The Effect of Stand Structure on Soil Physico-Chemical and Biological Properties in a Primary Beech Forest
Abstract
1. Introduction
2. Materials and Methods
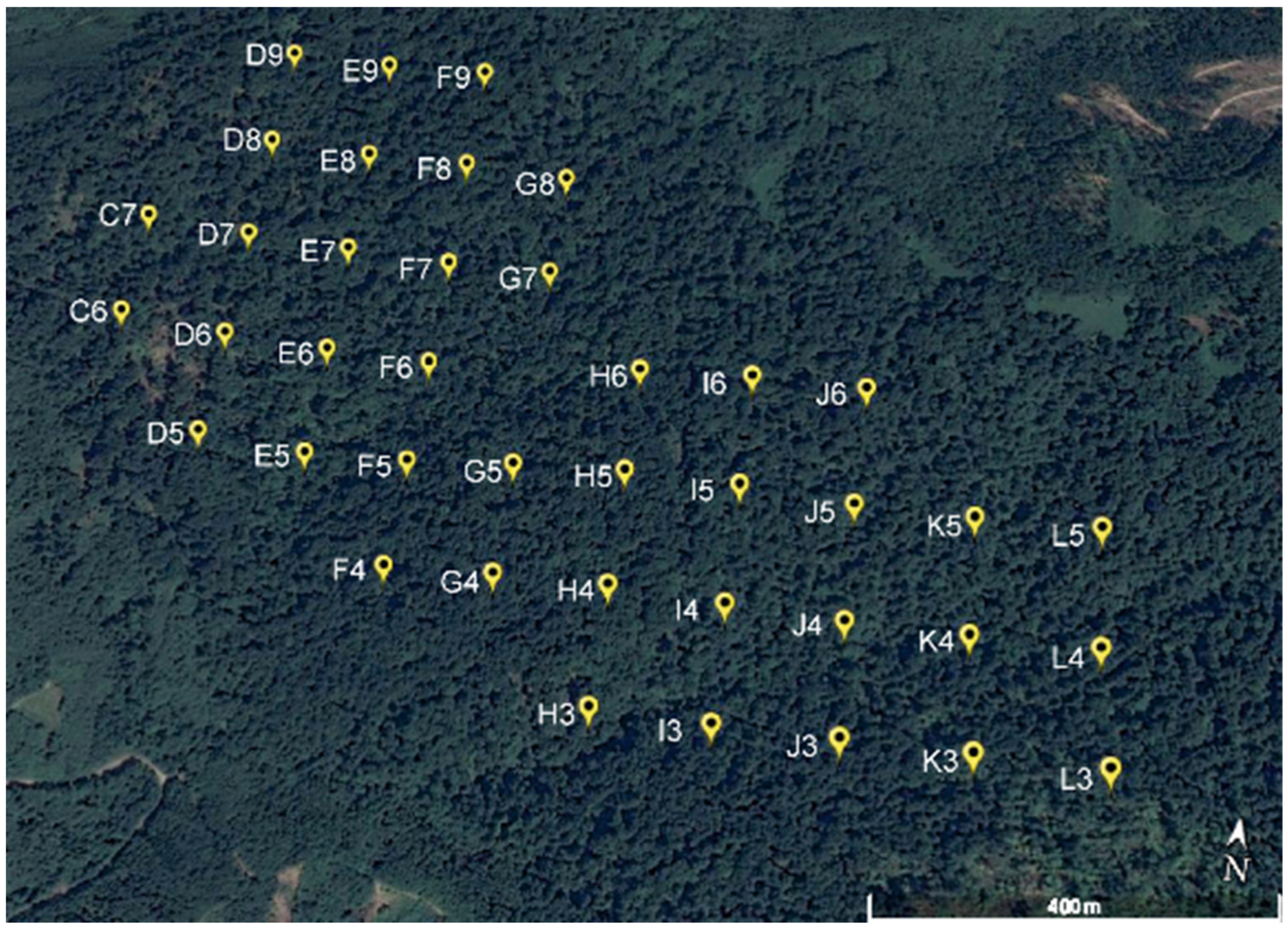
2.1. Site Description
2.2. Stand Structure Characteristics
2.3. Soil Properties and Microbial Analysis
2.4. Statistical Analysis
3. Results
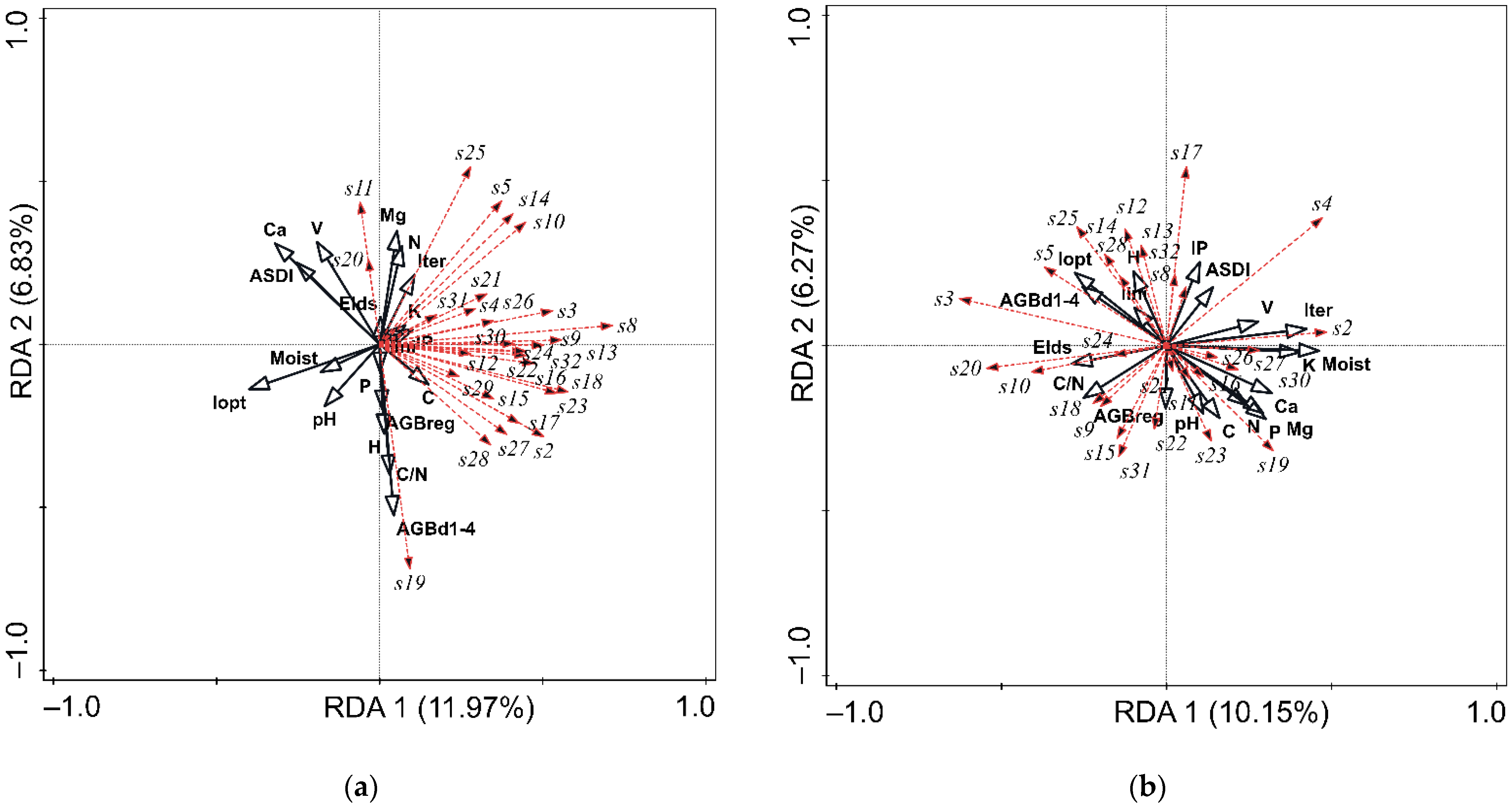
3.1. Spatial Variability of Stand Structure and Soil Biological Properties
3.2. Stand Structure as a Factor Influencing Soil Physico-Chemical and Biological Properties
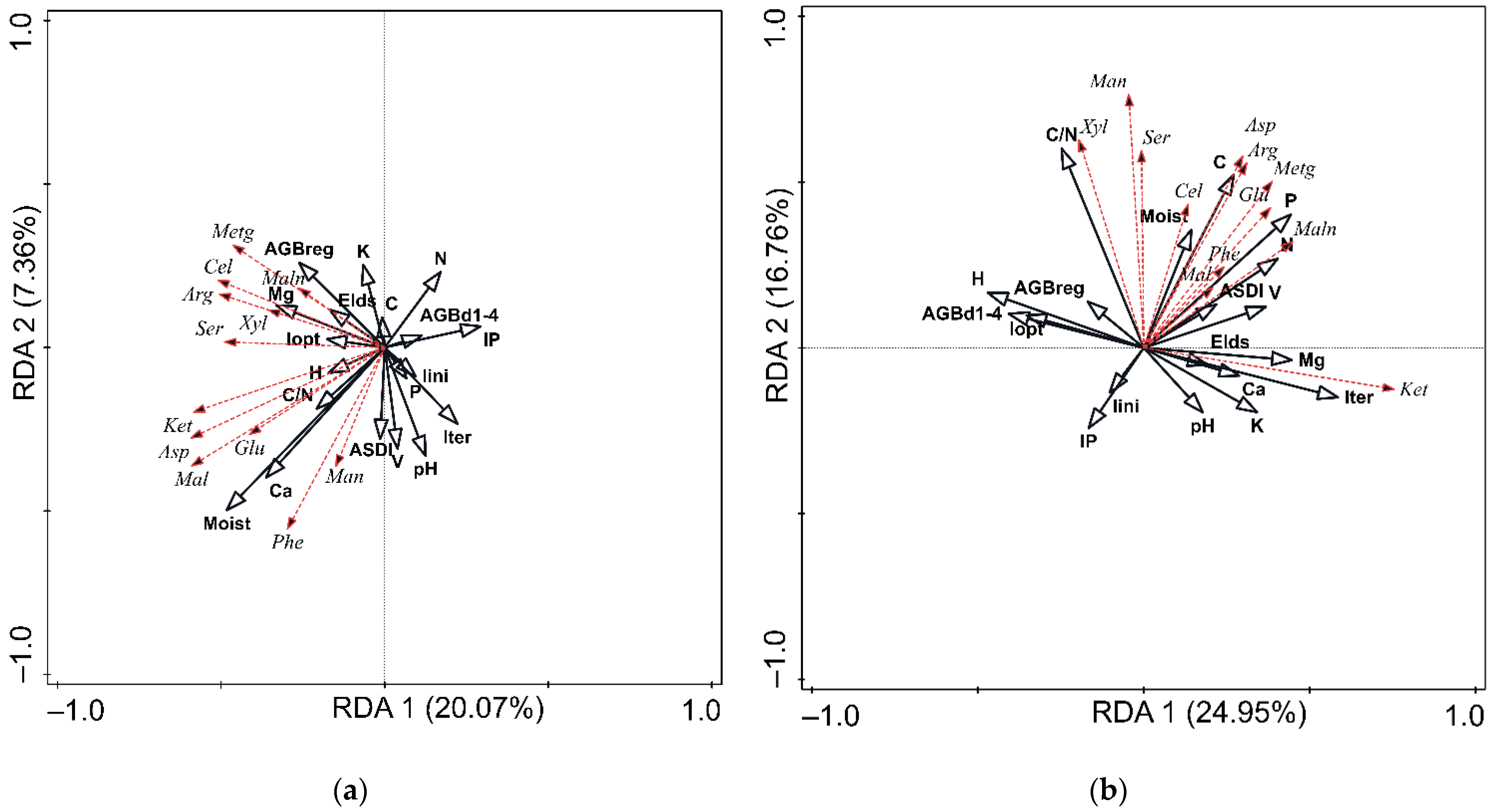
3.3. Stand Structure and Soil Physico-Chemical Properties as Factors Influencing Community-Level Physiological Profiles
4. Discussion
4.1. The Effect of Stand Structure on Soil Properties
4.2. Relationships between the Aboveground and Belowground Components
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the plant–soil system. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef]
- Miki, T.; Ushio, M.; Fukui, S.; Kondoh, M. Functional diversity of microbial decomposers facilitates plant coexistence in a plant-microbe-soil feedback model. Proc. Natl. Acad. Sci. USA 2010, 107, 14251–14256. [Google Scholar] [CrossRef]
- Pregitzer, C.C.; Bailey, J.K.; Hart, S.C. Soils as agents of selection: Feedbacks between plants and soils alter seedling survival and performance. Evol. Ecol. 2010, 24, 1045–1059. [Google Scholar] [CrossRef]
- Gibson, D.J. The maintenance of plant and soil heterogeneity in dune grassland. J. Ecol. 1988, 76, 497–508. [Google Scholar] [CrossRef]
- Pärtel, M.; Wilson, S.C. Root dynamics and spatial pattern in prairie and forest. Ecology 2002, 83, 1199–1203. [Google Scholar] [CrossRef]
- Stoyan, H.; De-Polli, H.; Böhm, S.; Robertson, G.P.; Paul, E.A. Spatial heterogeneity of soil respiration and related properties at the plant scale. Plant Soil 2000, 222, 203–214. [Google Scholar] [CrossRef]
- Wilkinson, S.C.; Anderson, J.M. Spatial patterns of soil microbial communities in a Norway spruce (Picea abies) plantation. Microb. Ecol. 2001, 42, 248–255. [Google Scholar] [CrossRef]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in forest ecosystem and landscape ecology. Bioscience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- Ujházy, K.; Križová, E.; Glončák, P.; Benčaťová, B.; Nič, J. Tree species and management effect on herb layer species composition in mountain fir-beech forests of the Western Carpathians. In The Carpathians: Integrating Nature and Society towards Sustainability; Kozak, J., Ostapowicz, K., Bytnerowicz, A., Wyżga, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.H.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Korpeľ, Š. Dynamika štruktúry a vývoj bukových prírodných lesov na Slovensku. Acta Fac. For. Zvolen 1987, 29, 59–85. (In Slovak) [Google Scholar]
