Growth and Mortality of Hybrid Poplar Short Rotation Culture (AF8 Clone) in Response to Clostera anastomosis L. (Lepidoptera: Notodontidae) Defoliation
Abstract
1. Introduction
2. Materials and Methods
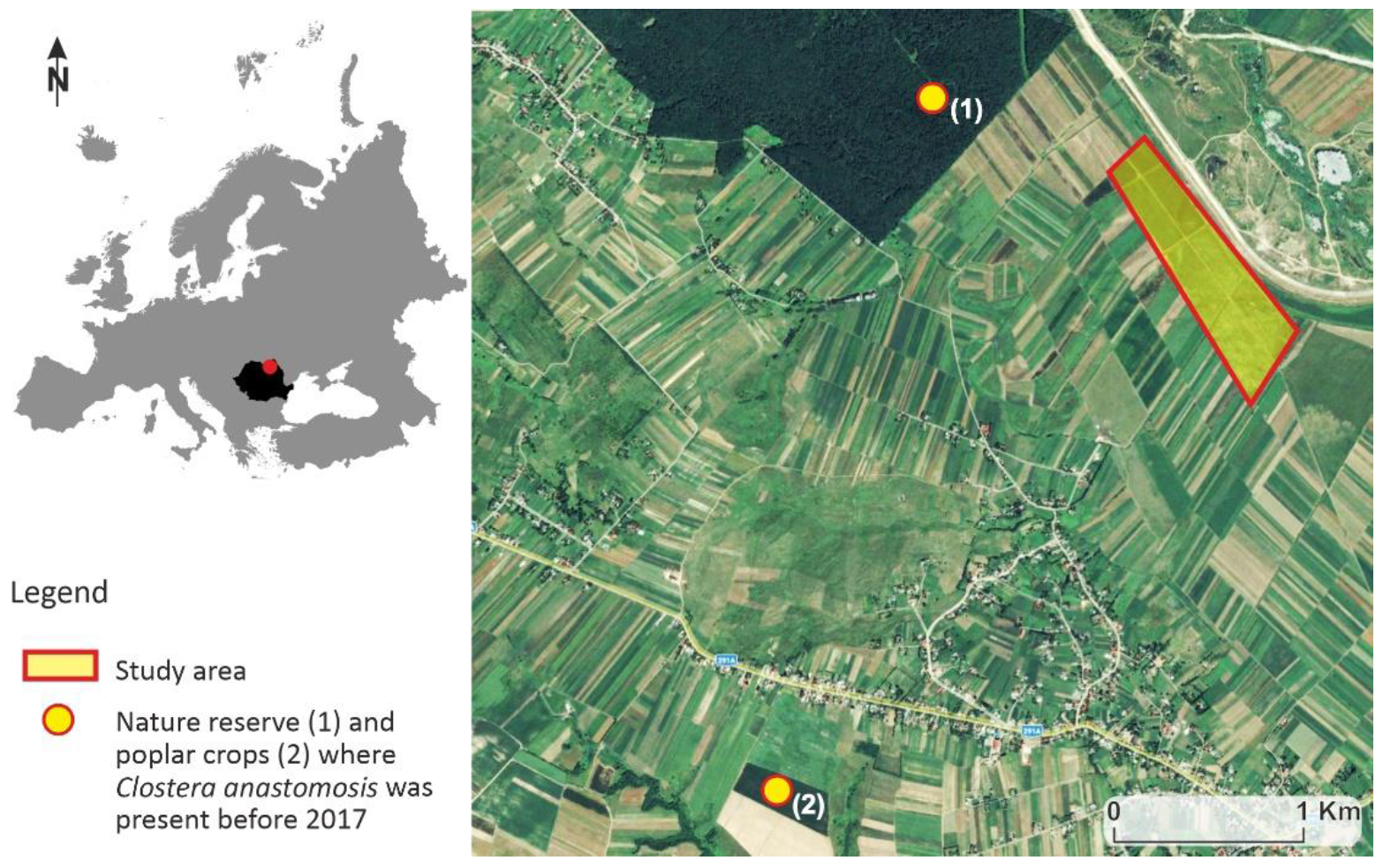
2.1. Study Area
2.2. C. anastomosis Outbreak
2.3. Trees Sampling and Growth Measurements
2.4. Aboveground Biomass Assessment
2.5. Assessment of Tree Mortality and Estimate of Biomass Loss
2.6. Data Analysis
3. Results
3.1. Defoliation Impact on Tree Growth
3.1.1. Annual Height Growth of Trees
3.1.2. Annual Radial Growth of Trees
3.1.3. The Impact of Defoliation on the Tree-level Aboveground Biomass
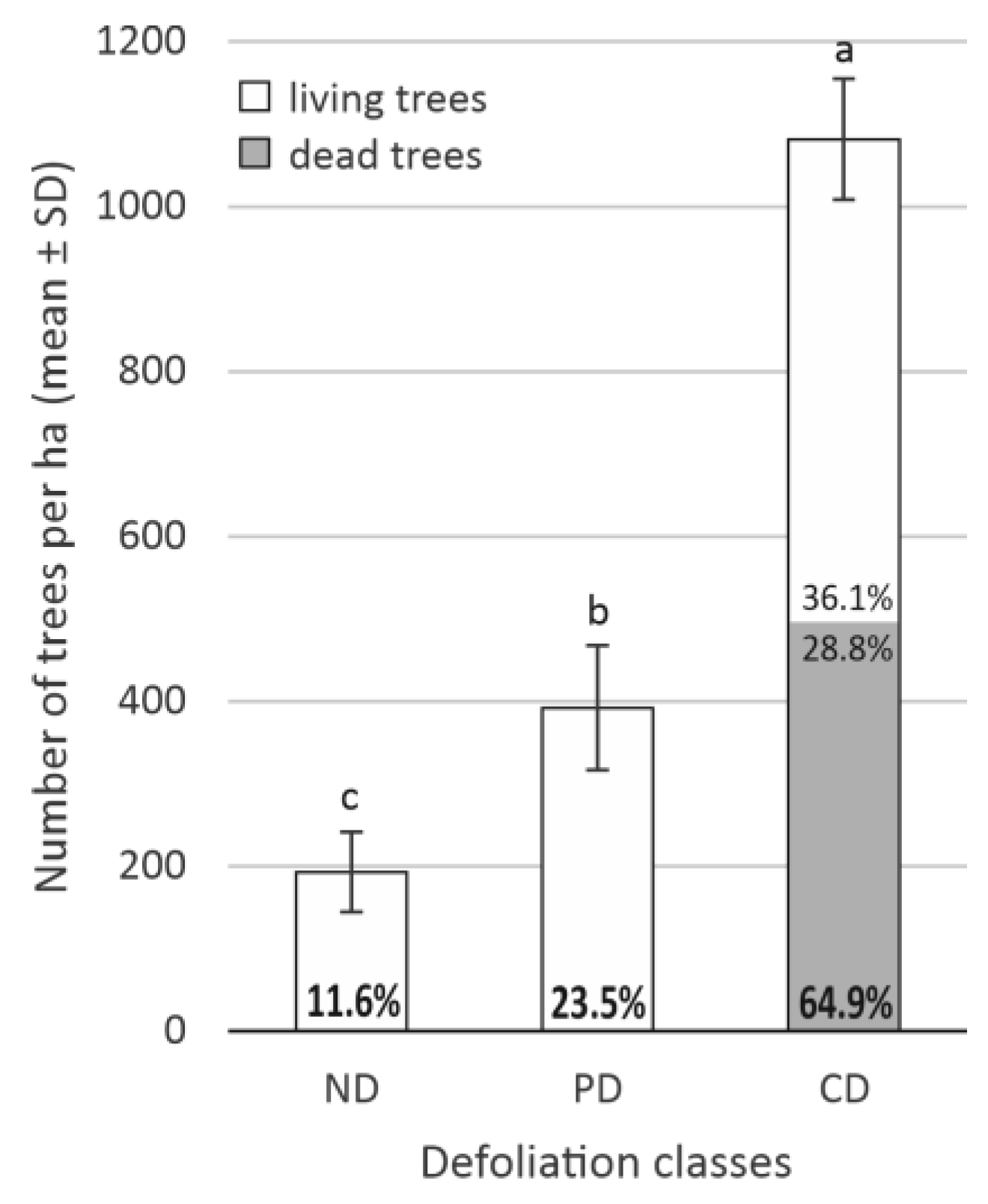
3.2. The Impact of Defoliation on Crop and Aboveground Biomass Losses
4. Discussion
4.1. Defoliation Impact on Individual Poplars’ Growth
4.2. The Impact of Defoliation on Poplar Crop and Aboveground Biomass Losses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Year | Shapiro–Wilk Test | Levene’s Test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Undefoliated | Partially Defoliated | Total Defoliated | |||||||
| W | p | W | p | W | p | F | p | ||
| Height Growth | 2014 | 0.9809 | 0.5901 | 0.9800 | 0.5540 | 0.9915 | 0.9742 | 1.1302 | 0.3258 |
| 2015 | 0.9565 | 0.0636 | 0.9702 | 0.2359 | 0.9887 | 0.9115 | 1.7519 | 0.1770 | |
