Effect of Platinum Nanoparticles (PtNPs) Pollution on the Biological Properties of Haplic Cambisols Eutric of the Caucasus Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Platinum Nanoparticles (PtNPs)
2.2. Study Object
2.3. Model Experiment
2.4. Biological Indicators
2.4.1. Total Number of Soil Bacteria
2.4.2. Enzymatic Activity
2.4.3. Phytotoxicity Analysis
2.5. Data Analysis
2.6. Statistical Analyses
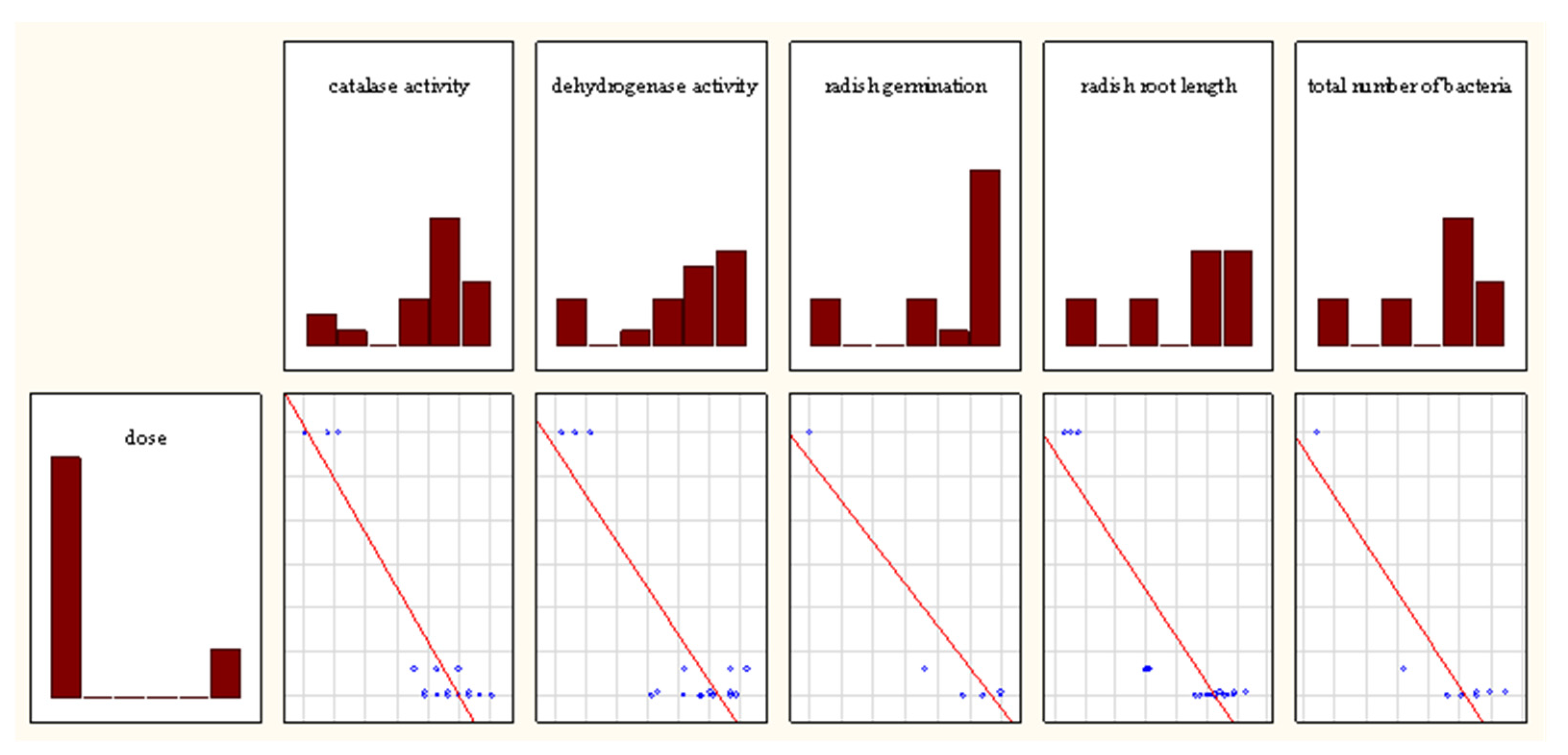
3. Results
3.1. Effect of PtNPs on the Enzymatic Activities of Haplic Cambisols Eutric
3.2. Effect of PtNPs on the Phytotoxicity of Haplic Cambisols Eutric
3.3. Effect of PtNPs on the Total Number of Bacteria in Haplic Cambisols Eutric
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, M.B.; Kelly, C.; Kabrick, J.; Schuler, J.L. Chapter 6-Temperate forests and soils. In Developments in Soil Science; Busse, M., Giardina, C.P., Morris, D.M., Page-Dumroese, D.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 36, pp. 83–108. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; 234p. [Google Scholar]
- Kowalska, J.B.; Waroszewski, J.; Gąsiorek, M.; Zadrożny, P.; Nicia, P. Deep subsoil storage of trace elements and pollution assessment in mountain podzols (Tatra mts., Poland). Forests 2021, 12, 291. [Google Scholar] [CrossRef]
- Figas, A.; Siwik-Ziomek, A.; Kobierski, M. Heavy metals and sulphur in needles of Pinus sylvestris L. and soil in the forests of city agglomeration. Forests 2021, 12, 1310. [Google Scholar] [CrossRef]
- Sidor, C.G.; Vlad, R.; Popa, I.; Semeniuc, A.; Apostol, E.; Badea, O. Impact of industrial pollution on radial growth of conifers in a former mining area in the Eastern Carpathians (Northern Romania). Forests 2021, 12, 640. [Google Scholar] [CrossRef]
- Caković, M.; Beloica, J.; Simić, S.B.; Miljković, P.; Lukić, S.; Baumgertel, A.; Schwaiger, F. Diffuse pollution and ecological risk assessment in Ludaš lake special nature reserve and Palić nature park (Pannonian Basin). Forests 2021, 12, 1461. [Google Scholar] [CrossRef]
- Ravindra, K.; Bencs, L.; Van Grieken, R. Platinum group elements in the environment and their health risk. Sci. Total Environ. 2004, 318, 1–43. [Google Scholar] [CrossRef]
- Komendova, R.; Zidek, J.; Berka, M.; Jemelkova, M.; Rezacova, V.; Conte, P.; Kucerik, J. Small-sized platinum nanoparticles in soil organic matter: Influence on water holding capacity, evaporation and structural rigidity. Sci. Total Environ. 2019, 694, 133822. [Google Scholar] [CrossRef]