- Korpeľ, Š. Die Urwälder der Westkarpaten; Gustav Fischer Verlag: Stuttgart, Germany, 1995; 310p. [Google Scholar]
- Pichler, V.; Hamor, F.; Vološčuk, I.; Sukharyuk, D. Outstanding Universal Value of the Ecological Processes in the Primeval Beech Forests of the Carpathians and Their Management as World Heritage Sites; VEDA: Bratislava, Slovakia, 2007; 62p. [Google Scholar]
- Trotsiuk, V.; Hobi, M.L.; Commarmot, B. Age structure and disturbance dynamics of the relic virgin beech forest Uholka (Ukrainian Carpathians). For. Ecol. Manag. 2012, 265, 181–190. [Google Scholar] [CrossRef]
- Hobi, M.L.; Ginzler, C.; Commarmot, B.; Bugmann, H. Gap pattern of the largest primeval beech forest of Europe revealed by remote sensing. Ecosphere 2015, 6, 76. [Google Scholar] [CrossRef]
- Feldmann, E.; Drössler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415, 38–46. [Google Scholar] [CrossRef]
- Zenner, E.K.; Peck, J.E.; Hobi, M.L.; Commarmot, B. The dynamics of structure across scale in a primeval European beech stand. Forestry 2015, 88, 180–189. [Google Scholar] [CrossRef]
- Barna, M.; Kulfan, J.; Bublinec, E. Buk a Bukové Ekosystémy Slovenska: Beech and Beech Ecosystems of Slovakia; Lesné ekosystémy Slovenska; VEDA Vydavateľstvo SAV: Bratislava, Slovakia, 2011; 634p. (In Slovak) [Google Scholar]
- Del Río, M.; Pretzsch, H.; Alberdi, I.; Bielik, K.; Bravo, F.; Brunner, A.; Condés, S.; Ducey, M.J.; Fonseca, T.; von Lüpke, N.; et al. Characterization of structure, dynamics, and productivity of mixed-species stands: Review and perspectives. Eur. J. Forest Res. 2016, 135, 23–49. [Google Scholar] [CrossRef]
- Leibundgut, H. Über Zweck und Methodik der Struktur und Zuwachsanalyse von Urwäldern. Schweiz. Z. Forstwes. 1959, 110, 111–124. (In German) [Google Scholar]
- Emborg, J.; Christensen, M.; Heilmann-Clausen, J. The structural dynamics of Suserup Skov. a near-natural temperate deciduous forest in Denmark. For. Ecol. Manag. 2000, 126, 173–189. [Google Scholar] [CrossRef]
- Král, K.; Vrška, T.; Hort, L.; Adam, D.; Šamonil, P. Developmental phases in a temperate natural spruce-fir-beech forest: Determination by a supervised classification method. Eur. J. For. Res. 2010, 129, 339–351. [Google Scholar] [CrossRef]
- Feldmann, E.; Glatthorn, J.; Hauck, M.; Leuschner, C. A novel empirical approach for determining the extension of forest development stages in temperate old-growth forests. Eur. J. For. Res. 2018, 137, 321–335. [Google Scholar] [CrossRef]
- Gömöryová, E.; Ujházy, K.; Martinák, M.; Gömöry, D. Soil microbial community response to variation in vegetation and abiotic environment in a temperate old-growth forest. Appl. Soil Ecol. 2013, 68, 10–19. [Google Scholar] [CrossRef]
