Porewater Sulfide: The Most Critical Regulator in the Degradation of Mangroves Dominated by Tides
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
2.2.1. Forest Structure
2.2.2. Hydrology
2.2.3. Biogeochemical Characteristics
- Soil porewater
- Soil
2.2.4. Microtopography Transect
2.3. Analysis
3. Results
3.1. Forest Structure
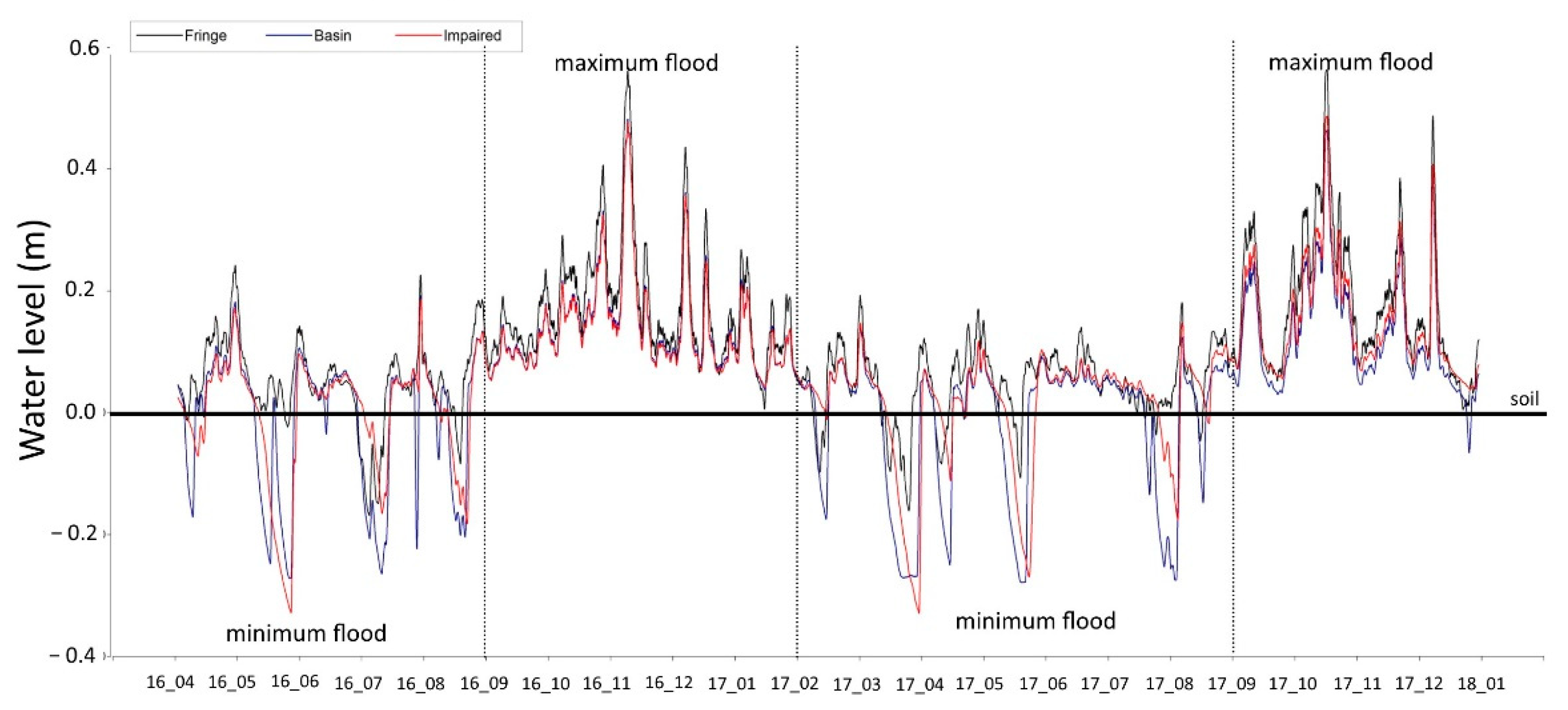
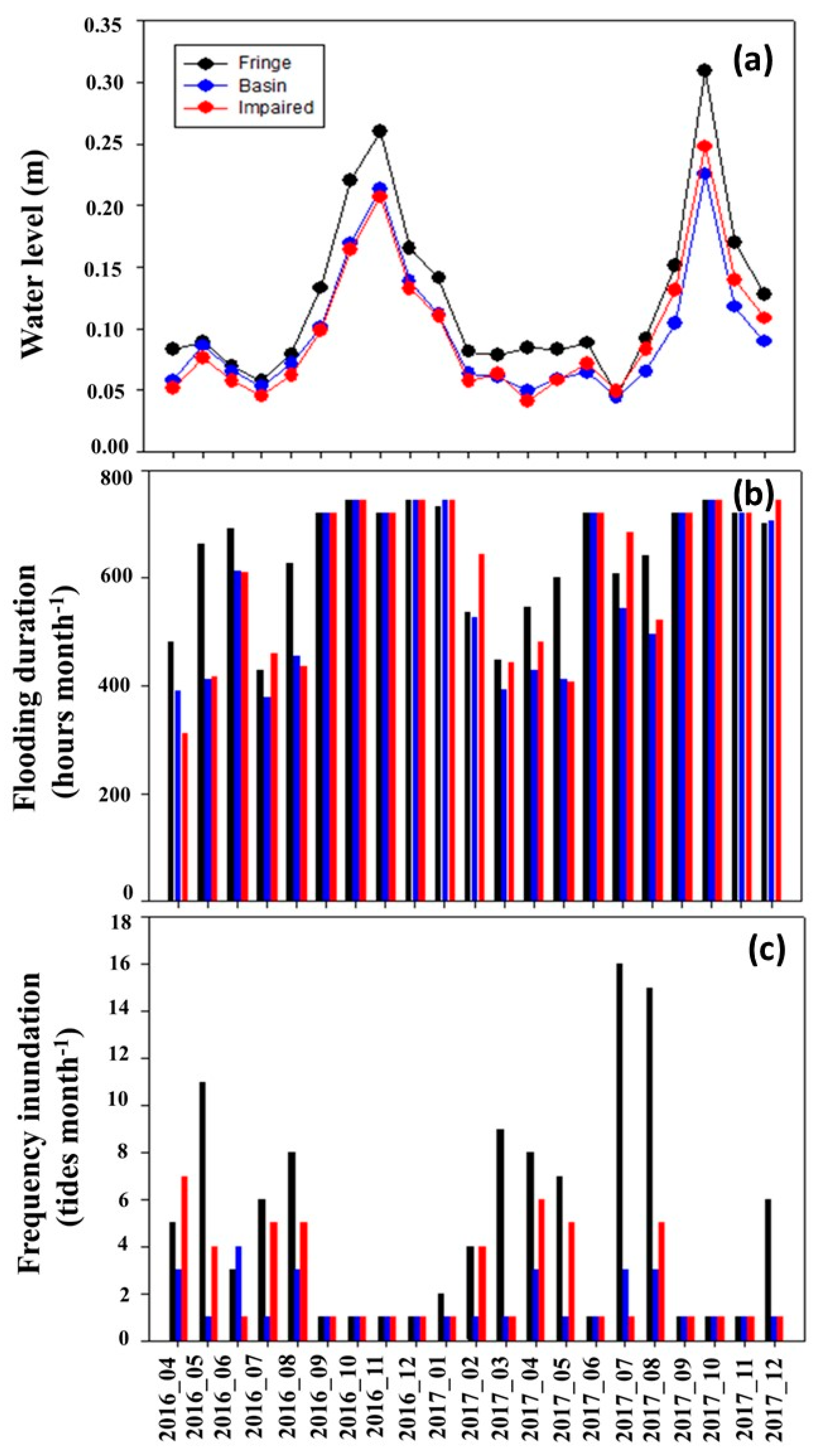
3.2. Flooding Levels and Hydroperiod
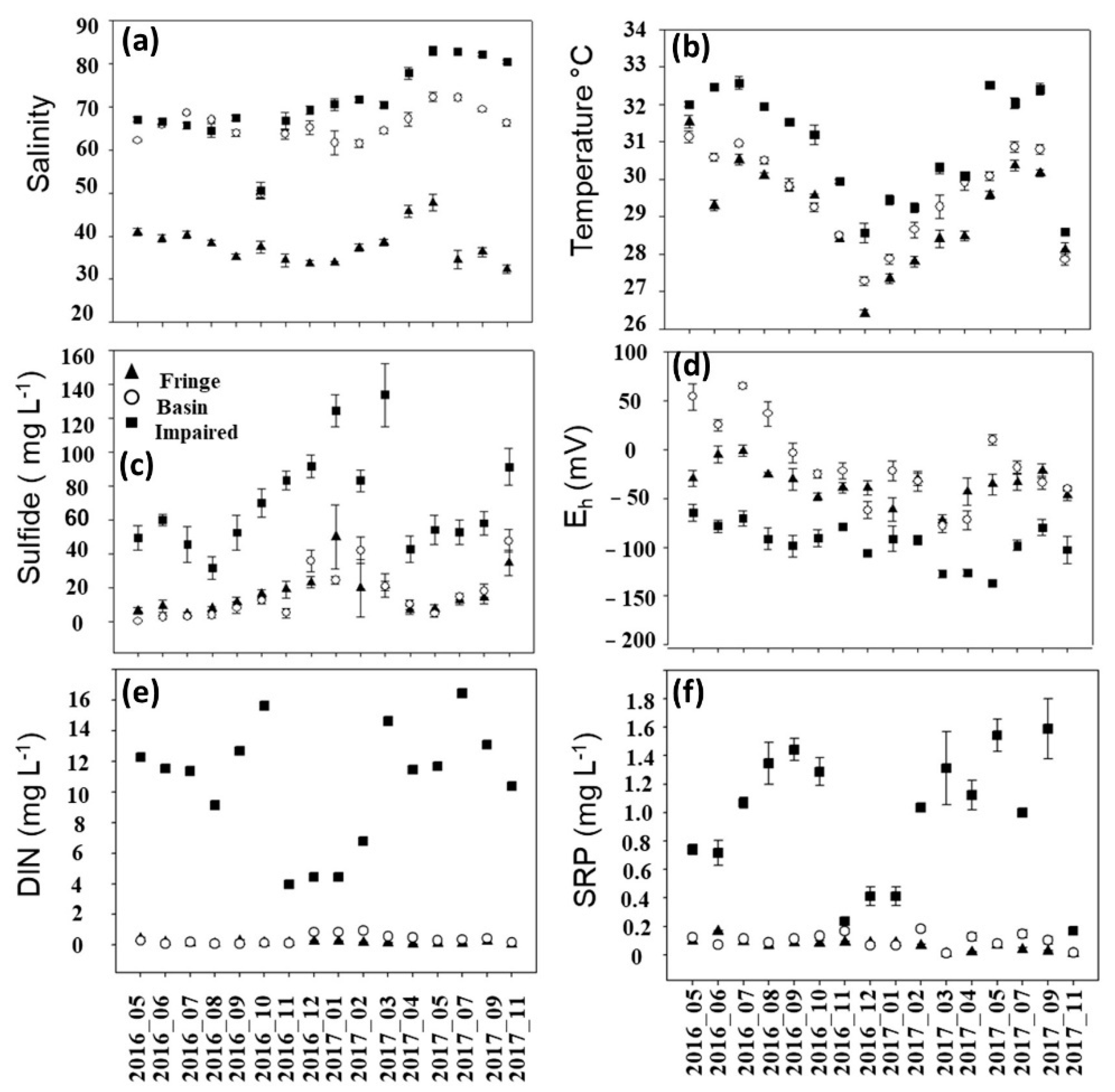
3.3. Biogeochemical Characteristics of Porewater
3.3.1. Spatio-Temporal
3.3.2. Seasonality
3.3.3. Soil
3.4. Microtopography Transect
