Proteomics Analysis and Identification of Proteins Related to Isoprenoid Biosynthesis in Cinnamomum camphora (L.) Presl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction of Essential Oil
2.3. Gas Chromatography-Mass Spectrometry Analysis
2.4. Protein Extraction and Digestion
2.5. High pH Reverse Phase Separation
2.6. Nano-HPLC-MS/MS Analysis
2.7. Data Analysis
2.8. Bioinformatic Analysis
3. Results
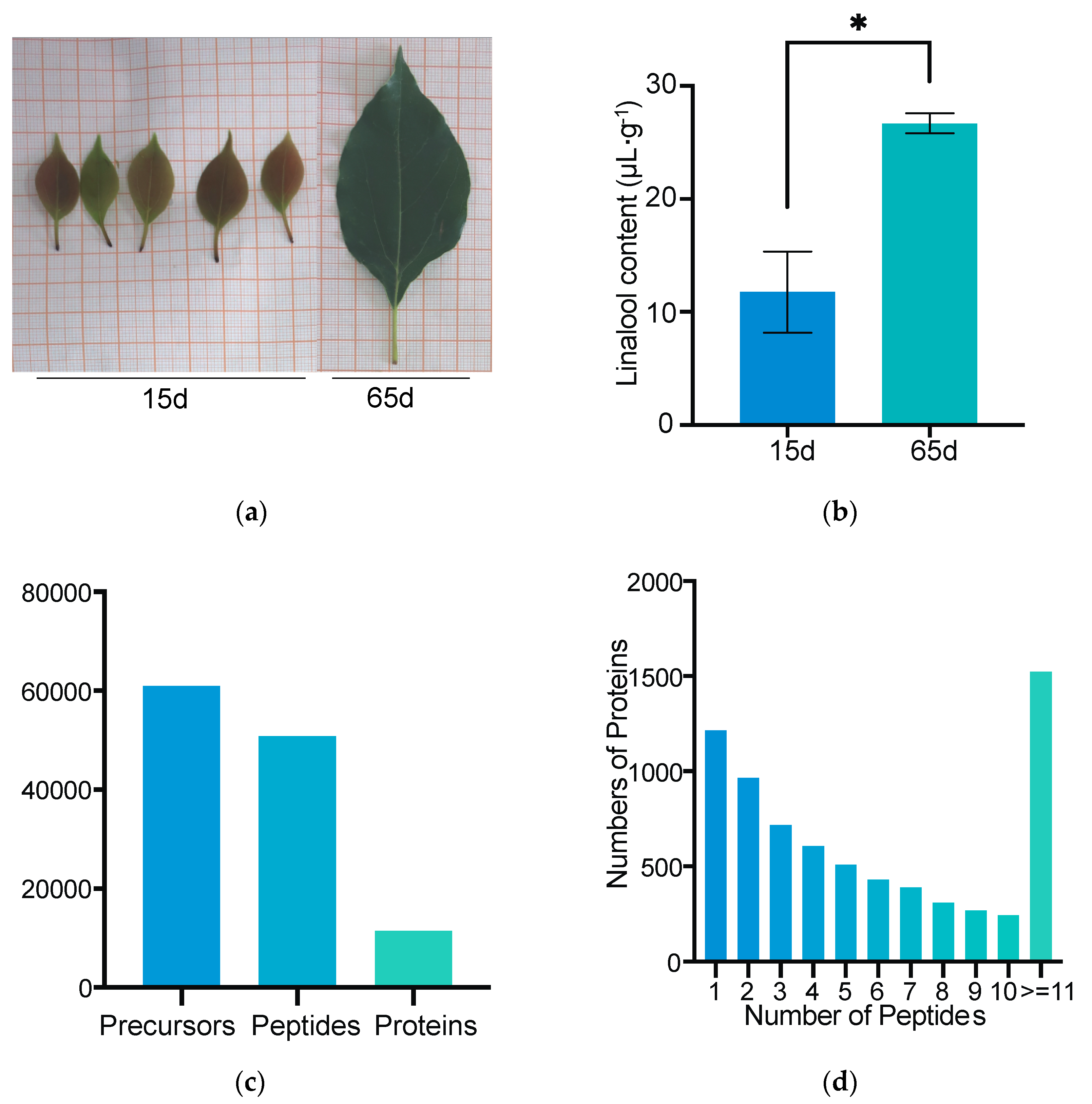
3.1. Linalool Content of Cinnamomum camphora Leaves at Different Stages
3.2. Quantitative Analysis and Functional Classification of Leaf Proteome
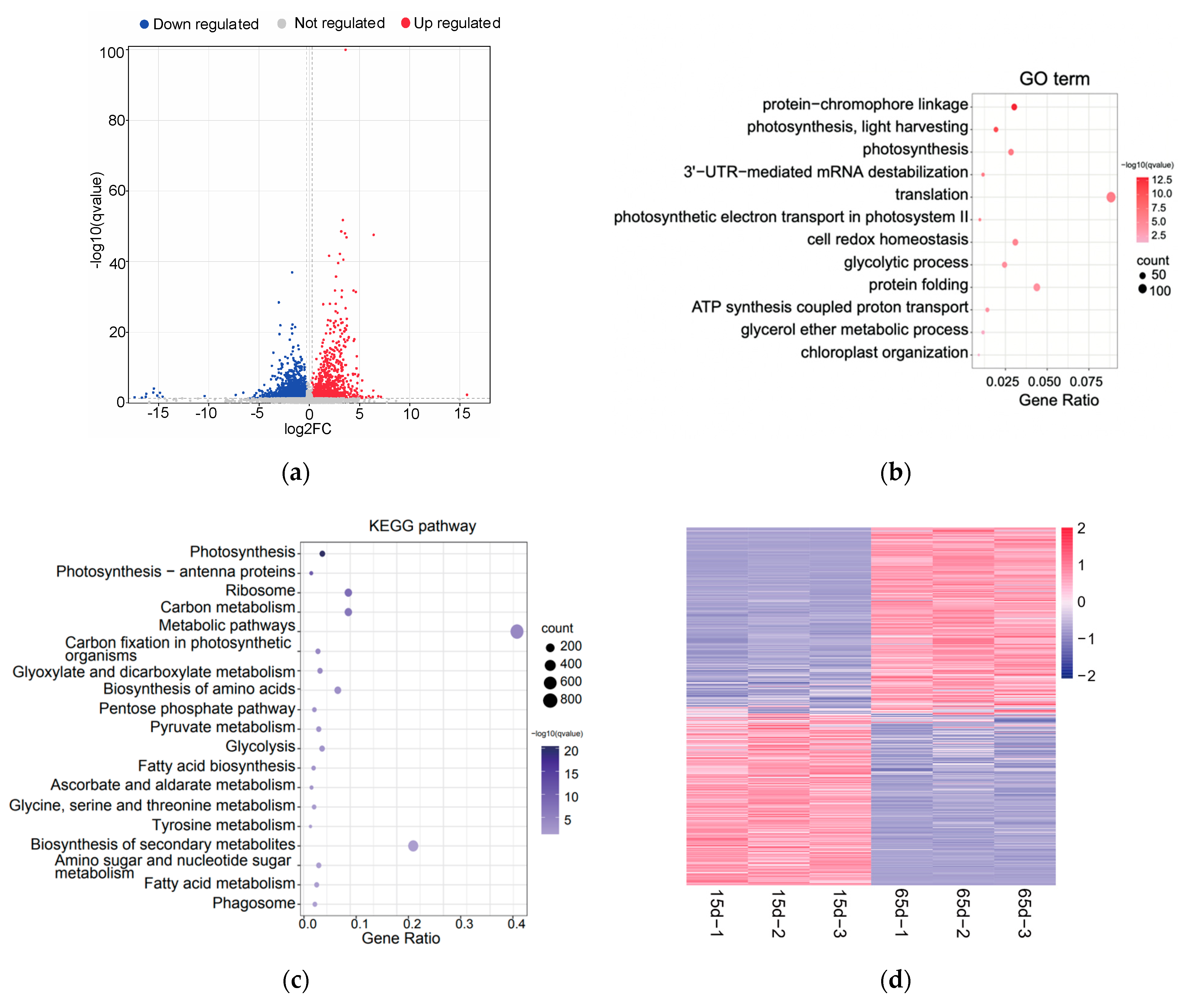
3.3. Identification and Enrichment of Differentially Expressed Proteins
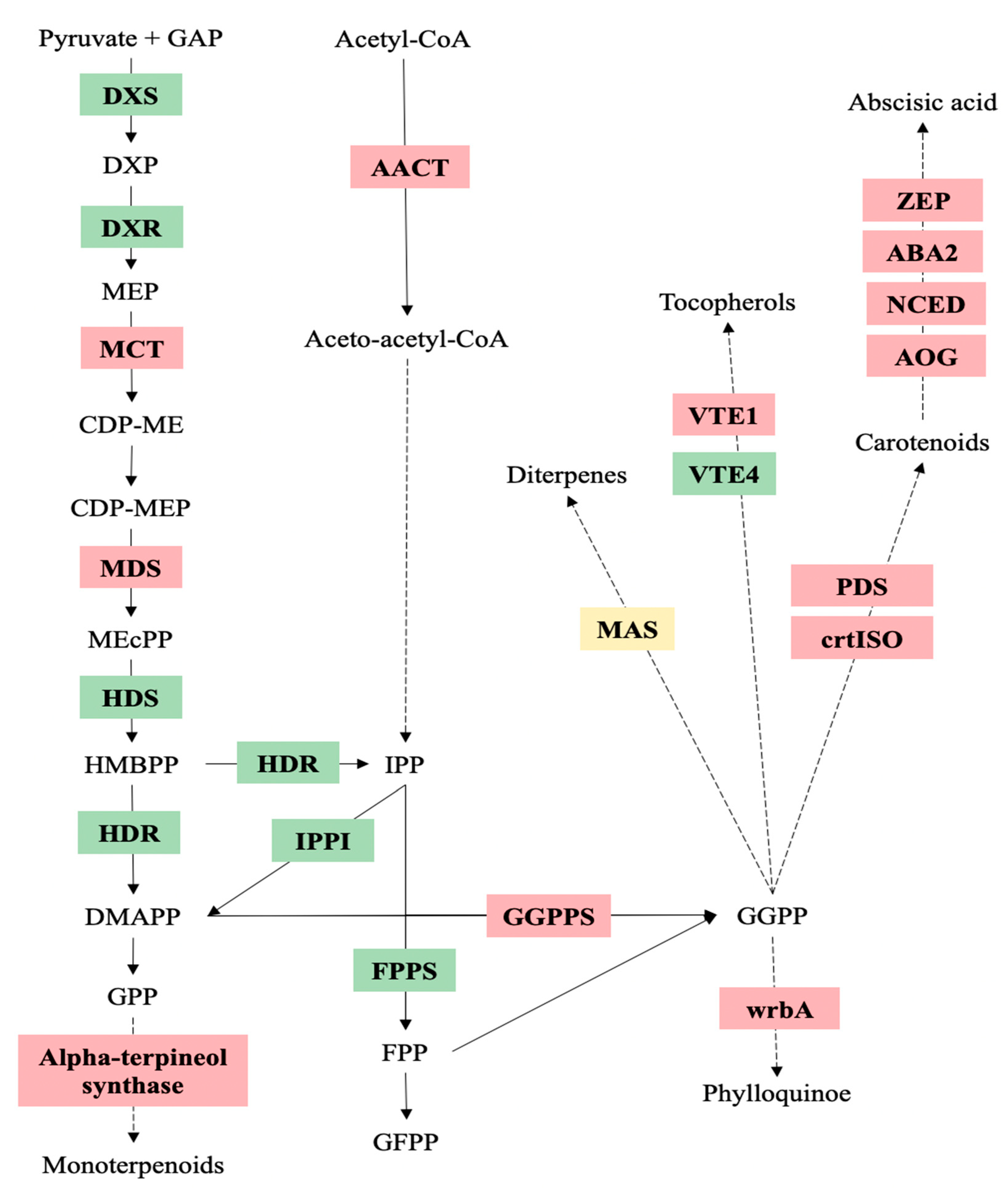
3.4. Proteins Related to Isoprenoid Biosynthesis
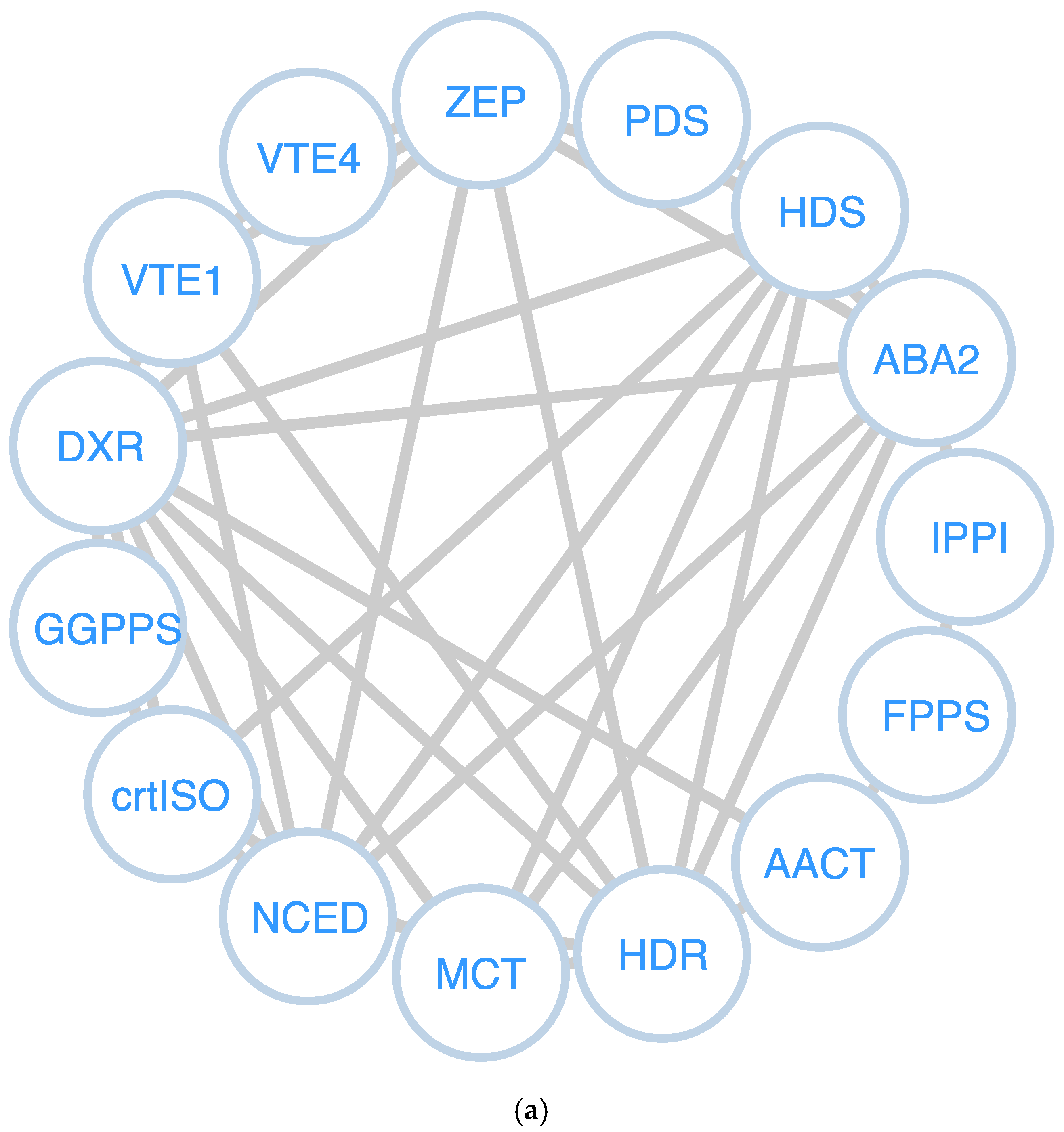
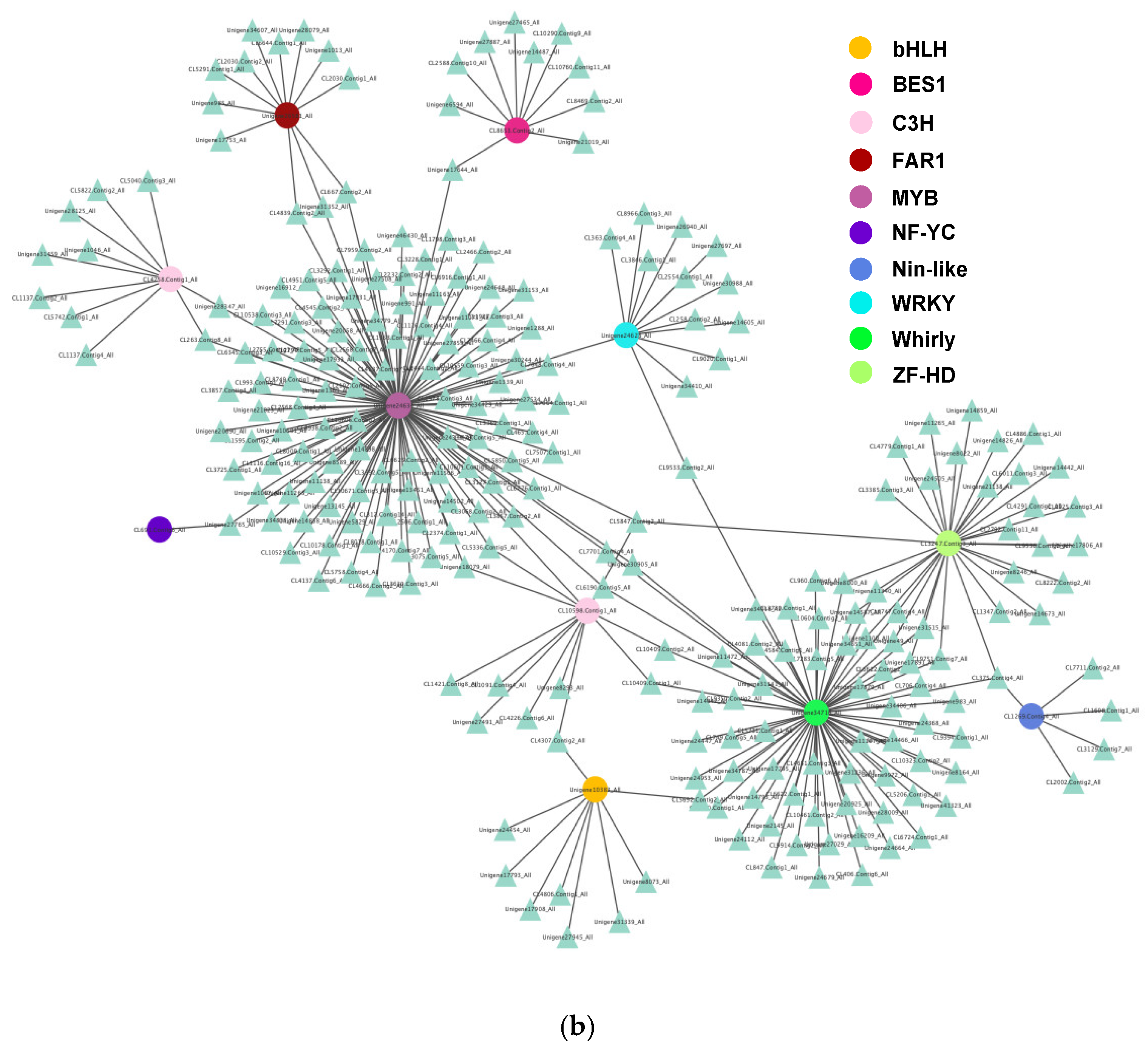
3.5. Analysis of Protein-Protein Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poudel, D.K.; Rokaya, A.; Ojha, P.K.; Timsina, S.; Satyal, R.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules 2021, 26, 5132. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Zhang, K.; Zhang, J.; Fu, J.; Li, J.; Wang, G.; Qiu, Z.; Wang, X.; Li, J. Antibacterial Activity of Cinnamomum camphora Essential Oil on Escherichia Coli During Planktonic Growth and Biofilm Formation. Front. Microbiol. 2020, 11, 561002. [Google Scholar] [CrossRef]
- Lv, C.; Hao, L.; Cui, X.; Yi, F.; Su, C. Study on the Composition and Physiological Activity of the Essential Oils and Extracts of Cinnamomum camphora Fruit. Chem. Biodivers. 2021, 18, e2100201. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhong, Y.; Wu, Y.; Li, Z.; Xu, L.-A.; Xu, M. Transcriptome Analysis and Identification of Genes Related to Terpenoid Biosynthesis in Cinnamomum camphora. BMC Genom. 2018, 19, 550. [Google Scholar] [CrossRef]