- Leuschner, C.; Feldmann, E.; Pichler, V.; Glatthorn, J.; Hertel, D. Forest management impact on soil organic carbon: A paired-plot study in primeval and managed European beech forests. For. Ecol. Manag. 2022, 512, e120163. [Google Scholar] [CrossRef]
- United Nations Educational; Scientific and Cultural Organization. Beech Primeval Forests of the Carpathians and the Ancient Beech Forests of Germany. 2011. Available online: http://whc.unesco.org/en/list/1133 (accessed on 14 October 2021).
- Meyer, P.; Ackermann, J.; Balcar, P.; Boddenberg, J.; Detsch, R.; Förster, B.; Fuchs, H.; Hoffmann, B.; Keitel, W.; Kölbel, M.; et al. Untersuchungen der Waldstruktur und ihrer Dynamik in Naturwaldreservaten. Arbeitskreis Naturwälder. In Bund-Länder-Arbeitsgemeinschaft Forsteinrichtung; IHW-Verlag: Eching, Germany, 2001. [Google Scholar]
- Annighöfer, P.; Ameztegui, A.; Ammer, C.; Balandier, P.; Bartsch, N.; Bolte, A.; Coll, L.; Collet, C.; Ewald, J.; Frischbier, N.; et al. Species-specific and generic biomass equations for seedlings and saplings of European tree species. Eur. J. For. Res. 2016, 135, 313–329. [Google Scholar] [CrossRef]
- Feldmann, E.; Glatthorn, J.; Ammer, C.; Leuschner, C. Regeneration Dynamics Following the Formation of Understory Gaps in a Slovakian Beech Virgin Forest. Forests 2020, 11, 585. [Google Scholar] [CrossRef]
- Prodan, M. Messung der Waldbestände; J. D. Sauerländer’s Verlag: Frankfurt am Main, Germany, 1951; p. 260. [Google Scholar]
- Petráš, R.; Pajtík, J. Sústava československých objemových tabuliek drevín. Lesn. Čas. 1991, 37, 49–56. (In Slovak) [Google Scholar]
- Long, J.N.; Daniel, T.W. Assessment of growing stock in uneven-aged stands. West. J. Appl. For. 1990, 5, 93–96. [Google Scholar] [CrossRef]
- Reineke, L.H. Perfecting a stand density index for even-aged forests. J. Agric. Res. 1933, 46, 627–638. [Google Scholar]
- Camino, R.D. Zur Bestimmung der Bestandeshomogenität. Allg. Forst. Jagd. Ztg. 1976, 147, 54–58. [Google Scholar]
- Kuuluvainen, T.; Pukkala, T. Effects of Scots pine seed trees on on the density of ground vegetation and tree seedlings. Silva Fenn. 1989, 23, 159–167. [Google Scholar] [CrossRef][Green Version]
- Goff, F.G.; West, D. Canopy-understory interaction effects on forest population structure. Forest Sci. 1975, 21, 98–108. [Google Scholar] [CrossRef]
- Khazijev, F.K.H. Fermentativnaja Aktivnost’ Počv; Metodičeskoje Posobje: Moscow, Russia, 1976; p. 262. [Google Scholar]
- Kandeler, E. Bestimmung der N-Mineralisation im anaeroben Brutversuch. In Bodenbiologische Arbeitsmethoden; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 160–161. [Google Scholar]
- Islam, K.R.; Weil, R.R. Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol. Fertil. Soils 1998, 27, 408–416. [Google Scholar] [CrossRef]
