Abstract
Leaf veins are the continuation of twigs, and the hydraulic system of a leaf is part of, and the continuation of, the hydraulic architecture of a tree. Previous studies have demonstrated that the vessel diameter of the widest part at the tree base is tightly related to the total stem length of a tree. Here, we demonstrate that: the vessel diameter of the narrowest part at the distal end of the tree (the terminal twigs) is closely correlated to the leaf size for an angiosperm tree. Consequently, the basic feature of the hydraulic system of an angiosperm tree may be predicted by two simple parameters: the stem length (or tree height) and the leaf size, with the tree height predicting the size of the widest vessel at the tree base and the leaf size predicting the size of the narrowest vessel at the terminal trig. Namely, there is an inherent coordination between the leaf size and the hydraulic architecture of the angiosperm tree. As leaves are replacing themselves every year, their responses to climate are direct and immediate, while the woody part of a tree is there for years and more, and thus can only respond passively to climate change. This may cause a mismatch between the woody part and leaf part of the hydraulic system, and thus endanger the hydraulic coordination between leaves and the woody part.
1. Introduction
Global climate change intensifies droughts and causes large scale dieback or even mortality of trees over natural forests or plantations [1,2,3]. The drought-induced dieback or mortality of trees are largely attributed to hydraulic failure resulted from xylem cavitation and embolization [4,5]. Angiosperm trees have successfully evolved the organ of xylem vessel, specialized in water transport, which can carry water efficiently from the roots underground to the leaves at the top of the tree [6]. In theory, xylem-conducting vessel should have evolved with not only efficiency but also resistance to hydraulic failure, in accordance with a widely recognized hypothesis named safety–efficiency tradeoff [7]. In fact, xylem vessels are on average indeed narrower (higher resistance to cavitation or embolization) in trees of dry or cold areas compared with trees in moist and warm areas, which ensures safety for trees in harsh environment and efficiency for trees in favorable conditions [8,9]. However, there is great dilemma in this theory of safety–efficiency tradeoff for the function of a xylem vessel: generally, there is only a weak correlation between safety and efficiency, and it cannot explain why in moist and warm areas, all branches have a low resistance to embolism but their vessel diameters vary in a large range [7].
One important reason for the poor correlation between resistance to embolism in xylem and its vessel diameter is that vessel diameters vary greatly even within an angiospermous tree. To ensure leaves in different part of the canopy get water supply equally, xylem vessels have evolved tapering upward from the tree base or trunk: large vessels of the tree trunk are with minimum resistance and the thin vessels at distal twigs have the most resistance for water transport [10,11,12,13,14,15]. Namely, by tapering the diameter of xylem vessel from the trunk base upwards, trees can minimize the adverse effect of height growth on pathway length resistance. In other words, the upwards tapering of xylem vessels provides an effective mechanism to supply the distal leaves with water, irrespective of the absolute root-to-leaf distance [16]. In a global analysis, Olson et al. [14] found that tree height was a dominant factor in determining xylem vessel diameter at the trunk base. In fact, the tapering of xylem vessels in angiosperm trees is a built-in or inherent feature evolved under environmental pressure, in an evolution requirement of maximizing water transport with minimum carbon cost [10,17,18,19]. Inferring reversely from the distal end of twigs, it is easy to understand why the higher the angiosperm trees, the larger the vessel diameter at the trunk base.
