Abstract
In order to explore the influence of climate warming on soil microbial metabolism in the ecosystem and reveal the relationship between soil microbial metabolism limitation and environmental factors, in this study, the effects of warming on soil enzyme activities and nutrient availability were investigated by setting underground heating cables at 2 °C and 4 °C soil warming in a typical Quercus acutissima forest in the northern subtropics, and enzyme stoichiometric models were used to evaluate the limits of soil microbial metabolism. The results showed that soil warming significantly increased the activities of β-1,4-glucosidase (BG) and L-leucine aminopeptidase (LAP), and significantly increased the contents of nitrate nitrogen (NO3−-N) and available phosphorus (AP) in soil. The soil warming increased soil microbial C limitation and alleviated soil microbial P limitation. Our study showed that the change of soil microbial C and P limitation caused by warming may cause a large amount of SOM decomposition in a short period, leading to a large fluctuation of soil carbon turnover, which is not conducive to the stability of the soil C pool. This study provides important insights linking microbial metabolism to soil warming and improves our understanding of C cycling in forest systems.
1. Introduction
Global climate change is one of the global issues that society is concerned about, and its most direct manifestation is global climate warming. Over the last century of observations, the average global temperature has risen by 1.07 °C [1]. According to the simulation results of the CMIP 5 model, by the end of the 21st century, the global average surface soil temperature may rise as high as 2.6~4.8 °C compared with the period from 1986 to 2005, which is most serious environmental problem facing mankind at present. As key biological factors in the terrestrial ecosystem, soil microorganisms play an important role in regulating soil nutrient cycling and are sensitive to soil temperature [2]. Soil microorganisms influence nutrient cycling by producing various enzymes that regulate the decomposition and mineralization of soil organic matter [3,4], which in turn provide energy and available nutrients for soil microbial metabolic activities [5,6]. Therefore, soil enzymes are key participants in microbial metabolism and soil organic matter decomposition, and soil enzymatic stoichiometry can be used as an important index to evaluate soil nutrient availability and soil microbial metabolism [7]. The carbon-metabolizing enzyme β-1,4-glucosidase (BG), nitrogen-metabolizing enzymes β-1,4-N-acetylglucosidase (NAG) and L-leucine aminopeptidase (LAP), and phosphorous-metabolizing enzyme acid phosphatase (ACP) are usually the main research objects [8]. Because these four enzymes can catalyze the generation of bioavailable terminal monomers [9], this process is closely related to nutrient cycling in the ecosystem. At present, there is no uniform conclusion on the response of soil enzyme activities to soil temperature change. Melillo et al. [10] showed that increasing soil temperature would enhance the activities of microorganisms and enzymes, accelerate the decomposition of soil organic matter, enhance soil respiration, and positively affect global change. Liu et al. [11] found that changes such as the decrease of soil water caused by the increase in soil temperature would reduce the availability of soil water and affect the metabolic activities and enzyme activities of soil microorganisms by limiting the diffusion loss of reaction substrates. Many studies showed that the soil enzyme activity was significantly affected by many temperature-related factors, including temperature increase amplitude, soil moisture content, and temperature increase duration [12,13,14].
Given that temperature is an important driver of ecosystem processes [15], climate warming is bound to affect soil enzyme activities and change ecosystem C, N, and P cycles, which in turn change the nutrient limitation of soil microorganisms [16]. Zheng et al. [17] found short-term soil warming decreased microbial C limitation and increased microbial P limitation by affecting soil available nutrients and soil water content. Therefore, with the background of global warming, it is of great significance to elucidate the variation characteristics of soil enzyme activities for studying soil nutrient availability, microbial nutrient metabolism, and soil C pool stability in specific regions. Moreover, Moorhead et al. [16,18] proposed quantifying soil microorganisms’ relative C limitation by vector length and the relative N and P limitation of soil microorganisms by vector angle, which can directly reflect the relative nutrient requirements of soil microorganisms. This method can help us intuitively understand the soil microbial metabolism in the ecosystem with the background of global warming [19] and reveal the relationship between soil microbial metabolism limitation and environmental factors [4]. It is helpful to elucidate the mechanism of soil C, N, and P cycling and thus improve our ability to predict soil C stocks under climate warming [20].
The existing soil warming experiments focus on temperate and tropical ecosystems, and few studies have been conducted in the northern subtropical region, which is particularly sensitive to climate change. To comprehensively assess the effects of climate warming on soil enzyme activities, soil nutrient availability, and soil microbial metabolism characteristics in the north subtropical forest ecosystem, this study took a Quercus acutissima forest in the Northern subtropical region as the research subject, and soil climate warming to increase soil temperature (warming by 2 °C, 4 °C) by burying heating cables. Therefore, we hypothesized that: (1) soil warming would have a positive effect on soil enzyme activities; (2) soil warming would decrease microbial C limitation and increase microbial P limitation; and (3) soil water content would be the key factor affecting microbial metabolism limitation.
2. Materials and Methods
2.1. Study Site
The study area is located in Zhenjiang, Jiangsu Province, which belongs to the north subtropical monsoon climate zone with four distinct seasons and sufficient light. The annual average temperature is 15.2 °C, the highest temperature is 39.6 °C, and the lowest temperature is −16.7 °C. The average annual precipitation is 1055.6 mm, with a great inter-annual variation. The average annual relative humidity is 79%. The study area is located in the hilly region of Jianghuai, and the terrain is mostly hilly and gentle. The soil layer thickness is generally 40~60 cm, and the soil pH is 4.5~5.0. The forest vegetation belongs to the east of the north subtropical region of China, and the zonal vegetation is a deciduous broad-leaved mixed forest with evergreen components. The test site is based on the Quercus acutissima forest. Shrubs mainly include Rosa multiflora, Fortunearia Sinensis, Callicarpa cathayana, Ilex cornuta, Symplocos paniculata, etc. Herbaceous plants mainly include Parthenocissus tricuspidata and Adiantum capillus-veneris [21].
