Abstract
In the western sector of the Alps, and particularly in the Aosta Valley, lichenological communities on broad-leaved trees have received very little attention, and information about lichen species associated with common ash (Fraxinus excelsior L.) are still scanty. Therefore, in this study, we analyzed epiphytic lichen communities of ash trees to estimate their composition, their conservation value, and their association with some key environmental variables. Our results show that lichen communities appear to be different in terms of occurrence and frequencies in different sampling sites. The tested environmental variables contribute to shape the lichen communities, which are significantly different (p < 0.05) in sites characterized by different elevation, solar radiation, and source vicinity. The conservation value of lichen species was assessed by comparing distributional data at the national and local level. Our findings show that, in the Italian Alps, ash trees represent an important substrate for locally, or even nationally, rare lichens: 14 recorded species were not previously known in the study area, and some (Lecanora impudens and Rinodina polyspora) were included in the red list of Italian epiphytic lichens.
1. Introduction
European broad-leaved forests have naturalistic, recreational, and historical values and are ecologically irreplaceable for many reasons, as they are key for carbon sequestration, provide stabilizing soils and slopes, and serve as significant habitats for many groups of organisms. The common ash, i.e., Fraxinus excelsior L., is a native tree species widespread all over Europe, with an enormous adaptation potential, being one of the most frequent broad-leaved tree species in managed forests. The common ash is linked to socio-economic, cultural, and ecological aspects in many countries, and is used for its high-quality timber, as fodder for livestock in rural areas during droughts, and even as an ornamental forage resource for cattle [1].
In the Alps, common ash populations play an important role in landscape structure and in ecological succession dynamics due to their capacity of colonizing heterogeneous sites. Here, common ash occurs as a secondary species in mixed broad-leaved forests mostly composed of maple, lime, and poplar, where they can reach an elevation of 1600–1800 m a.s.l. Populations of common ash are in general relatively young in the Alps (20–30 years old), resulting from heterogeneous management and abandonment, perpetrated through the years, of areas previously used as pastural grasslands. Moreover, common ash occurs on the slopes of river ravines and gorges in shaded canyons [2].
Climate and climate change stand among the key factors that can shape the geographic distribution of tree species within and across forest stands at the local, national, and global scale [3]. Climatic factors can exert their effects not only on the tree communities but also on other closely related species, including, for instance, lichens, fungi, animals, and so on [4,5,6]. The role of climate as a driving factor influencing biodiversity levels has been well documented at the global scale [7], yet detailed information on some key forest ecosystems may still be scanty, as in the case of the alpine forest stands hosting ash trees and their putatively associated lichen communities. Unravelling the association between biodiversity levels and climatic or climate-related environmental factors (such, as elevation, slope, and solar radiation) is pivotal to characterize lichen communities, with emphasis on their composition and conservation value. This is of the utmost importance also in consideration of the role of some lichen species that may serve as bioindicators in different environments, including forests [8]. Forest stands hosting common ash trees are likely to represent environments potentially rich in lichen biodiversity. For instance, ash bark, being smooth and sub-neutral, represents a preferential substrate for lichens, especially for some groups whose conservation is a priority or for some groups ecologically bound exclusively to ash populations [9,10].
Lichens are well-known indicators of ecosystem integrity and widely used in habitat protection and conservation, and in the framework of biodiversity programs. Lichen community composition is an important parameter for the assessment of environmental quality, typically air pollution [11]. Species richness, abundance, and various functional traits can be used for the assessment of the overall health status of the environment: high richness and the presence of rare species generally indicate a high environmental quality, whereas low richness and the presence of nitrophilous groups mirror more ecologically compromised environments [12,13]. In Europe, some monitoring programs specifically devoted to forests rely on lichens, organisms that are strictly dependent on forest dynamics [14,15].
The first studies of lichen epiphytes growing on ash have focused on analyzing previously collected records [16,17,18] in order to establish their conservation status and highlight the occurrence of rare or threatened species. However, to date, species richness, abundance, and the community composition in ash forests have not been explicitly addressed in alpine environments. Such information is urgent for conservation issues, such as estimating the likelihood of the extinction of lichen species and planning appropriate conservation and management options.
Within this framework, it is urgent to assess the status of lichen assemblages on ash trees in the north-western sector of the Alps, in order to have an up-to-date database to plan any future protection plans.
Our research was aimed at (1) exploring lichen communities associated with F. excelsior in Aosta Valley and their differences in relation to some key environmental variables assessed at the site level, and (2) appraising the local and regional lichen values in order to define the occurrence and rarity of lichen species.
2. Materials and Methods
2.1. Survey Area and Sampling
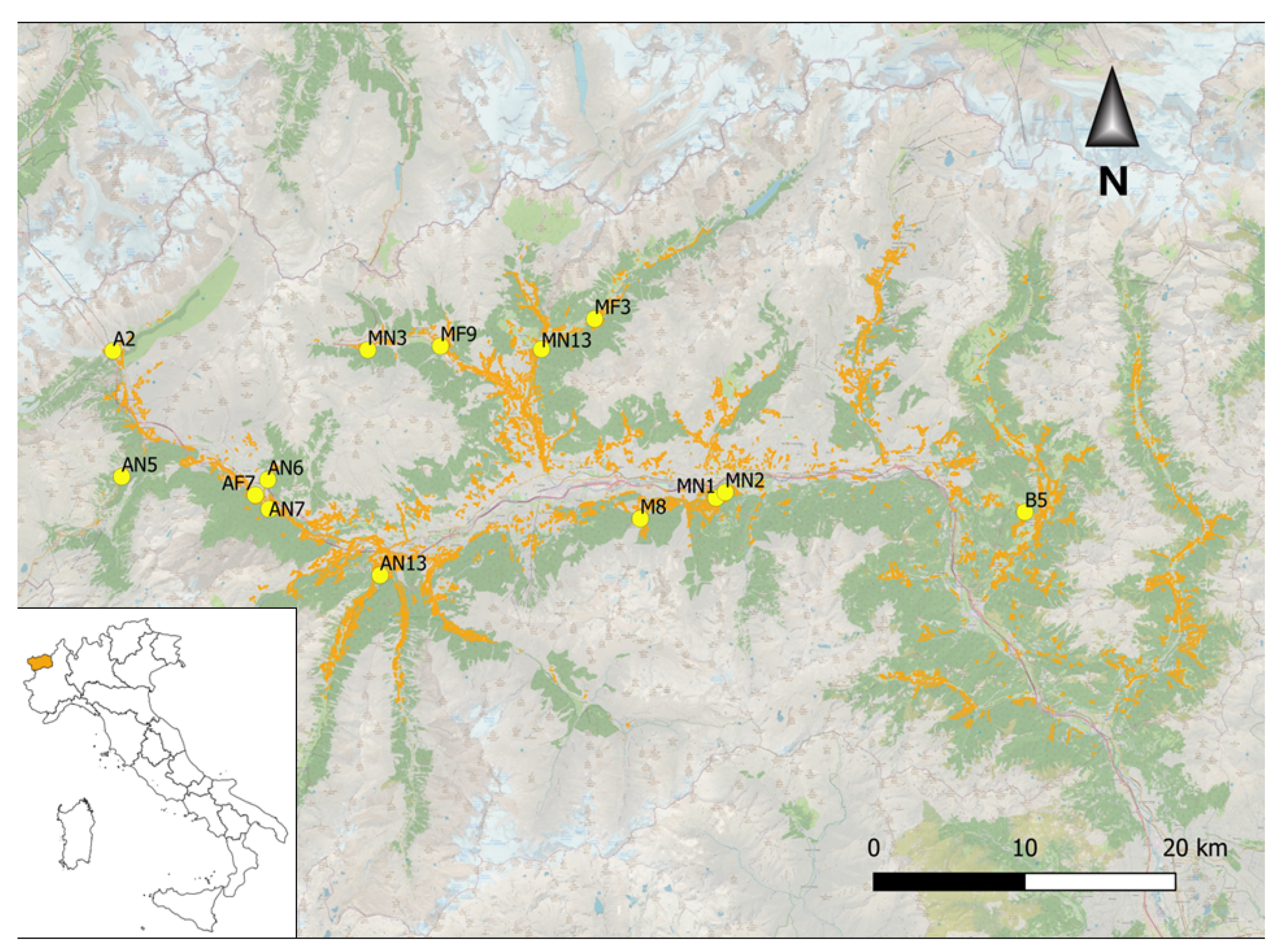
The survey area was located in the Aosta Valley, Northwest Italy (Figure 1); here, ash occurs along all the valley up to an elevation of 1600 m a.s.l. both in pasture edges and in closed ravines on steep slopes.
