact between crop residues and the soil microbiome compared with macroaggregates which lead to microaggregate owing to higher SOC decomposition than macroaggregates [13]. All in all, no consistent conclusions have been formulated, and different studies have shown that SOC decomposition rates within macroaggregates are higher than [14,15], lower than [16,17], or similar to [18] those within microaggregates. Furthermore, according to the C-quality temperature hypothesis that labile SOC with low activation energy for decomposition has a low Q10 [4], SOC within macroaggregates has lower Q10 values than that within microaggregates, which was confirmed by some studies in grasslands [19] and croplands [20]. However, some experiments reported macroaggregates with higher Q10 values than microaggregates [15,16,21]. Thus, exploring SOC decomposition and its Q10 in different size aggregates in forest ecosystems is unquestionably important to enhance the accuracy of predicting global soil C storage and C-climate feedback in Earth system models.
As one of the most effective management practices for enhancing soil fertility and increasing forest productivity, fertilization can affect SOC decomposition and Q10 [4,22,23,24] through directly or indirectly changing soil nutrient availability, SOC quantity and quality, microbial community composition and growth, and enzyme activity [23,25,26,27]. In fact, changes in soil nutrients, SOC quantity and quality, and microbial characteristics caused by fertilization vary with aggregate size [21,28]. For example, fertilizing N and P increased the SOC content and invertase activities in macroaggregates but had less effect on them in microaggregates in a cropland [29]. Some studies investigated the effects of N and P fertilization on SOC decomposition and its Q10 in bulk soils [21,23,30], but the information about how fertilization affected SOC decomposition in different size aggregates was still limited and most was in agricultural ecosystems [8,21,30,31]. N fertilization increased the Q10 in bulk soils and microaggregates but had no effect on the Q10 in macroaggregates under a wheat-based cropping system in Inceptisol [21]. Hence, investigating SOC decomposition and Q10 in different size aggregates and their responses to fertilization in forest ecosystems is very important to decrease the uncertainty for modeling C cycling.
In this study, we collected soils from a subtropical forest with long-term N and P addition to investigate SOC decomposition and its Q10 in three aggregate fractions with different sizes. SOC decomposition was measured during incubation at 18.0 and 23.0 °C and used in assessing the Q10 values of SOC decomposition, and soil chemical properties and key enzyme activities were also determined. Due to the substantial differences in C quality, and soil chemical properties and enzyme activities, we hypothesized that (1) SOC decomposition would increase with increasing aggregate size but Q10 would decrease, and (2) P fertilization would change the responses of SOC decomposition and Q10 in different size aggregates to N fertilization.
2. Materials and Methods
2.1. Study Site Description
This study was conducted at the Qianyanzhou Ecological Experimental Station, CAS (26°44′39″ N, 115°03′33″ E) in Taihe County, Jiangxi Province, subtropical China. This region has a subtropical monsoon climate with a mean annual temperature of 17.9 °C and a mean annual precipitation of 1475 mm. Native forests were evergreen broad-leaved plants and most have been transformed into Cunninghamia lanceolata, Pinus massoniana, or P. elliottii plantations since the 1980s. Soil with natural acidity and P limitation was derived from weathered red sandstone and classified as Ultisols according to the US Soil Taxonomy [32].
2.2. Field Experimental Design and Soil Sampling
This experiment with 18 plots (20 m × 20 m) was established in November 2011 in a 12-year-old pure C. lanceolata plantation with a density of 2100 trees hm−2. Before fertilization, the average tree height was 8.94 m, and the diameter at breast height was 10.3 cm [32], Additionally, soil properties were listed in Table S1 [33]. The dominant understory vegetation species at the site are Pteridium aquilinum, Parathelypteris chinensis, and Microlepia marginata. In this experiment, four treatments with three replications were included CT (no fertilization), N (50 kg N ha−1 year−1), P (50 kg P ha−1 year−1), and NP (50 kg N + 50 kg P ha−1 year−1). N and P fertilizers were NH4NO3 and NaH2PO4, and their mixture with sands was evenly spread on the soil surface. Approximately 60% of the total amounts of annual N and P fertilizers were applied in the growing season (March and June), and the remaining amounts were applied in the non-growing season (September and December).
In each plot, six soil cores (0–20 cm) with an auger (5.0 cm in diameter) were randomly collected after the litter layer was removed and then evenly mixed to a composite sample in June 2019. All soil samples were immediately transferred to the laboratory, passed through a 5 mm sieve by gently breaking up soil clods, and mixed thoroughly, and then organic residues and gravel were removed. Each soil sample was divided into two parts. One part was used for aggregate separation, and the other was passed through a 2 mm sieve, air-dried, and used to measure soil chemical properties.