| 2016 | 0.9694 | 0.2183 | 0.9628 | 0.1160 | 0.9151 | 0.0516 | 0.6170 | 0.5410 | |
| 2017 | 0.9692 | 0.2137 | 0.9675 | 0.1822 | 0.9616 | 0.1040 | 0.7032 | 0.4096 | |
| Radial growth | 2014 | 0.9640 | 0.1311 | 0.9509 | 0.0571 | 0.9756 | 0.3842 | 0.5744 | 0.5643 |
| 2015 | 0.9574 | 0.0690 | 0.9687 | 0.2046 | 0.9817 | 0.6237 | 0.1898 | 0.8373 | |
| 2016 | 0.9736 | 0.3234 | 0.9828 | 0.6739 | 0.9847 | 0.7597 | 0.7057 | 0.4954 | |
| 2017 | 0.6574 | 0.0504 | 0.9417 | 0.0257 | 0.9754 | 0.3767 | 3.0449 | 0.0592 | |
| Individual aboveground biomass | 2017 | 0.9654 | 0.1493 | 0.9687 | 0.2040 | 0.9794 | 0.5279 | 19.3594 | <0.0001 |
| 2017 * | 0.9687 | 0.2040 | 0.9724 | 0.2897 | 0.9794 | 0.5279 | 0.6449 | 0.0592 | |
| Total period | 0.9444 | 0.1103 | 0.9690 | 0.2103 | 0.9810 | 0.5951 | 1.9402 | 0.1473 | |
| Number of trees per hectare | 0.9688 | 0.8795 | 0.9456 | 0.6163 | 0.9269 | 0.4183 | 0.9373 | 0.4041 | |
References
- Long, H.; Li, X.; Wang, H.; Jia, J. Biomass resources and their bioenergy potential estimation: A review. Renew. Sustain. Energy Rev. 2013, 26, 344–352. [Google Scholar] [CrossRef]
- Verma, A.K.; Chettri, D.; Verma, A.K. Biomass, Bioenergy, and Biofuels. In Industrial Microbiology and Biotechnology; Verma, P., Ed.; Springer: Singapore, 2022; pp. 463–485. [Google Scholar]