- Insam, H. A new set of substrates proposed for community characterization in environmental samples. In Microbial Communities: Functional versus Structural Approaches; Insam, H., Rangger, A., Eds.; Springer: Heidelberg, Germany, 1997; pp. 260–261. [Google Scholar]
- Campbell, C.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. Reference Manual and User’s Guide to Canoco for Windows. Software for Canonical Community Ordination, version 4; Centre of Biometry: Wageningen, The Netherlands, 2002. [Google Scholar]
- Bach, L.H.; Grytnes, J.A.; Halvorsen, R.; Ohlson, M. Tree influence on soil microbial community structure. Soil Biol. Biochem. 2010, 42, 1934–1943. [Google Scholar] [CrossRef]
- Pawlik, Ł.; Šamonil, P. Biomechanical and biochemical effects recorded in the tree root zone—Soil memory. historical contingency and soil evolution under trees. Plant Soil 2018, 426, 109–134. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Choma, M.; Šamonil, P.; Kaštovská, E.; Bárta, J.; Tahovská, K.; Valtera, M.; Šantrůčková, H. Soil Microbiome Composition along the Natural Norway Spruce Forest Life Cycle. Forests 2021, 12, 410. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Bockheim, J.G. The effects of gap disturbance on nitrogen cycling and retention in late-successional northern hardwood–hemlock forests. Biogeochemistry 2007, 87, 231–245. [Google Scholar] [CrossRef]
- Metzger, J.C.; Schumacher, J.; Lange, M.; Hildebrandt, A. Neighbourhood and stand structure affect stemflow generation in a heterogeneous deciduous temperate forest. Hydrol. Earth Syst. Sci. 2019, 23, 4433–4452. [Google Scholar] [CrossRef]
- Hooper, D.U.; Bignell, D.E.; Brown, V.K.; Brussaard, L.; Dangerfield, J.M.; Wall, D.H.; Wardle, D.A.; Coleman, D.C.; Giller, K.E.; Lavelle, P.; et al. Interactions between aboveground and belowground biodiversity in terrestrial ecosystems: Patterns. mechanisms and feedbacks. Bioscience 2000, 50, 1049–1061. [Google Scholar] [CrossRef]
- Pietikainen, J.; Tikka, P.J.; Valkonen, S.; Isomaki, A.; Fritze, H. Is the soil microbial community related to the basal area of trees in a Scots pine stand? Soil Biol. Biochem. 2007, 39, 1832–1834. [Google Scholar] [CrossRef]
- Forrester, J.; Mladenoff, D.; Gower, S. Experimental manipulation of forest structure: Near-term effects on gap and stand scale C dynamics. Ecosystems 2013, 16, 1455–1472. [Google Scholar] [CrossRef]
- Bachofen, H.; Zingg, A. Effectiveness of structure improvement thinning on stand structure in subalpine Norway spruce (Picea abies (L.) Karst) stands. For. Ecol. Manag. 2001, 145, 137–149. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Zanini, M.; Dowgiallo, G.; Burrascano, S. Multiscale heterogeneity of topsoil properties in southern European old-growth forests. Eur. J. For. Res. 2015, 134, 911–925. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Griffiths, R.P.; Gray, A.N.; Spies, T.A. Soil properties in old-growth Douglas-Fir forest gaps in the western Cascade Mountains of Oregon. Northwest Sci. 2009, 84, 33–45. [Google Scholar] [CrossRef]