2.3. Soil Aggregate Fractionation and Incubation
Three soil aggregate fractions were obtained according to the modified dry-sieving method described by Jiang et al. [9] because it is gentler than conventional dry or wet sieving techniques. In brief, soil sample (100 g, dry weight) was placed in a Retsch AS200 Control (Retsch Technology, Dusseldorf, Germany) with two sieves (0.25 and 2 mm) stacked on each other for 2 min. After separation, mega-aggregates (>2.0 mm, MeA), macroaggregates (0.25–2.0 mm, MaA), and microaggregates (<0.25 mm, MiA) were obtained for future use. Aggregate fractions were divided into two parts: one was stored at 4 °C for measuring decomposition within 7 days, and the other was air-dried for measuring chemical properties.
Before incubation, the water content of the three aggregate fractions was adjusted to 50% water hold capacity (WHC) and then pre-incubated for 7 days. Soil samples (20 g dry weight) were placed in 500 mL glass bottles with sealed lids and then incubated at 18.0 °C (the average temperature of topsoil) and 23.0 °C for 30 days. During the incubation period, soil water content was maintained at 50% WHC by adding distilled water. The amount of CO2 derived from SOC decomposition was determined through alkali-trapping techniques with an automatic titrator (Mettler Toledo G20, Switzerland). A 50 mL beaker containing 15 mL of NaOH solution (0.10 M) was placed in each incubation Mason jar to trap CO2 and replaced with new beakers at intervals. The collected NaOH solution in each beaker was immediately transferred to a sample flask and sealed for CO2 quantification. CO2 derived from SOC decomposition was measured 1, 3, 7, 12, 16, 20, and 30 days after incubation [34].
2.4. Soil Chemical and Enzyme Activity Analysis
SOC and total N concentrations in three aggregate fractions were measured using a C/N analyzer (Elementar, Germany). Total P (TP) was determined using H2SO4–HClO4 digestion and molybdenum antimony colorimetric method [35]. Available P (AP) was extracted with sodium bicarbonate, and its concentration was determined using the molybdenum antimony colorimetric method [35]. Soil mineral N (i.e., NH4+-N and NO3−-N) and exchangeable cation (i.e., K+, Na+, Ca2+, and Mg2+) concentrations were determined according to standard methods [35]. Soil pH was assessed using a pH meter by slurry (soil:water ratio of 1:2.5) after shaking for 30 min [35]. Labile SOC was defined as organic carbon oxidized by 0.333 mol·L−1 potassium permanganate and determined using the method recommended by Blair et al. [36].
The activities of four hydrolases involved in C, N, and P cycles were determined: β-1,4-glucosidase (BG), β-N-acetyl-glucosaminidase (NaG), acid phosphatase (AcP), and leucine aminopeptidase (LAP). After incubation, enzyme activity was assayed using fluorogenically labeled substrates [37]. The used fluorometric substrates for BG, NaG, AP, and LAP were β-D-glucopyranoside, N-acetyl-β-D-glucosaminide, 4-methylumbelliferyl-phosphate, and L-leucine-7-amido-4-methylcoumarin hydrochloride. The detail process of assaying the enzyme activities was described in Razavi et al. [37]. In short, deionized water (50 mL) was added to 0.5 g of soil (dry weight), and the mixture was broken by low-energy sonication (40 J s−1 output energy) for 2 min. Then, 50 μL of the obtained soil suspension was added to 100 μL of substrate solution and 50 μL of buffer (pH: 6.50) in a 96-well microplate. After mixing, at 0, 1 and 2 h, fluorescence in the microplates was measured at an excitation wavelength of 355 nm, an emission wavelength of 460 nm, and a slit width of 25 nm. To make the curves of enzyme activities, enzyme activities at substrate concentrations of 0, 10, 20, 30, 50, 100, and 200 µmol g−1 were determined, and enzyme activity was expressed as MUF or AMC release in nmol per g dry soil per hour (nmol g−1 soil h−1) [38]. Enzyme activity (V) was fitted by using the Michaelis–Menten Equation [37].
where Vmax is the maximal rate of enzymatic activity at a given temperature, Km is the half saturation constant, and [S] is the substrate concentration.
V = Vmax [S]/(Km + [S])
2.5. Data Calculation and Statistic Analysis
In the present study, we only reported the cumulative SOC decomposition at 18.0 °C because this temperature reflects the average temperature of 0–5 cm soil in this region. To evaluate how SOC decomposition responds to climate warming, the Q10 of SOC decomposition was calculated with the following Equation [7,34]:
where C23 and C18 were the cumulative amounts of CO2 from SOC decomposition within 30 days at 23.0 and 18.0 °C, respectively.