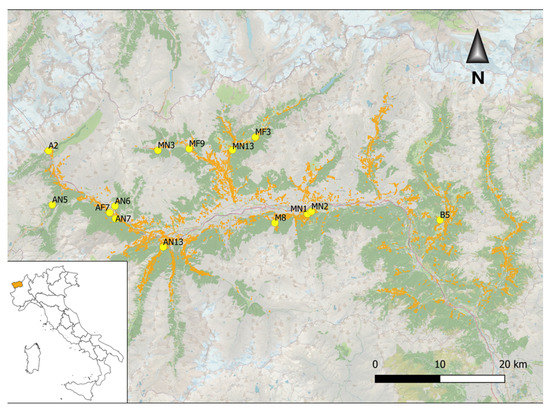
Figure 1.
Map showing the occurrence of forest stands including ashes (in orange) in Aosta Valley and location of the sampling sites (yellow points).
Investigations were carried out in 2021 at 14 sampling sites (named with letters A–P, see Table S1), surveying 3 trees for each site. Once the site coordinates were recorded in a random location within the forest hosting ash trees, the three trees nearest to the above coordinates were selected. The diameters at breast height (DBH) of the selected trees were measured (Table S2) and the same trees were inspected for lichen species occurrence and frequency.
2.2. Environmental Variables
For each site, the longitude and latitude were recorded with a Magellan MobileMapper GPS device (precision ±1 m) and the corresponding coordinates were used as input to derive elevation (el, m a.s.l.) and slope (sl, %). These were retrieved a posteriori from the digital terrain model of the Aosta Valley region. Similarly, solar radiation (sl, KWH/m2) was extrapolated using the regional geoportal navigator (see Data Availability Statement).
We used the distance between the sampled site points and the nearest occurring source of propagation units (i.e., spores, thallus fragments, soredia, isidia) as a continuous variable to assess the role of dispersal in the persistence of lichen communities in our environment. Using QGIS software [19] and the vegetation map of the Aosta Valley [2], we measured the linear distance between the sampling point (site) and the nearest polygon including broadleaves. The distance was measured along the four cardinal directions and the mean of the four was used as a parameter for source vicinity (sv, m). If other forest stands were present within 500 m from the sampling point, these were assumed to belong to the same population.
2.3. Lichen Survey
On each tree, the lichen survey was carried out using a 10 cm × 50 cm sampling grid divided into five contiguous squares and positioned at the N cardinal point, 100 cm above ground level. Species richness (number of different species) and frequency (occurrence within the squares) for each tree, and the relative frequency per site were calculated.
The most common lichen species were identified in the field, whereas critical species samples were collected and identified in the laboratory based on morphological and chemical characteristics. The collected material was deposited in the ORO herbarium. The nomenclature followed the information system on Italian lichens [20]. The conservation value of lichen species was assessed by comparing distributional data at the national [21] and local levels [22].
2.4. Overview on Lichen Traits
To assess the conservation value, in addition to data derived from lichen relevés, lichen species occurrences and frequencies were also discussed with reference to functional traits, as described in the database of Italian lichens [20]: it includes indications of biological traits and tolerances (classes 1–5). An overview of the traits of interest of this data set is shown in Table 1.

Table 1.
Functional traits datasets overview. EIVs = ecological indicator values according to Nimis, 2016 [20].
A comparison with already known information [20,22] and an examination of the rarity grade in the Italian montane ecoregion, as defined by Nimis, 2016 [20], were used to determine the commonness–rarity of the recorded lichen species.
2.5. Data Analysis
A principal component analysis (PCA) combined with a hierarchical cluster analysis (HCA) was run as described in Lione et al. [23] to assess whether sampling sites could be clustered based on the environmental and ecological variables collected, as previously described (i.e., el, sl, sv, sr, hereafter referred to as site variables). The site variables were centered and scaled prior to running the PCA [24]. The number of principal components (PCs) to retain was determined with the Kaiser criterion (KC) applied to the eigenvalues of the four PCs (PC1, PC2, PC3, and PC4) [25]. The variance explained by each retained PC was calculated as reported in [26]. In addition, Pearson’s coefficient (R), quantifying the correlation between each site variable, and the retained PC was calculated. Sites were plotted along with the site variables in the PC space defined by the retained PCs, and site principal coordinates were used to run the following HCA. The Euclidean distance and Ward D2 methods were combined to run the HCA algorithm and obtain the corresponding dendrogram [27,28]. Based on the dendrogram outcomes, two clusters of sites (C1 and C2, see results) sharing common underlying environmental and ecological characteristics were identified.
The presence and abundance of the lichen species were assessed at the within-cluster level. A Venn diagram visualization and the algebra of sets [24,29] were used for the partitioning of the species of lichens that were split among species present in both clusters (C1 ∩ C2, i.e., set intersection), species uniquely present in cluster 1 [C1/(C1 ∩ C2), i.e., set difference], and species included exclusively in cluster 2 [C2/(C1 ∩ C2)]. The relative frequency (%) of the species partitioned as described above was calculated along with the associated 95% confidence interval (CI95%), calculated as described in [30]. Partitioned frequencies were cross-tabulated and compared among the above sets with an overall χ2 test, followed by pairwise multiple comparisons conducted with a Yates-corrected χ2 test [31]. The p-values resulting from the multiple comparisons were corrected by using the Benjamini and Hochberg [32] method. A further contingency table was built by cross-tabulating the levels of two categorical variables, namely the species of lichens detected on ash trees (column variable) and the clusters of sites resulting from the PCA–HCA analyses (row variable). To assess the association between the levels of the column and row variables, the contingency table was analyzed with a χ2 test, the p-value of which was calculated by using a Monte Carlo method based on 104 iterations [33].