- Banaś, J.; Utnik-Banaś, K. Using Timber as a Renewable Resource for Energy Production in Sustainable Forest Management. Energies 2022, 15, 2264. [Google Scholar] [CrossRef]
- Jukka, L.; Miika, M.; Lauri, L.; Mirja, M.; Ville, U.; Lassi, L. A financial and environmental sustainability of circular bioeconomy: A case study of short rotation coppice, biochar and greenhouse production in southern Finland. Biomass Bioenergy 2022, 163, 106524. [Google Scholar] [CrossRef]
- Landgraf, D.; Carl, C.; Neupert, M. Biomass Yield of 37 Different SRC Poplar Varieties Grown on a Typical Site in North Eastern Germany. Forests 2020, 11, 1048. [Google Scholar] [CrossRef]
- Bouriaud, L.; Duduman, M.L.; Danila, I.-C.; Olenici, N.; Biris, I.-A.; Ciornei, I.; Barnoaiea, I.; Maciuca, A.; Savin, A.; Grosu, L. How to evaluate the sustainability of short-term cultures for biomass production? An application from NE Romania. Poljopr. I Sumar. 2015, 61, 7. [Google Scholar] [CrossRef]
- F.A.O. Poplars and Other Fast-Growing Trees—Renewable Resources for Future Green Economies. Synthesis of Country Progress Reports. In Proceedings of the 25th Session of the International Poplar Commission, Berlin/Heidelberg, Germany, 13–16 September 2016; Working Paper IPC/15. Forestry Policy and Resources Division; FAO: Rome, Italy, 2016; Available online: http://www.fao.org/forestry/ipc2016/en/ (accessed on 12 September 2022).