Q10 = (C23/C18)10/(23 − 18)
The normality and homoscedasticity of the measured variables were tested with the Shapiro–Wilk test and Levene’s test, respectively. Two-way ANOVA with least significant difference test was used in assessing the individual and interactive effects of soil aggregates and fertilization on cumulative SOC decomposition at 18.0 °C and its Q10 and enzyme activities. To explore the potential mechanisms of fertilization effect on cumulative SOC decomposition and Q10, we assessed the single-variable relationships of Q10 with the chemical properties and enzyme activities of the aggregate soils through Pearson’s correlation analysis. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). To further qualify the relative importance of key factors influencing SOC decomposition and Q10, we performed the classification random forests model analysis using the RandomForest package in R3.3.3 with default parameters, and detail information was described in Delgado-Baquerizo et al. [39].
3. Results
3.1. Effects of N and P Fertilization on SOC Decomposition
N and P fertilization significantly affected some chemical variables in the aggregates (Table 1) but had no effect on the proportions of different size aggregates in soil (Figure S1). The results of two-way ANOVA showed that aggregate size and fertilization significantly affected cumulative SOC decomposition over the 30-day incubation (p < 0.001: Figure 1). When not considering fertilization, cumulative SOC decomposition was 49.2% and 26.0% higher in MiA than in MeA and MaA, respectively. Alone, N fertilization significantly increased SOC decomposition by 39.4% in MaA and 23.7% in MiA relative to that in CT, but P fertilization had less effect on SOC decomposition. Cumulative SOC decomposition in MaA and MiA under NP fertilization was significantly lower than that under N fertilization (Figure 1), suggesting that P fertilization decreased the effects of N fertilization on SOC decomposition. However, no interaction effect of soil aggregate size and fertilization on SOC decomposition (p = 0.349) was observed.

Table 1.
Effects of N and P fertilization on chemical properties in different size aggregates in a Cunninghamia lanceolata forest.
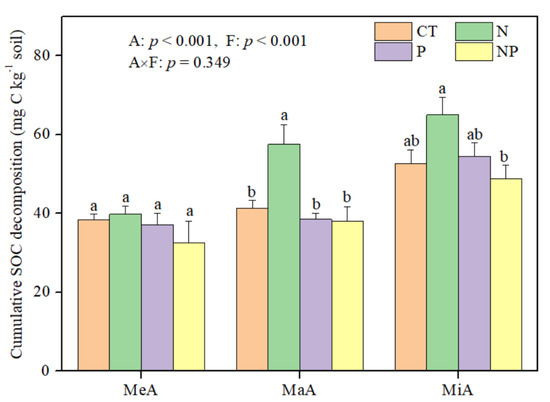
Figure 1.
Effects of N and P fertilization on the cumulative soil organic C (SOC) decomposition in different size aggregates during 30-day incubation at 18 °C in a subtropical forest. F and A represent fertilization and soil aggregate size, respectively. Different letters on the bars denote significant differences among fertilization treatments. MeA, MaA, and MiA represent mega-aggregates (>2.0 mm), macroaggregates (0.25–2.0 mm), and microaggregates (<0.25 mm). Data were represented as the mean with standard error.
3.2. Effects of N and P Fertilization on Q10 of SOC Decomposition
Soil aggregate size (p = 0.044) and fertilization (p < 0.001) significantly affected Q10, but their interaction effect (p = 0.804) was not detected (Figure 2). The averaged Q10 value in MiA (1.83) was significantly lower than that in MeA (2.29) and MaA (2.26), when not considering fertilization treatments (Figure 2), suggesting that Q10 had a trend to increase with increasing aggregate size. N and P fertilization had different effects on Q10, showing that sole N fertilization had no significant impact on Q10 in all aggregate fractions, whereas sole P fertilization increased Q10 by 46.7% and 46.6% in MeA and MaA relative to CT, respectively. The Q10 values in all size aggregates under NP fertilization were not significantly different from those under N fertilization, suggesting that P fertilization did not change the effects of N fertilization on Q10, but N fertilization changed the effects of P fertilization on Q10.
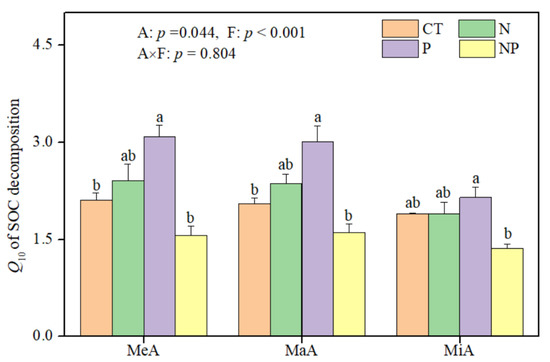
Figure 2.