- Kennedy, F.; Pitman, R. Factors affecting the nitrogen status of soils and ground flora in Beech woodlands. For. Ecol. Manag. 2004, 198, 1–14. [Google Scholar] [CrossRef]
- Glatthorn, J.; Pichler, V.; Hauck, M.; Leuschner, C. Effects of forest management on stand leaf area: Comparing beech production and primeval forests in Slovakia. For. Ecol. Manag. 2017, 389, 76–85. [Google Scholar] [CrossRef]
- Ullah, F.; Gilani, H.; Sanaei, A.; Hussain, K.; Ali, A. Stand structure determines aboveground biomass across temperate forest types and species mixture along a local-scale elevational gradient. For. Ecol. Manag. 2021, 486, 118984. [Google Scholar] [CrossRef]
- Tripler, C.E.; Kaushal, S.S.; Likens, G.E.; Todd Walter, M. Patterns in Potassium Dynamics in Forest Ecosystems. Ecol. Lett. 2006, 9, 451–466. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Viers, J.; Dupré, B.; Chabaux, F.; Gaillardet, J.; Audry, S.; Prokushkin, A.S.; Shirokova, L.S.; Kirpotin, S.N.; Lapitsky, S.A.; et al. Biogeochemistry of Carbon, Major and Trace Elements in Watersheds of Northern Eurasia Drained to the Arctic Ocean: The Change of Fluxes, Sources and Mechanisms under the Climate Warming Prospective. C. R. Geosci. 2012, 344, 663–677. [Google Scholar] [CrossRef]
- Klimek, B.; Chodak, M.; Jaźzwa, M.; Solak, A.; Tarasek, A.; Niklińska, M. The relationship between soil bacteria substrate utilisation patterns and the vegetation structure in temperate forests. Eur. J. For. Res. 2016, 135, 179–189. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Creamer, R.E.; Hannula, S.E.; Van Leeuwen, J.P.; Stone, D.; Rutgers, M.; Schmelz, R.M.; de Ruiter, P.C.; Hendriksen, N.B.; Bolger, T.; Bouffaud, M.L.; et al. Ecological network analysis reveals the inter-connection between soil biodiversity and ecosystem function as affected by land use across Europe. Appl. Soil Ecol. 2016, 97, 112–124. [Google Scholar] [CrossRef]
| Stand Structure Characteristics | Mean ± S.D. | Min. | Max. | Coefficient of Variation (%) |
|---|---|---|---|---|
| Volume (V) (m3·ha–1) | 658 ± 257.14 | 156.00 | 1168.00 | 39.10 |
| Aboveground woody biomass of regeneration (AGBreg) (kg ha–1) | 335 ± 460.80 | 0.00 | 2131.83 | 137.61 |
| Aboveground woody biomass of trees DBH 1–4 cm (AGBd1–4) (kg ha–1) | 756 ± 782.41 | 0.00 | 3613.79 | 103.45 |
| Aditive stand density index (ASDI) | 509.28 ± 144.34 | 221.00 | 833.00 | 28.34 |
| Coefficient of homogeneity (H) | 1.46 ± 0.20 | 1.21 | 2.16 | 13.75 |
| Tree influence potential (IP) | 121.16 ± 226.62 | 0.73 | 1315.48 | 187.05 |
| Initial stage index (Iini) | 0.82 ± 0.38 | 0.27 | 2.00 | 46.45 |