Statistical and mathematical analyses were conducted in R [34] by setting 0.05 as a significance threshold.
3. Results
A total of 45 lichen species were recorded on the sampled trees (coded 1–45, Table 2).

Table 2.
List of the species recorded on ash in Aosta Valley. Code: codes used for data analysis; status: indication about species recorded for the first time in the study area (^), confirmation of the species occurrence, specified only if older than a century (§), red listed species (*); rarity: rarity degree in the montane belt in Italy. The nomenclature of the taxa follows Nimis, 2016 [20].
Forty-six percent of these were crustose lichens, while 54% were foliose. No fruticose growth forms were recorded.
According to the different types of photobionts, the great majority (86%) had chlorococcoid green algae as a photosynthetic partner, 8% were in symbiosis with Trentepohlia, and 4% with cyanobacteria.
Out of the 45 species of lichens recorded in this study, 14 species were recorded for the first time in the Aosta Valley and two additional species had not been reported in the study area for over one century. Three species were considered threatened according to IUCN (International Union for Conservation of Nature) criteria. More than half of the species (64%) were thought to be rare (ranging from rather rare: 11 species, to extremely rare: five species) in the montane belt in Italy, while only 12 species fell within the range of common species.
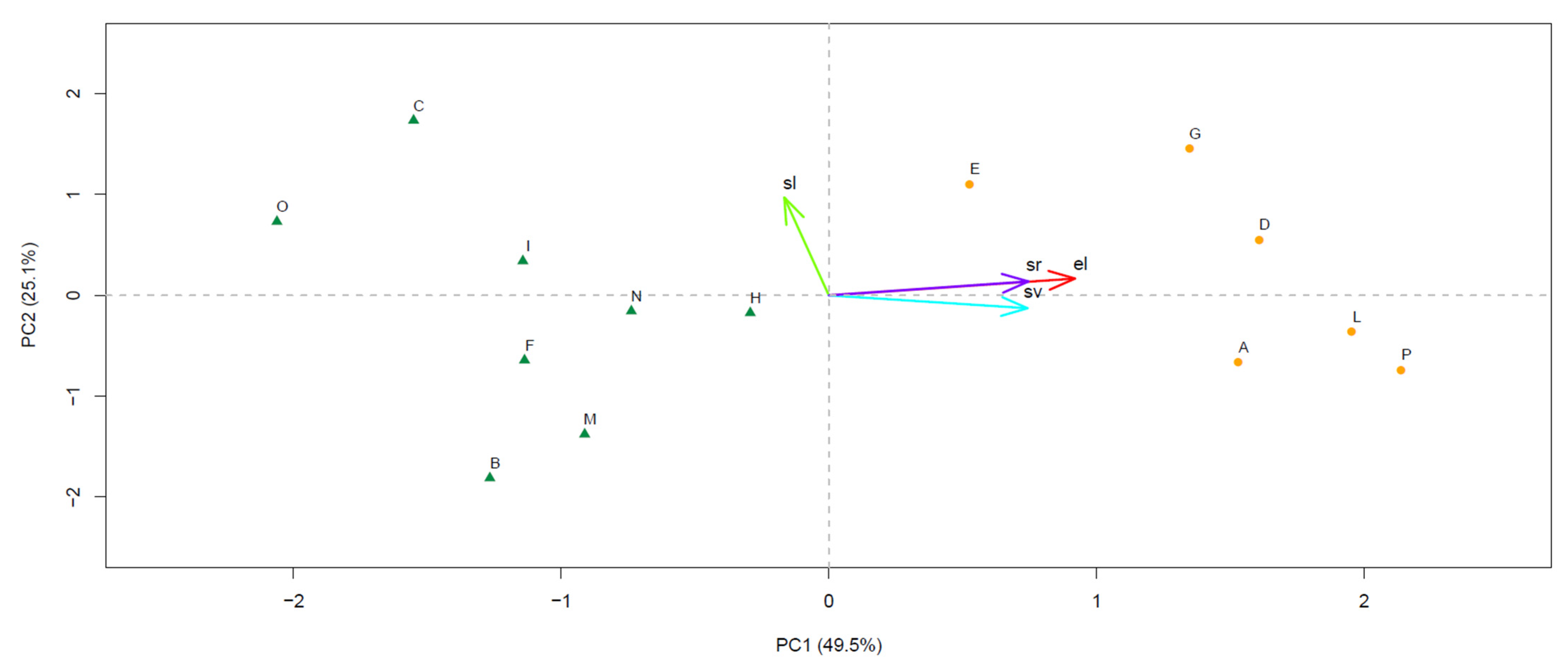
The PCA resulted in four PCs, the eigenvalues of which matched the Kaiser criterion in PC1 (eigenvalue, 1.981) and PC2 (eigenvalue, 1.005), while all other PCs displayed eigenvalues lower than one. PC1 and PC2 accounted for 49.5% and 25.1% of the total variance, respectively, resulting in a two-dimensional PC space associated with a cumulated variance explained at 74.6%. PC1 was positively correlated with el (R = 0.920) and followed in magnitude by sr (R = 0.746) and sv (R = 0.742), while the correlation with sl was milder and negative (R = −0.166). Conversely, PC2 was mostly correlated with sl (R = 0.971), while the correlation with the other site variables was lower than 0.2 in modulus (R = 0.167 for el, 0.138 for sr, and −0.128 for sv). The plot displaying the sampling sites in the PC space showed the presence of two distinct groups, one lying on the first and fourth quadrants (sites A, D, E, G, L, and P), and the other on the second and fourth quadrants (sites B, C, F, H, I, M, N, and O) (Figure 2).
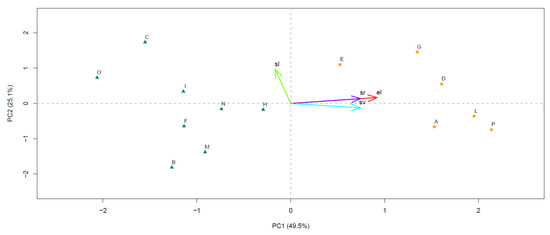
Figure 2.
Space defined by the principal components PC1 and PC2 retained from the principal component analysis (PCA). For each PC, the associated value of the explained variance (%) is reported. Sampling sites are plotted as dots, while site variables are displayed in a vector format. In both cases, the corresponding acronym is indicated (see main text for the acronym legends). Sites located in the first and fourth quadrants and those lying on the second and fourth quadrants are plotted with different symbols and colors.