- Guo, X.; Cui, M.; Deng, M.; Liu, X.; Huang, X.; Zhang, X.; Luo, L. Molecular Differentiation of Five Cinnamomum camphora Chemotypes Using Desorption Atmospheric Pressure Chemical Ionization Mass Spectrometry of Raw Leaves. Sci. Rep. 2017, 7, 46579. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and Isoprenoids: A Wealth of Compounds for Global Use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Kim, S.-M.; Min, J.-H.; Kwon, O.-K.; Park, M.-H.; Park, J.-W.; Ahn, H.I.; Hwang, J.-Y.; Oh, S.-R.; Lee, J.-W.; et al. Anti-Inflammatory Effects of Linalool on Ovalbumin-Induced Pulmonary Inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas Fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, E.M.; Semeih, M.Y. Phyto—Monoterpene Linalool as Precursor to Synthesis Epoxides and Hydroperoxides as Anti Carcinogenic Agents via Thermal and Photo Chemical Oxidation Reactions. Arab. J. Chem. 2019, 12, 966–973. [Google Scholar] [CrossRef]
- Pu, X.; Dong, X.; Li, Q.; Chen, Z.; Liu, L. An Update on the Function and Regulation of Methylerythritol Phosphate and Mevalonate Pathways and Their Evolutionary Dynamics. J. Integr. Plant Biol. 2021, 63, 1211–1226. [Google Scholar] [CrossRef]
- Bongers, M.; Perez-Gil, J.; Hodson, M.P.; Schrübbers, L.; Wulff, T.; Sommer, M.O.; Nielsen, L.K.; Vickers, C.E. Adaptation of Hydroxymethylbutenyl Diphosphate Reductase Enables Volatile Isoprenoid Production. eLife 2020, 9, e48685. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Qi, H.; Luan, X.; Xu, W.; Yu, F.; Zhong, Y.; Xu, M. The Chromosome-level Genome Sequence of the Camphor Tree Provides Insights into Lauraceae Evolution and Terpene Biosynthesis. Plant Biotechnol. J. 2021, 20, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, J.; Zhang, B.; Jin, X.; Zhang, H.; Jin, Z. Transcriptional Analysis of Metabolic Pathways and Regulatory Mechanisms of Essential Oil Biosynthesis in the Leaves of Cinnamomum camphora (L.) Presl. Front. Genet. 2020, 11, 598714. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhong, Y.; Yu, F.; Xu, M. Deep Sequencing Identifies MiRNAs and Their Target Genes Involved in the Biosynthesis of Terpenoids in Cinnamomum camphora. Ind. Crops Prod. 2020, 145, 111853. [Google Scholar] [CrossRef]
- Ni, Z.; Han, X.; Chen, C.; Zhong, Y.; Xu, M.; Xu, L.; Yu, F. Integrating GC-MS and SsRNA-Seq Analysis to Identify Long Non-Coding RNAs Related to Terpenoid Biosynthesis in Cinnamomum camphora. Ind. Crops Prod. 2021, 171, 113875. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Zhan, T.; Li, L.; Liu, S.; Huang, Y.; An, W.; Zheng, X.; Huang, S. Genome-Wide Identification and Functional Characterization of the Trans-Isopentenyl Diphosphate Synthases Gene Family in Cinnamomum camphora. Front. Plant Sci. 2021, 12, 708697. [Google Scholar] [CrossRef]
- Pappireddi, N.; Martin, L.; Wühr, M. A Review on Quantitative Multiplexed Proteomics. ChemBioChem 2019, 20, 1210–1224. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, Z.; Xu, S.; Xu, Q.; Zeng, X.; Jabu, D.; Yuan, H. Comparative Proteomics Analysis of Tibetan Hull-Less Barley under Osmotic Stress via Data-Independent Acquisition Mass Spectrometry. GigaScience 2020, 9, giaa019. [Google Scholar] [CrossRef]
