The Effect of Surface Treatment on the Antibacterial Properties of Wood and the Possibility to Detect the Antibacteriality with Fluorescence Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Material Selection and Specimen Preparation
2.2. Wood Specimen Surface Quality Verification by Roughness Measurements
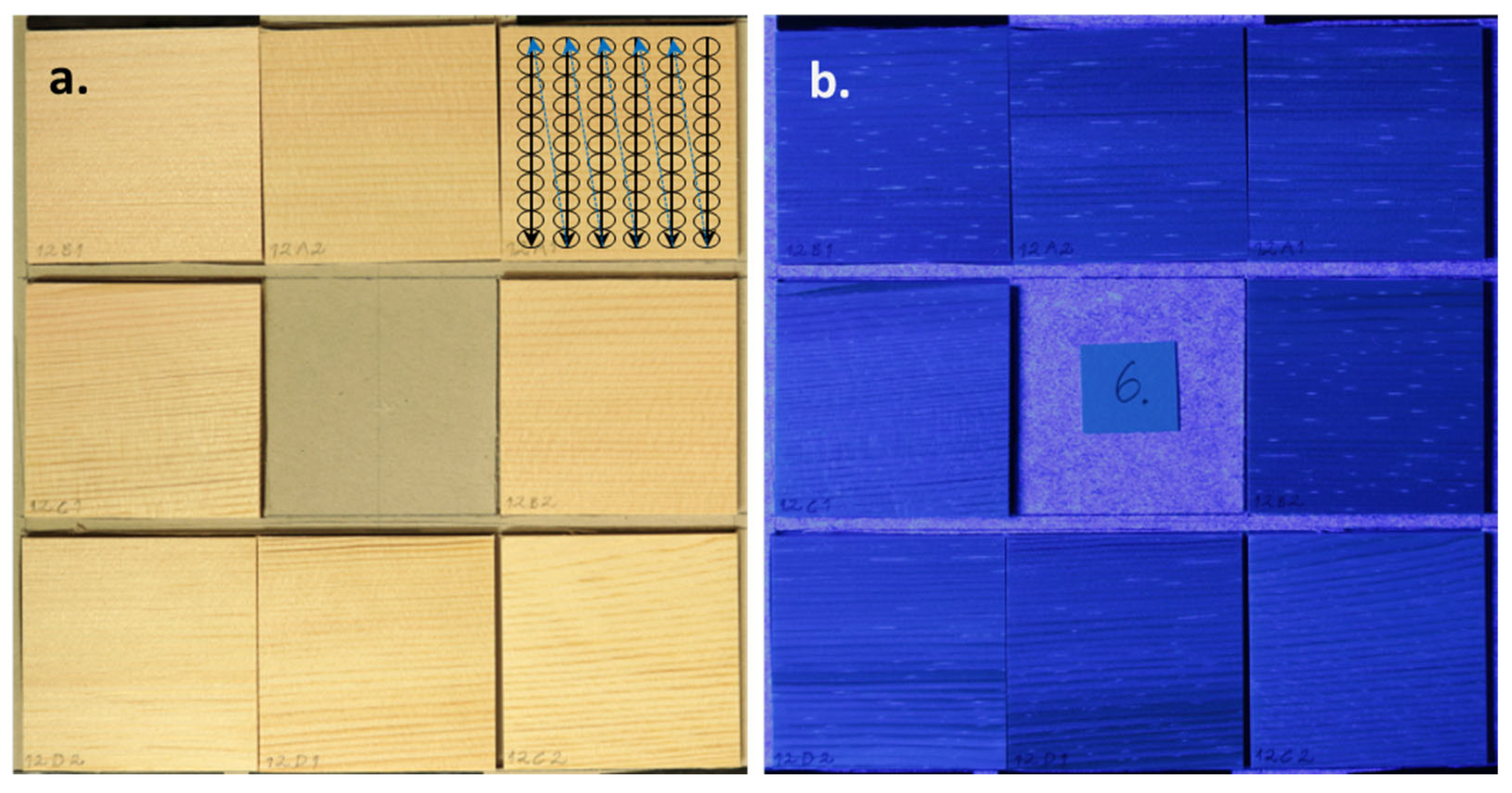
2.3. Selection of Scots Pine Heartwood Samples Based on Their UV-Excited Fluorescence