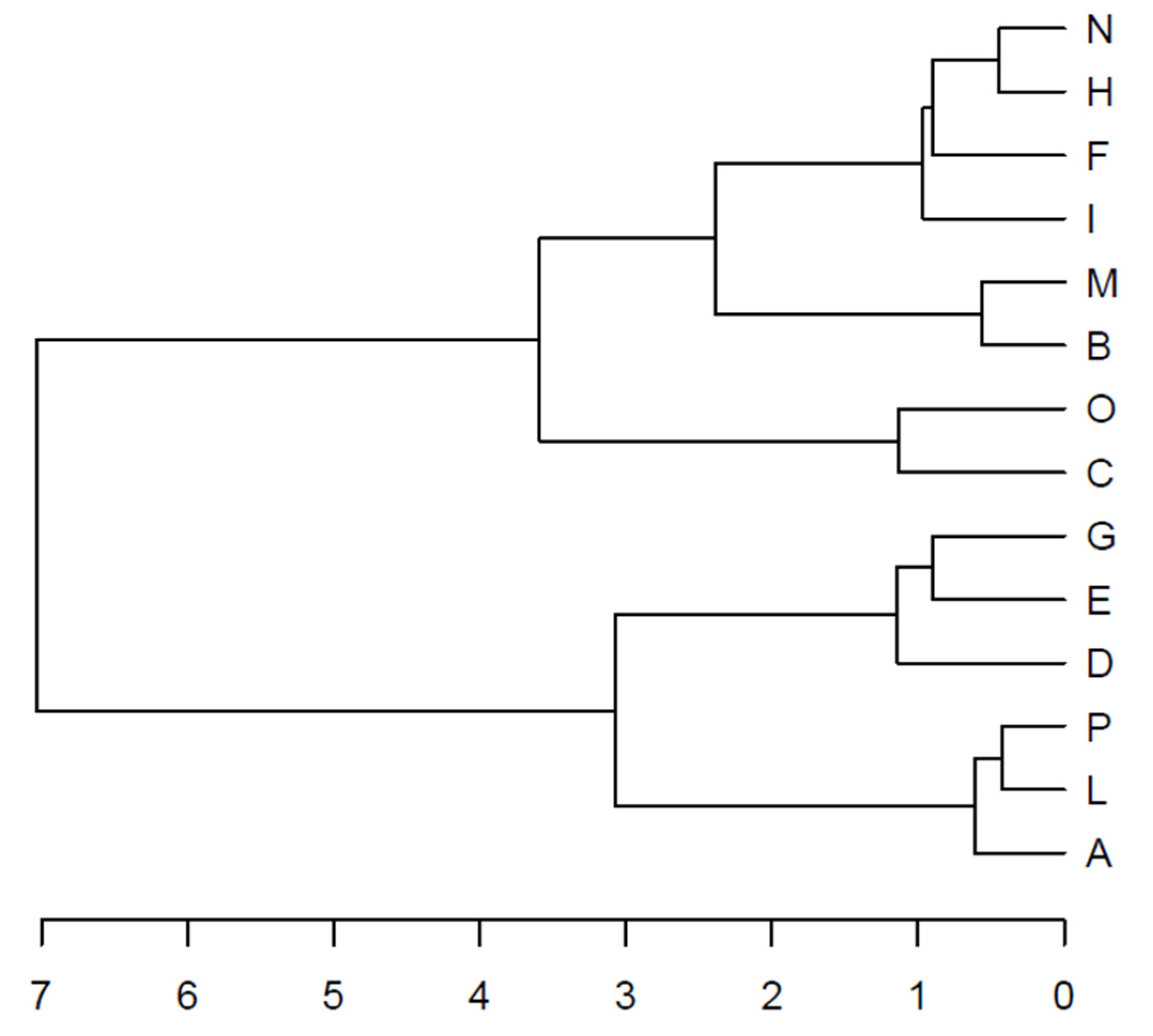
The HCA confirmed the presence of two clusters (C1 and C2) equivalent to the groups mentioned above (Figure 3).
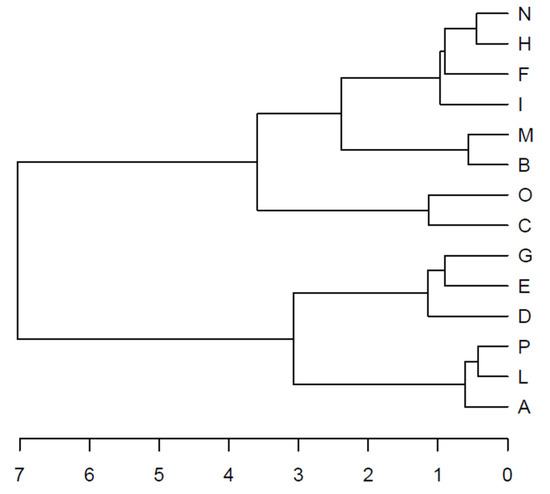
Figure 3.
Hierarchical cluster analysis (HCA) carried out on the site principal coordinates resulting from the retained components of the principal component analysis (PCA). The dendrogram shows the clustering of the sampling sites.
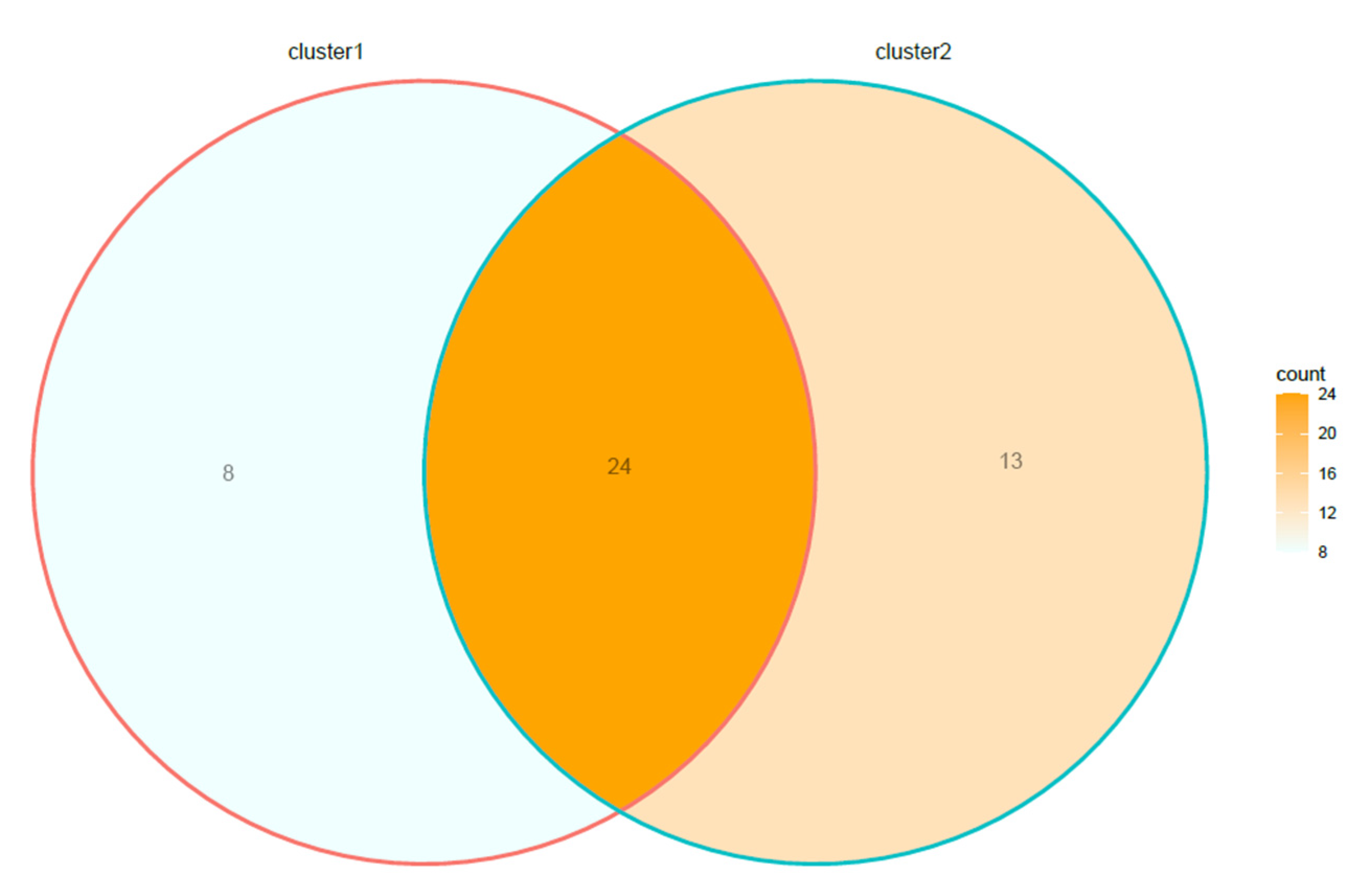
Overall, 17.7% (8.2–31.9% CI95%) of the species of lichens were detected exclusively in sites included in C1, 28.9% (17.2–44.0% CI95%) in the sites of C2, while 53.3% (38.7–68.2% CI95%) of the above species were shared between C1 and C2 (Figure 4).
