Knowledge about Plant Coexistence during Vegetation Succession for Forest Management on the Loess Plateau, China
Abstract
:1. Introduction
2. Materials and Methods
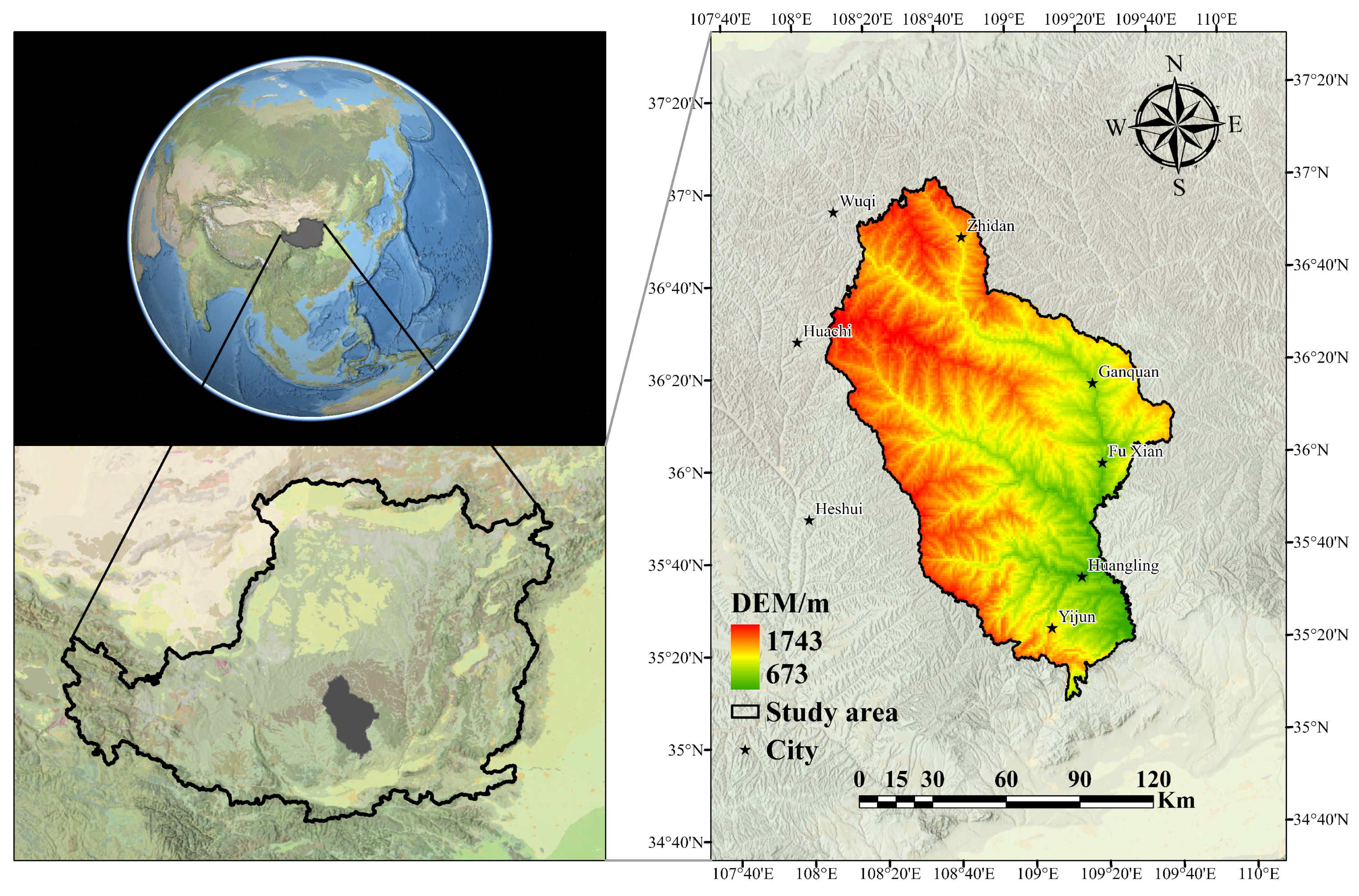
2.1. Study Area
2.2. Experimental Design and Investigation
2.3. Statistical Analyses
2.3.1. Species Selection
2.3.2. Ecological Niche Features
2.3.3. Overall Association
2.3.4. Interspecific Association
2.3.5. Strength of Associations
3. Results and Analysis
3.1. Importance Values and Ecological Niche Breadth of Dominant Plants
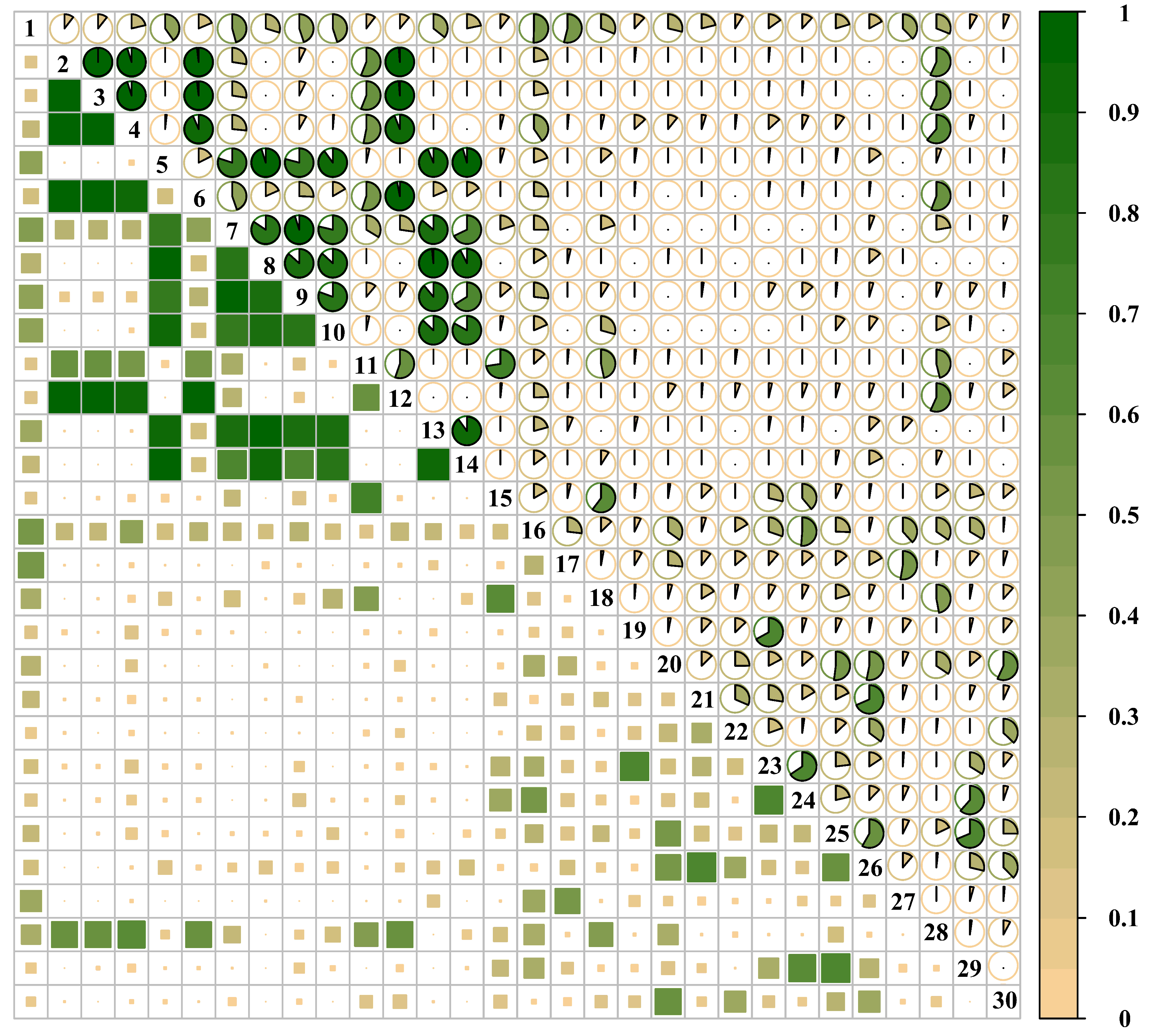
3.2. Niche Overlap
3.3. Overall Association Analysis
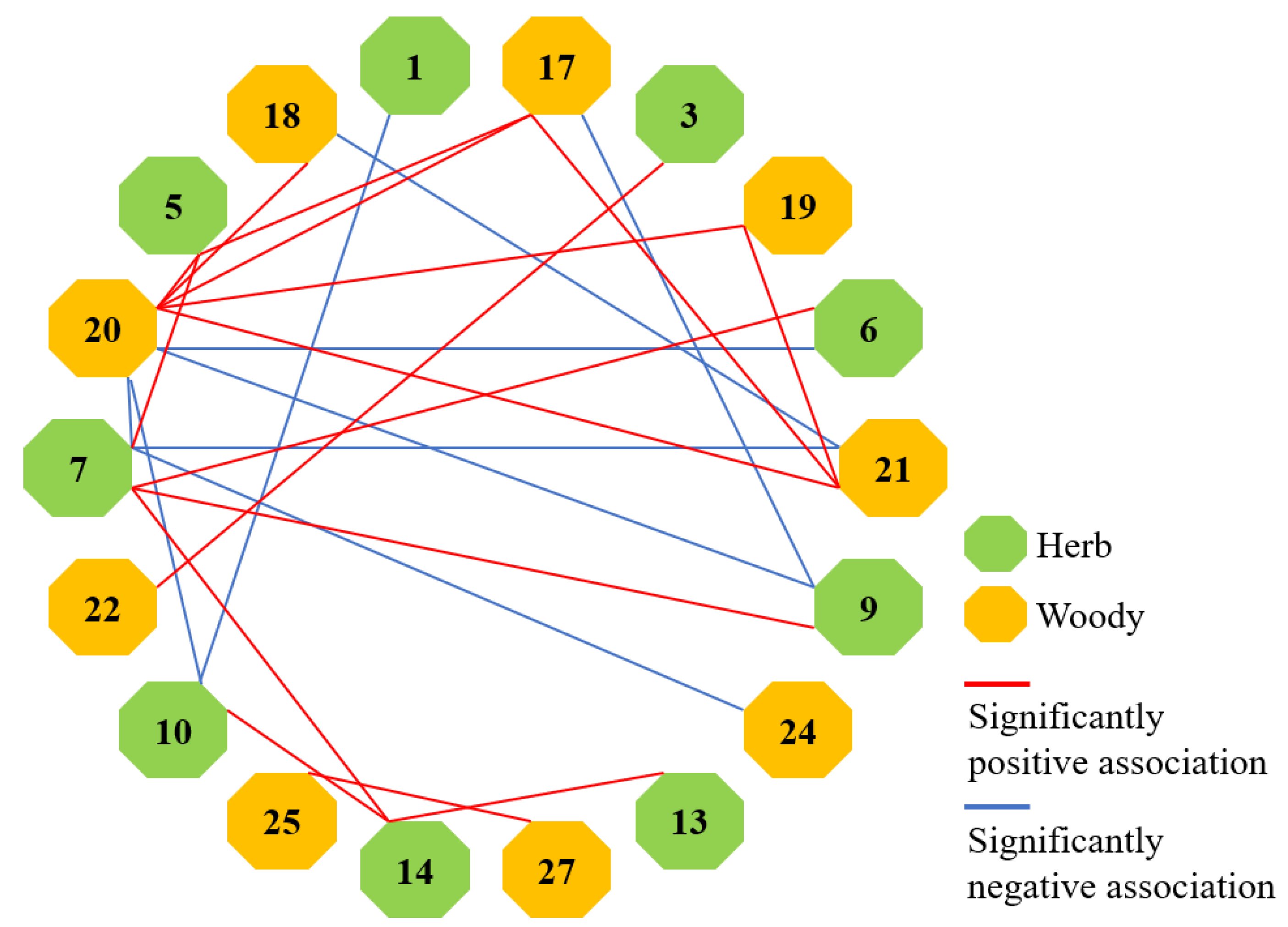
3.4. Species Association Analysis
3.4.1. Test of Species Associations
3.4.2. Strength of Species Associations
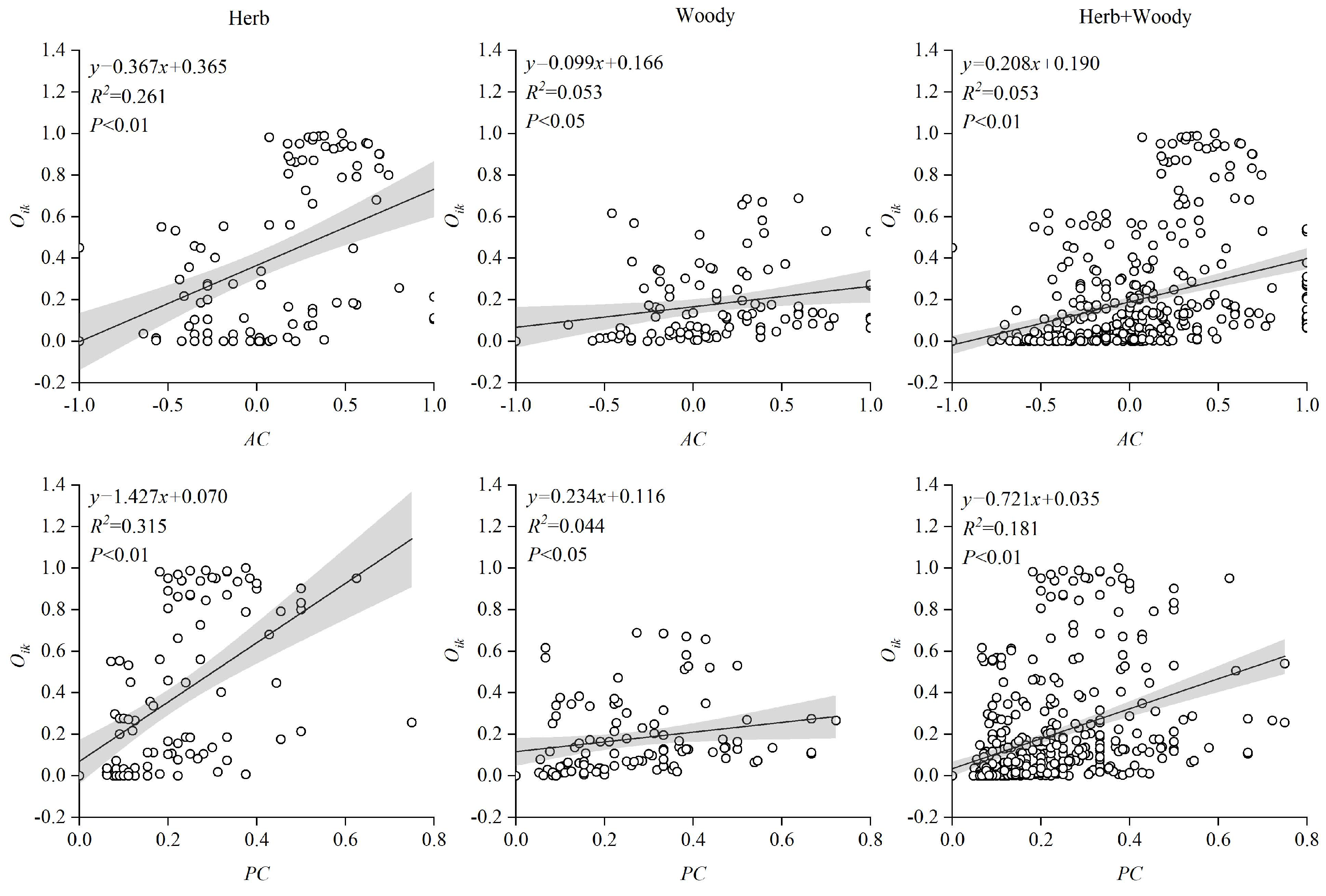
3.5. Regression Analysis between Association Strength and Niche Overlap
4. Discussion
4.1. Importance Values and Ecological Niches of Dominant Plants
4.2. Interspecific Association Strength and Niche Overlap Relationships
4.3. Inspirations and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D. Resource Competition and Community Structure. (MPB-17), Volume 17. In Resource Competition and Community Structure. (MPB-17); Princeton University Press: Princeton, NJ, USA, 2020; Volume 17. [Google Scholar] [CrossRef]
- Levine, J.M.; Bascompte, J.; Adler, P.B.; Allesina, S. Beyond pairwise mechanisms of species coexistence in complex communities. Nature 2017, 546, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Wang, X.; Yuan, Z.; Lin, F.; Ye, J.; Lin, G.; Hao, Z.; Bagchi, R. Tree species traits affect which natural enemies drive the Janzen-Connell effect in a temperate forest. Nat. Commun. 2020, 11, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bearup, D.; Nijs, I.; Wang, S.; Barabás, G.; Tao, Y.; Liao, J. Dispersal network heterogeneity promotes species coexistence in hierarchical competitive communities. Ecol. Lett. 2021, 24, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hui, C. How competitive intransitivity and niche overlap affect spatial coexistence. Oikos 2021, 130, 260–273. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Pocheville, A. The ecological niche: History and recent controversies. In Handbook of Evolutionary Thinking in the Sciences; Springer: Dordrecht, The Netherlands, 2015; pp. 547–586. [Google Scholar] [CrossRef]