- Silva, S.; Oliveira, H.; Craveiro, S.; Calado, A.; Santos, C. Pure anatase and rutile+anatase nanoparticles differently affect wheat seedlings. Chemosphere 2016, 151, 68–75. [Google Scholar] [CrossRef]
- Shara, S.; Shahsavaria, E.; Reithc, F.; Alghamdib, O.A.; Yamanib, H.A.; AlJudaibib, A.; Donnere, E.; Vasileiadisf, S.; Ball, A.S. Dose-related changes in respiration and enzymatic activities in soils amended with mobile platinum and gold. Appl. Soil Ecol. 2021, 157, 103727. [Google Scholar] [CrossRef]
- Hooda, P.S.; Miller, A.; Edwards, A. The distribution of automobile catalysts-cast platinum, palladium and rhodium in soils adjacent to roads and their uptake by grass. Sci. Total Environ. 2007, 384, 384–392. [Google Scholar] [CrossRef]
- Cicchella, D.; Fedele, L.; De Vivo, B.; Albanese, S.; Lima, A. Platinum group element distribution in the soils from urban areas of the Campania region (Italy). Geochem. Explor. Environ. Anal. 2008, 8, 31–40. [Google Scholar] [CrossRef]
- Zereini, F.; Wiseman, C.L.S. Platinum Metals in the Environment; Springer Verlag: Berlin/Heidelberg, Germany, 2015; 492p. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Mitra, A.; Sen, I. Anthrobiogeochemical Platinum, Palladium and Rhodium Cycles of Earth: Emerging Environmental Contamination. Geochim. Cosmochim. Acta 2017, 216, 417–432. [Google Scholar] [CrossRef]
- Morton, O.; Puchelt, H.; Hernández, E.; Lounejeva, E. Traffic-related platinum group elements (PGE) in soil from Mexico City. J. Geochem. Explor. 2001, 72, 223–227. [Google Scholar] [CrossRef]
- Gómez, B.; Gómez, M.; Sanchez, J.L.; Fernández, R.; Palacios, M.A. Platinum and rhodium distribution in airborne matter and road dust. Sci. Total Environ. 2001, 269, 131–144. [Google Scholar] [CrossRef]
- Grimaldi, M.; Bo, V.D.; Ferrari, B.; Roda, E.; De Luca, F.; Veneroni, P.; Barni, S.; Verri, M.; De Pascali, S.A.; Fanizzi, F.P.; et al. Long-term effects after treatment with platinum compounds, cisplatin and [Pt (O, O′-acac)(γ-acac)(DMS)]: Autophagy activation in rat B50 neuroblastoma cells. Toxicol. Appl. Pharmacol. 2019, 364, 1–11. [Google Scholar] [CrossRef]
- Rajendran, S.; Prabha, S.; Rathish, J.; Singh, G.; Al-Hashem, A. Chapter 12-Antibacterial activity of platinum nanoparticles. In Nanotoxicity. Prevention and Antibacterial Applications of Nanomaterials. Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–281. [Google Scholar] [CrossRef]