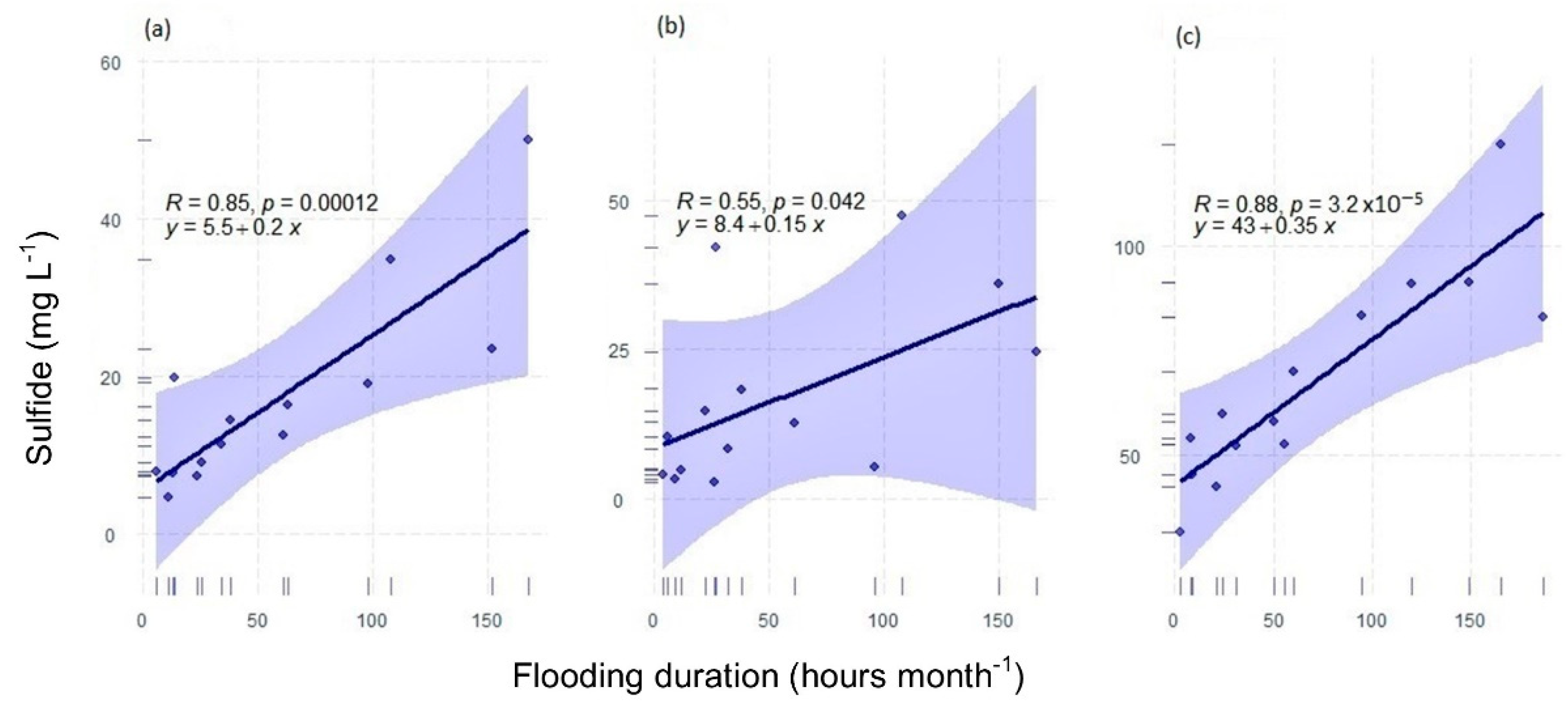
3.5. Relationship between Biogeochemical Variables and Flooding Period
4. Discussion
5. Conclusions
- The most critical regulator besides salinity in tidal-dominated mangroves was the sulfides, with the highest concentrations occurring at the end of the peak flood season.