- Pastore, A.I.; Barabás, G.; Bimler, M.D.; Mayfield, M.M.; Miller, T.E. The evolution of niche overlap and competitive differences. Nat. Ecol. Evol. 2021, 5, 330–337. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, Z.W.; Han, G.D. Effects of heavy grazing on the interspecific relationship of main plant species and community stability in a desert steppe. Chin. J. Ecol. 2019, 38, 2595–2602. (In Chinese) [Google Scholar]
- Gao, W.; Huang, Y.; Lin, J.; Huang, M.; Wu, X.; Lin, W.; Huang, S. Interspecific Correlations among Dominant Populations of Natural Forest of Endangered Species Betula fujianensis. Sci. Silvae Sin. 2021, 57, 1–14. [Google Scholar] [CrossRef]
- Jin, S.S.; Zhang, Y.Y.; Zhou, M.L.; Dong, X.M.; Chang, C.H.; Wang, T.; Yan, D.F. Interspecific Association and Community Stability of Tree Species in Natural Secondary Forests at Different Altitude Gradients in the Southern Taihang Mountains. Forests 2022, 13, 373. [Google Scholar] [CrossRef]
- Tokeshi, M. Species Coexistence: Ecological and Evolutionary Perspectives; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Adler, P.B.; Smull, D.; Beard, K.; Choi, R.T.; Furniss, T.; Kulmatiski, A.; Meiners, J.M.; Tredennick, A.T.; Veblen, K.E. Competition and coexistence in plant communities: Intraspecific competition is stronger than interspecific competition. Ecol. Lett. 2018, 21, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lü, Y.; Fu, B.; Comber, A.; Li, T.; Hu, J. Driving factors of land change in china’s loess plateau: Quantification using geographically weighted regression and management implications. Remote Sens. 2020, 12, 453. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, Y.; Wu, Z.; Lv, T. A bibliometric analysis on land degradation: Current status, development, and future directions. Land 2020, 9, 28. [Google Scholar] [CrossRef]
- Hua, F.; Wang, X.; Zheng, X.; Fisher, B.; Wang, L.; Zhu, J.; Tang, Y.; Yu, D.W.; Wilcove, D.S. Opportunities for biodiversity gains under the world’s largest reforestation programme. Nat. Commun. 2016, 7, 12717. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Xu, B.C. Discussion on some issues about returning Farmland to Forest or Grassland on Loess Plateau in new era. Bull. Soil Water Conserv. 2019, 39, 3. (In Chinese) [Google Scholar]