- Bloch, K.; Pardesi, K.; Satriano, C.; Ghosh, S. Bacteriogenic Platinum Nanoparticles for Application in Nanomedicine. Front. Chem. 2021, 9, 624344. [Google Scholar] [CrossRef]
- Ávila, M.I.A.; Toledo-Carrillo, E.; Dutta, J. Improved chlorate production with platinum nanoparticles deposited on fluorinated activated carbon cloth electrodes. Clean. Eng. Technol. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Lévay, K.; Kárpáti, T.; Hegedűs, L. Selective hydrogenation of benzonitrile and its homologues to primary amines over platinum. J. Ind. Eng. Chem. 2021, 101, 279–292. [Google Scholar] [CrossRef]
- Jung, T.K.; Joh, D.W.; Lee, S.Y.; Choi, M.S.; Hyun, S.K.; Lee, H.S. Mechanical alloying of platinum with 5% ZrO2 nanoparticles for glass making tools. Trans. Nonferrous Met. Soc. China 2014, 24, 99–105. [Google Scholar] [CrossRef]
- Birke, M.; Rauch, U.; Stummeyer, J.; Lorenz, H.; Keilert, B. A review of platinum group element (PGE) geochemistry and a study of the changes of PGE contents in the topsoil of Berlin, Germany, between 1992 and 2013. J. Geochem. Explor. 2018, 187, 72–96. [Google Scholar] [CrossRef]
- Marcheselli, M.; Sala, L.; Mauri, M. Bioaccumulation of PGEs and other traffic related metals in populations of the small mammal Apodemus sylvaticus. Chemosphere 2010, 80, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Alt, F.; Eschnauer, H.R.; Mergler, B.; Messerschmidt, J.; Tolg, G.; Fresenius, J. A contribution to the ecology and enology of platinum. Anal. Chem. 1997, 357, 1013. [Google Scholar] [CrossRef]
- Orecchio, S.; Amorello, D. Platinum levels in urban soils from Palermo (Italy); Analytical method using voltammetry. Microchem. J. 2011, 99, 283–288. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622. [Google Scholar] [CrossRef] [PubMed]
- Maye, M.M.; Lim, I.I.S.; Luo, J.; Rab, Z.; Rabinovich, D.; Liu, T.B.; Zhong, C.J.; Am, J. Mediator-template assembly of nanoparticles. Chem. Soc. 2005, 127, 1519–1529. [Google Scholar] [CrossRef]
- Seckin, H.; Tiri, R.N.E.; Meydan, I.; Aygun, A.M.; Gunduz, K.; Sen, F. An environmental approach for the photodegradation of toxic pollutants from wastewater using Pt–Pd nanoparticles: Antioxidant, antibacterial and lipid peroxidation inhibition applications. Environ. Res. 2022, 208, 112708, 0013-9351. [Google Scholar] [CrossRef]