2.2. Experimental Treatments
The study plots were set in Jurong City, Jiangsu Province, and three sample plots were arranged in a Q. acutissima forest with relatively uniform topography and slope, with a distance of more than 10 m from each other. Four 3 × 3 m subplots were set in each sample plot, a total of 12 subplots, with a spacing of more than 3 m between the subplots. Four treatments (soil temperature increase of 2 °C, soil temperature increase of 4 °C, disturbed control, and blank control) were randomly applied to four subplots in each sample plot. In December 2019, the same heating cables were laid in parallel in each plot. The heating power per unit area of the soil was calculated according to the plot area, which was arranged in parallel with a depth of 10 cm and a spacing of 20 cm and surrounded in the outermost circle to ensure the uniformity of warming in the plot. The temperature sensor buried at 10 cm was used to collect and compare the soil temperature data of the control field and the warming sample field. The temperature controller scanned every 10 min, and the relay switch was controlled by the temperature controller to warm the soil. The cables were arranged in the same way as before but without heating. Three months after the cable was laid out, it was powered on for warming (March 2020) to reduce the impact of soil disturbance.
2.3. Soil Sample Collection
In March 2021 (1 year of warming), soil samples ranging from 0 to 10 cm were collected in each subplot after removing surface litter according to the 5-point sampling method. Soil samples from 5 points in each plot were evenly mixed to collect a total of 12 bags of soil samples. The samples were quickly frozen with dry ice and brought back to the laboratory. Gravel and plant roots were picked out and divided into two parts after a 2 mm sieve. One part was stored in a −20 °C refrigerator for long-term determination of soil enzyme activities and available nutrients, and the other part was dried naturally for determination of soil physical and chemical properties.
2.4. Determination of Soil Physical and Chemical Properties and Enzyme Activities
Soil TN and SOC contents were determined by an elemental analyzer (Vario EL iii, Elementar, Frankfurt, Germany). Soil TP content was prepared by H2SO4-H2O2 de-boiling solution and measured by molybdenum-antimony anticolorimetric method. Soil water content was determined after oven-drying 10 g of fresh soil at 105 °C for 48 h. Soil pH was measured by a pH meter (soil and water ratio 2.5:1, Sartorius Gmb H: Gottingen, Germany). Soil DOC concentration was extracted with 0.5 M K2SO4 and shaken for 60 min at 200 rpm on a reciprocal shaker. The extracts were filtered through a Millipore 0.45-μm filter and then measured using a TOC analyzer (Shimadzu TOC-L, Kyoto, Japan). Soil NO3−-N concentration was quantified by spectrophotometry, and soil NH4+-N was determined by (indophenol blue) colorimetric method [22]. The activities of BG, NAG, LAP, and ACP in soil were tested with the kit produced by Keming Biology (http://www.cominbio.com/index.html, accessed on 28 March 2022).
2.5. Analysis Methods
Based on the vector theory of enzyme chemistry proposed by Moorhead et al. [18,23], vector length (vector L) and vector angle (vector A) calculated by enzyme stoichiometric ratio were used in this study. Soil microbial nutrient limitation was analyzed by calculating vector length (vector L) and vector angle (vector A) of all data:
where X represents the relative activity of C and P metabolic enzymes and Y represents the relative activity of C and N metabolic enzymes. Vector L is the straight-line distance from the origin to the point (x,y), representing the degree of soil microbial C limitation. Vector A is the arctangent function of the extension line from origin to point (x,y), representing the limitation degree of soil microorganisms N and P. Vector angles greater than 45° indicate microbial P-limit, while vector angles less than 45° indicate N-limit.
X = (BG)/(BG + AP)
Y = (BG)/(BG + NAG + LAP)
Vector L = SQRT(X2 + Y2)
Vector A = DEGREES[ATAN2(X,Y)]
SPSS 21.0 (SPSS Inc., Chicago, IL, USA) and Canoco 5.0 were used for data analysis. One-way analysis of variance was used to analyze the differences in soil physical and chemical properties, soil enzyme activities, and enzyme stoichiometric ratios, and Pearson correlation analysis and redundancy analysis were performed. GraphPad Prism 9 was used for plotting.
3. Results
3.1. Effects of Soil Warming on Soil Physical and Chemical Properties
Table 1 showed that compared with T0 treatment, T2 and T4 treatments significantly reduced SOC content by 19.3% and 17.9% (both p < 0.05), respectively, and decreased SWC by 14.84% and 20.01%, respectively (both p < 0.05). Compared with the T0 treatment, T4 treatment significantly increased the contents of soil NO3−-N and AP by 18.82% and 98.58% (both p < 0.05). Soil N/P under T2 and T4 treatments significantly decreased compared with T0 treatment (p < 0.05). Moreover, soil TN, TP, NH4+-N, DOC, pH, and C/N had no significant response to soil warming (p > 0.05).

Table 1.
Soil physical and chemical properties contents under different treatments.
3.2. Changes in Soil Enzyme Activities and Soil Enzymatic Stoichiometry
In general, soil warming changed soil enzyme activities, but it depended on the type of enzyme. Specifically, compared with the T0 treatment, the activity of BG significantly increased under T2 and T4 treatments by 125.74% and 80.55% (p < 0.05, Figure 1a), while the activity of LAP only significantly increased under T4 treatment (p < 0.05, Figure 1b). Moreover, NAG and ACP activities did not respond to soil warming (p > 0.05, Figure 1c,d).