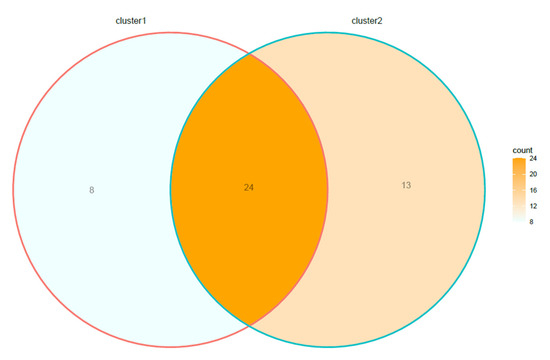
Figure 4.
Venn diagram showing the number of lichen species included in cluster 1, cluster 2, and in both clusters.
The frequency of species shared between clusters was significantly higher (p < 0.05) than that of the species included exclusively in C1 or C2, while the frequencies displayed by the two clusters were comparable (p > 0.05).
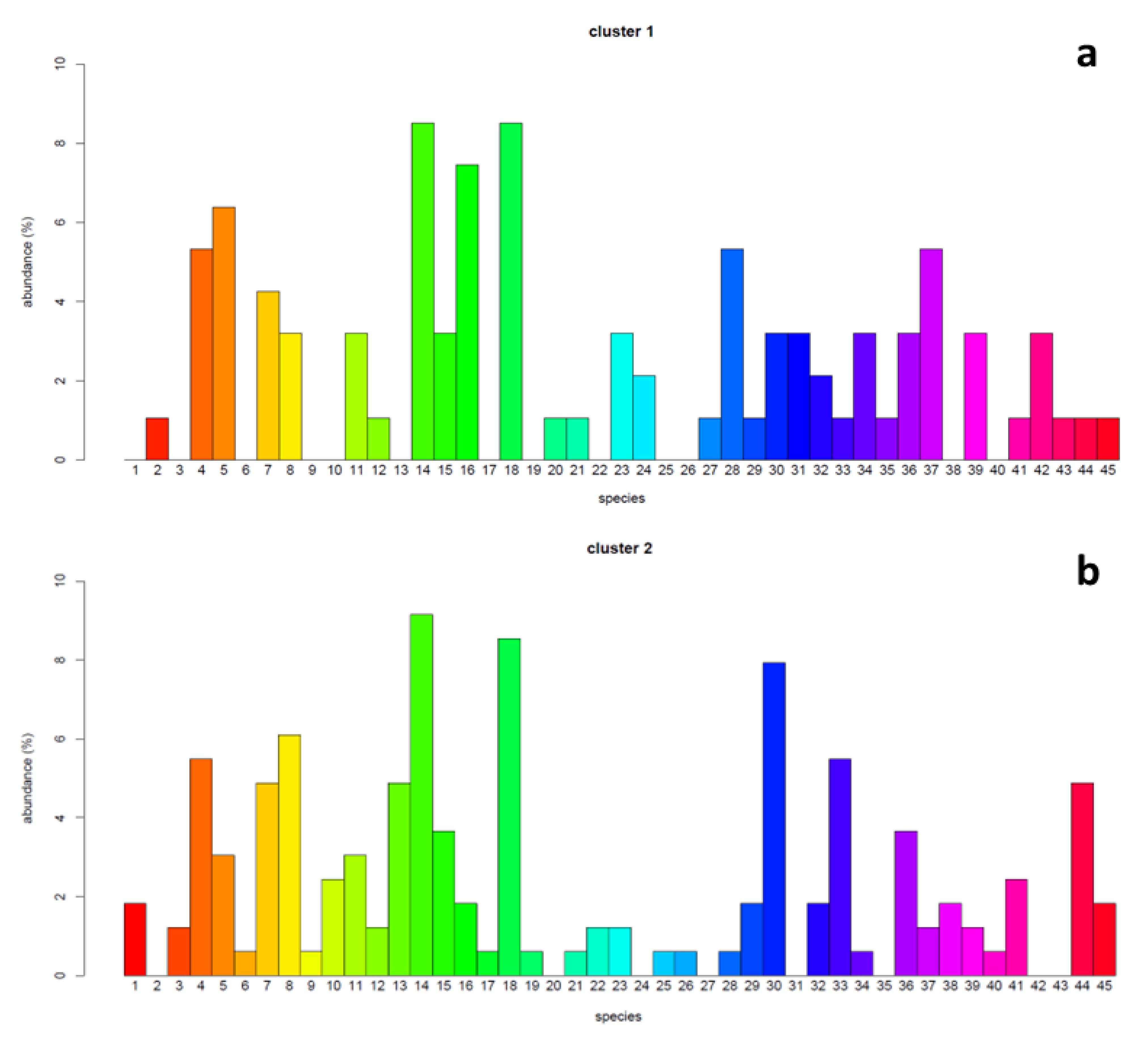
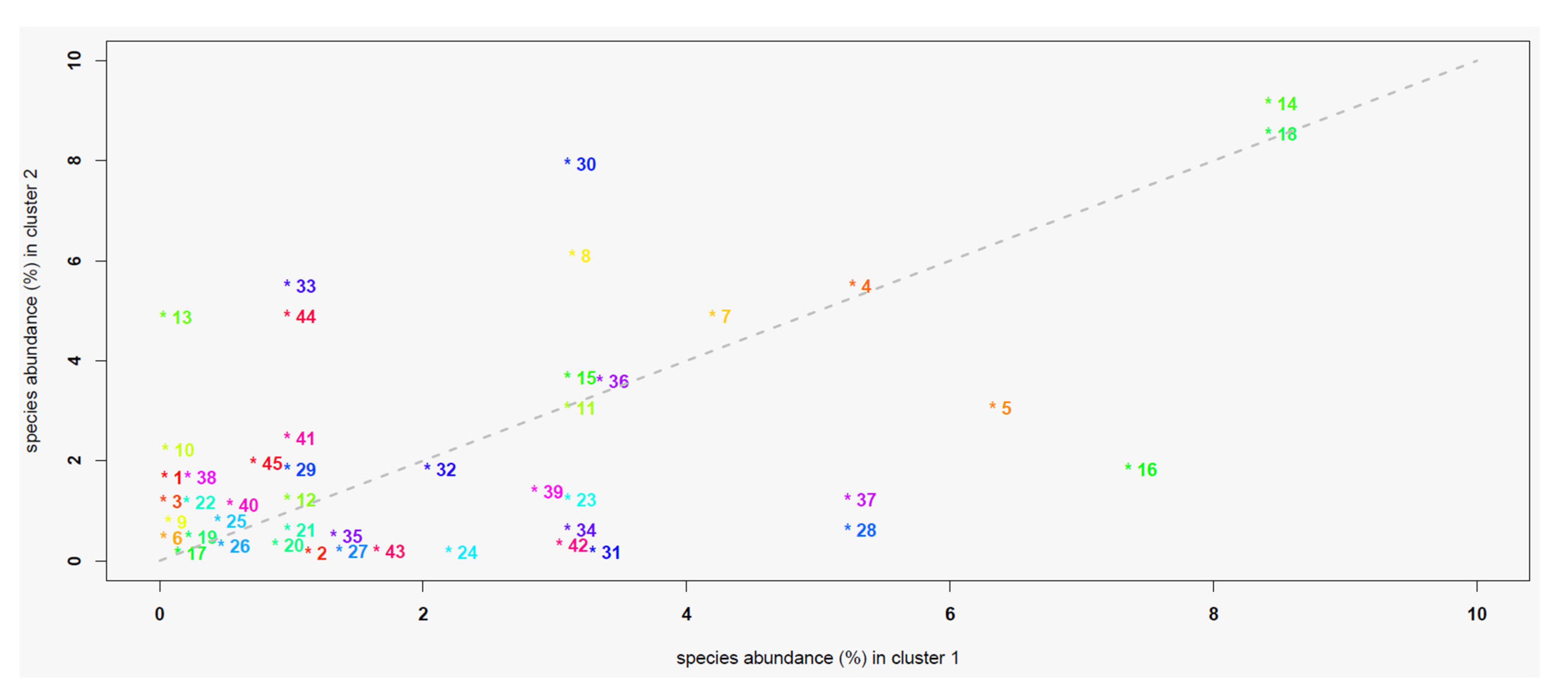
The χ2 test assessing the association between the species of lichens detected on ash trees and the site clusters resulting from the PCA–HCA analyses were significant (p < 0.05). The abundance of lichen species was variable within and between the clusters, with values ranging from 0 to 8.5% in C1 and from 0 to 9.1% in C2 (Figure 5). Depending on the case, some species showed abundances comparable between the two clusters, while others were more abundant in C1 than C2, or vice versa (Figure 6).
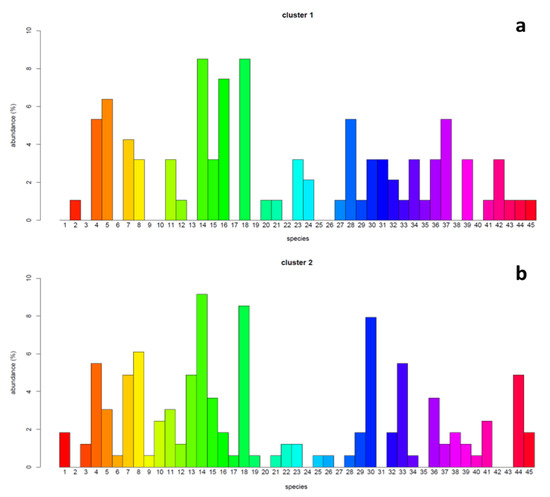
Figure 5.
Bar chart of the abundance (%) of the different species of lichens present in the two clusters of sites. The abundance is shown on the y-axis, while the x-axis reports the number of the corresponding species (see Table S2). Panel (a) refers to cluster 1, panel (b) to cluster 2.
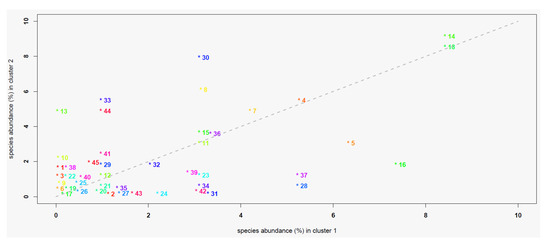
Figure 6.
Scatterplot of the relative abundance (%) of the lichen species in the two clusters of sites. The relative abundance of each species is reported on the x-axis for cluster 1 and on the y-axis for cluster 2. Points represent the corresponding lichen species (see main text for the acronym legend): species whose associated point lies in proximity of the bisector line display comparable abundances between the different clusters, those lying over the line are more abundant in cluster 2 than in cluster 1, while the remaining ones are more abundant in cluster 1 than in cluster 2.
4. Discussion