- Lushchaeva, I.V.; Morgalev, Y.N. Effect of Platinum Nanoparticles on Biological Activity of Humus-Accumulated Horizons. Adv. Mater. Res. 2015, 1085, 384–389. [Google Scholar] [CrossRef]
- Kołton, A.; Czaja, M.A. Influence of platinum ions on the germination and seedling root growth of different plant species. Geol. Geophys. Environ. 2014, 40, 343–348. [Google Scholar] [CrossRef]
- Rahman, M.S.; Chakraborty, A.; Mazumdar, S.; Nandi, N.C.; Bhuiyan, M.N.I.; Alauddin, S.M.; Khan, I.A.; Hossain, M.J. Effects of poly(vinylpyrrolidone) protected platinum nanoparticles on seed germination and growth performance of Pisum sativum. Nano-Struct. Nano-Objects 2020, 21, 100408. [Google Scholar] [CrossRef]
- Astafurova, T.P.; Morgalev, Y.N.; Borovikova, G.V.; Zotikova, A.P.; Verkhoturova, G.S.; Zaitseva, T.A.; Postovalova, V.M.; Morgaleva, T.A. Features of concentration dependence of the development of wheat seedlings in aqueous dispersed systems of platinum nanoparticles. Plant. Physiol. Genet. 2013, 45, 544–549. [Google Scholar]
- Asztemborska, M.; Steborowski, R.; Kowalska, J.; Bystrzejewska- Piotrowska, G. Accumulation of aluminium by plants exposed to nano-and microsized particles of Al2O3. Int. J. Environ. Res. 2015, 9, 109–116. [Google Scholar] [CrossRef]
- Savignan, L.; Faucher, S.; Chéry, P.; Lespes, G. Platinum group elements contamination in soils: Review of the current state. Ecotoxicol. Environ. Safe. 2021, 222, 112459. [Google Scholar] [CrossRef] [PubMed]
- Djingova, R.; Kovacheva, P.; Wagner, G.; Markert, B. Distribution of platinum group elements and other traffic related elements among different plants along some highways in Germany. Sci. Total Environ. 2003, 308, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Fatoki, O.S. Anthropogenic platinum enrichment in the vicinity of mines in the bushveld igneous complex, South Africa. Water Air Soil Pollut. 2013, 224, 1395. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Fedorenko, A.; Mandzhieva, S.; Sushkova, S.; Lysenko, N.D.V.; Azarov, A.; Chokheli, V. Destructive effect of copper oxide nanoparticles on ultrastructure of chloroplast, plastoglobules and starch grains in spring barley (Hordeum sativum). Int. J. Agric. Biol. 2019, 21, 171–174. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Tsepina, N.; Minnikova, T.; Kazeev, K.