- Xie, X.; Yan, Y.; Liu, T.; Chen, J.; Huang, M.; Wang, L.; Chen, M.; Li, X. Data-Independent Acquisition Proteomic Analysis of Biochemical Factors in Rice Seedlings Following Treatment with Chitosan Oligosaccharides. Pestic. Biochem. Physiol. 2020, 170, 104681. [Google Scholar] [CrossRef]
- Cai, Y.-F.; Zhang, L.; Peng, L.-C.; Li, S.-F.; Song, J.; Xie, W.-J.; Wang, J.-H. Key Proteins and Metabolic Pathways Involved in 24-Epibrasionlide Improving Drought Tolerance of Rhododendron Delavayi Franch. Horticulturae 2021, 7, 501. [Google Scholar] [CrossRef]
- Riebel, M.; Fronk, P.; Distler, U.; Tenzer, S.; Decker, H. Proteomic Profiling of German Dornfelder Grape Berries Using Data-Independent Acquisition. Plant Physiol. Biochem. 2017, 118, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, T.; Ji, L.; Dong, Y.; Sidoli, S.; Yuan, Z.; Cai, C.; Chen, J.; Tang, Y.; Shen, Q.; et al. Comprehensive Map of the Artemisia Annua Proteome and Quantification of Differential Protein Expression in Chemotypes Producing High versus Low Content of Artemisinin. Proteomics 2020, 20, 1900310. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, W.B. In Vitro Estimation of Superfluid Critical Extracts of Some Plants for Their Antimicrobial Potential, Phytochemistry, and GC–MS Analyses. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Korpinen, R.I.; Välimaa, A.-L.; Liimatainen, J.; Kunnas, S. Essential Oils and Supercritical CO2 Extracts of Arctic Angelica (Angelica archangelica L.), Marsh Labrador Tea (Rhododendron tomentosum) and Common Tansy (Tanacetum vulgare)—Chemical Compositions and Antimicrobial Activities. Molecules 2021, 26, 7121. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, A.; Zenda, T.; Liu, S.; Liu, X.; Wang, Y.; Li, J.; Duan, H. DIA (Data Independent Acquisition) Proteomic Based Study on Maize Filling-Kernel Stage Drought Stress-Responsive Proteins and Metabolic Pathways. Biotechnol. Biotechnol. Equip. 2020, 34, 1198–1214. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, H.; Wen, S.; Qiu, F.; Liu, X. Effects of Harvest Season and Storage Time on the Essential Oil of the Linalool Chemotype of Cinnamomum camphora. J. Essent. Oil Bear. Plants 2019, 22, 1379–1385. [Google Scholar] [CrossRef]
- Mergner, J. Proteomic and Transcriptomic Profiling of Aerial Organ Development in Arabidopsis. Sci. Data 2020, 7, 334. [Google Scholar] [CrossRef]
- Han, Y.; Wang, H.; Wang, X.; Li, K.; Dong, M.; Li, Y.; Zhu, Q.; Shang, F. Mechanism of Floral Scent Production in Osmanthus fragrans and the Production and Regulation of Its Key Floral Constituents, β-Ionone and Linalool. Hortic Res. 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Kondo, Y.; Fukuda, H. BES1 and BZR1 Redundantly Promote Phloem and Xylem Differentiation. Plant Cell Physiol. 2018, 59, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Jeon, S.J.; Hwang, S.M.; Hong, J.C.; Bahk, J.D. The C 3 H-Type Zinc Finger Protein GDS1/C3H42 Is a Nuclear-Speckle-Localized Protein That Is Essential for Normal Growth and Development in Arabidopsis. Plant Sci. 2016, 250, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhang, C.; Hou, X. Cloning and Functional Analysis of BcMYB101 Gene Involved in Leaf Development in Pak Choi (Brassica rapa ssp. Chinensis). Int. J. Mol. Sci. 2020, 21, 2750. [Google Scholar] [CrossRef]