However, the tapering end of the distal twigs is not the end of liquid water transport for trees. Liquid water continues moving on to the vessels in the petiole, the vein and the minor vein in leaf, and end up in the mesophyll cells, where water evaporates into gaseous phase and is diffused into the atmosphere through stomata. Therefore, the mesophyll cells of leaves are the real terminals for the liquid water transport in plant hydraulic architecture [6,20]. The hydraulic architecture of angiosperm trees absorbs water from soil and carries it from root to trunk, branches, twigs, petioles, veins and to the mesophyll cells to ensure the normal functioning of the mesophyll cells, and thus the tree. Although the hierarchical branching system of a tree is a three-dimensional fractal system while the fractal system of leaf veins is two-dimensional, the hydraulic architecture of an angiosperm tree and a leaf are rather similar: conducting vessels are narrowest at the distal end and widening towards the base to reach their maximum [13,15,20,21,22,23]. The only difference is that a tree grows on the ground, thus its base is at the ground surface, while a leaf grows on twigs, therefore its base is the petiole growing on the twigs. From this point of view, veins are the continuation of twigs, and the hydraulic architecture of a leaf is part of, and the continuation of, the hydraulic architecture of a tree. As Olson et al. [14] proved that the tree height could predict the xylem vessel diameter at the trunk base, based on the analogy between the hydraulic architecture of a tree and a leaf, and it may be inferred that the leaf size predicts the vessel diameter at the distal end of the twigs. Based on the reasoning above, our working hypothesis for the current study is that the leaf size can be used to predict the vessel diameter at the distal end of the tree twigs, and therefore an essential part in predicting the xylem diameter at the trunk base. As a result, the leaf size plays a significant role in predicting the whole hydraulic architecture of the angiosperm tree. As the leaf is the organ most directly sensing changes in the atmospheric environment [24,25], the effect of climate change on trees should also be firstly imposed on leaves and pass on to influence the coordination and the safety of the whole hydraulic architecture of the angiosperm tree.
2. Materials and Methods
2.1. Dataset for Anatomical Hydraulic Architecture in Angiosperm Stem
To understand the water transport processes to the leaves of angiosperm trees, we need not only the anatomical data of the conducting vessel in trunks and branches, but also those of leaves. The first part of the dataset we used came from an openly accessible data base of global trees [14]. We selected 407 angiosperm tree species from the data base, for which all parameters we needed were available. These 407 angiosperm species were from 19 communities, and their distribution spanned virtually the entire range of natural angiosperm-growth climates, as described by mean annual precipitation and temperature, as well as the world’s major vegetation types from the north temperate zone to the tropics, and from the tropics to the south temperate zone. All these 407 angiosperm species had available data on tree height, H, (or stem length, SL; Table 1), conducting vessel diameter at the tree base (VDb), vessel diameter at farthest twig (VDt) and wood density (WD). Here, the tree height (H) represented the length from the terminal tip of the farthest twig to the tree base at the ground surface. Therefore, here, the “height” and “stem length” were interchangeable, indicating a presumably closest proxy for the length of the water conductive path of a tree. VDb was determined at the outermost basal secondary xylem vessel, as low as possible above the buttress. VDt was measured at the terminal portion of the farthest twig from the base. All VDb and VDt in the data base were a hydraulically weighted mean diameter (Dh):
where is the natural vessel diameter in vessel n.

Table 1.
Definitions of traits and environmental variables studied and units used.
WD was the ratio between dry weight and volume of the wood, with wood volume determined by water displacement method. The climate data for the original habitat of each species were obtained from WorldClim v.1.4 (www.worldclim.org (accessed on 16 March 2021)). All anatomical parameters for each species in the data base were a mean value of that species. Of course, these vessel diameters were measured by different people, with different way of sampling, and errors might have been introduced when pooling them together. However, this is a data base with the largest number of species documented from different vegetation zones. General conclusions obtained from analyzing this data base should be applicable in general.
2.2. Dataset for Leaf Size and Water Conducting Vessel in the Vein
Water conducting in plants does not end at the farthest twig but at the mesophyll cells next to the stomata. Thus, the hydraulic architecture of a tree also includes the hydraulic architecture of the leaf, which was characterized in the current study by leaf size and conducting vessel diameter at both the petiole and the minor vein of the leaf. These data were taken from an openly accessible data base [23] that covers 36 evergreen dicotyledon (of angiosperm tree) species with simple leaves. The habitat of these species ranged from temperate, subtropical to tropical climates with humidity index (annual precipitation divided by annual potential evapotranspiration) ranging from 1.0 to 0.3. The mean annual precipitation ranged from 544 to 1353 mm. For the measurement on the leaf, fully expanded sunlit leaves were selected and detached from the twig, wrapped in a moist paper towel and then transferred to a formaldehyde–acetic-ethanol solution for sectioning. Leaf areas were measured using a flatbed scanner. Minor veins were defined as the most distal veins in the vascular network. All selected petiole and minor vein vessel radii were measured, and a mean hydraulically weighted radius was obtained for each sample. The mean hydraulically weighted radius (Rh) for each sample was calculated after Tyree and Zimmermann [6] (2002):
Rh, hydraulically weighted radius; R, calculated radius of an individual conduit; n, number of conduits measured in the sample.