- IPC. The Contribution of Poplars and Willows to Sustainable Forestry and Rural Development. Synthesis of Country Progress Reports; Working Paper IPC/3; IPC: Bonn, Germany, 2004. [Google Scholar]
- FAO. Improving lives with poplars and willows. Synthesis of country progress reports. In Proceedings of the 24th Session of the International Poplar Commission, Dehradun, India, 29 October–2 November 2012; Working Paper IPC/12. FAO: Rome, Italy, 2012. Available online: www.fao.org/forestry/ipc2012/en (accessed on 12 September 2022).
- Lindegaard, K.N.; Adams, P.W.R.; Holley, M.; Lamley, A.; Henriksson, A.; Larsson, S.; von Engelbrechten, H.-G.; Esteban Lopez, G.; Pisarek, M. Short rotation plantations policy history in Europe: Lessons from the past and recommendations for the future. Food Energy Secur. 2016, 5, 125–152. [Google Scholar] [CrossRef]
- Duduman, M.-L.; Lupaștean, D.; Nețoiu, C.; Tomescu, R. Research carried out in Romania on ecology and management of the poplar defoliator Clostera (Pygaera) anastomosis L. (Lepidoptera: Notodontidae). In Proceedings of the Biennial International Symposium Forest and Sustainable Development Brasov, Brașov, Romania, 25–27 October 2018; pp. 13–24. [Google Scholar]
- Dănilă, I.C. Cercetări Biometrice Privind Productivitatea Clonelor de Plop Hibrid în Culturi cu Ciclu Scurt de Producție din Nord-Estul României; Editura Universității „Ștefan cel Mare”: Suceava, România, 2019; p. 224. [Google Scholar]
- Tomescu, R.; Nef, L. Leaf eating insect damage on different poplar clones and sites. Ann. For. Sci. 2007, 64, 99–108. [Google Scholar] [CrossRef][Green Version]
- Tomescu, R.; Nețoiu, C. Insecte care Produc Vătămări Plopului și Salciei; Sivică, E., Ed.; Editura Silvică: București, Romania, 2009; p. 217. [Google Scholar]
- Lapietra, G.; Allegro, G. Insects damaging poplars in Italy during 198-89, control strategies and future perspectives. In Proceedings of the Working Party on Insects and Other Animal Pests, Buenos Aires, Argentina, 19–23 March 1990; p. 6. [Google Scholar]
- Helbig, C.E.; Müller, M.G.; Landgraf, D. Effects of Leaf Loss by Artificial Defoliation on the Growth of Different Poplar and Willow Varieties. Forests 2021, 12, 1224. [Google Scholar] [CrossRef]
- Delplanque, A. Les Insectes Associés aux Peupliers; Editions Memor: Bruxelles, Belgium, 1998; 421p. [Google Scholar]
- De Tillesse, V.; Nef, L.; Charles, J.; Hopkin, A.; Augustin, S. Damaging poplar Insects—Internationally Important Species; FAO: Rome, Italy, 2007. [Google Scholar]
- Pötzelsberger, E.; Gossner, M.M.; Beenken, L.; Gazda, A.; Petr, M.; Ylioja, T.; La Porta, N.; Avtzis, D.N.; Bay, E.; De Groot, M.; et al. Biotic threats for 23 major non-native tree species in Europe. Sci. Data 2021, 8, 210. [Google Scholar] [CrossRef]
- Stevens, M.; Kruger, E.; Lindroth, R. Variation in tolerance to herbivory is mediated by differences in biomass allocation in aspen. Funct. Ecol. 2008, 22, 40–47. [Google Scholar] [CrossRef]
- Christersson, L.; Ramstedt, M.; Forsberg, J. Pests, diseases and injuries in intensive short-rotation forestry. In Ecophysiology of Short Rotation-Forest Crops; Mitchell, C.P., Ed.; Elsevier Science Publisher: London, UK, 1992; pp. 185–216. [Google Scholar]
- Ciesla, W. Forest Entomology: A Global Perspective; Wiley: New York, NY, USA, 2011; p. 416. [Google Scholar]