Effects of N and P fertilization on temperature sensitivity (Q10) of soil organic carbon (SOC) decomposition in different size aggregates after over 30 days of incubation in a subtropical forest. F and A represent fertilization and soil aggregate size, respectively. Different letters on the bars denote significant differences among fertilization treatments. MeA, MaA, and MiA represent mega-aggregates (>2.0 mm), macroaggregates (0.25–2.0 mm), and microaggregates (<0.25 mm). Data were represented as the mean with standard error.
3.3. Effects of N and P Fertilization on Soil Enzyme Activities
Soil aggregate size, fertilization and their interaction significantly (p < 0.05) affected the activities of NaG (Figure 3b) and AcP (Figure 3d), but they had no effect on BG activity (Figure 3a). In addition, aggregate size and its interaction with fertilization significantly (p < 0.05) affected LAP activity (Figure 3c). N increased NaG activity in MaA and MiA (Figure 3b) and AcP in MiA (Figure 3d), but decreased LAP and AcP activities by 31.4% and 31.3% in MiA, respectively (Figure 3c,d). P and NP fertilization decreased AcP activity in all size aggregates (Figure 3d).
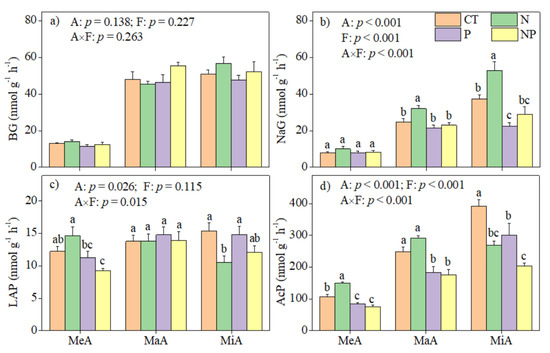
Figure 3.
Effects of N and P fertilization on the activities of (a) β-1,4-glucosidase (BG), (b) β-N-acetyl-glucosaminidase (NaG), (c) leucine aminopeptidase (LAP), and (d) acid phosphatase (AcP) at 18 °C at the end of incubation. F and A represent fertilization and soil aggregate size, respectively. Different letters on the bars denote significant differences among fertilization treatments. MeA, MaA, and MiA represent mega-aggregates (>2.0 mm), macroaggregates (0.25–2.0 mm), and microaggregates (<0.25 mm). Data were represented as the mean with standard error.
3.4. Factors Regulating SOC Decomposition and Q10
Cumulative SOC decomposition at 18 °C was positively related to the activities of BG, NaG, and AcP, and the concentrations of SOC, labile SOC and total N but negatively related to pH and exchangeable Na+ concentration (Figure 4a–d and Figure S2a,b). Q10 values were positively correlated with LAP activity, pH, and concentrations of exchangeable K+ and Mg2+ (Figure 4e–h). The results of random forests model analysis showed that total N, NAG activity and labile SOC concentration were the most important factors in regulating cumulative SOC decomposition at 18 °C, followed by SOC concentration, exchangeable Na+, AcP and BG activities (Figure 5a). LAP activity was the most important factor regulating the Q10 value of SOC decomposition, followed by exchangeable K+, Mg2+ concentrations and pH (Figure 5b).
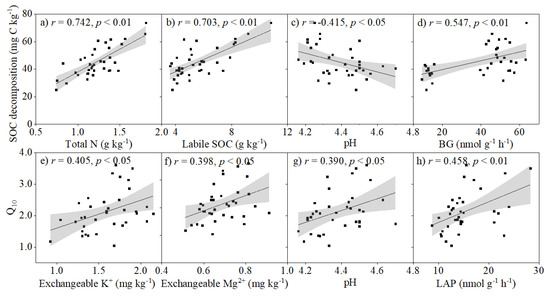
Figure 4.
Pearson’s correlation coefficients of key soil chemical properties and enzyme activities with (a–d) cumulative soil organic carbon (SOC) decomposition and (e–h) temperature sensitivity (Q10). BG and LAP represent β–1,4–glucosidase and leucine aminopeptidase, respectively. The shaded area represents 95% confidence interval.
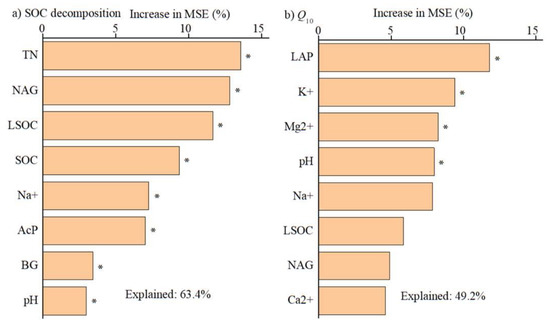
Figure 5.