2.3. Analysis Methods
In analyzing the contribution of various determinants to VDb, an OLS (ordinary least squares) method was employed instead of SMA (standard major axis regression), for the fact that stem length and leaf size can be more precisely measured than vessel diameter and what concerned us most was the relative importance of various determinants to VDb, not the scaling exponents. The relationships among stem length, leaf size and vessel diameter are in fact relationships in allometry. In order to analyze these relationships with a simple linear model, all data were log-transformed.
To compare the relative importance of climatic factors and SL in determining VDb, normalized data were employed, namely, the observed value minus the mean, divided by the standard error; this way, all variables were transformed to a dataset distributed between (0, 1). Then, a multiple regression was employed and squared standardized coefficients (β2) were obtained. It reflected the relative importance of independent variables to the dependent variable. Meanwhile, other methods were also employed to compare the relative importance of various determinants to VDb. For instance, we calculated the correlations between subject variables and the residual variation of VDb after SL and other variables had been taken into account through a multiple linear regression. If a significant correlation was obtained between the subject variable and the residual variation of VDb, it meant that that variable had a significant impact on VDb. Otherwise, it meant that that variable did not have a significant impact on VDb, or its impact had been represented or covered up by other variables.
When considering the role of the leaf size (LS), the linear correlation analysis between Log10 (LS) and the corresponding vessel radius in the petiole (Rp), Log10 (Rp), was performed. Then, the residual errors of that linear regression were correlated to the conduit radius of minor veins defined as the most distal veins in the vascular network of a leaf. This was to verify the hypothesis of the hydraulic optimality models such as that of West, Brown and Enquist (WBE) [10]: the radius of the most distal veins in the vascular network of a leaf is a constant, not related to, and not varying with, the leaf size. If this was proved to be true, leaf size alone would be sufficient to predict Rp (conducting vessel radius in petiole), and the variation in conducting vessel radius of minor veins could be neglected. All the above-mentioned data analyses and figures were done in Matlab 2017.
3. Results
Based on the prediction of the WBE model, the diameter of the conducting vessel at the tree base (VDb) should be well correlated to the tree height (SL) and the conducting vessel diameter at the farthest twig (VDt) of the tree. As expected, when log10 (SL) or log10 (VDt) were plotted against log10 (VDb), significant linear relationships were obtained (Figure 1a). Furthermore, SL explained 63% of the variations in VDb (Figure 1a) and VDt explained 48% of the variations in VDb (Figure 1b). Namely, SL and VDt were both major determinants for VDb.
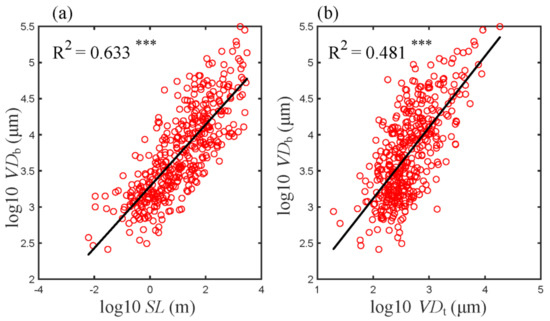
Figure 1.
Log−log scaling relationships between VDb and SL (a) and VDt (b). R2 is indicated in each panel. Asterisks indicate levels of significance (*** p < 0.001). Abbreviations for traits are the same as in Table 1.