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Mazarji, M.; Singh, R.K.; Rajput, V.D. Influence of Silver Nanoparticles on the Biological Indicators of Haplic Chernozem. Plants 2021, 10, 1022. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Baez-Bernal, D.; Castro Insúa, J.; García, M.I. Effects of mussel shell addition on the chemical and biological properties of a Cambisol. Chemosphere 2011, 86, 1117–1121. [Google Scholar] [CrossRef]
- Valkov, V.F.; Kazeev, K.S.; Kolesnikov, S.I. Soil Science: Textbook for Universities; ICC “Mart”: Rostov n/D, Russia; Publishing Center “Mart”: Moscow, Russia, 2004; 496p. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010; p. 548. [Google Scholar]
- Kolesnikov, S.I.; Timoshenko, A.N.; Kazeev, K.S.; Akimenko, Y.V.; Myasnikova, M.A. Ecotoxicity of copper, nickel, and zinc nanoparticles assessment on the basis of biological indicators of chernozems. Eurasian Soil Sci. 2019, 52, 982–987. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Kazeev, K.S.; Akimenko, Y.V. Development of regional standards for pollutants in the soil using biological parameters. Environ. Monit. Assess. 2019, 191, 544. [Google Scholar] [CrossRef]
- Zvyagintsev, D.G.; Bab’eva, I.P.; Zenova, G.M. Biologiya Pochv (Soil Biology); Moskow State University: Moscow, Russia, 2005; p. 445. ISBN 5211049837. [Google Scholar]
- Kolesnikov, S.I.; Kazeev, K.S.; Tatosyan, M.L.; Valkov, V.F. Influence of oil and oil products contamination on biological state of common chernozem. Soil Sci. 2006, 5, 616–620. [Google Scholar]
- Galstyan, A.S. On the stability of soil enzymes. Soil Sci. 1982, 4, 108–110. [Google Scholar]
- Kazeev, K.S.; Kolesnikov, S.I.; Valkov, V.F. Biological Diagnosis and Indication of Soils: Methodology and Methods of Research; Publishing House Growth: Rostov N/A, Russia; Unta: Moscow, Russia, 2003; 204p. [Google Scholar]
- Manikandana, M.; Wua, H.-F.; Hasana, N. Cell population-based mass spectrometry using platinum nanodots for algal and fungal studies. Biosens. Bioelectron. 2012, 35, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.; Ghosh, P.S.; Rotello, V.M.; Knapp, M.J. Disruption of protein-protein interactions using nanoparticles: Inhibition of cytochrome C peroxidase. Chem. Commun. 2006, 7, 1390–1392. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Nagy, K.L.; Marcus, M.A.; Lanson, M.; Geoffroy, N.; Jacquet, T.; Kirpichtchikova, T. Formation of metallic copper nanoparticles at the soil–root interface. Environ. Sci. Technol. 2008, 42, 1766–1772. [Google Scholar] [CrossRef]