- Kou, P.; Xu, Q.; Jin, Z.; Yunus, A.P.; Luo, X.; Liu, M. Complex anthropogenic interaction on vegetation greening in the Chinese Loess Plateau. Sci. Total Environ. 2021, 778, 146065. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Q.; Feng, X.; Shi, W.; Fu, B.; Lü, Y.; Liu, Y. The synergistic effects of afforestation and the construction of check-dams on sediment trapping: Four decades of evolution on the Loess Plateau, China. Land Degrad. Dev. 2019, 30, 622–635. [Google Scholar] [CrossRef]
- Jin, F.; Yang, W.; Fu, J.; Li, Z. Effects of vegetation and climate on the changes of soil erosion in the Loess Plateau of China. Sci. Total Environ. 2021, 773, 145514. [Google Scholar] [CrossRef]
- Wang, Y.H.; Bonell, M.; Feger, K.H.; Yu, P.; Xiong, W.; Xu, L. Changing forestry policy by integrating water aspects into forest/vegetation restoration in dryland areas in China. Bull. Chin. Acad. Sci. 2012, 26, 59–67. [Google Scholar] [CrossRef]
- Sun, G.; Zhou, G.; Zhang, Z.; Wei, X.; McNulty, S.G.; Vose, J.M. Potential water yield reduction due to forestation across China. J. Hydrol. 2006, 328, 548–558. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Hasi, E.; Li, J. Desertification in China: An assessment. Earth-Sci. Rev. 2008, 88, 188–206. [Google Scholar] [CrossRef]
- Li, S.; Liang, W.; Fu, B.; Lü, Y.; Fu, S.; Wang, S.; Su, H. Vegetation changes in recent large-scale ecological restoration projects and subsequent impact on water resources in China’s Loess Plateau. Sci. Total Environ. 2016, 569, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.A.; Wang, Y.; Xia, Y.; Jia, X. Soil drought and water carrying capacity for vegetation in the critical zone of the Loess Plateau: A review. Vadose Zone J. 2018, 17, 170077. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Fu, B.; Lü, Y.; Liu, Y.; Wu, X. Integrating vegetation suitability in sustainable revegetation for the Loess Plateau, China. Sci. Total Environ. 2021, 759, 143572. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.; Li, Y.; Härdtle, W.; Von Oheimb, G.; Yang, X.; et al. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef]