- The maximum flooding season was from September to January, and the minimum flooding season was from February to August for Laguna de Terminos.
- In this context, the fringe mangroves are connected to the central water bodies. In contrast, the black mangrove predominates in inner basin sites, which are more fragile because they depend on the superficial flow and are distant to the primary water sources.
- The interruption of water flow will cause tree death and soil deterioration as regulators such as lower redox potential, lower sulfide concentration, and high salinity become critical with prolonged flooding and evaporation.
- Increased hydrological connectivity through the hydrological restoration of areas affected by soil salinity and sulfides can contribute to the natural establishment and growth of mangroves. Our results can support decisions on mangrove management or restoration strategies.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayden, H.L.; Granek, E.F. Coastal sediment elevation change following anthropogenic mangrove clearing. Estuar. Coast. Shelf Sci. 2015, 165, 70–74. [Google Scholar] [CrossRef]
- McKee, K.L. Soil Physicochemical Patterns and Mangrove Species Distribution—Reciprocal Effects? J. Ecol. 1993, 81, 477. [Google Scholar] [CrossRef]
- Twilley, R.R.; Rivera-Monroy, V.H. Developing performance measures of mangrove wetlands using simulation models of hydrology, nutrient biogeochemistry, and community dynamics. J. Coast. Res. 2005, 21, 79–93. [Google Scholar] [CrossRef]
- Krauss, K.W.; Doyle, T.W.; Twilley, R.R.; Rivera-Monroy, V.H.; Sullivan, J.K. Evaluating the relative contributions of hydroperiod and soil fertility on growth of south Florida mangroves. Hydrobiologia 2006, 569, 311–324. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; Mckee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Pérez-Ceballos, R.; Zaldívar-Jiménez, A.; Canales-Delgadillo, J.; López-Adame, H.; López-Portillo, J.; Merino-Ibarra, M. Determining hydrological flow paths to enhance restoration in impaired mangrove wetlands. PLoS ONE 2020, 15, e0227665. [Google Scholar] [CrossRef]
- Alongi, D.M. Present state and future of the worlds mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Fiedler, S.; Vepraskas, M.J.; Richardson, J.L. Soil Redox Potential: Importance, Field Measurements, and Observations. Adv. Agron. 2007, 94, 1–54. [Google Scholar] [CrossRef]
- Fink, D.F.; Mitsch, W.J. Hydrology and nutrient biogeochemistry in a created river diversion oxbow wetland. Ecol. Eng. 2007, 30, 93–102. [Google Scholar] [CrossRef]
- Schoeneberger, P.J.; Wysocki, D.A. Hydrology of soils and deep regolith: A nexus between soil geography, ecosystems and land management. Geoderma 2005, 126, 117–128. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; Osti-Sáenz, J.; Chan-Keb, C.A.; Chan-Canul, E.; Gómez-Ramírez, D.; Requena-Pavón, G.; Reyes-Castellanos, J. Programa Regional para la Caracterización y el Monitoreo de Ecosistemas de Manglar del Golfo de México y Caribe Mexicano: Campeche. Universidad Autónoma Campeche. Centro de Ecología Pesquería y Oceanografía del Golfo de México. Informe final SNIB-CONABIO. Proyecto FN010, D.F. México. 2012. Available online: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfFN010.pdf (accessed on 12 August 2022).