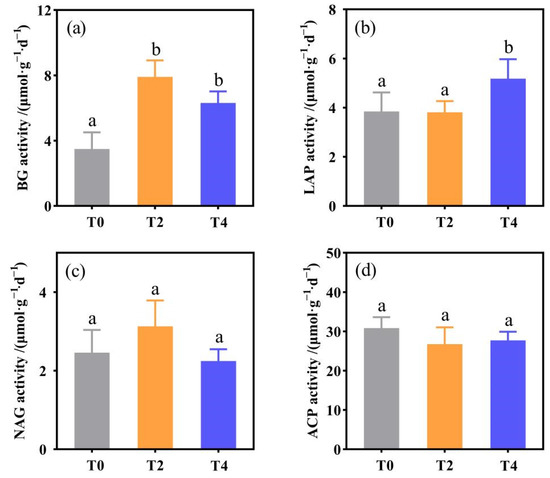
Figure 1.
Changes in soil enzyme activities under different treatments: (a) the activity of BG (β-1,4-glucosidase); (b) the activity of NAG (β-1,4-N-acetylglucosidase); (c) the activity of LAP (L-leucine aminopeptidase); (d) the activity of ACP (acid phosphatase). T0: control without warming; T2: 2 °C warming treatment; T4: 4 °C warming treatment. Lowercase letters represent significant differences in soil enzyme activity under different treatments (p < 0.05).
Soil enzymatic stoichiometry reflects the relative requirements of soil microorganisms for C, N, and P acquisition. In this study, BG: (NAG+LAP), BG: AP, and (NAG+LAP): AP increased with soil warming. Specifically, BG: (NAG+LAP) under T2 treatment was significantly higher than that under T0 treatment (p < 0.05, Figure 2a). Compared with the T0 treatment, soil warming significantly increased BG: AP (p < 0.05), but had no significant effect on (NAG+LAP): AP (Figure 2b,c).
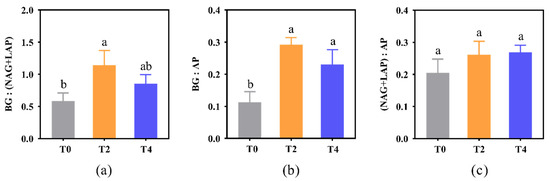
Figure 2.
Changes in soil enzymatic stoichiometry under different treatments. (a) ratio of C metabolizing enzyme to N metabolizing enzymes; (b) ratio of C metabolizing enzyme to P metabolizing enzyme; (c) ratio of N metabolizing enzymes to P metabolizing enzyme. BG: β-1,4-glucosidase; NAG: β-1,4-N-acetylglucosidase; LAP: L-leucine aminopeptidase; ACP: acid phosphatase. T0: control without warming; T2: 2 °C warming treatment; T4: 4 °C warming treatment. Lowercase letters represent significant differences in soil enzyme activity under different treatments (p < 0.05).
3.3. Associations of Microbial C and P Limitation with other Factors
The results of correlation analysis further showed that SOC, SWC, and microbial P limitation were significantly negatively correlated with microbial C limitation (p < 0.05, Figure 3a,b,d). AP was positively correlated with microbial C limitation (p < 0.05, Figure 3c). Furthermore, AP and NO3−-N were significantly negatively correlated with microbial P limitation (p < 0.05, Figure 3e,f).
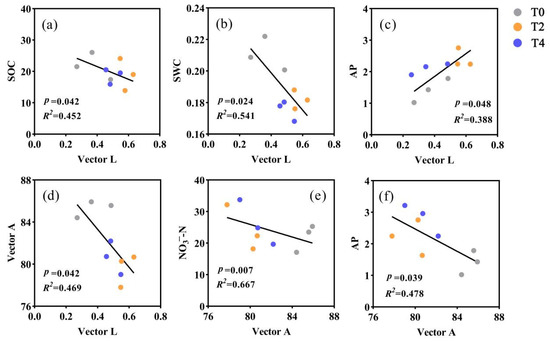
Figure 3.
Relationships between microbial C limitation with SOC (a), SWC (b), AP (c), and microbial P limitation (d). Relationships between microbial P limitation with NO3−-N (e), AP (f). T0: control without warming; T2: 2 °C warming treatment; T4: 4 °C warming treatment.
3.4. Response of Soil Enzyme Activity and Microbial C, P Limitation to Environmental Factors
Dimensionality reduction of the factors involved in this study was carried out by principal components, and the results (Figure 4a) showed that the first two axes of principal components explained 63.70% of the total variance contribution rate. There was a significant difference in PC1 between the T0 treatment and the two warming treatments. SWC, AP, and BG were the main difference factors, but there was no significant difference between T2 and T4 treatment. Among them, SWC, C/P, SOC, C/N, AP, NO3−-N, NAG, and BG have the highest contribution to the two principal components (Figure 4b).
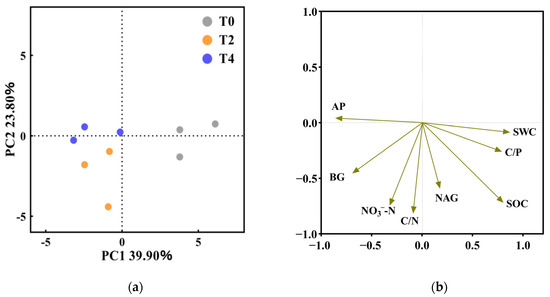
Figure 4.