Our results showed a significant association of lichen communities with elevation and solar radiation. Sites from cluster 2 were clearly distinct from those included in cluster 1, while the former were located at a lower elevation, ranging from 700 to 1100 m a.s.l, and the latter included forest stands growing at an elevation over 1100 m a.s.l. The same pattern was detected for solar radiation: cluster 1 displayed solar radiation values higher than 1000 KW/m2, while the values of cluster 2 were substantially below this threshold (see Table S1). The association between elevation and solar radiation has been reported elsewhere, suggesting that elevation may be a good proxy for light availability in mountain environments [35]. Our results suggested that elevation and solar radiation were the environmental variables most associated with the differences detected between the lichen communities characterizing the two clusters of sites. Although we cannot exclude that other geographic or environmental variables may have been associated with the frequency and abundance of the lichen species detected in this study, the dimension reduction and the coordinate analysis carried out on the virtual space defined by the site environmental variables have already proved its reliability for the ecological characterization of sampling sites [23]. According to the database of Italian lichens [20], the majority of species exclusive for cluster 1 show high EIVs for light tolerance (>4), while most species included in cluster 2 appear to be less heliophilous (Table 3).

Table 3.
Relative frequencies of exclusive species (f) in each detected cluster for the light EIVs (ecological indicator values), according to Nimis, 2016 [20]. For cluster 1, a value of 0.75 of the species had a high EIV for light tolerance (>4), for cluster 2, 0.42.
Lichen assemblages were also associated with the source vicinity parameter. Studies on the impact of a tree species’ abundance on lichen biodiversity at the landscape level [36] indicated that the occurrence of many different tree species may serve as potential alternative substrata. Because the extent of propagules and the availability of suitable substrata in their proximity have been demonstrated to have a positive impact on lichen species richness [37], the presence of suitable alternative tree hosts with similar bark features and wood structures can act as a reservoir for lichen propagation forms assisting in the maintenance of lichen populations in sites where ash is less abundant.