In addition to SL and VDt, the wood density (WD) and environmental factors such as temperature (mean annual temperature, MAT) and precipitation (mean annual precipitation, MAP) were also proposed to be related to VDb [9]. To quantify the relative importance of SL, VDt, MAT, MAP and WD to VDb, a multiple regression analysis was performed on standardized data from all related variables. Squared standardized coefficients (β2) were obtained, which indicated the relative importance of each variable to the subject variable (VDb in this case). The β2 of SL, VDt, MAT, MAP and WD with respect to VDb were 0.370, 0.084, 0.012, 0.014 and 0.003, respectively (Figure 2). SL and VDt were still the two most influential variables as determinants of VDb, and the other variables contributed little to the variations in VDb (Figure 2).
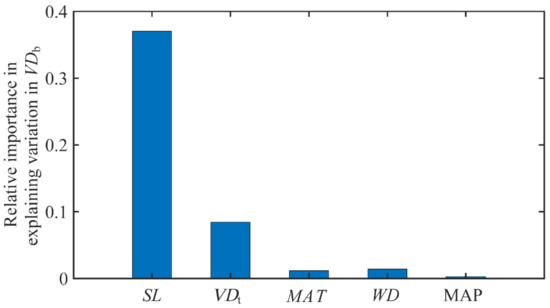
Figure 2.
Relative importance of two climatic variables, VDt and SL, as well as WD in explaining VDb variation. All these variables were standardized before performing a multiple linear regression, so that the squared standardized coefficients (β2) depict the relative importance of explanatory variables. Abbreviations for traits and environmental variables are the same as in Table 1.
To further evaluate the importance of each variable, an analysis on residual variations was performed. First, a multiple linear regression was performed on SL and VDt with respect to VDb, and the obtained residual variations in VDb were linearly correlated to T (Figure 3a). However, it was found that (Figure 3a) merely a weak correlation was obtained, and MAT only explained 0.1% of the residual variations in VDb (Figure 3a). When the contribution of SL and MAT to VDb was eliminated following the same analysis procedure, the residual variation of VDb was still significantly related to VDt, and VDt still explained 11.4% of the residual variation in VDb (Figure 3b). Evidently, VDt is a non-negligible variable in determining VDb.
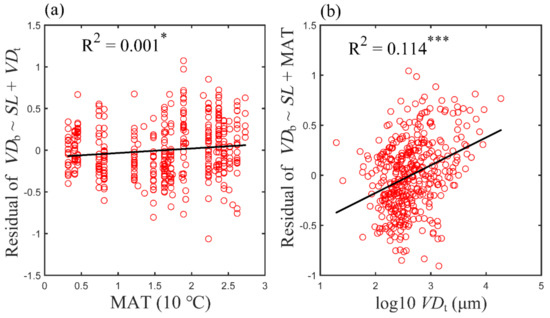
Figure 3.
Relationship between residual variation in VDb and MAT (a) and VDt (b): (a) after eliminating the contribution of SL and VDt; (b) after eliminating the contribution of SL and MAT. R2 is indicated in each panel. Asterisks indicate levels of significance (* p < 0.05; *** p < 0.001). Abbreviations for traits and environmental variables are the same as in Table 1.
When only the contribution of SL to VDb was eliminated through linear regression, the residual variations of VDb were found to be closely correlated to the other variables except MAP (Figure 4). VDt explained most of the residual variation of VDb (19%); MAT explained about 9% and WD explained approximately 10%; MAP was not significantly related to the residual variations in VDb and thus did not explain its variation. Evidently, Figure 4 demonstrates that, beside SL, VDt was the most influential variable to VDb.