2.4. Preparation and Spreading of Bacterial Suspension
2.5. Estimation of Bacterial Viability on the Surfaces
2.6. Statistical Analyses
3. Results
3.1. Surface Roughness
3.2. Growth of Bacterial Colonies on Low- and High-Fluorescence Scots Pine Heartwood Samples
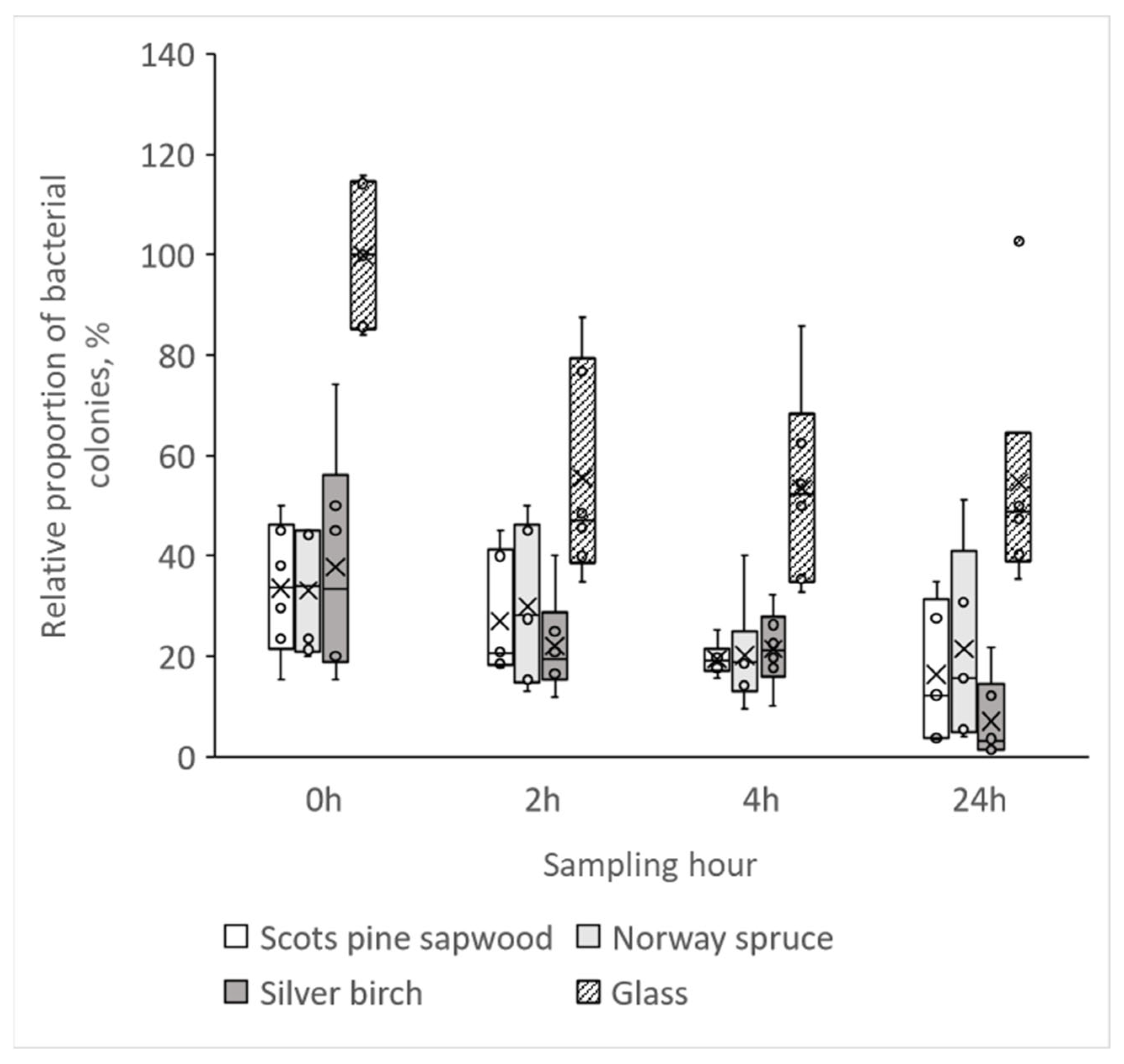
3.3. Growth of Bacterial Colonies on Untreated Wood Materials