- Gagnon, Z.E.; Newkirk, C.; Hicks, S. Impact of platinum group metals on the environment: A toxicological, genotoxic and analytical chemistry study. J. Environ. Sci. Health 2006, 41, 397–414. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S. The influence of silicon on the growth of barley, photosynthesis and ultrastructure in chromium-stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef]
- Yadav, G.; Shrivastava, P.K.; Singh, V.P.; Prasad, S.M. The intensity of light changes the degree of arsenic toxicity in seedlings Helianthus annuus L. Biol. Trace Elem. Res. 2014, 158, 410–421. [Google Scholar] [CrossRef]
- Xiong, Z.T.W.H. Copper toxicity and bioaccumulation in Chinese cabbage (Brassica pekinensis Rupr.). Environ. Toxicol. 2005, 20, 188–194. [Google Scholar] [CrossRef]
- Stejskal, K.; Šupálková, V.; Zítka, O.; Húska, D.; Sures, B.; Beklová, M.; Pikula, J.; Horna, A.; Havel, L.; Kizek, R. Affecting of various plant models by cisplatin. Listy Cukrov. A Reparske 2007, 123, 328–329. [Google Scholar]
- Babula, P.; Adam, V.; Opatrilova, R.; Zehnalek, J.; Havel, L.; Kizek, R. Uncommon heavy metals, metalloids and their plant toxicity: A review. Environ. Chem. Lett. 2008, 6, 189–213. [Google Scholar] [CrossRef]
- Schäfer, J.; Hannker, D.; Eckhardt, J.-D.; Stüben, D. Uptake of traffic-related heavy metals and platinum group elements (PGE) by plants. Sci. Total Environ. 1998, 215, 59–67. [Google Scholar] [CrossRef]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health Concerns of Various Nanoparticles: A Review of Their in Vitro and in Vivo Toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; He, L.; Ma, B.; Chen, T. High-drug-loading mesoporous silica nanorods with reduced toxicity for precise cancer therapy against nasopharyngeal carcinoma. Adv. Funct. Mater. 2017, 27, 1703313. [Google Scholar] [CrossRef]
- Zhang, K. Integration of er stress, oxidative stress and the inflammatory response in health and disease. Int. J. Clin. Exp. Med. 2010, 3, 33–40. [Google Scholar] [PubMed]