Principal component analysis (PCA) of soil and enzyme activity factors under different treatments. T0: control without warming; T2: 2 °C warming treatment; T4: 4 °C warming treatment. (a) Different colors represent different treatments, the farther the distance between points represents the greater the sample difference; (b) According to the quality of variables in principal component analysis, eight variables with greater contribution were selected.
RDA analysis was conducted with soil enzyme activity and microbial C, P limitation as response variables, and soil physical and chemical properties as explanatory variables, respectively (Figure 5). The explanation rate of the first two ranking axes reached 77.76%, the first axis explained 57.79% of the variables, and the second axis explained 19.97% of the variables. SWC (p = 0.008) was the significant factor affecting soil, with explanatory rates of 44.6%.
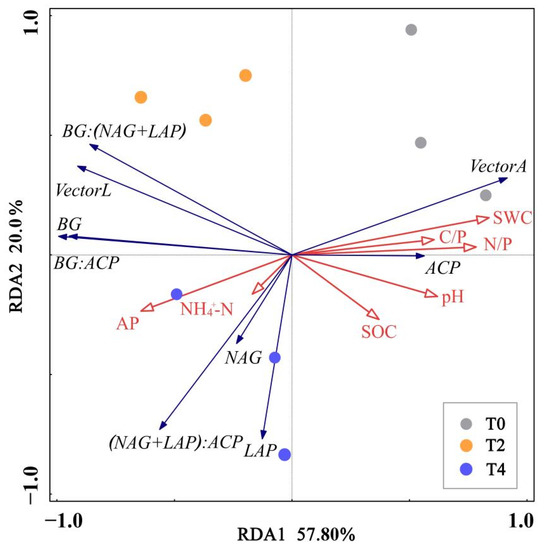
Figure 5.
Redundancy analysis (RDA) of soil properties, soil enzyme activities, and microbial C, P limitation. T0: control without warming; T2: 2 °C warming treatment; T4: 4 °C warming treatment. SWC: soil water content; SOC: soil organic carbon; NH4+-N: ammonium nitrogen; AP: available phosphorus; C/P: SOC/TP; N/P: TN/TP; BG: β-1,4-glucosidase; NAG: β-1,4-N-acetylglucosidase; LAP: L-leucine aminopeptidase; ACP: acid phosphatase.
4. Discussion
4.1. Effects of Soil Warming on Soil Enzyme Activities
Soil enzymes are mainly derived from soil microorganisms, plant root exudates, soil animal exudates, and their residues. As reported, soil enzymes are affected by soil temperature [24], soil water content [25], and nutrient availability [26]. The C-metabolizing enzyme BG is associated with a relatively unstable carbon pool and is a major component of cellulase, mainly involved in the hydrolysis of glycosidic bonds between atomic groups in cellulose [27]. In our study, soil warming increased the activity of BG, which is consistent with the results of Caitlin et al. [28]. Moreover, we found that BG activity was significantly negatively correlated with SOC and SWC (Table A1), indicating that the SOC decomposition rate and SWC level may be the main factors affecting its activity under warming conditions [29]. Previous studies have shown that increasing temperature and decreasing SWC will accelerate the cycling rate of unstable carbon and promote the decomposition of SOC [15,30,31]. Qi et al. [32,33] found that temperature change could significantly change soil enzyme activities by affecting the soil water-driven SOC decomposition rate, which was similar to our findings.
The response of soil enzyme activities to different temperature gradients was also different. Specifically, LAP activity increased significantly only under T4 treatment, while NAG and ACP activities did not change significantly. On the one hand, some hydrolases were not sensitive to small temperature increases [33]. On the other hand, since the samples of this experiment were collected in March, the temperature was relatively low, which would lead to the passivation of some soil enzymes, and the seasonal variation of temperature had a significant impact on enzymes [19,34]. However, these results are in contrast to a recent meta-analysis. Chen et al. [35] studied the effects of soil warming on soil enzymes and found that soil warming only significantly affected oxidase but did not have a consistent and significant effect on hydrolase. One of the author’s explanations was that soil hydrolases mainly degrade unstable reaction substrates, and soil warming leads to the decrease of substrate availability, which will gradually inhibit soil enzyme activities [36]. Although warming can directly increase enzyme activity by increasing enzyme kinetics [37], the effects of these two directions cancel each other, resulting in an insignificant response to warming.
Soil pH is also an important factor affecting soil enzyme activities and their stoichiometric ratios [38]. In this study, pH was significantly negatively correlated with BG activity and BG/AP (Table A1), which is consistent with the results of Sinsabaugh et al. [39] on enzyme activities at a global ecological scale. This may be because pH regulates soil enzyme activities by influencing the soil microbial community structure, the binding state between enzymes and soil particles, soil nutrients, and soil functions [40]. In addition, the warming time of this experiment is relatively short, and long-term warming experiments usually exceed 10 years [35,41,42]. In the future, we will focus on exploring the coupling relationship between soil enzyme activities and seasonal dynamic changes in temperature and duration of warming.
4.2. Effects of Soil Warming on Soil Nutrient Availability
The results of this study revealed that the T2 and T4 treatments increased the contents of AP and NO3−-N in soil and improved soil N and P availability in the Q. acutissima forest in the north subtropical region [14,43]. Many studies found that the availability of soil N increased with the increase of soil temperature, because the increasing temperature increases the activities of soil N metabolizing enzymes and related microorganisms, accelerates the mineralization rate of soil N, and promotes the accumulation of soil available N [44,45], which is consistent with the results of our study. However, during the whole experimental period, the response of AP to soil warming was different from the results of previous studies. In contrast to soil N, P supply in soil is controlled by a combination of biotic and abiotic (adsorption/desorption and dissolution) processes [46]. Usually, soil warming promotes the uptake of phosphorus by surface plants, increases the content of litter phosphorus, and reduces soil available phosphorus [41,47]. However, as the Q. acutissima forest in the experimental area of this study belongs to an overmature forest, short-term warming of 1 year may not affect its growth.