- Chen, R.; Twilley, R.R. A gap dynamic model of mangrove forest development along gradients of soil salinity and nutrient resources. J. Ecol. 1998, 86, 37–51. [Google Scholar] [CrossRef]
- Echeverría-Ávila, S.; Pérez-Ceballos, R.; Zaldívar-Jiménez, A.; Canales-Delgadillo, J.; Brito-Pérez, R.; Merino-Ibarra, M.; Vovides, A. Regeneración natural de sitios de manglar degradado en respuesta a la restauración hidrológica. Madera y Bosques 2019, 25, e2511754. [Google Scholar] [CrossRef]
- Osland, M.J.; Day, R.H.; Hall, C.T.; Feher, L.C.; Armitage, A.R.; Cebrian, J.; Dunton, K.H.; Hughes, A.R.; Kaplan, D.A.; Langston, A.K.; et al. Temperature thresholds for black mangrove (Avicennia germinans) freeze damage, mortality and recovery in North America: Refining tipping points for range expansion in a warming climate. J. Ecol. 2019, 108, 654–665. [Google Scholar] [CrossRef]
- Lamers, L.P.M.; Govers, L.L.; Janssen, I.C.J.M.; Geurts, J.J.M.; Van der Welle, M.E.W.; Van Katwijk, M.M.; Van der Heide, T.; Roelofs, J.G.M.; Smolders, A.J.P. Sulfide as a soil phytotoxin—A review. Front. Plant Sci. 2013, 4, 268. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.L.; Mendelssohn, I.A.; Hester, M.W. Reexamination of Pore Water Sulfide Concentrations and Redox Potentials Near the Aerial Roots of Rhizophora mangle and Avicennia germinans. Am. J. Bot. 1988, 75, 1352. [Google Scholar] [CrossRef]
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove Forests: One of the World’s Threatened Major Tropical Environments. Bioscience 2001, 51, 807. [Google Scholar] [CrossRef]
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Chang. Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef]
- Valderrama-Landeros, L.H.; Rodríguez-Zúñiga, M.T.; Troche-Souza, C.; Velázquez-Salazar, S.; Villeda-Chávez, E.; Alcántara-Maya, J.A.; Vázquez-Balderas, B.I.; Cruz-López, M.I.; Ressl, R. Manglares de México: Actualización y Exploración de los Datos del Sistema de Monitoreo 1970/1980–2015, 1st ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Ciudad de México, Mexico, 2017; p. 128. [Google Scholar]
- López-Portillo, J.; Zaldívar-Jiménez, A.; Lara-Domínguez, A.N.; Pérez-Ceballos, R.; Bravo-Mendoza, M.; Núñez-Álvarez, N.; Aguirre-Franco, L. Hydrological Rehabilitation and Sediment Elevation as Strategies to Restore Mangroves in Terrigenous and Calcareous Environments in Mexico. In Wetland Carbon and Environmental Management, 1st ed.; Krauss, K.W., Zhu, Z., Stagg, C.L., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 173–190. [Google Scholar]
- Canales-Delgadillo, J.; Perez-Ceballos, R.; Merino-Ibarra, M.; Cardoza-Cota, G.; Cardoso-Mohedano, J.G. The effect of mangrove restoration on avian assemblages of a coastal lagoon in southern Mexico. PeerJ 2019, 7, e7493. [Google Scholar] [CrossRef]
- Villalobos-Zapata, G.J.; Yáñez-Arancibia, A.; Day, J.W., Jr.; Lara-Domínguez, A. Ecología y manejo de los manglares en la Laguna de Términos, Campeche, México. In Ecosistemas de Manglar en América Tropical; Yáñez-Arancibia, A., Lara-Domínguez, A.L., Eds.; Instituto de Ecología A.C.: Xalapa, Mexico; UICN/ORMA: San José, Costa Rica; NOAA/NMFS: Silver Spring, MO, USA, 1999; pp. 263–274. [Google Scholar]
- Arriaga, L.; Espinoza, J.M.; Aguilar, C.; Martínez, E.; Gómez, L.; Loa, E. Regiones terrestres prioritarias de México. Escala de trabajo 1:1,000,000; Comisión Nacional para el Conocimiento y uso de la Biodiversidad: Mexico City, Mexico, 2000; p. 611. [Google Scholar]
- Yáñez-Arancibia, A.; Day, J.W., Jr. Ecological characterization of Terminos Lagoon, a tropical lagoon-estuarine system in the southern Gulf of Mexico. Oceanol. Acta 1982, 5, 431–440. [Google Scholar]
- Instituto Nacional de Ecología. Programa de Manejo del Área de Protección de Flora y Fauna Laguna de Términos; INE-SEMARNAP: Mexico City, Mexico, 1997; p. 166. [Google Scholar]
- Coronado-Molina, C.A.; Alvarez-Guillen, H.; Day, J.W.; Reyes, E.; Perez, B.C.; Vera-Herrera, F.; Twilley, R. Litterfall dynamics and nutrient cycling in mangrove forest of Southern Everglades, Florida and Terminos Lagoon, Mexico. Wetl. Ecol. Manag. 2000, 20, 123–136. [Google Scholar] [CrossRef]
- Escudero, M.; Silva, R.; Mendoza, E. Beach erosion driven by natural and human activity at Isla del Carmen Barrier Island, Mexico. J. Coast. Res. 2014, 71, 62–74. [Google Scholar] [CrossRef]