- Palmeros-Suárez, P.A.; Massange-Sánchez, J.A.; Martínez-Gallardo, N.A.; Montero-Vargas, J.M.; Gómez-Leyva, J.F.; Délano-Frier, J.P. The Overexpression of an Amaranthus Hypochondriacus NF-YC Gene Modifies Growth and Confers Water Deficit Stress Resistance in Arabidopsis. Plant Sci. 2015, 240, 25–40. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54 and WRKY70 Transcription Factors Are Involved in Brassinosteroid-Regulated Plant Growth and Drought Response. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-Y.; Kim, O.-K.; Kim, S.-G.; Yang, M.-S.; Park, C.-M. Nuclear Import and DNA Binding of the ZHD5 Transcription Factor Is Modulated by a Competitive Peptide Inhibitor in Arabidopsis. J. Biol. Chem. 2011, 286, 1659–1668. [Google Scholar] [CrossRef]
- Jang, S.; Cho, J.-Y.; Do, G.-R.; Kang, Y.; Li, H.-Y.; Song, J.; Kim, H.-Y.; Kim, B.-G.; Hsing, Y.-I. Modulation of Rice Leaf Angle and Grain Size by Expressing OsBCL1 and OsBCL2 under the Control of OsBUL1 Promoter. Int. J. Mol. Sci. 2021, 22, 7792. [Google Scholar] [CrossRef]
- Seo, H.; Kim, S.-H.; Lee, B.-D.; Lim, J.-H.; Lee, S.-J.; An, G.; Paek, N.-C. The Rice Basic Helix–Loop–Helix 79 (OsbHLH079) Determines Leaf Angle and Grain Shape. Int. J. Mol. Sci. 2020, 21, 2090. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, M.; Liu, Y.; Wang, B.; Wei, H.; Kong, D.; Wang, H. Arabidopsis FHY3 and FAR1 Function in Age Gating of Leaf Senescence. Front. Plant Sci. 2021, 12, 770060. [Google Scholar] [CrossRef]