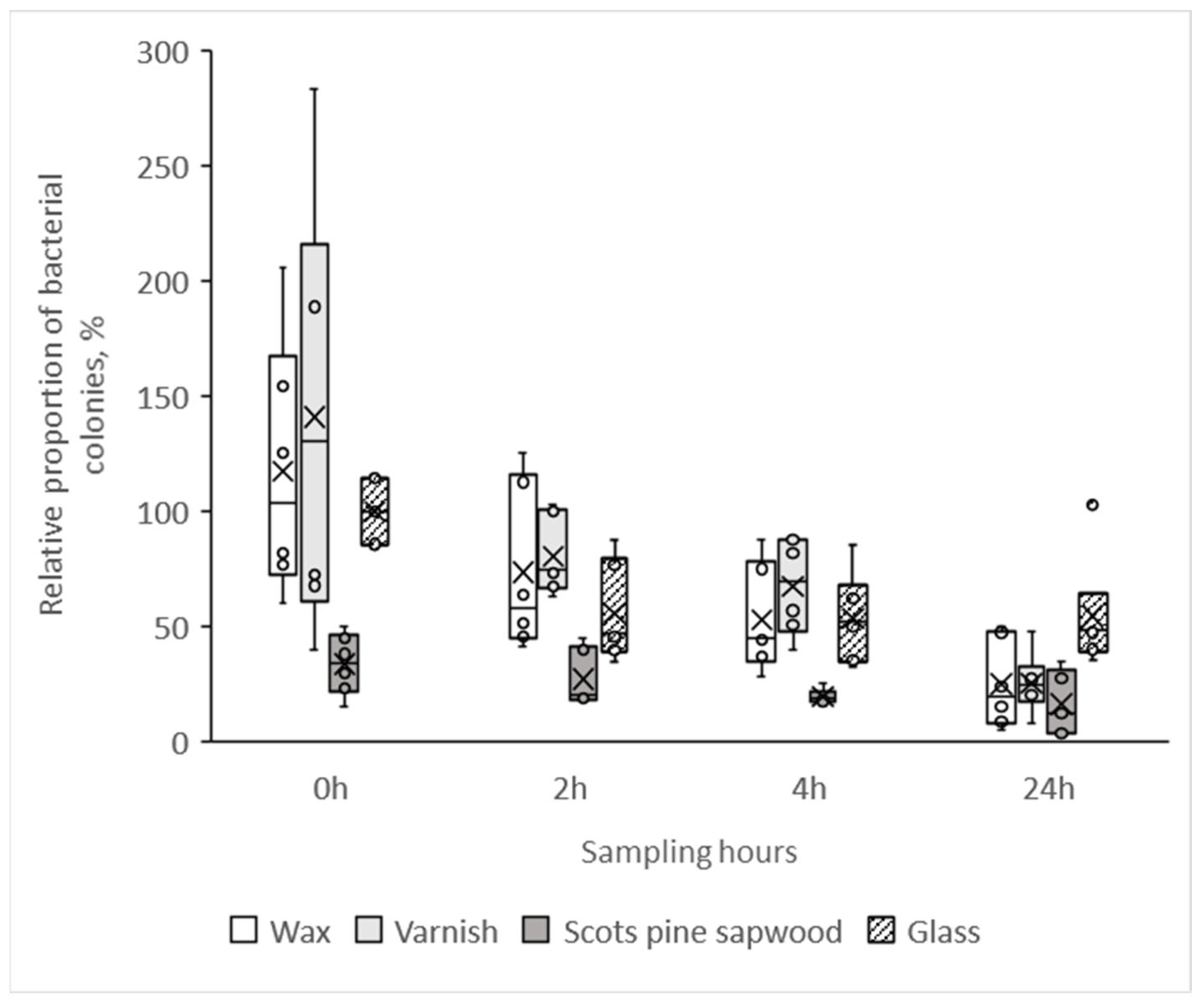
3.4. Growth of Bacterial Colonies on the Untreated and the Surface Treated Scots Pine Heartwood Specimen
4. Discussion
5. Conclusions
- -
- Both surface coatings, wax and varnish, had a similar number of bacteria at each time point as the glass reference, which clearly shows that these coatings inhibit the antibacterial properties of wood.