The correlation results (Table A2) showed that AP and SWC were significantly negatively correlated, indicating that there was some coupling relationship between them. The decrease of SWC may be caused by warming, which is not conducive to the migration and diffusion of P components in the soil [48] and can reduce the leaching loss of slow-available P in the surface soil [49,50], which provides a possibility for the increase of soil AP.
4.3. Effects of Soil Warming on Soil Microbial Nutrient Limitation
Soil microbes can regulate soil nutrient availability, and the change in soil temperature will affect the soil microbial community and activity, thereby affecting the process of nutrient absorption and release [41]. Therefore, there is a close relationship between soil microbial nutrient limitation and soil nutrient availability. However, the limiting factors of soil microbial nutrients under warming conditions in different regions are still unclear. In this study, the lnBG: ln(NAG+LAP): lnACP of soil in the north subtropical Q. acutissima forest was about 1:1.57:2.89, which was different from the global average of 1:1:1 [39,41], indicating that N and P elements available to soil microorganisms in the study area were relatively lacking. A serious north subtropical soil microbial P limit has been confirmed, because the soil P element mainly comes from rock weathering, and the rainfall erosion and soil erosion soil P element loss [51], and in the north subtropical acid soil metal ion binding ability is strong, and availability P element is easily incorporated into the stability of the closed state storage P. The utilization rate of the P element is reduced [50,51]. The soil lnBG: ln(NAG+LAP): lnACP after warming treatment was about 1:1.02:1.71, which gradually tended to be balanced compared with T0 treatment, indicating that warming alleviated the relative N and P limitations of microorganisms in the north subtropical forest system. The analysis showed that BG: ACP was significantly negatively correlated with TN/TP and soil pH (Table A1), which was consistent with Guan’s findings [52]. The decreasing trend of TN/TP was induced by soil warming, which further proved that warming alleviated phosphorus limitation in the study area, However, soil P limitation still exists in the study area (Figure 6).
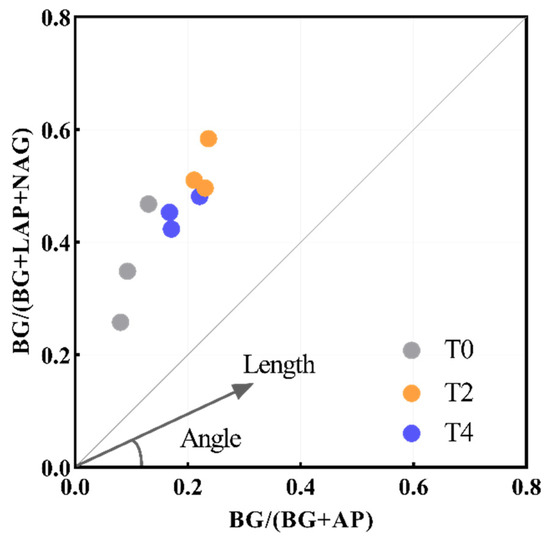
Figure 6.
The variation of vector length and angle. T0: control without warming; T2: 2 °C warming treatment; T4: 4 °C warming treatment.
Soil enzymatic stoichiometry can reflect soil microbial nutrient acquisition ability, nutrient resource utilization, and microbial nutrient limitation [5], and sometimes can be used as a biological index to measure soil fertility [53]. Our results revealed that BG: ACP and BG:(NAG+LAP) were significantly increased compared with the T0 treatment. According to the principle of resource allocation, the relative input of soil microorganisms to C metabolic enzymes was greater than that of N and P metabolic enzymes due to warming [54]. In addition, the significant increase of vector L due to warming also indicates the transition of soil microorganisms to the C-limit (Figure 6). Linear regression analysis showed that SOC and SWC were significantly correlated with vector L. The relative increase of soil microbial C limitation may be related to the significant decomposition of SOC caused by warming [32,55]. The results of PCA and RDA showed that SWC is the most important factor affecting soil enzyme activity and soil microbial nutrient limitation among soil physical and chemical properties, and it is also the main factor distinguishing significant differences in soil enzyme activity and soil microbial nutrient limitation under different temperature changes. Therefore, warming may change the decomposition of SOC driven by SWC, thereby indirectly changing soil enzyme activities and microbial metabolic limitation [30,33]. Linear regression analysis showed that AP and NO3−-N was significantly negatively correlated with vector A, and AP was significantly positively correlated with vector L(p < 0.05, Figure 3c,e,f), indicating that the limitation of soil microorganisms C and P was strongly affected by the available nutrients of the soil, which was similar to Cui’s results [56].
In this study, there were significant changes in soil microbial C and P limitations, and there was a significant negative correlation between them (p < 0.05, Figure 3d), reflecting the coupling relationship between soil microbial C and P limitations under warming conditions, which jointly play an important role in soil nutrient cycling [6]. It is generally believed that soil microbial C and P limitations will accelerate the decomposition of SOM to produce more available nutrients [56], which will stimulate the decomposition of SOC. In this study, soil microbial C limitation was significantly correlated with SOC (p < 0.05, Figure 3a), indicating that the limitation of soil microorganisms C may be detrimental to SOC assimilation by soil microorganisms [57,58,59]. In addition, changes in soil microbial C and P limitations caused by soil warming may cause a large amount of SOM decomposition in a short period and affect soil C sequestration. Under the current global warming, it will be more difficult to accurately estimate global soil carbon storage in the short term.