- Rivera-Monroy, V.H.; Madden, C.J.; Day, J.W.; Twilley, R.R.; Vera-Herrera, F.; Alvarez-Guillén, H. Seasonal coupling of a tropical mangrove forest and an estuarine water column: Enhancement of aquatic primary productivity. Hydrobiologia 1998, 379, 41–53. [Google Scholar] [CrossRef]
- Feller, I.C.; McKee, K.L.; Whigham, D.F.; O’Neill, J.P. Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 2003, 62, 145–175. [Google Scholar] [CrossRef]
- Pool, D.J.; Snedaker, S.; Lugo, A. Structure of mangrove forests in Florida, Puerto Rico, Mexico, and Costa Rica. Biotropica 1977, 9, 195–212. [Google Scholar] [CrossRef]
- Snedaker, S.C.; Snedaker, J.G. The Mangrove Ecosystem: Research Methods; Unesco: Bungay, UK, 1984; p. 251. [Google Scholar]
- Valdez-Hernández, J.I. Aprovechamiento Forestal de manglares en el estado de Nayarit costa Pacifica de México. Madera Y Bosques 2002, 8, 129–145. [Google Scholar] [CrossRef][Green Version]
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, M.T.; Pérez-Ceballos, R.; Zaldívar-Jiménez, A.; Lara-Domínguez, A.; Teutli-Hernández, C.; Herrera-Silveira, J. Muestreo de variables hidrológicas, fisicoquímicas y del sedimento. In Métodos para la caracterización de los manglares mexicanos: Un enfoque espacial multi-escala; Rodríguez Zúñiga, M.T., Villeda-Chávez, E., Vázquez-Lule, A.D., Bejarano, M., Cruz-López, M.I., Olguín, M., Villela-Gaytán, S.A., Flores, R., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Ciudad de México, Mexico, 2018; pp. 131–169. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd ed.; WILEY-VCH Verlag GmbH: Mexico City, Mexico, 1999. [Google Scholar] [CrossRef]
- Kirkwood, D. Sanplus Segmented Flow Analyzer and Its Applications. Seawater Analysis; Skalar: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Rabenhorst, M.C.; Hively, W.D.; James, B.R. Measurements of Soil Redox Potential. Soil Sci. Soc. Am. J. 2009, 73, 668–674. [Google Scholar] [CrossRef]
- Chen, R.; Twilley, R.R. Patterns of Mangrove Forest Structure and Soil Nutrient Dynamics Along the Shark River Estuary, Florida. Estuaries 1999, 22, 955–970. [Google Scholar] [CrossRef]
- Castañeda-Moya, E.; Twilley, R.R.; Rivera-Monroy, V.H. Allocation of biomass and net primary productivity of mangrove forests along environmental gradients in the Florida Coastal Everglades, USA. For. Ecol. Manag. 2013, 307, 226–241. [Google Scholar] [CrossRef]
- Konare, H.S.; Yost, R.M.; Doumbia, W.; McCarty, G.; Jarju, A.; Kablan, R. Loss on ignition: Measuring soil organic carbon in soils of the Sahel, West Africa. Afr. J. Agric. Res. 2010, 5, 3088–3095. [Google Scholar]
- Pérez-Ceballos, R.; Echeverría-Ávila, S.; Zaldívar-Jiménez, A.; Zaldívar-Jiménez, T.; Herrera-Silveira, J. Contribution of microtopography and hydroperiod to the natural regeneration of Avicennia germinans in a restored mangrove forest. Ciencias Mar. 2017, 43, 55–67. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Pub. Co.: Reading, MA, USA, 1977. [Google Scholar]
- Di Rienzo, J.; Balzarini, M.; Robledo, C.; Casanoves, F.; Gonzales, L.; Tablada, E. InfoStat Software Estadístico; FCA Universidad Nacional de Córdoba: Córdoba, Argentina, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; p. 3. Available online: https://www.R-project.org/ (accessed on 12 August 2022).
- Nickerson, N.H.; Thibodeau, F.R. Association between pore water sulfide concentrations and the distribution of mangroves. Biogeochemistry 1985, 1, 183–192. [Google Scholar] [CrossRef]
- Marchand, C.; Lallier-Vergès, E.; Baltzer, F. The composition of sedimentary organic matter in relation to the dynamic features of a mangrove-fringed coast in French Guiana. Estuar. Coast. Shelf Sci. 2003, 56, 119–130. [Google Scholar] [CrossRef]
- Lyimo, T.L.; Mushi, D. Sulfide Concentration and Redox Potential Patterns in Mangrove Forests of Dar es Salaam: Effects on Avicennia marina and Rhizophora mucronata Seedling Establishment. West. Indian Ocean J. Mar. Sci. 2007, 4, 163–174. [Google Scholar] [CrossRef]
- Coull, B.C. Role of meiofauna in estuarine soft-bottom habitats. Aust. J. Ecol. 1999, 24, 327–343. [Google Scholar] [CrossRef]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef]
- Day, J.W., Jr.; Coronado-Molina, C.; Vera-Herrera, F.R.; Twilley, R.R.; Rivera-Monroy, V.H.; Alvarez-Guillen, H.; Day, R.; Conner, W. A 7 year record of above-ground net primary production in a southeastern Mexican mangrove forest. Aquat. Bot. 1996, 55, 39–60. [Google Scholar] [CrossRef]