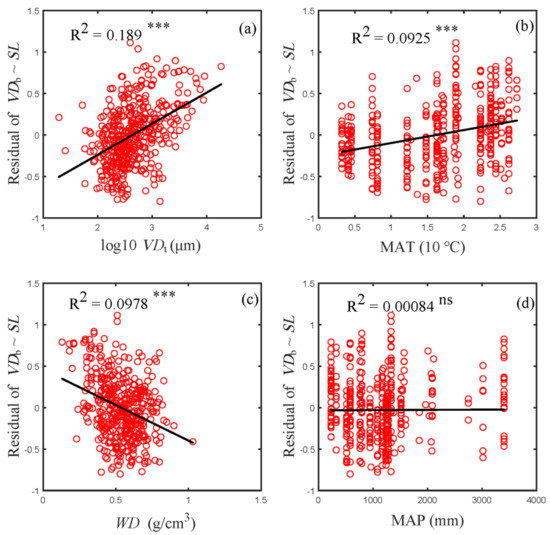
Figure 4.
Relationship between residual variation in VDb and VDt (a), MAT (b), WD (c) and MAP (d). Residual variation of VDb was obtained after eliminating the contribution of SL to VDb through linear regression. R2 is indicated in each panel. Asterisks indicate levels of significance (ns, p > 0.05; *** p < 0.001). Abbreviations for traits and environmental variables are the same as in Table 1.
Figure 5 demonstrates that the size of the conducting vessel of the petiole was strongly correlated to the leaf size, with the leaf size explaining 83% of the variation in the conducting vessel size in the petiole (Figure 5a). Furthermore, the residual variation in the vessel size of the petiole, after eliminating the contribution of the leaf size through a linear regression, was not significantly related to the vessel size in minor veins of the leaf (Figure 5b). In fact, the WBE model also assumed that the vessel size in a minor vein was not related to leaf size: it was set as constant in the model and was proved here to be true [10]. Furthermore, as climatic factors were not contributing much to the conducting vessel size (Figure 4b,d), the leaf size must be the dominated factor in determining the conducting vessel size of the petiole (Figure 5a).
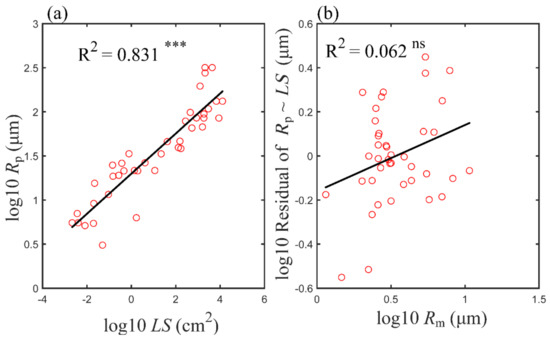
Figure 5.
Log−log scaling relationship between leaf size (LS) and the hydraulic radius of petiole vessels, Rp (a), and the residual variation of Rp (after eliminating the contribution of LS through linear regression) vs. the hydraulic radius of minor vein vessels, Rm (b). R2 is indicated in each panel. Asterisks indicate levels of significance (ns, p > 0.05; *** p < 0.001). Abbreviations for traits are the same as in Table 1.
4. Discussion
We found that the vessel diameter at the farthest end of the distal twig (VDt) was closely associated with the vessel diameter at the tree base (VDb; Figure 1, Figure 2, Figure 3 and Figure 4); simultaneously, the leaf size coordinated with the conducting vessel diameter at the petiole for angiosperm trees (Figure 5). As the vessel in the petiole is a direct smooth extension of the vessel in the twig from which it grows out, the vessel diameter at the connecting point of these two parts can be taken as one, and thus a petiole is not different from a twig in this regard [14,15]. Therefore, the leaf size can be considered the only determinant of VDt (Figure 5), which in turn coordinated well with VDb (Figure 1, Figure 2, Figure 3 and Figure 4) and thus the architecture of the hydraulic architecture of angiosperm trees. The overall results proved that our primary hypothesis was true. The hydraulic architecture of an angiosperm tree is an interdependent, built-in system shaped during evolution. The effect of climatic factors such MAT and MAP were already built-in during evolution and thus may not be able to be seen directly (Figure 2, Figure 3 and Figure 4). The main feature of this hydraulic architecture may be predicted by two simple characters of an angiosperm tree: height (Figure 1, Figure 2, Figure 3 and Figure 4) and leaf size (Figure 5), with tree height as a determinant of the diameter of the widest part of xylem vessels at the tree base, and leaf size as a determinant of the diameter of the narrowest part of the xylem vessels at the distal end of the twigs. This finding should not come as a big surprise, considering that a leaf’s hydraulic architecture is rather similar to that of a tree: a leaf can be considered as a “mini tree” in this sense. The only difference is that a tree grows on the ground and a leaf grows on a twig.