- Kulman, H.M. Effects of Insect Defoliation on Growth and Mortality of Trees. Annu. Rev. Entomol. 1971, 16, 289–324. [Google Scholar] [CrossRef]
- Reichenbacker, R.R.; Schultz, R.C.; Hart, E.R. Artificial Defoliation Effect on Populus Growth, Biomass Production, and Total Nonstructural Carbohydrate Concentration. Environ. Entomol. 1996, 25, 632–642. [Google Scholar] [CrossRef]
- Duduman, M.L.; Lupaștean, D.; Pînzanu, Ș.I.; Ilașcă, A.; Dănilă, I. Treatment efficacy of Clostera anastomosis L. caterpillars control in postdormancy phase. Bucov. For. 2015, 15, 167–176. [Google Scholar]
- Tomescu, R.; Nețoiu, C. Pyagera (Clostera) anastomosis Linnaeus, 1758 (Lepidoptera, Notodontidae)—an insect with an increased damaging potential of poplars and willows. In Proceedings of the Biennial International Symposium, Forest and Sustainable Development, Braşov, Romania, 15–16 October 2010; pp. 217–228. [Google Scholar]
- Pașcovici, V.; Nemeș, I. Studii privind sistematica, bioecologia și răspândirea geografică a speciei Clostera (=Pygaera) anastomosis L. (Lep. Notodontidae), din R.S. România. Stud. Și Comunicări De Ocrotirea Nat. 1973, III, 451–462. [Google Scholar]
- Arru, G.M. Pygaera anastomosis (L.) (Lepidoptera Notodontidae)—Studio morfologico ed etologico. Boll. Di Zool. Agrar. E Di Bachic. 1965, 2, 207–272. [Google Scholar]
- Tang, F.; Wang, Y.-y.; Gao, X.-w. In vitro inhibition of carboxylesterases by insecticides and allelochemicals in Micromelalopha troglodyta (Graeser)(Lepidoptera: Notodontidae) and Clostera anastomosis (L.)(Lepidoptera: Notodontidae). J. Agric. Urban Entomol. 2008, 25, 193–203. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Pașcovici, V. Clostera (Pygaera) anastomosis L., un defoliator periculos al monoculturilor de plop cu vegetația slăbită. Rev. Pădurilor 1973, 88, 308–311. [Google Scholar]
- Singh, A.P. Poplar leaf defoliators, Clostera spp. (Lepidoptera: Notodontidae). In Poplars and Willows Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; CABI; FAO: Rome, Italy, 2014; pp. 488–490. [Google Scholar]
- Mikloš, I. Kvaliteta hrane kao jedan od uzroka masovnih pojava topolina čupavog prelca (Pygaera anastomosis L.) u nasadima euroameričkih topola. Šumarski List 1971, 95, 53–83. [Google Scholar]
- Mikloš, I. Pygaera anastomosis L.—Novi štetnik na topolama. Šumarski List 1960, 84, 368–370. [Google Scholar]
- Kailidis, D.S. Das Pappelinsektenproblem in Griechenland. Anz. Für Schädlingskunde 1969, 42, 167–171. [Google Scholar] [CrossRef]
- Chaudhry, M.I.; Admad, I. Biology of Ichthyura anastomosis Steph., a common hairy defliator of poplars in Pakistan. Pak. J. For. 1974, 24, 55–68. [Google Scholar]
- Özay, F.Ș.; Güler, N.; Uluer, K.; Selek, F. Investigation on Pygaera (Clostera) anastomosis L. which is harmfull on poplars. Teknġk Bülten 2000, 191, 1–19. [Google Scholar]
- Kamata, N. Outbreaks of forest defoliating insects in Japan, 1950–2000. Bull. Entomol. Res. 2002, 92, 109–117. [Google Scholar] [CrossRef]
- Li, H.-X.; Wang, Z.-Y.; Guo, S.-P.; Xie, S.-P. Bioassay of Clostera anastomosis granulosis virus. J. For. Res. 2006, 17, 50–52. [Google Scholar] [CrossRef]