- -
- The relative proportion of viable bacteria was lower on all untreated wood specimens, also on birch, compared to the number of bacteria on the glass surface, showing that all studied wood species have antibacterial properties.
- -
- The relative proportion of viable bacteria at the 24 h time point was the lowest on the high fluorescence Scots pine heartwood.
- -
- This study showed that the fluorescence method for predicting the antibacterial properties of Scots pine heartwood was successfully applied.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsunetsugu, Y.; Miyazaki, Y.; Sato, H. Physiological effects in humans induced by the visual stimulation of room interiors with different wood quantities. J. Wood Sci. 2007, 53, 11–16. [Google Scholar] [CrossRef]
- Nyrud, A.Q.; Bringslimark, T. Is interior wood use psychologically beneficial? A review of psychological responses toward wood. Wood Fiber Sci. 2010, 42, 202–218. [Google Scholar]
- Vainio-Kaila, T. Antibacterial Properties of Scots Pine and Norway Spruce. Ph.D. Thesis, Aalto University, Espoo, Finland, 2017; p. 179. [Google Scholar]
- Milling, A.; Kehr, R.; Wulf, A.; Smalla, K. Survival of bacteria on wood and plastic particles: Dependence on wood species and environmental conditions. Holzforschung 2005, 59, 72–81. [Google Scholar] [CrossRef]
- Milling, A.; Smalla, K.; Kehr, R.; Wulf, A. The use of wood in practice—A hygienic risk? Holz als Roh-und Werkst. 2005, 63, 463–472. [Google Scholar] [CrossRef]
- Munir, M.T.; Pailhories, H.; Eveillard, M.; Aviat, F.; Lepelletier, D.; Belloncle, C.; Federighi, M. Antimicrobial Characteristics of Untreated Wood: Towards a Hygienic Environment. Health 2019, 11, 152–170. [Google Scholar] [CrossRef][Green Version]
- Ak, N.O.; Cliver, D.O.; Kaspar, C.W. Cutting boards of plastic and wood contaminated experimentally with bacteria. J. Food Prot. 1994, 57, 16–22. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Kyyhkynen, A.; Rautkari, L.; Siitonen, A. Antibacterial effects of extracts of pinus sylvestris and picea abies against staphylococcus aureus, enterococcus faecalis, escherichia coli, and streptococcus pneumoniae. BioResources 2015, 10, 7763–7771. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Zhang, X.; Hänninen, T.; Kyyhkynen, A.; Johansson, L.-S.; Willför, S.; Österberg, M.; Siitonen, A.; Rautkari, L. Antibacterial effects of wood structural components and extractives from pinus sylvestris and picea abies on methicillin-resistant Staphylococcus aureus and Escherichia coli O157: H7. BioResources 2017, 14, 7601–7614. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and lipophilic extractives in Scots pine knots and stemwood. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Eckerman, C.; Holmbom, B. Lignans and lipophilic extractives in Norway spruce knots and stemwood. Holzforschung 2003, 57, 27–36. [Google Scholar] [CrossRef]