5. Conclusions
Based on a 1-year field warming experiment, we found that soil warming significantly altered enzyme activity, but it depended on the type of enzyme. Warming significantly increased the activities of BG and LAP, and the changes of these enzymes were directly mediated by warming temperature, seasonal temperature, and SWC. Warming significantly reduced SOC content and soil SWC but significantly increased AP and NO3−-N contents, and increased soil available N and P. After one year of short-term warming, soil microbial N and P limitations were alleviated due to the improvement of soil available nutrients, but soil microbial P limitation still existed in the north subtropical Quercus acutissima forest. The enhancement of soil microbial C limitation was mainly attributed to the decomposition and loss of SOC driven by SWC changes caused by warming, which indirectly changed soil microbial C limitation. In addition, the change of soil microbial C and P limitations caused by soil warming will cause a large amount of SOM decomposition in a short period, which is not conducive to soil C sequestration. Under the current climate warming, accurate estimation of global soil carbon storage will become more difficult.
Author Contributions
Conceptualization, J.W. and M.Z.; methodology, J.W.; software, J.W. and M.Z.; validation, J.W. and M.Z.; formal analysis, J.W.; investigation, J.W., X.W. and J.K.; resources, J.W.; data curation, J.W.; writing—original draft preparation, J.W.; writing—review and editing, L.C., H.H. and M.Z.; visualization, J.K.; supervision, L.C. and H.H.; project administration, L.C. and H.H.; funding acquisition, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the positioning research project of forest ecological system in Yangtze River delta of National Forestry and Grassland Administration, grant number 2021132068; Special fund project for technology innovation on Carbon Peak Carbon-neutral in 2021, Jiangsu Province, grant number BE2022305, and Technical support and collaboration project from Wuxi Water Conservancy Bureau, grant number 2107116.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Correlation analysis of soil enzyme activities and environmental factors.
Table A1.
Correlation analysis of soil enzyme activities and environmental factors.
| SOC | TN | TP | SWC | PH | NO3−-N | NH4+-N | AP | DOC | C/N | C/P | N/P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BG | −0.84 * | −0.398 | −0.021 | −0.642 * | −0.717 * | 0.436 | 0.279 | 0.442 | 0.046 | 0.353 | −0.356 | −0.56 |
| LAP | 0.043 | 0.157 | 0.148 | −0.093 | −0.424 | 0.202 | −0.188 | 0.405 | −0.047 | −0.506 | −0.126 | 0.116 |
| NAG | 0.372 | 0.158 | 0.368 | 0.102 | 0.143 | 0.2 | 0.856 ** | −0.249 | 0.048 | 0.572 | 0.001 | −0.22 |
| ACP | 0.539 | 0.562 | 0.101 | 0.252 | 0.652 | −0.155 | 0.306 | −0.733 * | −0.798 * | 0.236 | 0.903 ** | 0.881 ** |
| CEs/NEs | −0.439 | −0.57 | −0.259 | −0.61 | −0.662 | 0.256 | 0.068 | 0.384 | 0.053 | 0.333 | −0.332 | −0.543 |
| CEs/PEs | −0.365 | −0.51 | −0.036 | −0.642 | −0.814 ** | 0.44 | 0.15 | 0.607 | 0.302 | 0.241 | −0.587 | −0.760 * |
| NEs/PEs | −0.062 | −0.121 | 0.274 | −0.183 | −0.595 | 0.379 | 0.188 | 0.579 | 0.474 | −0.145 | −0.618 | −0.572 |
** and * indicate significant difference at p < 0.01 and p < 0.05. CEs/NEs: BG/(NAG+LAP); CEs/PEs: BG/ACP; NEs/PEs: (NAG+LAP): ACP.

Table A2.
Correlation analysis between environmental factors.
Table A2.
Correlation analysis between environmental factors.
| SOC | TN | TP | pH | SWC | NO3−-N | NH4+-N | AP | DOC | C/N | C/P | N/P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOC | 1 | 0.961 ** | 0.844 ** | −0.157 | 0.651 | 0.431 | 0.383 | −0.408 | −0.119 | 0.394 | 0.625 | 0.442 |
| TN | 1 | 0.807 ** | −0.085 | 0.660 | 0.3736 | 0.154 | −0.387 | −0.087 | 0.124 | 0.619 | 0.553 | |
| TP | 1 | −0.405 | 0.261 | 0.721 * | 0.234 | 0.050 | 0.300 | 0.334 | 0.122 | −0.031 | ||
| pH | 1 | 0.428 | −0.825 ** | −0.023 | −0.590 | −0.141 | −0.286 | 0.231 | 0.372 | |||