The discovery on ash of three previously unknown or rarely found species (Arthonia radiata, Arthonia ruana, and Pseudoschismatomma rufescens) that have a symbiotic relationship with Trentepholia algae accounted for 8% of the total lichens recorded. When comparing the known distribution of lichens with Trentepohlia in the study area, only six species were previously known, all occurring in less than three localities and all, except one, were recorded more than one century ago and are considered very rare [22]. The newly recorded species were unlikely to have gone unnoticed because they are common in Italy, occurring in all the administrative regions except the one under study [20]. Lichens in Western Europe are responding to climate change, and this is particularly true for species that contain Trentepohlia as phycobionts that are most rapidly increasing in forests [38]. Our findings supported this observation, showing that epiphytic species with Trenthepolia appeared to colonize new areas as a result of global warming. The low occurrence of epiphytic lichens with cyanobacteria recorded on ash in Aosta Valley is not surprising considering the xeric condition of Aosta Valley, since these cyanolichens rely on liquid water to perform photosynthesis and therefore are very sensitive to dry conditions [4].
As already demonstrated in other countries [9,10,39], ash trees represent an important substrate for locally, or even nationally, rare lichens: most of the species recorded on ash are considered rare in the montane ecoregion in Italy. Among the species listed for the first time in the region, Athallia cerinelloides is uncommon in the Italian Alps [40]. It has a more northern distribution than A. cerinella and can colonize twigs of conifers. This feature may help the spread and persistence of these species in areas in which conifers represent the majority of wooded vegetation.
Two different Lecania species, inconspicuous crustose lichens often unnoticed, were recorded for the first time on ash in Aosta Valley: Lecania cyrtellina, which appears to be rare in the western part of Italy, and L. naegelii. L. cyrtellina, which is only distantly related to L. cyrtella [41] but shares many macroscopical morphological similarities with this last one [42], and thus may not have been recognized by previous authors, while L. naegelii, more peculiar, colonizes bark of deciduous species in humid conditions and is rare in the study area.
In Aosta Valley, ash hosts some red-listed lichen species such as Rinodina polyspora, a critically endangered species previously considered regionally extinct [43] and the vulnerable—according to IUCN categories—Lecanora impudens, for which this finding broadens the known range of its occurrence in Italy to the western Alps. Presently, these notable species were only reported on ash in Aosta Valley and are extremely rare in the Italian Alps [40]. Many endangered lichen species are stenotopic, and the dynamics of their population are closely connected to the dynamics of their substrata [44]; hence, the conservation of forest stand hosting ash trees may play a key role for the conservation of lichen biodiversity.
5. Conclusions
Lichen communities on ash in Aosta Valley were mostly shaped by elevation and solar radiation. Our results outlined that the composition of lichen communities was also associated with the source vicinity, suggesting that the presence of alternative tree hosts may have had a positive impact on species richness. The surveys conducted in this study revealed the presence of some lichens associated with Trentepholia, previously unknown in the study area. The newfound occurrence of Trentepholia-associated lichens was likely a consequence of climate change, and in particular the rise of temperatures. This hypothesis is also supported by the finding of species rare in the montane ecoregion, but common in the submediterranean one. Even if the concepts of “commonness” and “rareness” are difficult to define without an expert assessment and long-term study confirmation of the species’ occurrence, the finding of many submediterranean species, newly recorded or not recorded before the 21st century, represents an example of the altitudinal shift in biodiversity, occurring throughout the world due to global warming. Rare species were also found, including some inconspicuous crustose lichens often unnoticed: two red-listed species, Rinodina polyspora, which had been considered extinct at the regional level, and Lecanora impudens, a vulnerable species according to IUCN. Therefore, ash populations in Aosta Valley host peculiar lichen communities, the conservation of which is critical for the conservation of biodiversity of lichen assemblages.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13081288/s1; Table S1: sites codes; Table S2: Diameter at breast height (DBH) of investigated trees.
Author Contributions
Conceptualization, D.I. and S.O.; methodology, D.I. and S.O.; data analyses G.L. and S.O.; investigation, S.O. and D.I.; writing—original draft preparation, D.I. and S.O.; writing—review and editing, D.I., G.L. and S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Altitude and exposure data were retrieved from the digital terrain model of the Valle d’Aosta region (https://mappe.partout.it/pub/geonavitg/geodownload.asp?carta=DTM0508, accessed on 26 May 2022); solar radiation data were retrieved from the regional geoportal navigator (Navigatore Cartografico SCT-3.31.0, https://mappe.regione.vda.it/pub/geoCartoSCT/, accessed on 1 June 2022).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- FRAXIGEN. Ash Species in Europe: Biological Characteristics and Practical Guidelines for Sustainable Use; Oxford Forestry Institute, University of Oxford: Oxford, UK, 2005; ISBN 9780850741637. [Google Scholar]
- Camerano, P.; Terzuolo, P.G.; Varese, P. I Tipi Forestali Della Valle d’Aosta; Compagnia delle Foreste: Arezzo, Italy, 2000. [Google Scholar]
- Iverson, L.R.; Prasad, A.M. Potential changes in tree species richness and forest community types following climate change. Ecosystems 2001, 4, 186–199. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Palmqvist, K.; Solhaug, K.A.; Holien, H.; Hilmo, O.; Nybakken, L.; Myhre, L.C.; Ohlson, M. Growth of epiphytic old forest lichens across climatic and successional gradients. Can. J. For. Res. 2007, 37, 1832–1845. [Google Scholar] [CrossRef]
- Rodenhouse, N.L.; Christenson, L.M.; Parry, D.; Green, L.E. Climate change effects on native fauna of northeastern forests. Can. J. For. Res. 2009, 39, 249–263. [Google Scholar] [CrossRef]
- Oita, S.; Ibáñez, A.; Lutzoni, F.; Miadlikowska, J.; Geml, J.; Lewis, L.A.; Hom, E.F.Y.; Carbone, I.; U’Ren, J.M.; Arnold, A.E. Climate and seasonality drive the richness and composition of tropical fungal endophytes at a landscape scale. Commun. Biol. 2021, 4, 313. [Google Scholar] [CrossRef] [PubMed]
- Wieczynski, D.J.; Boyle, B.; Buzzard, V.; Duran, S.M.; Henderson, A.N.; Hulshof, C.M.; Kerkhoff, A.J.; McCarthy, M.C.; Michaletz, S.T.; Swenson, N.G.; et al. Climate shapes and shifts functional biodiversity in forests worldwide. Proc. Natl. Acad. Sci. USA 2019, 116, 587–592. [Google Scholar] [CrossRef]
- Miller, J.E.; Villella, J.; Stone, D.; Hardman, A. Using lichen communities as indicators of forest stand age and conservation value. For. Ecol. Manag. 2020, 475, 118436. [Google Scholar] [CrossRef]
- Moe, B.; Botnen, A.A. Quantitative Study of the Epiphytic Vegetation on Pollarded Trunks of Fraxinus Excelsior at Havrå, Osterøy, Western Norway. Plant Ecol. 1997, 129, 157–177. [Google Scholar] [CrossRef]
- Ellis, C.J.; Coppins, B.J.; Eaton, S.; Simkin, J. Implications of Ash Dieback for Associated Epiphytes. Conserv. Biol. 2013, 27, 899–901. [Google Scholar] [CrossRef]
- Nimis, P.L.; Scheidegger, C.; Wolseley, P.A. Monitoring with LichensMonitoring Lichens. Monitoring with Lichens—Monitoring Lichens. In NATO Science Series; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Dordrecht, NL, USA, 2002; Volume 7. [Google Scholar] [CrossRef]
- Ellis, C.J.; Asplund, J.; Benesperi, R.; Branquinho, C.; Di Nuzzo, L.; Hurtado, P.; Martínez, I.; Matos, P.; Nascimbene, J.; Pinho, P.; et al. Functional Traits in Lichen Ecology: A Review of Challenge and Opportunity. Microorganisms 2021, 9, 766. [Google Scholar] [CrossRef]
- Abas, A. A Systematic Review on Biomonitoring Using Lichen as the Biological Indicator: A Decade of Practices, Progress and Challenges. Ecol. Indic. 2021, 121, 107–197. [Google Scholar] [CrossRef]
- Stofer, S.; Catalayud, V.; Ferretti, M.; Fischer, R.; Giordani, P.; Keller, C.; Stapper, N.; Scheidegger, C. Epiphytic Lichen Monitoring within the EU/ICP Forests Biodiversity Test-Phase on Level II Plots. ForestBIOTA (Forest Biodiversity Test-Phase Assessments). 2003. Available online: www.forestbiota.org (accessed on 26 May 2022).