Importance of key factors influencing (a) cumulative soil organic carbon decomposition and (b) temperature sensitivity (Q10) based on random forests model analysis. * represents significant effect at p < 0.05. SOC and LSOC denote soil organic C, and labile SOC by KMnO4, respectively. K+, Na+, Ca2+ and Mg2+ denote exchangeable K, Na, Ca and Mg cations, respectively. TN denotes total nitrogen. BG, NAG, AcP and LAP denote β-1,4-glucosidase, β-N-acetyl-glucosaminidase, acid phosphatase and leucine aminopeptidase, respectively.
4. Discussion
4.1. Soil Organic Carbon Decomposition and Its Responses to N and P Fertilization in Different Aggregates
Differences in SOC decomposition at 18 °C between aggregate fractions were observed, showing that MiA had a higher SOC decomposition rate than MeA and MaA (Figure 1), which was in agreement with some previous observation [16,17]. This result did not support our first hypothesis that SOC decomposition will increase with aggregate size. Higher SOC decomposition in the small aggregates than in the large aggregates was due to lower physical protection of SOC within small aggregates than that within large aggregates [5] and the higher SOC and labile SOC concentrations in small aggregates (Table 1 and Table S2). The physical protection of aggregates to SOC was mainly attributed to the formation of a physical barrier that restricts microbial access to SOC [5]; however, microaggregates before forming macroaggregates have much weaker physical protection to SOC within microaggregates than SOC within macroaggregates [5]. Thus, SOC decomposition in the small aggregates was higher than that in the large aggregates. In our study, higher SOC decomposition in MiA than MaA was in part attributed to lower C:N ratio of soil organic matter in MiA than in MaA (Table 1) because soil organic matter with low C:N ratio is preferentially utilized by microorganisms [34]. The other potential mechanisms that small aggregates had higher SOC decomposition than large aggregates need to be further explored.
N fertilization increased SOC decomposition in MaA and MiA (Figure 1). This finding was partly consistent with some previous observations that N fertilization increased SOC decomposition in >0.053 mm aggregates in a highland agroecosystem [8] and in macro- and microaggregates in a subtropical cropland [21]. These results suggested that responses of SOC decomposition to N fertilization varied among different size aggregates and our results implied that N fertilization was not in favor of SOC sequestration in MaA and MiA. N fertilization generally enhances fresh organic matter inputs by increasing litter production and rhizodeposition [23,40], resulting in higher SOC concentrations. Thus, increased concentrations of total and labile SOC in MaA and MiA after N fertilization (Table 1) were responsible for the promotion of N fertilization to SOC decomposition in MaA and MiA.
Lower SOC decomposition in MaA and MiA under NP than those under N (Figure 1) implied that P fertilization changed the effects of N fertilization on SOC decomposition in the different size aggregates. We attributed these results to P-fertilization-induced decreases in total and labile SOC concentrations in MaA and MiA under N fertilization (Table 1). In addition, soils fertilized with P and N fertilizer exhibited changes in enzyme activities (Figure 3), and these changes were partly responsible for the alteration of the influences of N fertilization on SOC decomposition following P fertilization, which was supported the strong correlations between SOC decomposition and enzyme activities (Figure 4 and Figure S2).
4.2. Temperature Sensitivity of Soil Organic Carbon Decomposition in Different Aggregates and its Responses to N and P Fertilization
Unlike our hypothesis that Q10 would decrease with increasing aggregate size, we found that SOC within large aggregates (MeA and MaA) had higher averaged Q10 values than that within small aggregates (MiA) (Figure 2). Our results were in agreement with some previous studies that macroaggregates had higher Q10 values than microaggregates in croplands [16,21] and forests [15], although they were opposite to the C-quality temperature hypothesis that decomposition of recalcitrant SOC is more sensitive to temperature change than that of labile SOC [4]. One reason was that the C-quality temperature hypothesis is generally applicable when SOC decomposition is only limited by C quality [4]; however, Q10 is usually simultaneously affected by several factors such as C quality and nutrient availability [34,41], and in the present study, Q10 was significantly related to LAP activity, pH, exchangeable K+ and Mg2+ concentrations (Figure 4e–h). The higher Q10 in large aggregates than that of SOC associated with small aggregates implied that SOC within the large aggregates will have higher risk of C loss in response to climate warming and generate positive feedback for global warming.