- Gong, C.; Tan, Q.; Xu, M.; Liu, G. Mixed-species plantations can alleviate water stress on the Loess Plateau. For. Ecol. 2020, 458, 117767. [Google Scholar] [CrossRef]
- Wang, K.B.; Ren, Z.P.; Deng, L.; Zhou, Z.C.; Shangguan, Z.P.; Shi, W.Y.; Chen, Y.P. Profile distributions and controls of soil inorganic carbon along a 150-year natural vegetation restoration chronosequence. Soil Sci. Soc. Am. J. 2016, 80, 193–202. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.; Zhu, G.; Liu, Y.; Chen, L.; Shangguan, Z.P. Changes of soil carbon in five land use stages following 10 years of vegetation succession on the Loess Plateau, China. Catena 2018, 171, 185–192. [Google Scholar] [CrossRef]
- Deng, L.; Kim, D.G.; Peng, C.; Shangguan, Z.P. Controls of soil and aggregate-associated organic carbon variations following natural vegetation restoration on the Loess Plateau in China. Land Degrad. Dev. 2018, 29, 3974–3984. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- West, D.C.; Shugart, H.H.; Botkin, D.F. (Eds.) Forest Succession: Concepts and Application; Springer Science and Business Media: Berlin, Germany, 2012. [Google Scholar]
- Zou, H.Y.; Liu, G.B.; Wang, H.S. The vegetation development in North Ziwuling forest region in last fifty years. Acta Botan. Boreali-Occiden. Sin. 2002, 22, 1–8. (In Chinese) [Google Scholar]
- Fan, W.Y.; Wang, X.A.; Guo, H. Analysis of plant community successional series in the Ziwuling area on the Loess Plateau. Acta Ecol. Sin. 2006, 26, 706–714. (In Chinese) [Google Scholar]
- Cheng, J.; Cheng, J.; Shao, H.; Zhao, L.; Yang, X. Soil seed banks and forest succession direction reflect soil quality in Ziwuling Mountain, Loess Plateau, China. Clean–Soil Air Water 2012, 40, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Cowles, H.C. The Ecological Relations of the Vegetation on the Sand Dunes of Lake Michigan. Part I.-Geographical Relations of the Dune Floras. Bot. Gaz. 1899, 27, 95–117. [Google Scholar] [CrossRef]