| SWC | 1 | −0.240 | 0.240 | −0.832 ** | −0.314 | 0.123 | 0.763 * | 0.705 * | ||||
| NO3−-N | 1 | 0.233 | 0.464 | 0.221 | 0.308 | −0.146 | −0.282 | |||||
| NH4+-N | 1 | −0.322 | −0.407 | 0.861 ** | 0.376 | 0.012 | ||||||
| AP | 1 | 0.422 | −0.187 | −0.797 * | −0.710 * | |||||||
| DOC | 1 | −0.140 | −0.692 * | −0.628 | ||||||||
| C/N | 1 | 0.206 | −0.234 | |||||||||
| C/P | 1 | 0.902 ** | ||||||||||
| N/P | 1 |
** and * indicate significant difference at p < 0.01 and p < 0.05.
References
- Pörtner, H.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC Sixth Assessment Report; Intergovernmental Panel on Climate: Geneva, Switzerland, 2022. [Google Scholar]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Zhou, H.; Ganjurjav, H.; Wang, X. Climate change and human activities altered the diversity and composition of soil microbial community in alpine grasslands of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2016, 562, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Rosinger, C.; Rousk, J.; Sandén, H. Can enzymatic stoichiometry be used to determine growth-limiting nutrients for microorganisms?-A critical assessment in two subtropical soils. Soil Biol. Biochem. 2019, 128, 115–126. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Chen, W.; Wu, Y.; Qiao, L.; Yan, Z.; Liu, G.; Xue, S. Long-term warming does not affect soil ecoenzyme activity and original microbial nutrient limitation on the Qinghai—Tibet Plateau. Soil Ecol. Lett. 2022, 4, 383–398. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Wang, Y.; Cheng, H.; An, S.; Chang, S.X. Soil extracellular enzyme stoichiometry reflects the shift from P-to N-limitation of microorganisms with grassland restoration. Soil Biol. Biochem. 2020, 149, 107928. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Zhang, Y.; Li, P.; Zhang, X. Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Jicha, T.M.; Cotter, A.M.; Trebitz, A.S.; Danz, N.P. Sediment microbial enzyme activity as an indicator of nutrient limitation in Great Lakes coastal wetlands. Freshw. Biol. 2006, 51, 1670–1683. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Huang, C.; Wang, K.; Liu, Q.; Liu, Y.; Hai, X.; Shangguan, Z. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steudler, P.A.; Aber, J.D.; Newkirk, K.; Lux, H.; Bowles, F.P.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Wan, S. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Glob. Change Biol. 2009, 15, 184–195. [Google Scholar] [CrossRef]
- Zuccarini, P.; Asensio, D.; Ogaya, R.; Sardans, J.; Peñuelas, J. Effects of seasonal and decadal warming on soil enzymatic activity in a P-deficient Mediterranean shrubland. Glob. Change Biol. 2020, 26, 3698–3714. [Google Scholar] [CrossRef] [PubMed]
- Brockett, B.F.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, R.; Xiong, P.; Wan, C.; Cao, G.; Liu, Q. Initial soil responses to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China: Nutrient availabilities, microbial properties and enzyme activities. Appl. Soil Ecol. 2010, 46, 291–299. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Wang, Y.; Xu, Z.; Han, H.; Li, L.; Wan, S. Warming and increased precipitation have differential effects on soil extracellular enzyme activities in a temperate grassland. Sci. Total Environ. 2013, 444, 552–558. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Change Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Chen, Y.; Zhang, J.; Li, H.; Wang, L.; Chen, Q. Short-term warming shifts microbial nutrient limitation without changing the bacterial community structure in an alpine timberline of the eastern Tibetan Plateau. Geoderma 2020, 360, 113985. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Seasonal patterns of microbial extracellular enzyme activities in an arctic tundra soil: Identifying direct and indirect effects of long-term summer warming. Soil Biol. Biochem. 2013, 66, 119–129. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M. Temperature and soil organic matter decomposition rates–synthesis of current knowledge and a way forward. Glob. Change Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, H.; Wang, J.; Wang, X.; Tian, Z.; Deng, W.; Wu, C.; Zhu, L.; Lu, Q.; Feng, Y. Effects of nitric acid rain stress on soil nitrogen fractions and fungal communities in a northern subtropical forest, China. Sci. Total Environ. 2023, 856, 158904. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, H.; Wang, J.; Zhu, Z.; Feng, Y. Nitric Acid Rain Increased Bacterial Community Diversity in North Subtropical Forest Soil. Forests 2022, 13, 1349. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- Menichetti, L.; Reyes Ortigoza, A.L.; García, N.; Giagnoni, L.; Nannipieri, P.; Renella, G. Thermal sensitivity of enzyme activity in tropical soils assessed by the Q10 and equilibrium model. Biol. Fertil. Soils 2015, 51, 299–310. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.; Li, Y.; Liu, S.; Chu, G.; Xu, Z.; Liu, J. Warming effects on biomass and composition of microbial communities and enzyme activities within soil aggregates in subtropical forest. Biol. Fertil. Soils 2016, 52, 353–365. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Li, J.; Zhou, X.; Cao, J.; Wang, R.W.; Wang, Y.; Shelton, S.; Jin, Z.; Walker, L.M. Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Glob. Change Biol. 2017, 23, 1328–1337. [Google Scholar] [CrossRef]
- Looby, C.I.; Treseder, K.K. Shifts in soil fungi and extracellular enzyme activity with simulated climate change in a tropical montane cloud forest. Soil Biol. Biochem. 2018, 117, 87–96. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- García-Palacios, P.; Crowther, T.W.; Dacal, M.; Hartley, I.P.; Reinsch, S.; Rinnan, R.; Rousk, J.; Van den Hoogen, J.; Ye, J.; Bradford, M.A. Evidence for large microbial-mediated losses of soil carbon under anthropogenic warming. Nat. Rev. Earth Environ. 2021, 2, 507–517. [Google Scholar] [CrossRef]