Namely, for a single angiosperm tree, the conducting vessel diameter at any point may be simply predicted by its distance to the farthest leaf and the leaf size itself [18] (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5). The same rules would apply over all tree species if the vessel diameters were normalized to eliminate the effect of vessel diameter variations in absolute values across species [11,13,15,18] (Figure 1, Figure 2, Figure 3 and Figure 4). This means that the variation of conducting vessel diameters is common: it occurs within a tree at different position and among trees with different height or leaf size. Therefore, although the conducting vessel size was proposed to be the result of trade-off between safety and efficiency for water transport, this trade-off relation was proved to be weak [7]. For instance, although there was study at the branch-scale showing that bigger vessels were indeed subjected to embolism earlier than smaller vessels in a single stem segment [14], a study at the whole-tree-scale indicated otherwise: the resistance to embolism was not significantly different among different-size vessels at branches of different sizes or ages [26]. A study on xylem vessels in leaf also indicated that there was no significant correlation between vessel diameter and resistance to embolism [19]. Namely, more and more pieces of evidence indicate that the diameter of water conducting vessels is not directly related to the resistance to embolism. In fact, within a tree, the vessel diameter at the trunk is always much bigger than that in the leaf. If the vessel diameter determined the resistance to embolism, when drought occurred, the trunk would be subjected to embolism first. This contradicts the hydraulic segmentation hypothesis [6]) and the known fact that facing a grave drought, trees always tend to sacrifice (subjected to embolism first) easily replaceable small branches or foliage, to reduce overall water loss and thus to preserve or protect the tree trunk.
The evolution goal of the plant hydraulic architecture is to transport water from soil to leaf in a most efficient and safest way, optimizing a tapering vessel system sending water to every mesophyll cell with the least carbon investment [10,27]. The farthest end of the distal twig is not the destination of the liquid water transport of a tree’s hydraulic architecture; the mesophyll cell is. That way, the hydraulic architecture of a tree makes mesophyll cells water-saturated: a water condition just like their evolutionary ancestors in the ocean [3,28]. The water-saturated mesophyll cells can thus perform the most fundamental function of a tree, photosynthesis, at the top of the tree where sunlight is abundant. Obviously, this is only true if the leaf is considered as an integrated part of the hydraulic architecture, and the mesophyll cell is considered the destination of liquid water transport, as we did in the current study; then, the hydraulic architecture of a tree can be understood fully in a systematic way.
As the mesophyll cell is the site of photosynthesis and the site for water to evaporate into the air through the stomata, the drought resistance of a tree should be directly represented by the worst water condition (lowest water potential) that the mesophyll cell can endure. This lowest water potential is termed the turgor loss point (TLP) of the leaf, and a large number of experimental evidence has suggested that TLP is highly related to the drought resistance of a tree [19]. In fact, since the water-transporting function of xylem is to ensure a sufficient water supply to the mesophyll cell, the xylem resistance to embolism must match the need of a leaf to maintain its water potential above the TLP: a low resistance to embolism would put the mesophyll cell at risk [29,30], while a high resistance to embolism would require a high carbon investment [19,31], which is a disadvantage when competing with others. Namely, the TLP or osmotic adjustment ability of the foliage directly represent the drought resistance of a tree; the xylem vessel size does not. A xylem vessel is getting bigger and bigger from the mesophyll cell to the tree trunk to get water into the mesophyll cell and to minimize resistance for water transport, with an embolism resistance at each part of the xylem system conforming to the need of maintaining the leaf water potential above the TLP [6,31,32]. A study on sugar maple indicated that although the vessel sizes at branches of different ages or the trunk were different, the resistance to embolism was rather similar [26]. A drought experiment on grape vine revealed that when the xylem of foliage was in deep embolism, that in branches was still intact and without significant embolism [5]. It is no doubt that xylem vessels in leaves are much smaller than those in branches, but they were much more vulnerable to embolism! This agreed well with the vulnerability segmentation hypothesis [6] but contradicted the theoretic prediction of lager vessel being more vulnerable to embolism [7,9].