- Blois, J.L.; Williams, J.W.; Fitzpatrick, M.C.; Jackson, S.T.; Ferrier, S. Space can substitute for time in predicting climate-change effects on biodiversity. Proc. Natl. Acad. Sci. USA 2013, 110, 9374–9379. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, K.B.; Chen, M.L.; Shangguan, Z.P.; Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. Catena 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Liu, X.F.; Wang, Z.L.; Zhao, S.W. Soil organic carbon fractions and sequestration across a 150-yr secondary forest chronosequence on the Loess Plateau, China. Catena 2015, 133, 303–308. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Hai, X.; Li, J.; Shangguan, Z.P.; Peng, C.; Deng, L. Long-term forest succession improves plant diversity and soil quality but not significantly increase soil microbial diversity: Evidence from the Loess Plateau. Ecol. Eng. 2020, 142, 105631. [Google Scholar] [CrossRef]
- Li, J. Flora of China. Harv. Pap. Bot. 2007, 13, 301–302. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource Partitioning in Ecological Communities: Research on how similar species divide resources helps reveal the natural regulation of species diversity. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Song, Y.C. Vegetation Ecology; East China Normal University Press: Shanghai, China, 2001. (In Chinese) [Google Scholar]
- Colwell, R.K.; Futuyma, D.J. On the measurement of niche breadth and overlap. Ecology 1971, 52, 567–576. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Schluter, D. A variance test for detecting species associations, with some example applications. Ecology 1984, 65, 998–1005. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Dai, J.; Liu, H.; Xu, C.; Qi, Y.; Zhu, X.; Zhou, M.; Liu, B.; Wu, Y. Divergent hydraulic strategies explain the interspecific associations of co-occurring trees in forest–steppe ecotone. Forests 2020, 11, 942. [Google Scholar] [CrossRef]
- Grieg-Smith, P. Quantitative Plant Ecology (Studies in Ecology); Blackwell Science Ltd: Hoboken, NJ, USA, 1983. [Google Scholar]
- Zhang, J.T. Quantitative Ecology; Science Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Zhang, J.L. Spaa: Species Association Analysis, R Package Version 0.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Wei, T.; Simko, V.R. Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 20 August 2022).