Unlike our hypothesis, sole N fertilization had less effect on Q10 in all size aggregates (Figure 2), which differed from some previous observations that N fertilization had various effects on Q10 in different size aggregates [8,21,30]. For example, Ghosh et al. [21] reported that N fertilization increased Q10 in microaggregates but had no effect on Q10 in macroaggregates in a subtropical cropland. Q10 was controlled by a series of soil environmental factors, such as SOC quantity and quality, nutrient availability, and microbial community and enzyme activity [34,41]. The inconsistency was attributed to the different changes in soil properties caused by N fertilization among various experiments. In our study, sole N fertilization had less effect on soil chemical properties (Table 1) and BG and LAP activities (Figure 3) in different size aggregates. In contrast to N fertilization, P fertilization significantly increased Q10 in MeA and MaA (Figure 2). This was attributed to the decrease in SOC contents and the increase in total P contents following P fertilization (Table 1), which caused the shift of microbial P limitation to C limitation [42]. In MeA, MaA and MiA, similar Q10 values under NP fertilization and N fertilization (Figure 2) indicated that P fertilization had less modification on the effect of N fertilization on Q10 in all size aggregates, which did not support our hypothesis that P fertilization would change the response of Q10 to N fertilization.
Some field and laboratory experiments demonstrated that the influences of fertilization on SOC decomposition and its Q10 in bulk soils are strongly related to the forms of N and P fertilizers [43,44] through affecting plant growth, rhizodeposition, microbial biomass and activity, and SOC quantity and quality [26,45]. In our study, NH4NO3 and NaH2PO4 were used for N and P fertilization, respectively. Thus, our results obtained from the long-term fertilization experiment should be interpreted with caution, and whether N and P fertilization affect SOC decomposition and Q10 in different aggregates occurs in different sites and forest ecosystems should be determined.
5. Conclusions
N fertilization accelerated SOC decomposition in small aggregates, whereas P fertilization increased Q10 in large aggregates, suggesting that N and P fertilization had different effects on SOC decomposition and Q10, but these effects were related to soil aggregate size. Furthermore, P fertilization changed the influences of N fertilization on SOC decomposition in small aggregates, while N fertilization decreased the responses of Q10 to P fertilization in all size aggregates. Total and labile SOC and soil enzyme activities were important factors of regulating SOC decomposition and its Q10. Overall, this study provides solid evidence of the different effects of N and P fertilization on the decomposition and Q10 of SOC associated with different size aggregates. Our findings highlighted the importance of considering differences in the responses of SOC decomposition in different size aggregates to anthropogenic N and P inputs in most C cycling models in the prediction of SOC dynamics and its feedback to environmental changes in terrestrial ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14010072/s1, Table S1: Basic physicochemical characteristics of the soil from the treatment plots before fertilization. There are no significant differences of any soil properties. Table S2: Results (p values) of one-way ANOVA for N and P fertilization on the chemical variables in different aggregate fractions; Figure S1: N and P fertilization had no effect on soil aggregate fractions in a subtropical forest. MeA, MaA and MiA represent mega-aggregates (>2.0 mm), macroaggregates (0.25–2.0 mm) and microaggregates (<0.25 mm); Figure S2: Pearson’s correlation coefficients of enzyme activities (a,b) and soil chemical properties (c,d) with cumulative soil organic carbon (SOC) decomposition. AcP and NAG represent acid phosphatase and β-N-acetyl-glucosaminidase, respectively. The shaded area represents 95% confidence interval.
Author Contributions
Q.W. designed this experiment, Q.W. and J.L. wrote this paper, J.L. measured and collected experimental data, X.Z. provided some suggestions to improve the paper quality, and S.L. collected soil samples. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 31830015 and 32171752.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.
Acknowledgments
The authors would like to acknowledge Qianyanzhou Ecological Experimental Station in Jiangxi Province for providing the experimental plots.
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships that are relevant to the content of this article.