It can be seen that the leaf, seemingly negligible in the huge hydraulic architecture of an angiosperm tree, plays a key role not only in coordinating the hydraulic architecture, but also in determining the drought resistance of the tree. Leaves grow on twigs just like trees grow on the ground, and the minihydraulic architecture of a leaf is a direct extension of the hydraulic architecture of the tree. These two systems match each other and merge into one. However, with global climate changes going on, a mismatch between these two systems may occur: the leaves are replacing themselves every year, but the main hydraulic architecture of a tree is there for years or tens of years and even more. This mismatch may have catastrophic consequences on trees. A study on the leaf as an integrated part of the tree’s hydraulic architecture would give much more insight on how to predict this consequence and on finding ways to avoid it.
Author Contributions
X.C. and Y.L. conceived and designed the study. X.C., Z.-Y.W. and Y.L. wrote the paper. X.-J.Z. and J.-B.X. reviewed critically and edited the manuscript significantly. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grants nos.: 41730638, 31770651, 31901280).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created in this study.
Acknowledgments
We are grateful to the many people who have contributed to this study, especially Lu Shitong, Chen Sen, and Ye Linfeng for technical support in data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Meyer, C.W.; et al. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.L.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Venturas, M.D.; Sperry, J.S.; Hacke, U.G. Plant xylem hydraulics: What we understand, current research, and future challenges. J. Integr. Plant Biol. 2017, 59, 356–389. [Google Scholar] [CrossRef] [PubMed]
- Rood, S.B.; Patiño, S.; Coombs, K.; Tyree, M.T. Branch sacrifice: Cavitation-associated drought adaptation of riparian cottonwoods. Trees 2000, 14, 0248–0257. [Google Scholar] [CrossRef]
- Hochberg, U.; Windt, C.W.; Ponomarenko, A.; Zhang, Y.-J.; Gersony, J.; Rockwell, F.E.; Holbrook, N.M. Stomatal Closure, Basal Leaf Embolism, and Shedding Protect the Hydraulic Integrity of Grape Stems. Plant Physiol. 2017, 174, 764–775. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Gleason, S.M.; Westoby, M.; Jansen, S.; Choat, B.; Hacke, U.G.; Pratt, R.B.; Bhaskar, R.; Brodribb, T.J.; Bucci, S.J.; Zanne, A.E.; et al. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytol. 2016, 209, 123–136. [Google Scholar] [CrossRef]
- Pfautsch, S.; Harbusch, M.; Wesolowski, A.; Smith, R.; Macfarlane, C.; Tjoelker, M.G.; Reich, P.B.; Adams, M.A. Climate determines vascular traits in the ecologically diverse genus Eucalyptus. Ecol. Lett. 2016, 19, 240–248. [Google Scholar] [CrossRef]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2017, 40, 831–845. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the structure and allometry of plant vascular systems. Nature 1999, 400, 664–667. [Google Scholar] [CrossRef]
- Mencuccini, M.; Hölttä, T.; Petit, G.; Magnani, F. Sanio’s laws revisited. Size-dependent changes in the xylem architecture of trees. Ecol. Lett. 2007, 10, 1084–1093. [Google Scholar] [CrossRef]
- Petit, G.; Anfodillo, T.; Mencuccini, M. Tapering of xylem conduits and hydraulic limitations in sycamore (Acer pseudoplatanus) trees. New Phytol. 2008, 177, 653–664. [Google Scholar] [CrossRef]
- Carrer, M.; von Arx, G.; Castagneri, D.; Petit, G. Distilling allometric and environmental information from time series of conduit size: The standardization issue and its relationship to tree hydraulic architecture. Tree Physiol. 2015, 35, 27–33. [Google Scholar] [CrossRef]