- Giordani, P.; Brunialti, G.; Nascimbene, J.; Gottardini, E.; Cristofolini, F.; Isocrono, D.; Matteucci, E.; Paoli, L. Aspects of biological diversity in the CONECOFOR plots. III. Epiphytic lichens. Ann. Ist. Sper. Selv. 2006, 30, 43–50. [Google Scholar]
- Edwards, B. A Preliminary Assessment of the Importance of Ash Trees for Epiphytic Lichens in the British Isles, Version 1.0. Report of British Lichen Society; British Lichen Society: London, UK, 2012. [Google Scholar]
- Lõhmus, A.; Runnel, K. Ash Dieback Can Rapidly Eradicate Isolated Epiphyte Populations in Production Forests: A Case Study. Biol. Conserv. 2014, 169, 185–188. [Google Scholar] [CrossRef]
- Łubek, A.; Kukwa, M.; Czortek, P.; Jaroszewicz, B. Impact of Fraxinus Excelsior Dieback on Biota of Ash-Associated Lichen Epiphytes at the Landscape and Community Level. Biodivers. Conserv. 2020, 29, 431–450. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project. 2022. Available online: http://www.qgis.org (accessed on 26 May 2022).
- Nimis, P.L. ITALIC—The Information System on Italian Lichens. Version 5.0; University of Trieste, Dept. of Biology: Trieste, Italy, 2016; Available online: https://italic.units.it/ (accessed on 5 May 2022).
- Nascimbene, J.; Nimis, P.L.; Ravera, S. Evaluating the Conservation Status of Epiphytic Lichens of Italy: A Red List. Plant Biosyst. 2013, 147, 898–904. [Google Scholar] [CrossRef]
- Piervittori, R.; Isocrono, D. I Licheni Della Valle d’Aosta—1: Indagine Bibliografica e Aspetti Storici (1764–1998). Monografie 1; Museo Regionale di Scienze Naturali: St. Pierre, Italy, 1999. [Google Scholar]
- Lione, G.; Giordano, L.; Sillo, F.; Gonthier, P. Testing and modelling the effects of climate on the incidence of the emergent nut rot agent of chestnut Gnomoniopsis castanea. Plant Pathol. J. 2015, 64, 852–863. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book, 2nd ed.; John Wiley & Sons: Chichester, UK, 2013; ISBN 9780470510247. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; Sage Publication Ltd.: London, UK, 2009. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Gao, C.H.; Yu, G.; Cai, P. ggVennDiagram: An intuitive, easy-to-use, and highly customizable R package to generate Venn diagram. Front. Genet. 2021, 12, 1598. [Google Scholar] [CrossRef]
- Blaker, H. Confidence curves and improved exact confidence intervals for discrete distributions. Can. J. Stat. 2000, 28, 783–798. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Hope, A.C. A simplified Monte Carlo significance test procedure. J. R. Stat. Soc. Ser. B Stat. Methodol. 1968, 30, 582–598. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 27 October 2019).
- Chmura, D.; Żarnowiec, J.; Staniaszek-Kik, M. Altitude Is a Better Predictor of the Habitat Requirements of Epixylic Bryophytes and Lichens than the Presence of Coarse Woody Debris in Mountain Forests: A Study in Poland. Ann. For. Sci. 2022, 79, 7. [Google Scholar] [CrossRef]
- Farmer, A.M.; Bates, J.W.; Bell, J.N.B. Comparisons of Three Woodland Sites in NW Britain Differing in Richness of the Epiphytic Lobarion Pulmonariae Community and Levels of Wet Acidic Deposition. Ecography 1991, 14, 85–91. [Google Scholar] [CrossRef]
- Paltto, H.; Thomasson, I.; Nordén, B. Multispecies and Multiscale Conservation Planning: Setting Quantitative Targets for Red-Listed Lichens on Ancient Oaks. Conserv. Biol. 2010, 24, 758–768. [Google Scholar] [CrossRef]
- Aptroot, A.; van Herk, C.M. Further evidence of the effects of global warming on lichens, particularly those with Trentepohlia phycobionts. Environ. Pollut. 2007, 146, 293–298. [Google Scholar] [CrossRef]
- Thor, G.; Johansson, P.; Jönsson, M.T. Lichen Diversity and Red-Listed Lichen Species Relationships with Tree Species and Diameter in Wooded Meadows. Biodivers. Conserv. 2010, 19, 2307–2328. [Google Scholar] [CrossRef]
- Nimis, P.L.; Hafellner, J.; Roux, C.; Clerc, P.; Mayrhofer, H.; Martellos, S.; Bilovitz, P.O. The lichens of the Alps—An annotated checklist. MycoKeys 2018, 31, 1–634. [Google Scholar] [CrossRef]
- Næsborg, R.R.; Ekman, S.; Tibell, L. Molecular phylogeny of the genus Lecania (Ramalinaceae, lichenized Ascomycota). Mycol. Res. 2007, 111, 581–591. [Google Scholar] [CrossRef]
- Næsborg, R.R. Taxonomic revision of the Lecania cyrtella group based on molecular and morphological evidence. Mycologia 2008, 100, 397–416. [Google Scholar] [CrossRef]
- Jüriado, I.; Liira, J.; Paal, J.; Suija, A. Tree and Stand Level Variables Influencing Diversity of Lichens on Temperate Broad-Leaved Trees in Boreo-Nemoral Floodplain Forests. Biodivers. Conserv. 2008, 18, 105. [Google Scholar] [CrossRef]
- Ravera, S.; Bianchi, E.; Brunialti, G.; Ciotti, R.; Di Nuzzo, L.; Isocrono, D.; Gheza, G.; Giordani, P.; Guttová, A.; Malíček, J.; et al. Studia Lichenologica in Italy. I. New records of red-listed species. Borziana 2021, 2, 87–108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).