| Optimum stage index (Iopt) | 0.77 ± 0.44 | 0.00 | 2.00 | 57.19 |
| Terminal stage index (Iter) | 0.55 ± 0.41 | 0.00 | 2.00 | 75.44 |
| Evenness (EIds) | 84.79 ± 15.41 | 40.88 | 99.73 | 18.18 |
| Soil Properties | Mean ± S.D. | Min. | Max. | Coefficient of Variation (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Horizons | O-Hor | A-Hor | O-Hor | A-Hor | O-Hor | A-Hor | O-Hor | A-Hor |
| Soil biological properties | ||||||||
| Catalase activity (mL O2.min–1 g–1) | 16.48 ± 4.54a | 3.23 ± 0.70b | 6.84 | 1.34 | 31.12 | 4.23 | 27.55 | 21.64 |
| Basal respiration (µg CO2.g–1 h–1) | 23.17 ± 6.41a | 0.51 ± 0.24b | 8.89 | 0.16 | 42.74 | 1.34 | 27.65 | 47.86 |
| Microbial biomass (µg C g–1) | 6,617 ± 3,615a | 735 ± 269b | 990 | 329 | 17,395 | 1364 | 54.63 | 36.64 |
| N-mineralization (µg NH4+–N g–1 d–1) | 26.17 ± 11.01a | 1.25 ± 0.40b | 8.16 | 0.67 | 63.98 | 2.17 | 42.09 | 32.26 |
| Hill’s index | 13.55 ± 2.01a | 10.55 ± 1.82b | 9.29 | 5.71 | 17.18 | 13.67 | 14.84 | 17.21 |
| Richness | 23.40 ± 2.49a | 19.00 ± 2.29b | 19.00 | 14.00 | 28.00 | 25.00 | 10.64 | 12.04 |
| Cmic/C | 179.89 ± 96.85a | 200.84 ± 76.74a | 30.37 | 107.97 | 413.19 | 505.53 | 53.84 | 38.21 |
| BR/Cmic | 0.50 ± 0.54a | 0.07 ± 0.02b | 0.12 | 0.03 | 3.43 | 0.14 | 106.24 | 32.38 |
| BR/C | 0.63 ± 0.17a | 0.14 ± 0.05b | 0.22 | 0.05 | 1.04 | 0.50 | 27.51 | 55.49 |
| Physico-chemical properties | ||||||||
| Soil Moisture (% w/w) | 243.72 ± 73.47a | 44.58 ± 10.06b | 32.43 | 28.59 | 415.04 | 75.54 | 30.15 | 22.57 |
| pH-KCl | 5.46 ± 0.23a | 4.80 ± 0.32b | 4.81 | 4.15 | 6.09 | 5.54 | 4.28 | 6.61 |
| N (%) | 1.51 ± 0.25a | 0.26 ± 0.08b | 1.04 | 0.12 | 1.91 | 0.45 | 16.82 | 30.98 |
| C (%) | 37.22 ± 4.55a | 3.86 ± 1.33b | 28.20 | 1.78 | 45.90 | 8.37 | 12.23 | 34.45 |
| Mg (mg kg–1) | 574 ± 86a | 77 ± 44b | 400 | 23 | 776 | 229 | 15.11 | 56.93 |
| Ca (mg kg–1) | 3,525 ± 385a | 673 ± 420b | 2,849 | 79 | 4528 | 1,593 | 10.93 | 62.35 |
| K (mg kg–1) | 906 ± 312a | 122 ± 59b | 471 | 44 | 1805 | 282 | 34.51 | 48.44 |
| P (mg kg–1) | 81.12 ± 22.11a | 4.87 ± 1.57b | 49.00 | 2.25 | 132.00 | 8.21 | 27.26 | 32.16 |
| C/N | 25.11 ± 3.73a | 15.10 ± 2.08b | 17.91 | 11.98 | 32.46 | 19.27 | 14.87 | 13.75 |
| Community level physiological profiles (CLPPs) | ||||||||
| α-Ketoglutaric acid (Ket) (µg CO2 -C g−1 h−1) | 4260 ± 1360a | 403 ± 184b | 1176 | 138 | 7861 | 928 | 31.94 | 45.73 |
| Arginine (Arg) (µg CO2 -C g−1 h−1) | 1786 ± 801a | 131 ± 65b | 402 | 18 | 3777 | 319 | 44.88 | 49.82 |
| Asparagine (Asp) (µg CO2 -C g−1 h−1) | 2682 ± 1347a | 109 ± 63b | 455 | 24 | 6385 | 265 | 50.24 | 57.98 |
| Cellulose (Cel) (µg CO2 -C g−1 h−1) | 1051 ± 791a | 50 ± 20b | 156 | 23 | 3129 | 115 | 75.30 | 40.99 |
| Malic acid (Mal) (µg CO2 -C g−1 h−1) | 2358 ± 678a | 385 ± 114b | 930 | 186 | 3680 | 758 | 28.76 | 29.70 |