- Gu, L.; Dou, L.; Guo, Y.; Wang, H.; Li, L.; Wang, C.; Ma, L.; Wei, H.; Yu, S. The WRKY Transcription Factor GhWRKY27 Coordinates the Senescence Regulatory Pathway in Upland Cotton (Gossypium hirsutum L.). BMC Plant Biol. 2019, 19, 116. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Liu, D.; Huang, M.; Ma, J.; Li, Z.; Li, M.; Sui, S. CpWRKY71, a WRKY Transcription Factor Gene of Wintersweet (Chimonanthus praecox), Promotes Flowering and Leaf Senescence in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 5325. [Google Scholar] [CrossRef] [PubMed]
- Janack, B.; Sosoi, P.; Krupinska, K.; Humbeck, K. Knockdown of WHIRLY1 Affects Drought Stress-Induced Leaf Senescence and Histone Modifications of the Senescence-Associated Gene HvS40. Plants 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, M.; Tian, L.; Wang, S.; Wu, L.; Ku, L.; Zhang, J.; Song, X.; Liu, H.; Chen, Y. Global Transcriptome Analysis of the Maize (Zea mays L.) Inbred Line 08LF during Leaf Senescence Initiated by Pollination-Prevention. PLoS ONE 2017, 12, e0185838. [Google Scholar] [CrossRef]
- Chenge-Espinosa, M.; Cordoba, E.; Romero-Guido, C.; Toledo-Ortiz, G.; León, P. Shedding Light on the Methylerythritol Phosphate (MEP)-Pathway: Long Hypocotyl 5 (HY5)/Phytochrome-Interacting Factors (PIFs) Transcription Factors Modulating Key Limiting Steps. Plant J. 2018, 96, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Schluttenhofer, C.; Yuan, L. Regulation of Specialized Metabolism by WRKY Transcription Factors. Plant Physiol. 2015, 167, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, Q.; Wang, C.; Liang, J.; Liu, L.; Wang, Q. ZmWRKY79 Positively Regulates Maize Phytoalexin Biosynthetic Gene Expression and Is Involved in Stress Response. J. Exp. Bot. 2018, 69, 497–510. [Google Scholar] [CrossRef]