- Luan, J.; Liu, S.; Chang, S.X.; Wang, J.; Zhu, X.; Liu, K.; Wu, J. Different effects of warming and cooling on the decomposition of soil organic matter in warm–temperate oak forests: A reciprocal translocation experiment. Biogeochemistry 2014, 121, 551–564. [Google Scholar] [CrossRef]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Schindlbacher, A.; Wang, J.; Yang, Y.; Song, Z.; You, Y.; Shi, Z.; Li, Z.; Chen, L. Experimental warming reduced topsoil carbon content and increased soil bacterial diversity in a subtropical planted forest. Soil Biol. Biochem. 2019, 133, 155–164. [Google Scholar] [CrossRef]
- Campbell, J.L.; Mitchell, M.J.; Groffman, P.M.; Christenson, L.M.; Hardy, J.P. Winter in northeastern North America: A critical period for ecological processes. Front. Ecol. Environ. 2005, 3, 314–322. [Google Scholar] [CrossRef]
- Meng, C.; Tian, D.; Zeng, H.; Li, Z.; Chen, H.Y.; Niu, S. Global meta-analysis on the responses of soil extracellular enzyme activities to warming. Sci. Total Environ. 2020, 705, 135992. [Google Scholar] [CrossRef]
- Rinnan, R.; Michelsen, A.; Bååth, E. Long-term warming of a subarctic heath decreases soil bacterial community growth but has no effects on its temperature adaptation. Appl. Soil Ecol. 2011, 47, 217–220. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Finzi, A.C. Seasonal variation in the temperature sensitivity of proteolytic enzyme activity in temperate forest soils. J. Geophys. Res. Biogeosci. 2012, 117, 1018. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition. Biogeochemistry 2014, 117, 23–37. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Wang, J.; Zhang, W. Bioactive Characteristics of Soil Microorganisms in Different-aged Orange(Citrus reticulate) Plantations. Agric. Sci. Technol. 2012, 13, 1277–1281. [Google Scholar]
- Hu, W.; Tan, J.; Shi, X.; Lock, T.R.; Kallenbach, R.L.; Yuan, Z. Nutrient addition and warming alter the soil phosphorus cycle in grasslands: A global meta-analysis. J. Soils Sediments 2022, 22, 2608–2619. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Peñuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Change Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Ganjurjav, H.; Wang, X.; Su, X.; Wu, X. Soil bacterial and fungal diversity differently correlated with soil biochemistry in alpine grassland ecosystems in response to environmental changes. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, Z.; Fu, G. A meta-analysis of the effects of experimental warming on soil carbon and nitrogen dynamics on the Tibetan Plateau. Appl. Soil Ecol. 2015, 87, 32–38. [Google Scholar] [CrossRef]
- Geng, Y.; Baumann, F.; Song, C.; Zhang, M.; Shi, Y.; Kühn, P.; Scholten, T.; He, J. Increasing temperature reduces the coupling between available nitrogen and phosphorus in soils of Chinese grasslands. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Santos, F.; Abney, R.; Barnes, M.; Bogie, N.; Ghezzehei, T.A.; Jin, L.; Moreland, K.; Sulman, B.N.; Berhe, A.A. The role of the physical properties of soil in determining biogeochemical responses to soil warming. In Ecosystem Consequences of Soil Warming; Elsevier: Amsterdam, The Netherlands, 2019; pp. 209–244. [Google Scholar]
- Wang, Q.K.; Wang, S.L.; Liu, Y.X. Responses to N and P fertilization in a young Eucalyptus dunnii plantation: Microbial properties, enzyme activities and dissolved organic matter. Appl. Soil Ecol. 2008, 40, 484–490. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Pendall, E.; Morgan, J.A.; Blumenthal, D.M.; Carrillo, Y.; LeCain, D.R.; Follett, R.F.; Williams, D.G. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol. 2012, 196, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci Total Environ 2018, 612, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, W.; Liu, H.; Xu, X.; Zeng, H. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol. Indic. 2019, 101, 453–464. [Google Scholar] [CrossRef]
- Jiang, Y.; Rocha, A.V.; Rastetter, E.B.; Shaver, G.R.; Mishra, U.; Zhuang, Q.; Kwiatkowski, B.L. C–N–P interactions control climate driven changes in regional patterns of C storage on the North Slope of Alaska. Landsc. Ecol. 2016, 31, 195–213. [Google Scholar] [CrossRef]
- Guan, P.; Yang, J.; Yang, Y.; Wang, W.; Zhang, P.; Wu, D. Land conversion from cropland to grassland alleviates climate warming effects on nutrient limitation: Evidence from soil enzymatic activity and stoichiometry. Glob. Ecol. Conserv. 2020, 24, e1328. [Google Scholar] [CrossRef]
- Li, J.; Shangguan, Z.; Deng, L. Dynamics of soil microbial metabolic activity during grassland succession after farmland abandonment. Geoderma 2020, 363, 114167. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Rieke, E.L.; Cappellazzi, S.B.; Cope, M.; Liptzin, D.; Mac Bean, G.; Greub, K.L.; Norris, C.E.; Tracy, P.W.; Aberle, E.; Ashworth, A. Linking soil microbial community structure to potential carbon mineralization: A continental scale assessment of reduced tillage. Soil Biol. Biochem. 2022, 168, 108618. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2021, 458, 7–20. [Google Scholar] [CrossRef]
- Nishina, K.; Ito, A.; Beerling, D.J.; Cadule, P.; Ciais, P.; Clark, D.B.; Falloon, P.; Friend, A.D.; Kahana, R.; Kato, E. Quantifying uncertainties in soil carbon responses to changes in global mean temperature and precipitation. Earth Syst. Dyn. 2014, 5, 197–209. [Google Scholar] [CrossRef]
- Exbrayat, J.; Pitman, A.J.; Zhang, Q.; Abramowitz, G.; Wang, Y. Examining soil carbon uncertainty in a global model: Response of microbial decomposition to temperature, moisture and nutrient limitation. Biogeosciences 2013, 10, 7095–7108. [Google Scholar] [CrossRef]
- Cui, L.; Li, X.; Lin, J.; Guo, G.; Zhang, X.; Zeng, G. The mineralization and sequestration of soil organic carbon in relation to gully erosion. Catena 2022, 214, 106218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).