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Álvarez-Yépiz, J.C.; Búrquez, A.; Dovčiak, M. Ontogenetic shifts in plant–plant interactions in a rare cycad within angiosperm communities. Oecologia 2014, 175, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; O’Hara, K.L.; Li, W.Z.; Gong, Z.W. Spatial patterns and interspecific associations among trees at different stand development stages in the natural secondary forests on the Loess Plateau, China. Ecol. Evol. 2019, 9, 6410–6421. [Google Scholar] [CrossRef]
- Fielding, A.P.; Pantel, J.H. Eco-evolutionary feedbacks and the maintenance of metacommunity diversity in a changing environment. Genes 2020, 11, 1433. [Google Scholar] [CrossRef]
- Tsafack, N.; Wang, X.; Xie, Y.; Fattorini, S. Niche overlap and species co-occurrence patterns in carabid communities of the northern Chinese steppes. ZooKeys 2021, 1044, 929–949. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, B.; Chen, Y.; Jia, H.; Wei, Q.; Ye, Y. How do similarities in spatial distributions and interspecific associations affect the coexistence of Quercus species in the Baotianman National Nature Reserve, Henan, China. Ecol. Evol. 2018, 8, 2580–2593. [Google Scholar] [CrossRef]
- Stubbs, W.J.; Bastow, W.J. Evidence for limiting similarity in a sand dune community. J. Ecol. 2004, 92, 557–567. [Google Scholar] [CrossRef]
- Nathan, R. Long-distance dispersal of plants. Science 2006, 313, 786–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petritan, I.C.; Marzano, R.; Petritan, A.M.; Lingua, E. Overstory succession in a mixed Quercus petraea–Fagus sylvatica old growth forest revealed through the spatial pattern of competition and mortality. For. Ecol. Manag. 2014, 326, 9–17. [Google Scholar] [CrossRef]
- Ma, F.; Pan, G.; Li, X.; Han, Y. Interspecific relationship and canonical correspondence analysis within woody plant communities in the karst mountains of Southwest Guangxi, southern China. J. Beijing For. Univ. 2017, 39, 32–44. [Google Scholar] [CrossRef]
- Kikuzawa, K.; Onoda, Y.; Wright, I.J.; Reich, P.B. Mechanisms underlying global temperature-related patterns in leaf longevity. Glob. Ecol. Biogeogr. 2013, 22, 982–993. [Google Scholar] [CrossRef]
- Tilman, D. Competition and biodiversity in spatially structured habitats. Ecology 1994, 75, 2–16. [Google Scholar] [CrossRef]
- Pedersen, R.Ø.; Bollandsås, O.M.; Gobakken, T.; Næsset, E. Deriving individual tree competition indices from airborne laser scanning. For. Ecol. Manag. 2012, 280, 150–165. [Google Scholar] [CrossRef]
- Wu, S.; Wen, L.; Dong, S.; Gao, X.; Xu, Y.; Li, S.; Dong, Q.; Wessell, K. The Plant Interspecific Association in the Revegetated Alpine Grasslands Determines the Productivity Stability of Plant Community Across Restoration Time on Qinghai-Tibetan Plateau. Front. Plant Sci. 2022, 13, 850854. [Google Scholar] [CrossRef]
- Shao, L.Y.; Zhang, G.F. Niche and interspecific association of dominant tree populations of Zelkova schneideriana communities in eastern China. Bot. Sci. 2021, 99, 823–833. [Google Scholar] [CrossRef]
- Gu, L.; Gong, Z.W.; Li, W.Z. Niches and interspecific associations of dominant populations in three changed stages of natural secondary forests on Loess Plateau, PR China. Sci. Rep. 2017, 7, 6604. [Google Scholar] [CrossRef]
- Fu, B.; Wang, S.; Liu, Y.; Liu, J.; Liang, W.; Miao, C. Hydrogeomorphic ecosystem responses to natural and anthropogenic changes in the Loess Plateau of China. Annu. Rev. Earth Planet. Sci. 2017, 45, 223–243. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Huang, J.; Wen, Z.; Du, S. Afforestation and climatic niche dynamics of black locust (Robinia pseu-doacacia). For. Ecol. Manag. 2018, 407, 184–190. [Google Scholar] [CrossRef]