- Poret, N.; Twilley, R.R.; Rivera-Monroy, V.H.; Coronado-Molina, C. Belowground decomposition of mangrove roots in Florida coastal everglades. Estuaries Coasts 2007, 30, 491–496. [Google Scholar] [CrossRef]
- Santos-Ramirez, J. Efecto de las Condiciones Ambientales Sobre la Descomposición de Raíces del Mangle Avicennia germinans en Sitios Con Restauración de Isla del Carmen, Campeche. Master’s Thesis, Universidad Autónoma del Carmen, Ciudad del Carmen, Campeche, Mexico, 24 November 2017. [Google Scholar]
- Moreno-Casasola, P.; Warner, B. Breviario Para Describir, Observar Y Manejar Humedales, 3rd ed.; Serie Costa Sustentable no 1; RAMSAR, Instituto de Ecología, A.C., CONANP, US Fish and Wildlife Service, US State Department: Xalapa, Mexico, 2009; p. 406. [Google Scholar]
- Cruz-Lucas, M.A. Topografía y Factores Ambientales Relacionados a las Comunidades Vegetales en un Humedal. Master’s Thesis, Universidad Veracruzana, Veracruz, Mexico, 27 October 2010. [Google Scholar]
- Ramírez-Barrón, E. Distribución de las especies de manglar en relación a la microtopografía y la manipulación experimental del hidroperiodo para abatir la salinidad intersticial en una marisma hipersalina, en el estero de Urías: Mazatlán Sinaloa. Master´s Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, August 2014. [Google Scholar]
- Flores-Verdugo, F.; Moreno-Casasola, P.; Agraz-Hernández, M.; López-Rosas, H.; Benitez-Pardo, D. La topografía y el hidroperíodo: Dos factores que condicionan la restauración de los humedales costeros. Boletín La Soc. Botánica México 2007, 80, 33–47. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; del Río-Rodríguez, R.E.; Chan-Keb, C.A.; Osti-Saenz, J.; Muñiz-Salazar, R. Nutrient removal efficiency of Rhizophora mangle (L.) seedlings exposed to experimental dumping of municipal waters. Diversity 2018, 10, 16. [Google Scholar] [CrossRef]
- Alongi, D.M. Early growth responses of mangroves to different rates of nitrogen and phosphorus supply. J. Exp. Mar. Bio. Ecol. 2011, 397, 85–93. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wattayakorn, G.; Boyle, S.; Tirendi, F.; Payn, C.; Dixon, P. Influence of roots and climate on mineral and trace element storage and flux in tropical mangrove soils. Biogeochemistry 2004, 69, 105–123. [Google Scholar] [CrossRef]
- Krauss, K.W.; Demopoulos, A.W.J.; Cormier, N.; From, A.S.; McClain-Counts, J.P.; Lewis, R.R. Ghost forests of Marco Island: Mangrove mortality driven by belowground soil structural shifts during tidal hydrologic alteration. Estuar. Coast. Shelf Sci. 2018, 212, 51–62. [Google Scholar] [CrossRef]
- Flores Verdugo, F.J.; Agraz Hernández, C.; Benítez Pardo, D. Ecosistemas acuáticos costeros: Importancia, retos y prioridades para su conservación. In Perspectivas Sobre Conservación de Ecosistemas Acuáticos en México, 1st ed.; Sánchez, O., Herzig, M., Peters, E., Márque-Huitzil, R., Zambrano, L., Eds.; Secretaría de Medio Ambiente y Recursos Naturales Instituto Nacional de Ecología U.S. Fish & Wildlife Service Unidos para la Conservación, A.C.; Universidad Michoacana de San Nicolás Hidalgo: Morelia, Mexico, 2007; pp. 147–166. Available online: https://docplayer.es/12780089-Ecosistemas-acuaticos-costeros-importancia-retos-y-prioridades-para-su-conservacion.html (accessed on 13 June 2022).
- Reddy, K.R.; De Laune, R.D. Biogeochemistry of Wetlands, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 800. [Google Scholar]
- Flores-Verdugo, F.J.; Agraz-Hernández, C.M.; Benítez-Pardo, D. Creación y Restauración de Ecosistemas de Manglar: Principios Básicos. In Manejo Costero Integral: El Enfoque Municipal; Moreno-Casasola, P., Peresbarbosa, E., Travieso, A.C., Eds.; Instituto de Ecología: Xalapa, Mexico, 2005; pp. 1093–1110. [Google Scholar]
- Krauss, K.W.; Mckee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R.; Chen, L. How mangrove forests adjust to rising sea level. New Phytol. 2013, 202, 19–34. [Google Scholar] [CrossRef]
- Kristensen, E.; Alongi, D.M. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron, and sulfur biogeochemistry in mangrove sediment. Limnol. Oceanogr. 2006, 51, 1557–1571. [Google Scholar] [CrossRef]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, biomass, and productivity of mangrove forests: A review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Jiang, G.F.; Goodale, U.M.; Liu, Y.Y.; Hao, G.Y.; Cao, K.F. Salt management strategy defines the stem and leaf hydraulic characteristics of six mangrove tree species. Tree Physiol. 2017, 37, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Feller, I.C.; Lovelock, C.E.; McKee, K.L. Nutrient addition differentially affects ecological processes of Avicennia germinans in nitrogen versus phosphorus limited mangrove ecosystems. Ecosystems 2007, 10, 347–359. [Google Scholar] [CrossRef]