| Methylglucamine (Metg) (µg CO2 -C g−1 h−1) | 2105 ± 928a | 122 ± 50b | 252 | 32 | 4440 | 234 | 44.11 | 41.33 |
| Phenylalanine (Phe) (µg CO2 -C g−1 h−1) | 1094 ± 414.44a | 80 ± 34b | 242 | 10 | 1939 | 148 | 37.88 | 42.97 |
| Serine (Ser) (µg CO2 -C g−1 h−1) | 3550 ± 1716.59a | 163 ± 88b | 1139 | 45 | 11,215 | 412 | 48.35 | 54.30 |
| Mannose (Man) (µg CO2 -C g−1 h−1) | 3154 ± 1392.23a | 176 ± 91b | 752 | 40 | 8036 | 524 | 44.14 | 52.10 |
| Glutamine (Glu) (µg CO2 -C g−1 h−1) | 1215 ± 394.65a | 157 ± 66b | 345 | 37 | 1988 | 349 | 32.49 | 42.36 |
| Malonic acid (Maln) (µg CO2 -C g−1 h−1) | 1969 ± 1174a | 106 ± 79b | 327 | 6 | 6115 | 358 | 59.61 | 75.10 |
| Xylose (Xyl) (µg CO2 -C g−1 h−1) | 3926 ± 1390a | 121 ± 69b | 692 | 13 | 6606 | 316 | 35.41 | 57.24 |
| V | AGBreg | AGBd1–4 | ASDI | H | IP | Iini | Iopt | Iter | EIds | |
|---|---|---|---|---|---|---|---|---|---|---|
| Soil biological properties | ||||||||||
| Cat | −0.163 | 0.130 | 0.095 | −0.180 | −0.071 | 0.022 | 0.025 | −0.197 | −0.059 | −0.023 |
| BR | 0.158 | 0.168 | −0.107 | 0.256 | 0.340* | −0.005 | 0.169 | 0.234 | −0.065 | −0.153 |
| Cmic | 0.104 | 0.404 * | −0.026 | 0.121 | −0.016 | −0.032 | −0.037 | 0.137 | −0.056 | 0.166 |
| Cmic/C | 0.061 | 0.420 ** | −0.010 | 0.089 | 0.018 | −0.030 | −0.014 | 0.167 | −0.107 | 0.139 |
| BR/Cmic | −0.218 | −0.179 | 0.058 | −0.129 | 0.281 | −0.037 | 0.314 | 0.079 | −0.160 | −0.238 |
| BR/C | −0.031 | 0.114 | −0.052 | 0.104 | 0.470 ** | −0.008 | 0.284 | 0.270 | −0.201 | −0.226 |
| N−min | 0.137 | 0.340 * | −0.093 | 0.176 | 0.024 | −0.017 | −0.104 | 0.248 | −0.129 | 0.001 |
| Hill index | −0.088 | −0.027 | 0.251 | −0.085 | −0.175 | 0.262 | −0.128 | 0.052 | −0.020 | 0.113 |
| Richness | 0.110 | 0.010 | 0.150 | 0.108 | −0.143 | 0.322 * | −0.068 | 0.101 | 0.047 | 0.150 |
| Physico-chemical properties | ||||||||||
| Moisture | 0.099 | 0.114 | −0.116 | 0.150 | 0.232 | −0.010 | 0.133 | 0.135 | 0.001 | −0.008 |
| pH | 0.325 * | −0.305 | 0.019 | 0.392 * | 0.046 | 0.323 * | 0.170 | 0.095 | 0.055 | 0.089 |
| N | 0.366 * | 0.142 | 0.159 | 0.332 * | 0.055 | 0.105 | −0.205 | 0.229 | 0.250 | 0.229 |
| C | 0.194 | 0.166 | 0.217 | 0.225 | 0.253 | 0.311 | −0.081 | 0.301 | −0.081 | 0.038 |
| C/N | −0.289 | −0.059 | −0.028 | −0.219 | 0.143 | 0.123 | 0.190 | −0.019 | −0.365 * | −0.245 |
| Mg | 0.291 | −0.077 | 0.021 | 0.268 | −0.134 | 0.095 | −0.162 | 0.067 | 0.203 | 0.332 * |
| Ca | 0.243 | −0.164 | −0.102 | 0.187 | −0.117 | −0.003 | −0.088 | −0.142 | 0.367 * | 0.240 |
| K | 0.401 * | −0.071 | −0.025 | 0.446 ** | 0.030 | 0.454 ** | −0.081 | 0.295 | 0.067 | 0.256 |
| P | 0.437 ** | 0.205 | −0.012 | 0.444 ** | 0.092 | 0.225 | −0.266 | 0.392 * | 0.031 | 0.085 |
| V | AGBreg | AGBd1–4 | ASDI | H | IP | Iini | Iopt | Iter | EIds | |