- Ștefănescu, M.; Nițescu, C.; Simionescu, A.; Iliescu, G. Starea Fitosanitară a Pădurilor și Culturilor Forestiere din R.S. România; Editura Ceres-București: Bucharest, Romania, 1980; p. 527. [Google Scholar]
- Rubio, A.; Loetti, V.; Bellocq, I. Effect of defoliation intensity and timing on the growth of Populus alba and Salix babylonica × Salix alba. Bosque 2013, 34, 353–358. [Google Scholar] [CrossRef]
- Fransen, J.J.; Houtzagers, G. Loss of increment as a result of defoliation, and the seasonal growth of Poplars. Ned. Boschb. Tijdschr. 1946, 18, 36–39. [Google Scholar]
- Joly, R. Influence des défoliateurs forestiers sur l’accroissement. Rev. For. Française 1959, 1959, 775–784. [Google Scholar] [CrossRef]
- Kamilovski, M. Determining the best time to destroy defoliators of poplar. God. Zborn. Zemi. Sum. Fak. Univ. Skopje 1966, 19, 157–186. [Google Scholar]
- Hodson, A. The response of aspen (Populus tremuloides) to artificial defoliation! Mich. Entomol. Soc. 1981, 14, 167–169. [Google Scholar]
- Bassman, J.; Myers, W.; Dickmann, D.; Wilson, L. Effects of simulated insect damage on early growth of nursery-grown hybrid poplars in northern Wisconsin. Can. J. For. Res. 1982, 12, 1–9. [Google Scholar] [CrossRef]
- Moulinier, J.; Lorenzetti, F.; Bergeron, Y. Growth and mortality of trembling aspen (Populus tremuloides) in response to artificial defoliation. Acta Oecologica 2014, 55, 104–112. [Google Scholar] [CrossRef]
- Coyle, D.R.; McMillin, J.D.; Hall, R.B.; Hart, E.R. Cottonwood leaf beetle (Coleoptera: Chrysomelidae) defoliation impact on Populus growth and above-ground volume in a short-rotation woody crop plantation. Agric. For. Entomol. 2002, 4, 293–300. [Google Scholar] [CrossRef]
- Krause, S.C.; Raffa, K.F. Defoliation tolerance affects the spatial and temporal distributions of larch sawfly and natural enemy populations. Ecol. Entomol. 1996, 21, 259–269. [Google Scholar] [CrossRef]
- Hartley, S.E.; Lawton, J.H. Effects of Different Types of Damage on the Chemistry of Birch Foliage, and the Responses of Birch Feeding Insects. Oecologia 1987, 74, 432–437. [Google Scholar] [CrossRef]
- Kosola, K.; Dickmann, D.; Paul, E.; Parry, D. Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 2001, 129, 65–74. [Google Scholar] [CrossRef]
- Schäfer, P.; Saleh, M.; Yu, R.; Zhang, X.; Thomas, F.M. Decrease in growth increment of Populus euphratica upon defoliation by Lepidopteran larvae in a Central-Asian floodplain forest. J. Arid. Environ. 2017, 146, 99–102. [Google Scholar] [CrossRef]
- Duduman, M.-L.; Lupaștean, D. Cercetări Privind Monitorizarea și Controlul Populațiilor de Insecte ce Produc Vătămări Culturilor de Plop cu Ciclu Scurt de Producție—Raport de Cercetare, Beneficiar F.E. AGRAR Dornești; Universitatea ”Ștefan cel Mare”: Suceava, Romania, 2015; p. 15. [Google Scholar]
- Clinovschi, F.; Palaghianu, C. Studiu asupra structurii vegetatiei lemnoase din Rezervatia Zamostea-Lunca. An. Univ. Stefan Cel Mare Suceava-Sect. Silvic. 2007, 9, 19–28. [Google Scholar]
- Popovici, O.G.; Cenușă, R.L.; Savin, A. Vegetation conditions of "Zamostea-Luncă" Reserve. Rev. De Silvic. Și Cinegetică 2014, 19, 142–147. [Google Scholar]
- Google Maps. Zvoriștea Village, Suceava County, Romania. Available online: https://earth.google.com/web/@47.85737964,26.26597711,273.94124377a,3225.27234918d,35y,0h,0t,0r (accessed on 10 October 2022).