- Du, S.; Wang, Y.L.; Kume, T.; Zhang, J.G.; Otsuki, K.; Yamanaka, N.; Liu, G.B. Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agric. For. Meteorol. 2011, 151, 1–10. [Google Scholar] [CrossRef]
- Cao, S.; Sun, G.; Zhang, Z.; Chen, L.; Feng, Q.; Fu, B.; McNulty, S.; Shankman, D.; Tang, J.; Wang, Y.; et al. Greening china naturally. Ambio 2011, 40, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Lu, N.; Fu, B.; Wang, J.; Li, Z.; Fang, W.; Liu, J.; Wang, C.; Zhang, L. Evapotranspiration partitioning and its implications for plant water use strategy: Evidence from a black locust plantation in the semi-arid Loess Plateau, China. For. Ecol. Manag. 2018, 424, 428–438. [Google Scholar] [CrossRef]
- Wei, J.S.; Li, Z.S.; Feng, X.Y.; Zhang, Y.; Chen, W.L.; Xing, W.U.; Jiao, L.; Wang, X.C. Ecological and physiological mecha-nisms of growth decline of Robinia pseudoacacia plantations in the Loess Plateau of China: A review. Chin. J. Appl. Ecology 2018, 29, 2433–2444. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. Responses of different physiological parameter thresholds to soil water availability in four plant species during prolonged drought. Agric. For. Meteorol. 2017, 247, 311–319. [Google Scholar] [CrossRef]
- Peng, S.; Ding, Y.; Wen, Z.; Chen, Y.; Cao, Y.; Ren, J. Spatiotemporal change and trend analysis of potential evapo-transpiration over the Loess Plateau of China during 2011–2100. Agric. For. Meteorol. 2017, 233, 183–194. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, M.; Yang, Y.; Zhao, X. Evaluating drought-induced mortality risk for Robinia pseudoacacia planta-tions along the precipitation gradient on the Chinese Loess Plateau. Agric. For. Meteorol. 2020, 284, 107897. [Google Scholar] [CrossRef]
- Usinowicz, J.; Chang-Yang, C.-H.; Chen, Y.-Y.; Clark, J.S.; Fletcher, C.; Garwood, N.C.; Chia-Hao, C.-Y.; Johnstone, J.; Lin, Y.; Metz, M.; et al. Temporal coexistence mechanisms contribute to the latitudinal gradient in forest diversity. Nature 2017, 550, 105–108. [Google Scholar] [CrossRef]

| Restoration Age (Year) | Succession Stage | Numbers of Sites | Representative Plants |
|---|---|---|---|
| 0 | Agricultural land | 6 | Viola collina Besser; Agropyron cristatum (L.) Gaertn. |
| 10 | Natural restoration of 10 years of grass | 6 | Bothriochloa ischaemum (L.) Keng; Artemisia chamaemelifolia Vill; Lespedeza bicolor Turcz. |
| 20 | Natural restoration of 20 years of grass | 6 | Artemisia codonocephala Diels; Agrimonia pilosa Ledeb; Lespedeza bicolor Turcz. |
| 40 | Shrub stage | 6 | Sophora davidii (Franch.) Pavol; Lespedeza bicolor Turcz; Agropyron cristatum (L.) Gaertn; Anemone chinensis Bunge. |
| 70 | Pioneer arbor stage | 6 | Betula platyphylla Sukaczev; Spiraea salicifolia L.; Lespedeza bicolor Turcz; Carex lanceolata Boott. |
| 120 | Sub-top stage | 6 | Pinus tabuliformis Carrière; Lespedeza bicolor Turcz; Carex lanceolata Boott. |
| 135 | Sub-top to top transition stage | 6 | Pinus tabuliformis Carrière; Quercus mongolica Fisch. Ex Ledeb; Lespedeza bicolor Turcz; Carex lanceolata Boott. |
| 150 | Top Stage | 6 | Quercus mongolica Fisch. Ex Ledeb; Lespedeza bicolor Turcz; Carex lanceolata Boott. |
| No. | Herb | IV/% | Bi | No. | Woody | IV/% | Bi |
|---|---|---|---|---|---|---|---|
| 1 | Carex lanceolata Boott | 14.43 | 12.80 | 16 | Lespedeza bicolor Turcz | 20.98 | 9.42 |
| 2 | Artemisia argyi H.Lév. & Vaniot | 8.7 | 1.07 | 17 | Quercus mongolica Fisch. Ex Ledeb | 11.41 | 5.25 |