- Söderberg, T.A.; Gref, R.; Holm, S.; Elmros, T.; Hallmans, G. Antibacterial activity of rosin and resin acids in vitro. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 199–205. [Google Scholar] [CrossRef]
- Bien-Aime, S.; Yu, W.; Uhrich, K.E. Pinosylvin-Based Polymers: Biodegradable Poly(Anhydride-Esters) for Extended Release of Antibacterial Pinosylvin. Macromol. Biosci. 2016, 16, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, L.E.; Holmbom, B.R. Antibacterial effects of knotwood extractives on paper mill bacteria. J. Ind. Microbiol. Biotechnol. 2004, 31, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, A.; Harju, A.M.; Venäläinen, M.; Saranpää, P.; Laakso, T. FT-NIR spectroscopy in predicting the decay resistance related characteristics of solid Scots pine (Pinus sylvestris L.) heartwood. Holzforschung 2008, 62, 284–288. [Google Scholar] [CrossRef]
- Munir, M.T.; Pailhoriès, H.; Aviat, F.; Lepelletier, D.; Le Pape, P.; Dubreil, L.; Irle, M.; Buchner, J.; Eveillard, M.; Federighi, M.; et al. Hygienic Perspectives of Wood in Healthcare Buildings. Hygiene 2021, 1, 2. [Google Scholar] [CrossRef]
- Antikainen, J.; Hirvonen, T.; Kinnunen, J.; Hauta-Kasari, M. Heartwood detection for Scotch pine by fluorescence image analysis. Holzforschung 2012, 66, 877–881. [Google Scholar] [CrossRef]
- Belt, T.; Venäläinen, M.; Harju, A. Non-destructive measurement of Scots pine heartwood stilbene content and decay resistance by means of UV-excited fluorescence spectroscopy. Ind. Crops Prod. 2021, 164, 113395. [Google Scholar] [CrossRef]
- Namvar, A.E.; Bastarahang, S.; Abbasi, N.; Ghehi, G.S.; Farhadbakhtiarian, S.; Arezi, P.; Hosseini, M.; Baravati, S.Z.; Jokar, Z.; Chermahin, S.G. Clinical characteristics of Staphylococcus epidermidis: A systematic review. GMS Hyg. Infect. Control 2014, 9, Doc23. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Routa, J.; Anttila, P.; Asikainen, A. Wood Extractives of Finnish Pine, Spruce and Birch—Availability and Optimal Sources of Compounds: A Literature Review; Natural Resources Institute: Helsinki, Finlabd, 2017; Available online: https://jukuri.luke.fi/handle/10024/540829 (accessed on 10 December 2022)ISBN 978-952-326-495-3.