|---|---|---|---|---|---|---|---|---|---|---|
| Soil biological properties | ||||||||||
| Cat | 0.238 | 0.149 | −0.294 | 0.199 | −0.163 | −0.250 | −0.049 | −0.092 | 0.046 | 0.076 |
| BR | 0.279 | 0.128 | 0.356 * | 0.248 | −0.042 | 0.051 | −0.161 | 0.070 | 0.018 | 0.018 |
| Cmic | 0.244 | 0.148 | 0.217 | 0.158 | −0.017 | 0.286 | −0.287 | −0.019 | 0.181 | 0.107 |
| Cmic/C | 0.166 | 0.089 | 0.271 | 0.120 | 0.158 | 0.098 | −0.071 | −0.046 | 0.149 | 0.137 |
| BR/Cmic | 0.186 | 0.026 | 0.167 | 0.219 | −0.033 | −0.051 | 0.072 | 0.098 | −0.106 | −0.067 |
| BR/C | 0.184 | 0.104 | 0.399 * | 0.169 | 0.094 | −0.036 | −0.018 | 0.021 | 0.030 | 0.071 |
| N−min | 0.175 | −0.035 | −0.097 | 0.125 | 0.007 | 0.109 | 0.045 | −0.310 | 0.194 | −0.042 |
| Hill index | −0.193 | 0.031 | 0.293 | −0.108 | 0.098 | 0.204 | −0.006 | 0.250 | −0.365 * | 0.061 |
| Richness | 0.082 | 0.124 | 0.199 | 0.195 | 0.333 * | 0.026 | 0.027 | 0.361 * | −0.305 | −0.034 |
| Physico-chemical properties | ||||||||||
| Moisture | −0.012 | 0.099 | 0.055 | −0.125 | −0.090 | 0.361 * | −0.185 | −0.288 | 0.200 | 0.025 |
| pH | −0.304 | −0.192 | −0.203 | −0.287 | 0.072 | −0.400 * | 0.420 ** | −0.441 ** | −0.001 | −0.074 |
| N | 0.106 | 0.051 | −0.026 | 0.145 | 0.099 | 0.014 | 0.025 | 0.071 | 0.062 | 0.238 |
| C | 0.018 | −0.006 | 0.002 | 0.094 | 0.200 | −0.004 | 0.082 | 0.106 | −0.046 | 0.124 |
| C/N | −0.167 | −0.187 | 0.057 | −0.070 | 0.232 | −0.039 | 0.207 | 0.028 | −0.197 | −0.223 |
| Mg | −0.026 | −0.187 | −0.150 | −0.001 | 0.060 | −0.241 | 0.291 | −0.278 | 0.116 | 0.095 |
| Ca | −0.208 | −0.155 | −0.070 | −0.213 | 0.110 | −0.228 | 0.300 | −0.400 * | 0.083 | 0.010 |
| K | 0.150 | −0.203 | −0.186 | 0.199 | −0.050 | 0.116 | 0.160 | −0.074 | 0.090 | 0.152 |
| P | 0.252 | 0.115 | −0.013 | 0.194 | 0.023 | −0.055 | −0.102 | −0.116 | 0.233 | 0.183 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Židó, J.; Šumichrast, L.; Kucbel, S.; Gömöryová, E. The Effect of Stand Structure on Soil Physico-Chemical and Biological Properties in a Primary Beech Forest. Forests 2022, 13, 1344. https://doi.org/10.3390/f13091344
Židó J, Šumichrast L, Kucbel S, Gömöryová E. The Effect of Stand Structure on Soil Physico-Chemical and Biological Properties in a Primary Beech Forest. Forests. 2022; 13(9):1344. https://doi.org/10.3390/f13091344
Chicago/Turabian StyleŽidó, Ján, Ladislav Šumichrast, Stanislav Kucbel, and Erika Gömöryová. 2022. "The Effect of Stand Structure on Soil Physico-Chemical and Biological Properties in a Primary Beech Forest" Forests 13, no. 9: 1344. https://doi.org/10.3390/f13091344
APA StyleŽidó, J., Šumichrast, L., Kucbel, S., & Gömöryová, E. (2022). The Effect of Stand Structure on Soil Physico-Chemical and Biological Properties in a Primary Beech Forest. Forests, 13(9), 1344. https://doi.org/10.3390/f13091344