- Valderrama, L.; Troche, C.; Rodriguez, M.T.; Marquez, D.; Vázquez, B.; Velázquez, S.; Vázquez, A.; Cruz, M.I.; Ressi, R. Evaluation of mangrove cover changes in Mexico during the 1970-2005 Period. Wetlands 2014, 34, 747–758. [Google Scholar] [CrossRef]
- Day, J.J.; Conner, W.; Ley-Lou, F.; Day, R.H.; Navarro, A.M. The Productivity and Composition of Mangrove Forest, Laguna De Terminos, Mexico. Aquat. Bot. 1987, 27, 267–284. [Google Scholar] [CrossRef]
- Cardona-Olarte, P.; Twilley, R.R.; Krauss, K.W.; Rivera-Monroy, V. Responses of neotropical mangrove seedlings grown in monoculture and mixed culture under treatments of hydroperiod and salinity. Hydrobiologia 2006, 569, 325–341. [Google Scholar] [CrossRef]
- Pérez-Castillo, V.F. Dinámica de C, N, P y Fe en Agua y Sedimentos en el Humedal Natural Ciénega de Tamasopo, S.L.P. Ph.D. Thesis, Facultad de Ciencias Químicas, Ingeniería y Medicina, Programas Multidisciplinarios de Posgrado en Ciencias Ambientales, Universidad Autónoma de San Luis Potosí, San Luis Potosí, Mexico, 2018. [Google Scholar]
- Jiménez, J.A.; Sauter, K. Structure and Dynamics of Mangrove Forests Along a Flooding Gradient. Estuaries 1991, 14, 49–56. [Google Scholar] [CrossRef]
- Yáñez–Espinosa, L.; Angeles, G.; López-Portillo, J.; Bárrales, S. Variación anatómica de la madera de Avicennia germinans en la laguna de la mancha, Veracruz, México. Boletín la Soc. Botánica México 2009, 15, 7–15. [Google Scholar] [CrossRef]
- Da-Cruz, C.C.; Mendoza, U.N.; Queiroz, J.B.; Da-Costa, N.; Lara, R.J. Distribution of mangrove vegetation along inundation, phosphorus, and salinity gradients on the Bragança Peninsula in Northern Brazil. Plant Soil 2013, 370, 393–406. [Google Scholar] [CrossRef]


| Site | Fringe Mangrove | Basin Mangrove | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | R. mangle | A. germinans | L. racemosa | A. germinans | ||||||||
| Development Stage | Tre | Sap | See | Tre | Sap | See | Tre | Sap | See | Tre | Sap | Seed |
| Density (ind ha−1) | 850 | 2400 | 2200 | 650 | 100 | 600 | 50 | 300 | 0 | 5550 | 2200 | 3100 |
| Height (m) | 7.9 | 1.01 | 0.334 | 8 | 1 | 0.325 | 2.01 | 1.21 | 0 | 5.8 | 1.09 | 0.26 |
| DBH (cm) | 10.4 | - | 13.5 | - | - | 1.5 | - | - | 7 | - | - | |
| Basal area (m2 ha−1) | 8.9 | - | - | 11.4 | - | - | 0.03 | - | - | 27.5 | - | - |
| IVI (%) | 69.7 | - | - | 28 | - | - | 2.3 | - | - | 100 | - | - |
| Zone | n | Coefficient | Std. Error | t Value | Pr (>|t|) |
|---|---|---|---|---|---|
| Fringe | 14 | 0.2 | 0.04 | 5.6 | 0.0001 |
| Basin | 14 | 0.15 | 0.07 | 2.3 | 0.0421 |
| Impaired | 14 | 0.35 | 0.05 | 6.4 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Ceballos, R.; Zaldívar-Jiménez, A.; Melgarejo-Salas, S.; Canales-Delgadillo, J.; López-Portillo, J.; Merino-Ibarra, M.; Celis-Hernandez, O.; Lara-Domínguez, A.L.; Ochoa-Gómez, J. Porewater Sulfide: The Most Critical Regulator in the Degradation of Mangroves Dominated by Tides. Forests 2022, 13, 1307. https://doi.org/10.3390/f13081307
Pérez-Ceballos R, Zaldívar-Jiménez A, Melgarejo-Salas S, Canales-Delgadillo J, López-Portillo J, Merino-Ibarra M, Celis-Hernandez O, Lara-Domínguez AL, Ochoa-Gómez J. Porewater Sulfide: The Most Critical Regulator in the Degradation of Mangroves Dominated by Tides. Forests. 2022; 13(8):1307. https://doi.org/10.3390/f13081307
Chicago/Turabian StylePérez-Ceballos, Rosela, Arturo Zaldívar-Jiménez, Sveidy Melgarejo-Salas, Julio Canales-Delgadillo, Jorge López-Portillo, Martín Merino-Ibarra, Omar Celis-Hernandez, Ana Laura Lara-Domínguez, and Jonathan Ochoa-Gómez. 2022. "Porewater Sulfide: The Most Critical Regulator in the Degradation of Mangroves Dominated by Tides" Forests 13, no. 8: 1307. https://doi.org/10.3390/f13081307
APA StylePérez-Ceballos, R., Zaldívar-Jiménez, A., Melgarejo-Salas, S., Canales-Delgadillo, J., López-Portillo, J., Merino-Ibarra, M., Celis-Hernandez, O., Lara-Domínguez, A. L., & Ochoa-Gómez, J. (2022). Porewater Sulfide: The Most Critical Regulator in the Degradation of Mangroves Dominated by Tides. Forests, 13(8), 1307. https://doi.org/10.3390/f13081307