- Okada, A.; Okada, K.; Miyamoto, K.; Koga, J.; Shibuya, N.; Nojiri, H.; Yamane, H. OsTGAP1, a bZIP Transcription Factor, Coordinately Regulates the Inductive Production of Diterpenoid Phytoalexins in Rice. J. Biol. Chem. 2009, 284, 26510–26518. [Google Scholar] [CrossRef]
- Yamamura, C.; Mizutani, E.; Okada, K.; Nakagawa, H.; Fukushima, S.; Tanaka, A.; Maeda, S.; Kamakura, T.; Yamane, H.; Takatsuji, H.; et al. Diterpenoid Phytoalexin Factor, a bHLH Transcription Factor, Plays a Central Role in the Biosynthesis of Diterpenoid Phytoalexins in Rice. Plant J. 2015, 84, 1100–1113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Zhang, F.; Chen, S.; Wang, K.; Xiang, G.; Liang, X.; An, J.; Li, K.; Liu, L. Proteomics Analysis and Identification of Proteins Related to Isoprenoid Biosynthesis in Cinnamomum camphora (L.) Presl. Forests 2022, 13, 1487. https://doi.org/10.3390/f13091487
Zhu C, Zhang F, Chen S, Wang K, Xiang G, Liang X, An J, Li K, Liu L. Proteomics Analysis and Identification of Proteins Related to Isoprenoid Biosynthesis in Cinnamomum camphora (L.) Presl. Forests. 2022; 13(9):1487. https://doi.org/10.3390/f13091487
Chicago/Turabian StyleZhu, Changsan, Fan Zhang, Silin Chen, Kun Wang, Ganju Xiang, Xiaojing Liang, Jiacheng An, Kaixiang Li, and Li Liu. 2022. "Proteomics Analysis and Identification of Proteins Related to Isoprenoid Biosynthesis in Cinnamomum camphora (L.) Presl" Forests 13, no. 9: 1487. https://doi.org/10.3390/f13091487
APA StyleZhu, C., Zhang, F., Chen, S., Wang, K., Xiang, G., Liang, X., An, J., Li, K., & Liu, L. (2022). Proteomics Analysis and Identification of Proteins Related to Isoprenoid Biosynthesis in Cinnamomum camphora (L.) Presl. Forests, 13(9), 1487. https://doi.org/10.3390/f13091487