References
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Hagerty, S.B.; Allison, S.D.; Schimel, J.P. Evaluating soil microbial carbon use efficiency explicitly as a function of cellular processes: Implications for measurements and models. Biogeochemistry 2018, 140, 269–283. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Arevalo, C.B.M.; Chang, S.X.; Bhatti, J.S.; Sidders, D. Mineralization Potential and Temperature Sensitivity of Soil Organic Carbon under Different Land Uses in the Parkland Region of Alberta, Canada. Soil Sci. Soc. Am. J. 2012, 76, 241–251. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Agren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M.; et al. Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward. Glob. Change Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Wei, X.R.; Ma, T.N.; Wang, Y.H.; Wei, Y.C.; Hao, M.D.; Shao, M.G.; Zhang, X.C. Long-term fertilization increases the temperature sensitivity of OC mineralization in soil aggregates of a highland agroecosystem. Geoderma 2016, 272, 1–9. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Jin, C.; Sun, B. Soil aggregate stratification of nematodes and ammonia oxidizers affects nitrification in an acid soil. Environ. Microbiol. 2014, 16, 3083–3094. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ghosh, A.; Zhang, Y.; Dalal, R.C.; Kopittke, P.M.; Jones, A.; Menzies, N.W. Land use affects temperature sensitivity of soil organic carbon decomposition in macroaggregates but not in bulk soils in subtropical Oxisols of Queensland, Australia. Soil Tillage Res. 2020, 198, 104566. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Cammeraat, E.; Perez-Cardiel, E.; Lasanta, T. Effects of secondary succession and afforestation practices on soil properties after cropland abandonment in humid Mediterranean mountain areas. Agric. Ecosyst. Environ. 2016, 228, 91–100. [Google Scholar] [CrossRef]
- Kan, Z.R.; Ma, S.T.; Liu, Q.Y.; Liu, B.Y.; Virk, A.L.; Qi, J.Y.; Zhao, X.; Lal, R.; Zhang, H.L. Carbon sequestration and mineralization in soil aggregates under long-term conservation tillage in the North China Plain. Catena 2020, 188, 12. [Google Scholar] [CrossRef]
- Manna, M.C.; Bhattacharyya, P.; Adhya, T.K.; Singh, M.; Wanjari, R.H.; Ramana, S.; Tripathi, A.K.; Singh, K.N.; Reddy, K.S.; Rao, A.S.; et al. Carbon fractions and productivity under changed climate scenario in soybean-wheat system. Field Crop. Res. 2013, 145, 10–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, N.N.; Liao, X.L.; Wang, Z.; Wei, X.R.; Jia, X.X. Long-term afforestation accelerated soil organic carbon accumulation but decreased its mineralization loss and temperature sensitivity in the bulk soils and aggregates. Catena 2021, 204, 10. [Google Scholar] [CrossRef]
- Kan, Z.R.; Liu, W.X.; Liu, W.S.; He, C.; Bohoussou, N.Y.; Dang, Y.P.; Zhao, X.; Zhang, H.L. Sieving soil before incubation experiments overestimates carbon mineralization but underestimates temperature sensitivity. Sci. Total Environ. 2022, 806, 10. [Google Scholar] [CrossRef]
- Sey, B.K.; Manceur, A.M.; Whalen, J.K.; Gregorich, E.G.; Rochette, P. Small-scale heterogeneity in carbon dioxide, nitrous oxide and methane production from aggregates of a cultivated sandy-loam soil. Soil Biol. Biochem. 2008, 40, 2468–2473. [Google Scholar] [CrossRef]
- Noellemeyer, E.; Frank, F.; Alvarez, C.; Morazzo, G.; Quiroga, A. Carbon contents and aggregation related to soil physical and biological properties under a land-use sequence in the semiarid region of central Argentina. Soil Tillage Res. 2008, 99, 179–190. [Google Scholar] [CrossRef]
- Yang, C.; Liu, N.; Zhang, Y.J. Effects of aggregates size and glucose addition on soil organic carbon mineralization and Q(10) values under wide temperature change conditions. Eur. J. Soil Biol. 2017, 80, 77–84. [Google Scholar] [CrossRef]
- Wang, J.; Chen, F.; Liu, Y. Respiration Characteristics of Different Sized Soil Aggregates and Their Contribution to Carbon Emissions. Plant Sci. J. 2014, 32, 586–593. [Google Scholar]
- Ghosh, A.; Bhattacharyya, R.; Dey, A.; Dwivedi, B.S.; Meena, M.C.; Manna, M.C.; Agnihortri, R. Long-term fertilization impact on temperature sensitivity of aggregate associated soil organic carbon in a sub-tropical inceptisol. Soil Tillage Res. 2019, 195, 12. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.J.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.A.; Wookey, P.A.; Agren, G.I.; Sebastia, M.T.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.K.; Zhang, W.D.; Sun, T.; Chen, L.C.; Pang, X.Y.; Wang, Y.P.; Xiao, F.M. N and P fertilization reduced soil autotrophic and heterotrophic respiration in a young Cunninghamia lanceolata forest. Agric. For. Meteorol. 2017, 232, 66–73. [Google Scholar] [CrossRef]