- Lai, L.; Li, S.-J.; Feng, J.; Mei, P.; Ren, Z.H.; Chang, Y.-L.; Liu, Y. Effects of Surface Charges on the Bactericide Activity of CdTe/ZnS Quantum Dots: A Cell Membrane Disruption Perspective. Langmuir 2017, 33, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Acc. Chem. Res. 2019, 52, 1632–1642, Epub 2019 Jun 3. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Liu, D.; Chen, J.; Yang, Z.; Dou, R.; Gao, X.; Wang, L. Effects of metal oxide nanoparticles on soil enzyme activities and bacterial communities in two different soil types. J. Soil. Sediment. 2018, 18, 211–221. [Google Scholar] [CrossRef]
- Galaktionova, L.; Gavrish, I.; Lebedev, S. Bioeffects of Zn and Cu Nanoparticles in Soil Systems. Toxicol. Environ. Health Sci. 2019, 11, 259–270. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Timoshenko, A.; Minnikova, T.; Tsepina, N.; Kazeev, K.; Akimenko, Y.; Zhadobin, A.; Shuvaeva, V.; Rajput, V.D.; Mandzhieva, S.; et al. Impact of Metal-Based Nanoparticles on Cambisol Microbial Functionality, Enzyme Activity, and Plant Growth. Plants 2021, 10, 2080. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in plant science: To make a long story short, Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef]
- Wang, W.; Ren, Y.; He, J.; Zhang, L.; Wang, X.; Cui, Z. Impact of copper oxide nanoparticles on the germination, seedling growth, and physiological responses in Brassica pekinensis L. Environ. Sci. Pollut. Res. 2020, 27, 31505–31515. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Sheikhbahaei, M.; Mohamadi, N.; Pabarja, A.; Abadi, M.F.S.; Tahroudi, M.H.M. Biosynthesis of Zinc Oxide Nanoparticles Using Hertia intermedia and Evaluation of its Cytotoxic and Antimicrobial Activities. BioNanoScience 2021, 11, 245–255. [Google Scholar] [CrossRef]
- Venzhik, Y.V.; Moshkov, I.E.; Dykman, L.A. Influence of Nanoparticles of Metals and Their Oxides on the Photosynthetic Apparatus of Plants. Biol. Bul. Russ. Acad. Sci. 2021, 48, 140–155. [Google Scholar] [CrossRef]
- Rajput, V.; Chaplygin, V.; Gorovtsov, A.; Fedorenko, A.; Azarov, A.; Chernikova, N.; Barakhov, A.; Minkina, T.; Maksimov, A.; Mandzhieva, S.; et al. Assessing the toxicity and accumulation of bulk- and nano-CuO in Hordeum sativum L. Environ. Geochem. Health 2020, 43, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, A.; Kolesnikov, S.; Varduni, V.; Ter-Misakyants, T.; Nevedomaya, E.; Kazeev, K. Assessment of Ecotoxicity of Copper Nanoparticles. Ecol. Indus. Russia 2021, 25, 61–65. (In Russian) [Google Scholar] [CrossRef]