- Alasia. AF8—Populus × Generosa; Alasia Green Forest: Cavallermaggiore, Italy, 1993. [Google Scholar]
- Larsson, L. CooRecorder and Cdendro Programs of the Coorecorder/Cdendropackage Version 7.6. Available online: http://www.cybis.se/forfun/dendro (accessed on 2 February 2018).
- Dănilă, I.-C.; Duduman, M.-L.; Palaghianu, C.; Bouriaud, L.; Bouriaud, O.; Coșofret, C.; Savin, A.; Scriban, R. PN III Tehno-Crops—Optimization of Short-Cycle Hybrid Poplar Technology for Superior Biomass Production; ”Ștefan cel Mare” University of Suceava, Forestry Faculty: Suceava, Romania, 2017; p. 27. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Nordman, E.E.; Robison, D.J.; Abrahamson, L.P.; Volk, T.A. Relative resistance of willow and poplar biomass production clones across a continuum of herbivorous insect specialization: Univariate and multivariate approaches. For. Ecol. Manag. 2005, 217, 307–318. [Google Scholar] [CrossRef]
- Nebeker, T.E.; Stone, W.D.; Beatty, T.L. Impact of herbivory by cottonwood leaf beetle on three selected cottonwood clones: Year 2 results. In Proceedings of the 13th Biennial Southern Silvicultural Research Conference, Memphis, TN, USA, 28 February–4 March 2005; Connor, K.F., Ed.; Gen. Tech. Rep. SRS–92. U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2006; pp. 420–423. [Google Scholar]
- Dănilă, I.-C.; Mititelu, C.; Palaghianu, C. Productivity of Short-Rotation Poplar Crops: A Case Study in the NE of Romania. Forests 2022, 13, 1089. [Google Scholar] [CrossRef]
- Dănilă, I.C.; Avăcăriței, D.; Savin, A.; Roibu, C.C.; Bouriaud, O.; Duduman, M.-L.; Bouriaud, L. Dinamica şi caracteristicile creşterii a şase clone de plop hibrid pe parcursul unui ciclu de producţie într-o plantație comparativă din Depresiunea Rădăuţi. Bucov. For. 2015, 15, 19–30. [Google Scholar]
- Schulze, E.D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M.; Arneth, A.; Zähle, S.; Sierra, C.; Schäfer, M. Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2019; p. 700. [Google Scholar]
- Ceulemans, R.; Deraedt, W. Production physiology and growth potential of poplars under short-rotation forestry culture. For. Ecol. Manag. 1999, 121, 9–23. [Google Scholar] [CrossRef]
- Niez, B.; Dlouha, J.; Moulia, B.; Badel, E. Water-stressed or not, the mechanical acclimation is a priority requirement for trees. Trees 2019, 33, 279–291. [Google Scholar] [CrossRef]
- M.A.P.A.M. Technical Rules for the Forest Protection; Technical rule No 6; Monitorul Oficial: Bucharest, Romania, 2003; p. 454. [Google Scholar]
- Krabel, D.; Meyer, M.; Solger, A.; Müller, R.; Carvalho, P.; Foulkes, J. Early root and aboveground biomass development of hybrid poplars (Populus spp.) under drought conditions. Can. J. For. Res. 2015, 45, 1289–1298. [Google Scholar] [CrossRef]
- Jing, D.W.; Xing, S.J.; Du, Z.Y.; Liu, F.C. Effects of drought stress on the growth, photosynthetic characteristics, and active oxygen metabolism of poplar seedlings. Ying Yong Sheng Tai Xue Bao 2013, 24, 1809–1816. [Google Scholar]
- Avăcăriței, D.; Savin, A.; Palaghianu, C.; Dănilă, I.-C. The effect of harrowing and weed control on biomass yields of hybrid poplar crops. Bucov. For. 2016, 16, 175–185. [Google Scholar] [CrossRef][Green Version]