| 3 | Agrimonia pilosa Ledeb | 7.53 | 1.07 | 18 | Sophora davidii (Franch.) Pavol | 5.67 | 4.36 |
| 4 | Miscanthus sinensis Andersson | 5.77 | 3.01 | 19 | Spiraea salicifolia L. | 5.55 | 2.11 |
| 5 | Artemisia chamaemelifolia Vill. | 5.17 | 3.49 | 20 | Pinus tabuliformis Carrière | 5.37 | 7.61 |
| 6 | Viola philippica Cav | 4.41 | 1.70 | 21 | Acer tataricum subsp. ginnala (Maxim.) Wesm | 4.34 | 3.68 |
| 7 | Potentilla chinensis Ser | 3.1 | 3.48 | 22 | Ostryopsis davidiana Decne | 4.32 | 2.67 |
| 8 | Bothriochloa ischaemum (L.) Keng | 2.67 | 2.27 | 23 | Betula platyphylla Sukaczev | 4 | 5.96 |
| 9 | Aster hispidus Thunb | 2.54 | 2.69 | 24 | Cotoneaster multiflorus Bunge | 3.63 | 3.34 |
| 10 | Artemisia lancea Vaniot | 2.21 | 3.81 | 25 | Lonicera japonica Thunb | 3.23 | 6.21 |
| 11 | Viola collina Besser | 2.18 | 3.38 | 26 | Campylotropis macrocarpa (Bunge) Rehder | 2.97 | 5.48 |
| 12 | Cyperus compressus L. | 1.84 | 1.45 | 27 | Koelreuteria paniculata Laxm | 2.75 | 1.43 |
| 13 | Sophora flavescens Aiton | 1.83 | 2.45 | 28 | Periploca sepium Bunge | 1.98 | 3.73 |
| 14 | Anemone chinensis Bunge | 1.76 | 2.23 | 29 | Rubus parvifolius L. | 1.84 | 2.29 |
| 15 | Agropyron cristatum (L.) Gaertn | 1.71 | 3.77 | 30 | Rhamnus utilis Decne | 1.52 | 1.54 |
| Life Form | Variance Ratio (VR) | Test Statistics (W) | χ2(0.95,N), χ2(0.05,N) | Test Results |
|---|---|---|---|---|
| Herb | 2.40 | 115.20 | 33.10, 65.17 | Significant association |
| Woody | 2.49 | 119.52 | 33.10, 65.17 | Significant association |
| Herb + Woody | 1.20 | 57.82 | 33.10, 65.17 | Not a significant association |
| Life Form | Positive Association (p) | Negative Association (N) | p/N |
|---|---|---|---|
| Herb | 85 | 20 | 4.25 |
| Woody | 78 | 27 | 2.89 |
| Herb + Woody | 317 | 118 | 2.69 |
| Index | Type | Strength of Association | Herb | Woody | Herb + Woody | |||
|---|---|---|---|---|---|---|---|---|
| Species Pair Number | % | Species Pair Number | % | Species Pair Number | % | |||
| AC | Positive association | AC ≥ 0.6 | 12 | 11.43 | 13 | 12.38 | 38 | 8.74 |
| 0.2 ≤ AC < 0.6 | 31 | 29.52 | 30 | 28.57 | 74 | 17.01 | ||
| 0 < AC < 0.2 | 18 | 17.14 | 20 | 19.05 | 99 | 22.76 | ||
| No association | AC = 0 | 0 | 0.00 | 1 | 0.95 | 3 | 0.69 | |
| Negative association | −0.2 ≤ AC < 0 | 8 | 7.62 | 18 | 17.14 | 59 | 13.56 | |
| −0.6 ≤ AC < −0.2 | 23 | 21.91 | 21 | 20.00 | 93 | 21.38 | ||
| AC < −0.6 | 13 | 12.38 | 2 | 1.91 | 69 | 15.86 | ||
| PC | 0.5 ≤ PC < 1 | 6 | 5.71 | 11 | 10.48 | 22 | 5.06 | |
| 0 < PC < 0.5 | 88 | 83.81 | 93 | 88.57 | 360 | 82.76 | ||
| PC = 0 | 11 | 10.48 | 1 | 0.95 | 53 | 12.18 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Zhang, X.; Xu, X.; Yi, H.; He, J.; He, L.; Sun, W. Knowledge about Plant Coexistence during Vegetation Succession for Forest Management on the Loess Plateau, China. Forests 2022, 13, 1456. https://doi.org/10.3390/f13091456
Tian Q, Zhang X, Xu X, Yi H, He J, He L, Sun W. Knowledge about Plant Coexistence during Vegetation Succession for Forest Management on the Loess Plateau, China. Forests. 2022; 13(9):1456. https://doi.org/10.3390/f13091456
Chicago/Turabian StyleTian, Qilong, Xiaoping Zhang, Xiaoming Xu, Haijie Yi, Jie He, Liang He, and Weinan Sun. 2022. "Knowledge about Plant Coexistence during Vegetation Succession for Forest Management on the Loess Plateau, China" Forests 13, no. 9: 1456. https://doi.org/10.3390/f13091456
APA StyleTian, Q., Zhang, X., Xu, X., Yi, H., He, J., He, L., & Sun, W. (2022). Knowledge about Plant Coexistence during Vegetation Succession for Forest Management on the Loess Plateau, China. Forests, 13(9), 1456. https://doi.org/10.3390/f13091456