- Jackson, M.T.; Prichard, H.M.; Sampson, J. Platinum-group elements in sewage sludge and incinerator ash in the United Kingdom: Assessment of PGE sources and mobility in cities. Sci. Total Environ. 2010, 408, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Zehnalek, J.; Adam, V.; Beklova, M.; Kizek, R. The effects on soil/water/plant/animal systems by platinum group elements. Cent. Eur. J. Chem. 2012, 10, 1369–1382. [Google Scholar] [CrossRef]
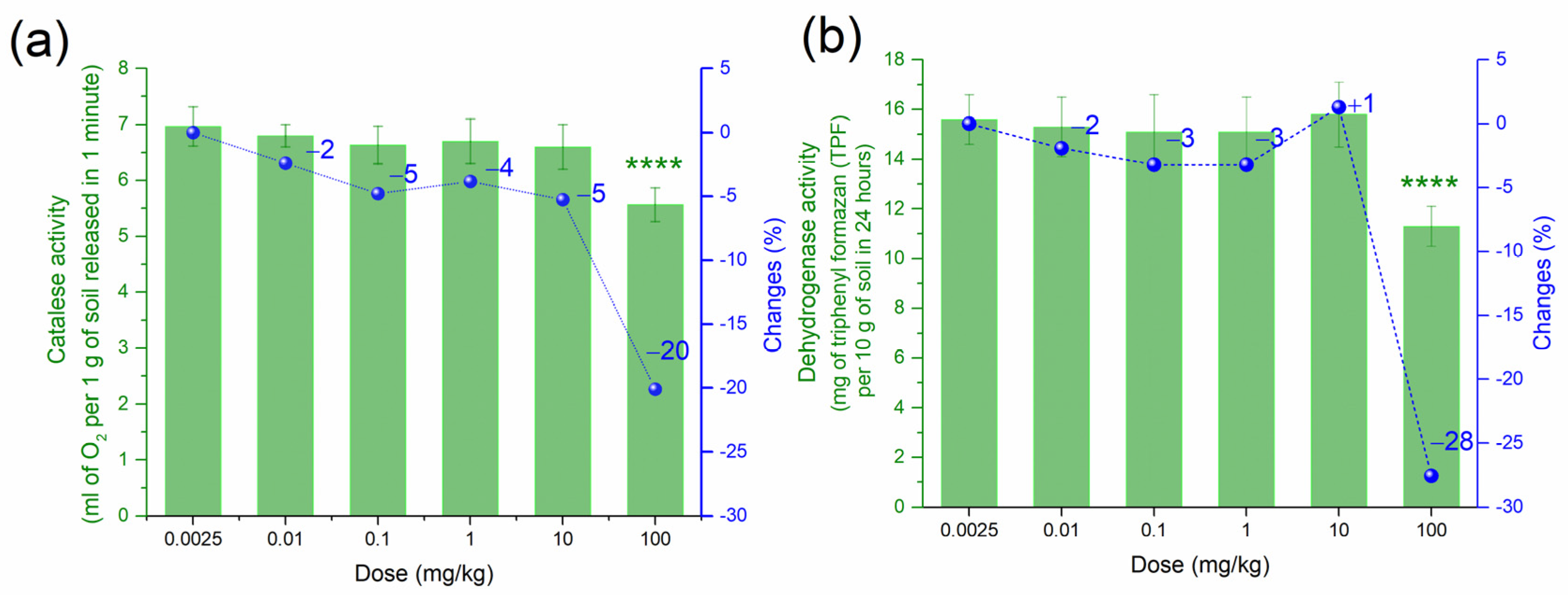
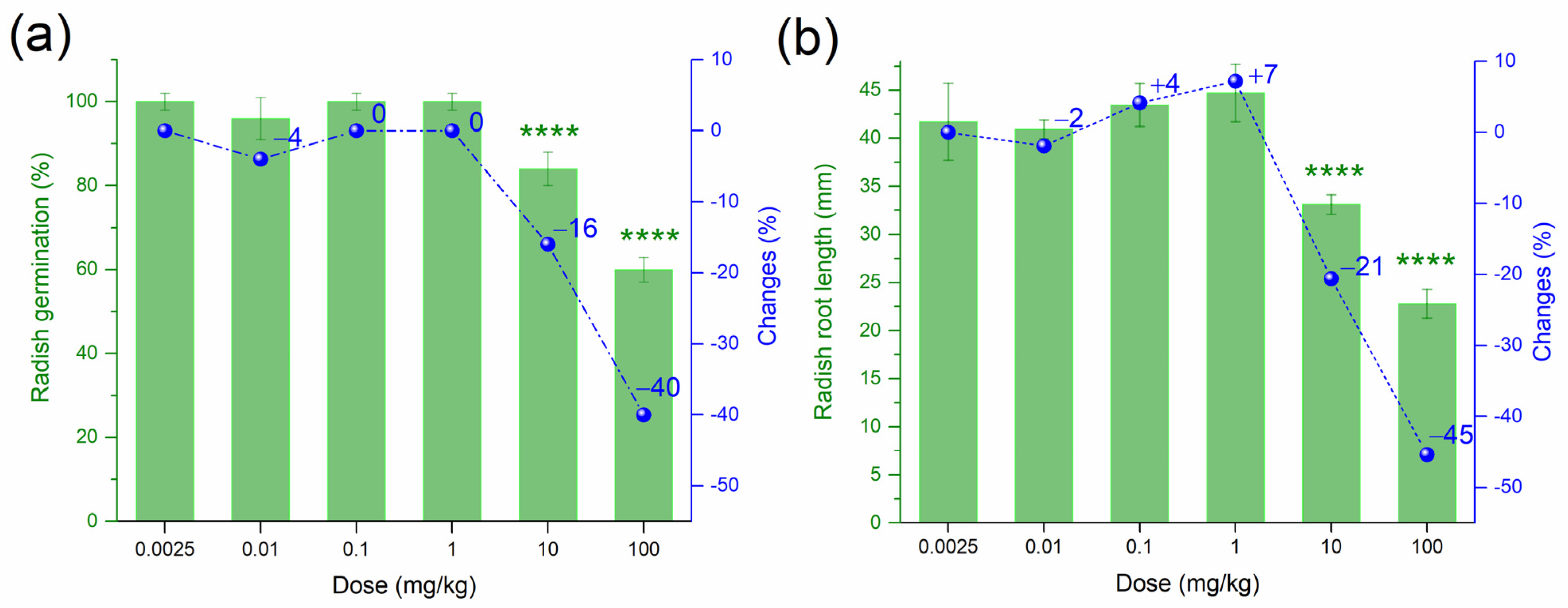
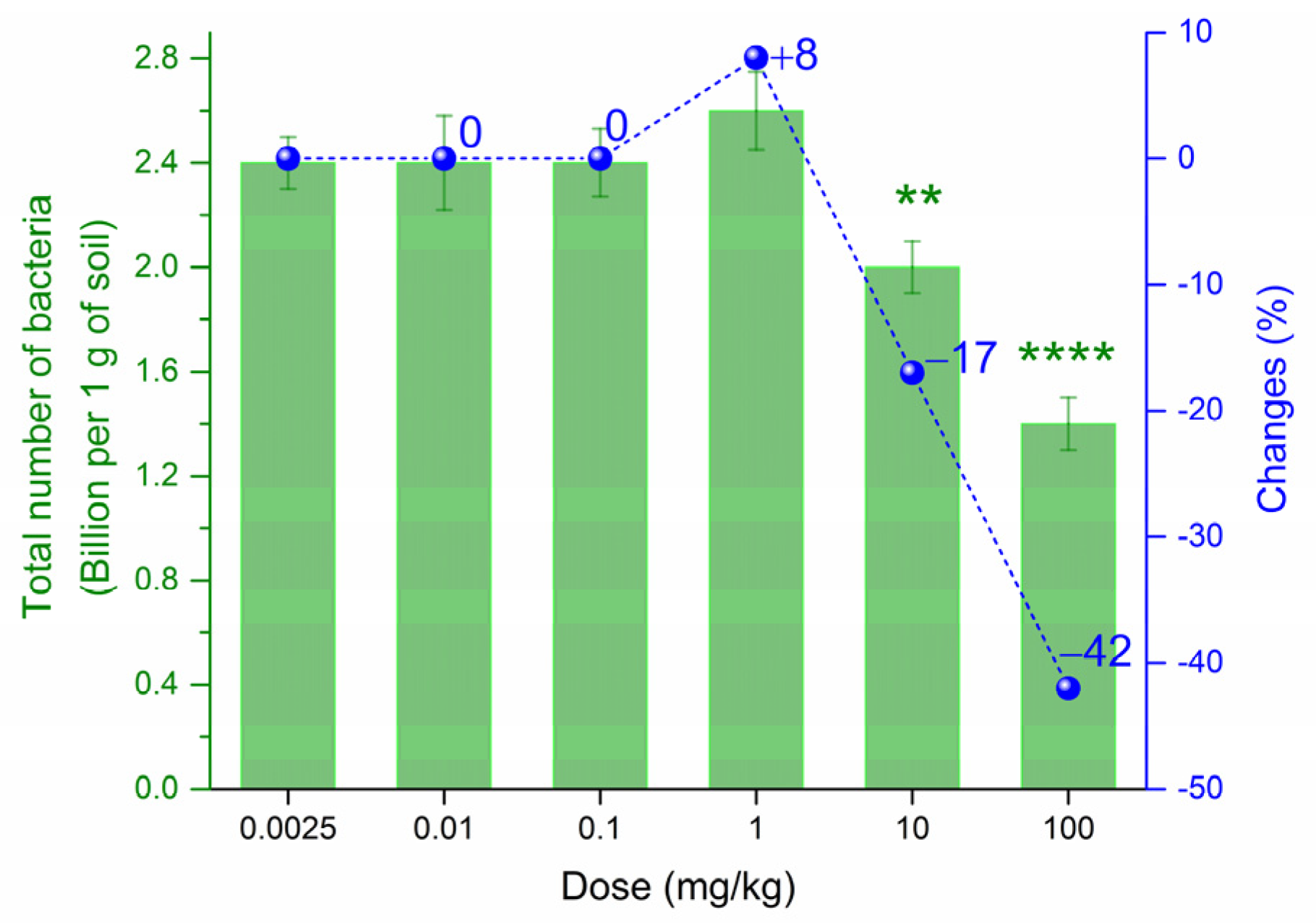
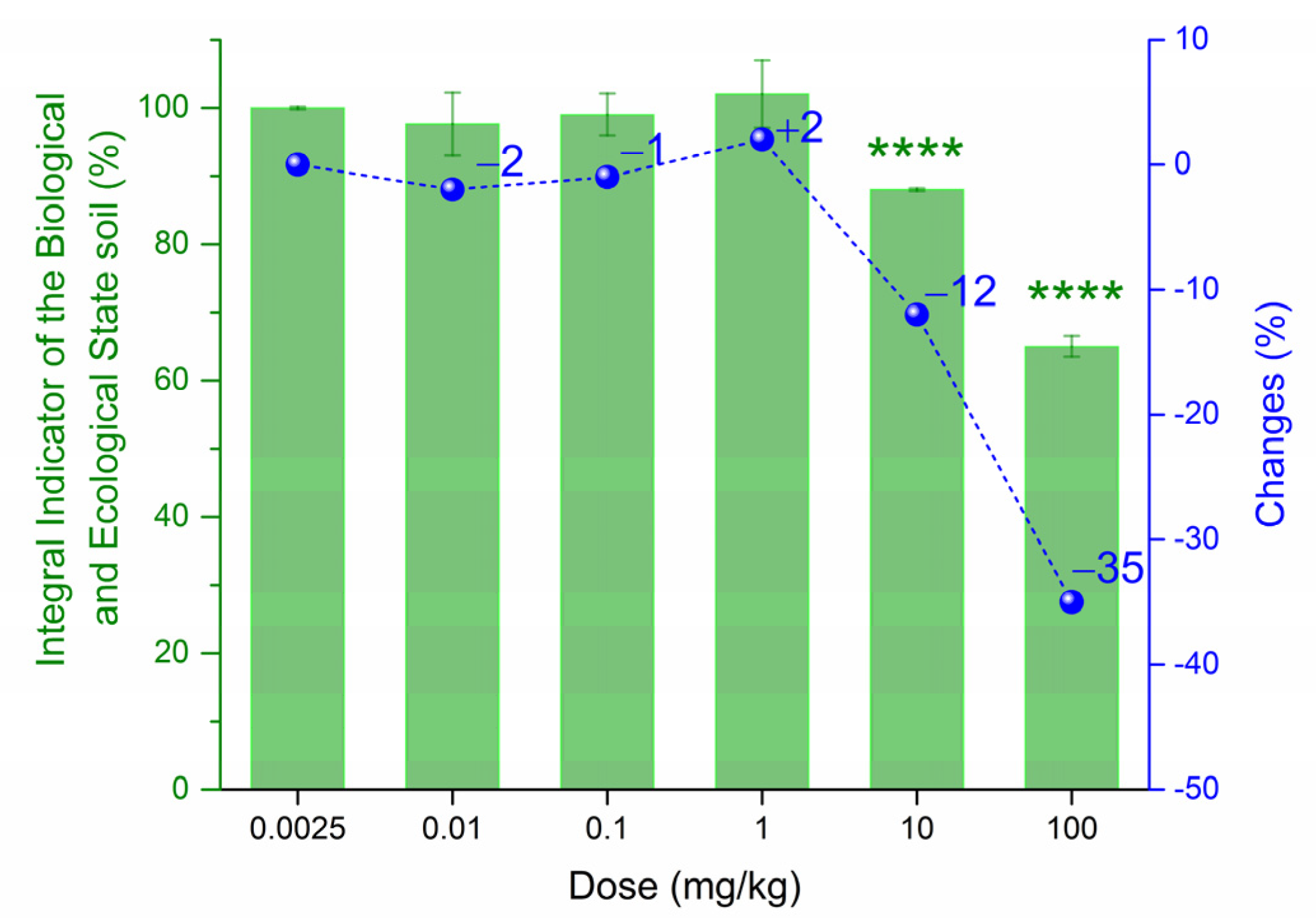
| Biological Indicator | Sensitivity 1 | Informativeness 2 |
|---|---|---|
| Catalase activity | 92 | −0.93 * |
| Dehydrogenases activity | 93 | −0.87 * |
| Germination of radish (R. sativus L.) seeds | 88 | −0.95 * |
| Length of radish (R. sativus L.) roots | 89 | −0.91 * |
| Total number of bacteria | 90 | −0.92 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnikov, S.; Timoshenko, A.; Kabakova, V.; Minnikova, T.; Tsepina, N.; Kazeev, K.; Minkina, T.M.; Shende, S.S.; Mandzhieva, S.S.; Tsitsuashvili, V.; et al. Effect of Platinum Nanoparticles (PtNPs) Pollution on the Biological Properties of Haplic Cambisols Eutric of the Caucasus Forests. Forests 2023, 14, 54. https://doi.org/10.3390/f14010054
Kolesnikov S, Timoshenko A, Kabakova V, Minnikova T, Tsepina N, Kazeev K, Minkina TM, Shende SS, Mandzhieva SS, Tsitsuashvili V, et al. Effect of Platinum Nanoparticles (PtNPs) Pollution on the Biological Properties of Haplic Cambisols Eutric of the Caucasus Forests. Forests. 2023; 14(1):54. https://doi.org/10.3390/f14010054
Chicago/Turabian StyleKolesnikov, Sergey, Alena Timoshenko, Victoria Kabakova, Tatiana Minnikova, Natalia Tsepina, Kamil Kazeev, Tatiana M. Minkina, Sudhir S. Shende, Saglara S. Mandzhieva, Victoria Tsitsuashvili, and et al. 2023. "Effect of Platinum Nanoparticles (PtNPs) Pollution on the Biological Properties of Haplic Cambisols Eutric of the Caucasus Forests" Forests 14, no. 1: 54. https://doi.org/10.3390/f14010054
APA StyleKolesnikov, S., Timoshenko, A., Kabakova, V., Minnikova, T., Tsepina, N., Kazeev, K., Minkina, T. M., Shende, S. S., Mandzhieva, S. S., Tsitsuashvili, V., & Sushkova, S. N. (2023). Effect of Platinum Nanoparticles (PtNPs) Pollution on the Biological Properties of Haplic Cambisols Eutric of the Caucasus Forests. Forests, 14(1), 54. https://doi.org/10.3390/f14010054