- Werner, C.; Haas, E.; Grote, R.; Gauder, M.; Graeff-Hönninger, S.; Claupein, W.; Butterbach-Bahl, K. Biomass production potential from Populus short rotation systems in Romania. GCB Bioenergy 2012, 4, 642–653. [Google Scholar] [CrossRef]
- Bell, A.C.; Clawson, S.; Watson, S. The long-term effect of partial defoliation on the yield of short-rotation coppice willow. Ann. Appl. Biol. 2006, 148, 97–103. [Google Scholar] [CrossRef]
- Bach, C.E. Effects of herbivory and genotype on growth and survivorship of sand-dune willow (Salix cordata). Ecol. Entomol. 1994, 19, 303–309. [Google Scholar] [CrossRef]






| Explanatory Variable | Dependent Variable | d.f. | Sum of Squares | Mean Squares | F | p |
|---|---|---|---|---|---|---|
| Defoliation class | Ih 2014 | 2 | 0.2114 | 0.1057 | 2.5829 | 0.0790 |
| Ih 2015 | 2 | 0.2997 | 0.1498 | 2.4438 | 0.0904 | |
| Ih 2016 | 2 | 0.4909 | 0.2454 | 1.5286 | 0.2203 | |
| Ih 2017 | 2 | 6.8723 | 3.4361 | 43.2759 | <0.0001 |
| Explanatory Variable | Dependent Variable | d.f. | Sum of Squares | Mean Squares | F | p |
|---|---|---|---|---|---|---|
| Defoliation class | Ir 2014 | 2 | 0.2138 | 0.1069 | 2.4753 | 0.0678 |
| Ir 2015 | 2 | 0.1487 | 0.0738 | 2.1426 | 0.1203 | |
| Ir 2016 | 2 | 0.5208 | 0.2604 | 1.6279 | 0.2348 | |
| Ir 2017 | 2 | 2598.1189 | 1299.0594 | 907.1418 | <0.0001 |
| Aboveground Biomass Acumulated in: | Explanatory Variable | DF | Sum of Squares | Mean Squares | F | p |
|---|---|---|---|---|---|---|
| 2014–2017 period | Defoliation class | 2 | 347.8928 | 173.9464 | 126.8460 | <0.0001 |
| 2017 | Defoliation class | 2 | 364.6105 | 182.3052 | 339.4080 | <0.0001 |
| Explanatory Variable | DF | Sum of Squares | Mean Squares | F | p |
|---|---|---|---|---|---|
| Defoliation class | 2 | 4,346,683.4600 | 2,173,341.7300 | 436.1694 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupaștean, D.; Isaia, G.; Dănilă, I.-C.; Coșofreț, C.; Scriban, R.E.; Duduman, M.-L. Growth and Mortality of Hybrid Poplar Short Rotation Culture (AF8 Clone) in Response to Clostera anastomosis L. (Lepidoptera: Notodontidae) Defoliation. Forests 2023, 14, 20. https://doi.org/10.3390/f14010020
Lupaștean D, Isaia G, Dănilă I-C, Coșofreț C, Scriban RE, Duduman M-L. Growth and Mortality of Hybrid Poplar Short Rotation Culture (AF8 Clone) in Response to Clostera anastomosis L. (Lepidoptera: Notodontidae) Defoliation. Forests. 2023; 14(1):20. https://doi.org/10.3390/f14010020
Chicago/Turabian StyleLupaștean, Daniela, Gabriela Isaia, Iulian-Constantin Dănilă, Cosmin Coșofreț, Ramona Elena Scriban, and Mihai-Leonard Duduman. 2023. "Growth and Mortality of Hybrid Poplar Short Rotation Culture (AF8 Clone) in Response to Clostera anastomosis L. (Lepidoptera: Notodontidae) Defoliation" Forests 14, no. 1: 20. https://doi.org/10.3390/f14010020
APA StyleLupaștean, D., Isaia, G., Dănilă, I.-C., Coșofreț, C., Scriban, R. E., & Duduman, M.-L. (2023). Growth and Mortality of Hybrid Poplar Short Rotation Culture (AF8 Clone) in Response to Clostera anastomosis L. (Lepidoptera: Notodontidae) Defoliation. Forests, 14(1), 20. https://doi.org/10.3390/f14010020