- Olson, M.E.; Soriano, D.; Rosell, J.A.; Anfodillo, T.; Donoghue, M.J.; Edwards, E.J.; León-Gómez, C.; Dawson, T.; Camarero Martínez, J.J.; Méndez-Alonzo, R.; et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. USA 2018, 115, 7551–7556. [Google Scholar] [CrossRef]
- Lechthaler, S.; Kiorapostolou, N.; Pitacco, A.; Anfodillo, T.; Petit, G. The total path length’s hydraulic resistance according to known anatomical patterns: What is the shape of the root-to-leaf tension gradient along the plant’s longitudinal axis? J. Theor. Biol. 2020, 502, 110369. [Google Scholar] [CrossRef]
- Enquist, B.J. Universal scaling in tree and vascular plant allometry: Toward a general quantitative theory linking plant form and function from cells to ecosystems. Tree Physiol. 2002, 22, 1045–1064. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Anfodillo, T.; Carraro, V.; Carrer, M.; Fior, C.; Rossi, S. Convergent tapering of xylem conduits in different woody species. New Phytol. 2006, 169, 279–290. [Google Scholar] [CrossRef]
- Blackman, C.J.; Brodribb, T.J.; Jordan, G.J. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 2010, 188, 1113–1123. [Google Scholar] [CrossRef]
- Cochard, H.; Nardini, A.; Coll, L. Hydraulic architecture of leaf blades: Where is the main resistance? Plant Cell Environ. 2004, 27, 1257–1267. [Google Scholar] [CrossRef]
- Mencuccini, M.; Martínez-Vilalta, J.; Hamid, H.A.; Korakaki, E.; Vanderklein, D. Evidence for age- and size-mediated controls of tree growth from grafting studies. Tree Physiol. 2007, 27, 463–473. [Google Scholar] [CrossRef]
- Coomes, D.A.; Heathcote, S.; Godfrey, E.R.; Shepherd, J.J.; Sack, L. Scaling of xylem vessels and veins within the leaves of oak species. Biol. Lett. 2008, 4, 302–306. [Google Scholar] [CrossRef]
- Gleason, S.M.; Blackman, C.J.; Gleason, S.T.; McCulloh, K.A.; Ocheltree, T.W.; Westoby, M. Vessel scaling in evergreen angiosperm leaves conforms with Murray’s law and area-filling assumptions: Implications for plant size, leaf size and cold tolerance. New Phytol. 2018, 218, 1360–1370. [Google Scholar] [CrossRef]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf Form and Photosynthesis. BioScience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Wilf, P.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Choat, B.; Lahr, E.C.; Melcher, P.J.; Zwieniecki, M.A.; Holbrook, N.M. The spatial pattern of air seeding thresholds in mature sugar maple trees. Plant Cell Environ. 2005, 28, 1082–1089. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A General Model for the Origin of Allometric Scaling Laws in Biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J.; Werber, J.R.; Elimelech, M. Capillary-driven desalination in a synthetic mangrove. Sci. Adv. 2020, 6, eaax5253. [Google Scholar] [CrossRef]
- Tyree, M.T.; Cochard, H.; Cruiziat, P.; Sinclair, B.; Ameglio, T. Drought-induced leaf shedding in walnut: Evidence for vulnerability segmentation. Plant Cell Environ. 1993, 16, 879–882. [Google Scholar] [CrossRef]
- Cardoso, A.A.; Batz, T.A.; McAdam, S.A.M. Xylem Embolism Resistance Determines Leaf Mortality during Drought in Persea americana. Plant Physiol. 2020, 182, 547–554. [Google Scholar] [CrossRef]
- Zhu, S.-D.; Chen, Y.-J.; Ye, Q.; He, P.-C.; Liu, H.; Li, R.-H.; Fu, P.-L.; Jiang, G.-F.; Cao, K.-F. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Felid, T.S.; Gleason, S.M.; Zanne, A.E.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).