- Katzenberger, R.H.; Rösel, A.; Vonberg, R.-P. Bacterial survival on inanimate surfaces: A field study. BMC Res. Notes 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Makison, C.; Swan, J. The Effect of Humidity on the Survival of MRSA on Hard Surfaces. Indoor Built Environ. 2006, 15, 85–91. [Google Scholar] [CrossRef]
- Välimaa, A.-L.; Honkalampi-Hämäläinen, U.; Pietarinen, S.; Willför, S.; Holmbom, B.; Von Wright, A. Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int. J. Food Microbiol. 2007, 115, 235–243. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.-E.; Eklund, P.; Willför, S.; Alakomi, H.-L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Venäläinen, M.; Julkunen-Tiitto, R.; Harju, A.M. Stilbene impregnation retards brown-rot decay of Scots pine sapwood. Holzforschung 2016, 70, 261–266. [Google Scholar] [CrossRef]
- Fries, A.; Ericsson, T.; Gref, R. High heritability of wood extractives in Pinus sylvestris progeny tests. Can. J. For. Res. 2000, 30, 1707–1713. [Google Scholar] [CrossRef]
- Karppanen, O.; Venäläinen, M.; Harju, A.M.; Laakso, T. The effect of brown-rot decay on water adsorption and chemical composition of Scots pine heartwood. Ann. For. Sci. 2008, 65, 610. [Google Scholar] [CrossRef]
- Verkasalo, E.; Roitto, M.; Möttönen, V.; Tanner, J.; Kumar, A.; Kilpeläinen, P.; Sikanen, L.; Ilvesniemi, H. Extractives of Tree Biomass of Scots Pine (Pinus sylvestris L.) for Biorefining in Four Climatic Regions in Finland—Lipophilic Compounds, Stilbenes, and Lignans. Forests 2022, 13, 779. [Google Scholar] [CrossRef]
- Himejima, M.; Hobson, K.R.; Otsuka, T.; Wood, D.L.; Kubo, I. Antimicrobial terpenes from oleoresin of ponderosa pine tree Pinus ponderosa: A defense mechanism against microbial invasion. J. Chem. Ecol. 1992, 18, 1809–1818. [Google Scholar] [CrossRef]
- Smith, E.; Williamson, E.; Zloh, M.; Gibbons, S. Isopimaric acid from Pinus nigra shows activity against multidrug-resistant and EMRSA strains of Staphylococcus aureus. Phyther. Res. 2005, 19, 538–542. [Google Scholar] [CrossRef]
- Hofmann, T.; Albert, L.; Németh, L.; Vršanská, M.; Schlosserová, N.; Voběrková, S.; Visi-Rajczi, E. Antioxidant and antibacterial properties of norway spruce (Picea abies h. karst.) and eastern hemlock (tsuga canadensis (l.) carrière) cone extracts. Forests 2021, 12, 1189. [Google Scholar] [CrossRef]
- Fyhrquist, P.; Virjamo, V.; Hiltunen, E.; Julkunen-Tiitto, R. Epidihydropinidine, the main piperidine alkaloid compound of Norway spruce (Picea abies) shows promising antibacterial and anti-Candida activity. Fitoterapia 2017, 117, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Virjamo, V.; Fyhrquist, P.; Koskinen, A.; Lavola, A.; Nissinen, K.; Julkunen-Tiitto, R. 1,6-Dehydropinidine Is an Abundant Compound in Picea abies (Pinaceae) Sprouts and 1,6-Dehydropinidine Fraction Shows Antibacterial Activity against Streptococcus equi Subsp. Equi. Molecules 2020, 25, 4558. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.; Ingraham, J.; Wheelis, M.; Painter, P. General Microbiology; MacMillan Education: London, UK, 1987. [Google Scholar]
- Hiltunen, E.; Pakkanen, T.T.; Alvila, L. Phenolic compounds in silver birch (Betula pendula Roth) wood. Holzforschung 2006, 60, 519–527. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kahkonen, M.; Heinonen, M.; Maatta-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Tomppo, L.; Tiitta, M.; Laakso, T.; Harju, A.; Venäläinen, M.; Lappalainen, R. Study of stilbene and resin acid content of Scots pine heartwood by electrical impedance spectroscopy (EIS). Holzforschung 2011, 65, 643–649. [Google Scholar] [CrossRef]