- Zhang, J.F.; Sayer, E.J.; Zhou, J.G.; Li, Y.W.; Li, Y.X.; Li, Z.A.; Wang, F.M. Long-term fertilization modifies the mineralization of soil organic matter in response to added substrate. Sci. Total Environ. 2021, 798, 13. [Google Scholar] [CrossRef] [PubMed]
- Contosta, A.R.; Frey, S.D.; Cooper, A.B. Soil microbial communities vary as much over time as with chronic warming and nitrogen additions. Soil Biol. Biochem. 2015, 88, 19–24. [Google Scholar] [CrossRef]
- Du, Y.H.; Guo, P.; Liu, J.Q.; Wang, C.Y.; Yang, N.; Jiao, Z.X. Different types of nitrogen deposition show variable effects on the soil carbon cycle process of temperate forests. Glob. Change Biol. 2014, 20, 3222–3228. [Google Scholar] [CrossRef]
- Geng, J.; Fang, H.J.; Cheng, S.L.; Pei, J. Effects of N deposition on the quality and quantity of soil organic matter in a boreal forest: Contrasting roles of ammonium and nitrate. Catena 2021, 198, 9. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, M.Y.; Bai, S.H.; Xu, Z.H.; Liu, Y.Q.; Chen, F.S.; Guo, X.M.; Zhang, L.; Luo, H.D.; Zhang, Q. Mineral fertilization and soil depth slightly affected aggregate structures despite significantly altered microbial properties in surface forest soils. J. Soils Sediments 2020, 20, 3615–3626. [Google Scholar] [CrossRef]
- Yu, H.Y.; Ding, W.X.; Luo, J.F.; Geng, R.L.; Ghani, A.; Cai, Z.C. Effects of long-term compost and fertilizer application on stability of aggregate-associated organic carbon in an intensively cultivated sandy loam soil. Biol. Fertil. Soils 2012, 48, 325–336. [Google Scholar] [CrossRef]
- Wankhede, M.; Ghosh, A.; Manna, M.C.; Misra, S.; Sirothia, P.; Rahman, M.M.; Bhattacharyya, P.; Singh, M.; Bhattacharyya, R.; Patra, A.K. Does soil organic carbon quality or quantity govern relative temperature sensitivity in soil aggregates? Biogeochemistry 2020, 148, 191–206. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Jiang, C.; Wu, M.; Li, Z. Organic Carbon Mineralization in Aggregate Fractions of Red Paddy Soil Under Different Fertilization Treatments. Sci. Agric. Sin. 2018, 51, 3325–3334. [Google Scholar]
- Tang, Y.C.; Zhang, X.Y.; Li, D.D.; Wang, H.M.; Chen, F.S.; Fu, X.L.; Fang, X.M.; Sun, X.M.; Yu, G.R. Impacts of nitrogen and phosphorus additions on the abundance and community structure of ammonia oxidizers and denitrifying bacteria in Chinese fir plantations. Soil Biol. Biochem. 2016, 103, 284–293. [Google Scholar] [CrossRef]
- Chen, F.S.; Niklas, K.J.; Liu, Y.; Fang, X.M.; Wan, S.Z.; Wang, H. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef]
- Wang, Q.K.; Liu, S.G.; Tian, P. Carbon quality and soil microbial property control the latitudinal pattern in temperature sensitivity of soil microbial respiration across Chinese forest ecosystems. Glob. Change Biol. 2018, 24, 2841–2849. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003. [Google Scholar]
- Blair, G.J.; Lefroy, R.D.B.; Lise, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Razavi, B.S.; Blagodatskaya, E.; Kuzyakov, Y. Nonlinear temperature sensitivity of enzyme kinetics explains canceling effect—a case study on loamy haplic Luvisol. Front. Microbiol. 2015, 6, 13. [Google Scholar] [CrossRef]
- German, D.P.; Chacon, S.S.; Allison, S.D. Substrate concentration and enzyme allocation can affect rates of microbial decomposition. Ecology 2011, 92, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Trivedi, P.; Osanai, Y.; Liu, Y.R.; Hamonts, K.; Jeffries, T.C.; Singh, B.K. Carbon content and climate variability drive global soil bacterial diversity patterns. Ecol. Monogr. 2016, 86, 373–390. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharyya, R.; Meena, M.C.; Dwivedi, B.S.; Singh, G.; Agnihotri, R.; Sharma, C. Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Tillage Res. 2018, 177, 134–144. [Google Scholar] [CrossRef]
- Meyer, N.; Welp, G.; Amelung, W. The Temperature Sensitivity (Q10) of Soil Respiration: Controlling Factors and Spatial Prediction at Regional Scale Based on Environmental Soil Classes. Glob. Biogeochem. Cycles 2018, 32, 306–323. [Google Scholar] [CrossRef]
- Camenzind, T.; Hattenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillig, M.C. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Coucheney, E.; Stromgren, M.; Lerch, T.Z.; Herrmann, A.M. Long-term fertilization of a boreal Norway spruce forest increases the temperature sensitivity of soil organic carbon mineralization. Ecol. Evol. 2013, 3, 5177–5188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.K.; Liu, S.G.; Wang, Y.P.; Tian, P.; Sun, T. Influences of N deposition on soil microbial respiration and its temperature sensitivity depend on N type in a temperate forest. Agric. For. Meteorol. 2018, 260, 240–246. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Change Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).