- Ericsson, T.; Fries, A.; Gref, R. Genetic correlations of heartwood extractives in pinus sylvestris progeny tests. For. Genet. 2001, 8, 73–79. [Google Scholar]
- ASipponen, A.; Laitinen, K. Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS 2011, 119, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Salmela, A.; Kulmala, I.; Karvinen, A.; Taillebot, V.; Weiss, P.; Gobert, T.; Berthier, A.; Guarnieri, V.; Raffestin, S.; Pasanen, P. Measurement and Simulation of Biocontamination in an Enclosed Habitat. Aerosol Sci. Eng. 2020, 4, 101–110. [Google Scholar] [CrossRef]

| Wood Species | Surface Treatment of Wood | Testing Methods | |||
|---|---|---|---|---|---|
| Surface Roughness | UV-Excited Fluorescence | Bacterial Viability on the Surfaces | |||
| Norway spruce | Sapwood | untreated | x | x | |
| Silver birch | Sapwood | untreated | x | x | |
| Scots pine | Sapwood | untreated | x | x | |
| Heartwood | untreated | x | x | ||
| Sapwood | Wax coating Akviwax Satin | x | x | ||
| Sapwood | Varnish Akvilac FD-J 10 | x | x | ||
| Fluorescence Group | Number of Samples | Imax | |
|---|---|---|---|
| Average, a.u. * | CV, % | ||
| Low fluorescence | 48 | 10,192 | 9 |
| Mixed fluorescence | 99 | 13,734 | 9 |
| High fluorescence | 52 | 17,988 | 13 |
| All samples | 199 | 13,991 | 23 |
| Wood Species | Surface Treatment | Image of Surface | Roughness, µm Ra (Sdev.) * |
|---|---|---|---|
| Norway spruce (Picea abies (L.) H. Karst.) | untreated |  | 9.5 (2.03) |
| Silver birch (Betula pendula Roth) | untreated |  | 11.8 (3.83) |
| Scots pine (Pinus sylvestris L.) | untreated |  | 13.7 (4.72) |
| Scots pine (Pinus sylvestris L.) | Wax coating Akviwax Satin, application rate 65–70 g/m2 |  | 11.6 (2.78) |
| Scots pine (Pinus sylvestris L.) | Varnish Akvilac FD-J 10), gloss 10, application rate 2 × 100 g/m2 |  | 13.0 (4.92) |
| Sampling Hour | Average Number of Bacterial Colonies | ||||||
|---|---|---|---|---|---|---|---|
| Glass | CV% | LF | CV% | HF | CV% | P a | |
| 0 h | 350 | 12 | 85 | 26 | 110 | 41 | 0.183 |
| 2 h | 260 | 11 | 53 | 36 | 109 | 33 | 0.021 |
| 24 h | 198 | 2 | 66 | 40 | 30 | 22 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vainio-Kaila, T.; Harju, A.; Rohumaa, A.; Paajanen, O.; Venäläinen, M.; Seppä, J.; Veijalainen, A.-M.; Pasanen, P. The Effect of Surface Treatment on the Antibacterial Properties of Wood and the Possibility to Detect the Antibacteriality with Fluorescence Method. Forests 2023, 14, 23. https://doi.org/10.3390/f14010023
Vainio-Kaila T, Harju A, Rohumaa A, Paajanen O, Venäläinen M, Seppä J, Veijalainen A-M, Pasanen P. The Effect of Surface Treatment on the Antibacterial Properties of Wood and the Possibility to Detect the Antibacteriality with Fluorescence Method. Forests. 2023; 14(1):23. https://doi.org/10.3390/f14010023
Chicago/Turabian StyleVainio-Kaila, Tiina, Anni Harju, Anti Rohumaa, Olli Paajanen, Martti Venäläinen, Julia Seppä, Anna-Maria Veijalainen, and Pertti Pasanen. 2023. "The Effect of Surface Treatment on the Antibacterial Properties of Wood and the Possibility to Detect the Antibacteriality with Fluorescence Method" Forests 14, no. 1: 23. https://doi.org/10.3390/f14010023
APA StyleVainio-Kaila, T., Harju, A., Rohumaa, A., Paajanen, O., Venäläinen, M., Seppä, J., Veijalainen, A.-M., & Pasanen, P. (2023). The Effect of Surface Treatment on the Antibacterial Properties of Wood and the Possibility to Detect the Antibacteriality with Fluorescence Method. Forests, 14(1), 23. https://doi.org/10.3390/f14010023






