Climate Change in the Provenance Regions of Romania over the Last 70 Years: Implications for Forest Management
Abstract
1. Introduction
2. Materials and Methods
3. Results
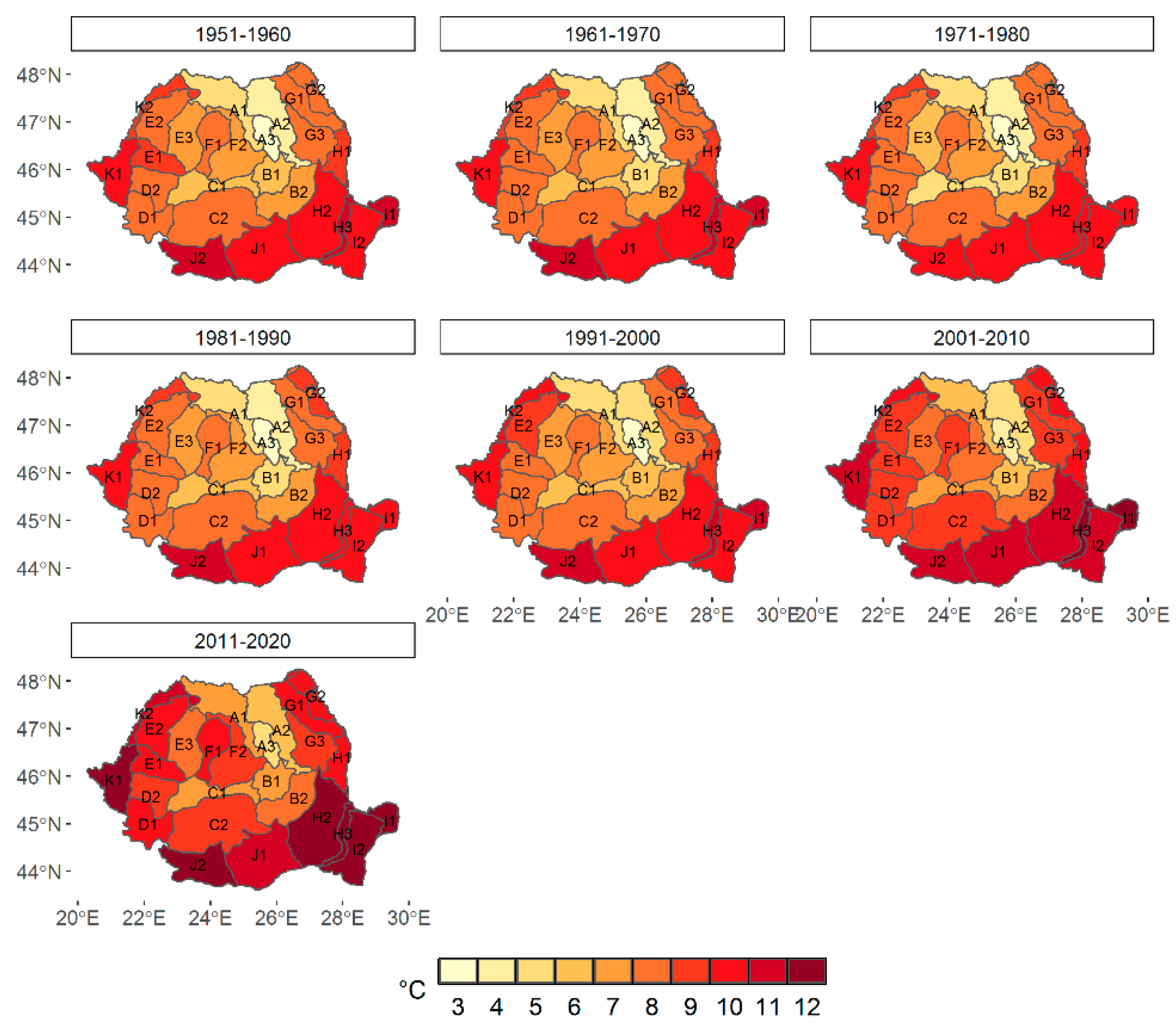
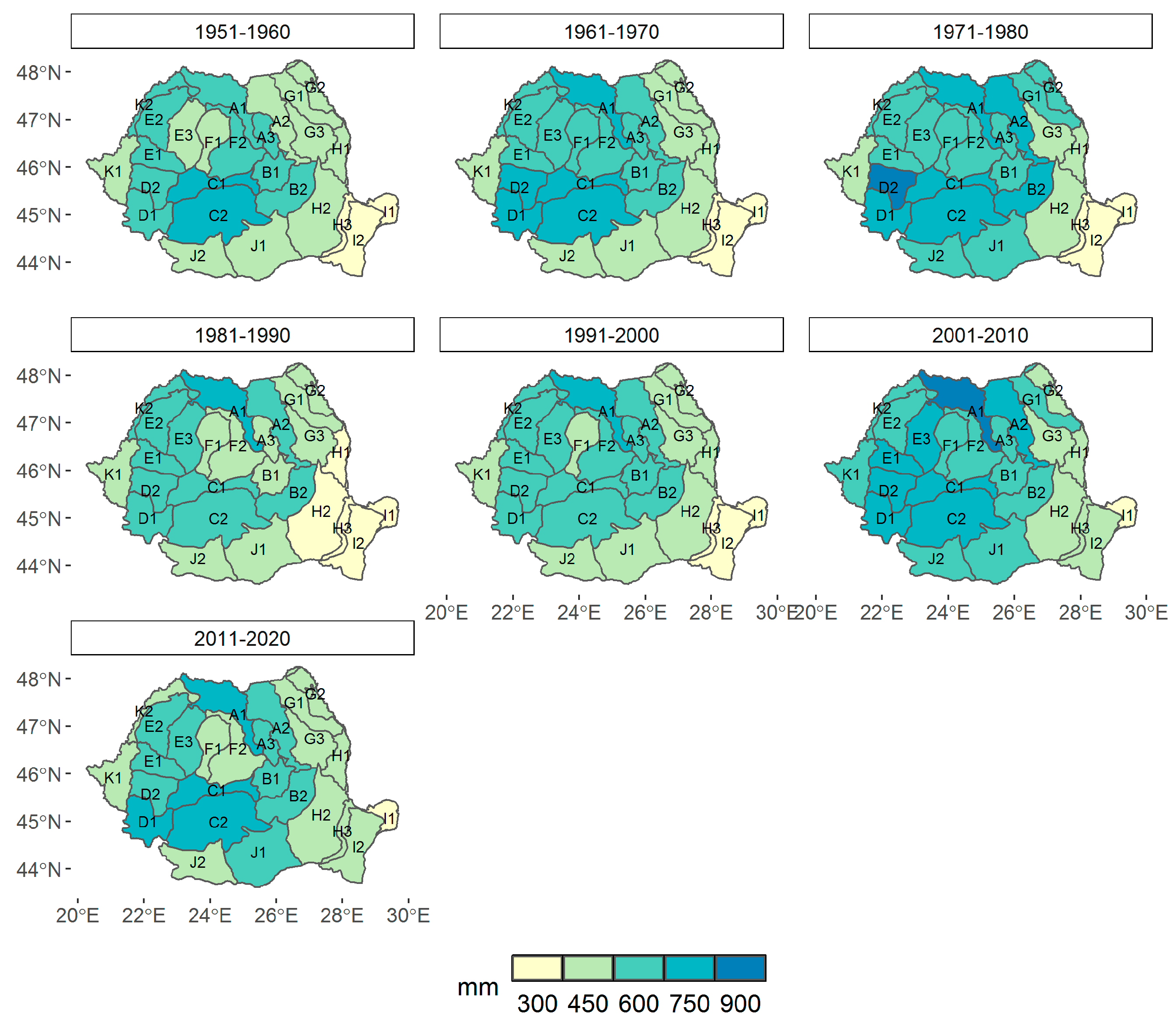
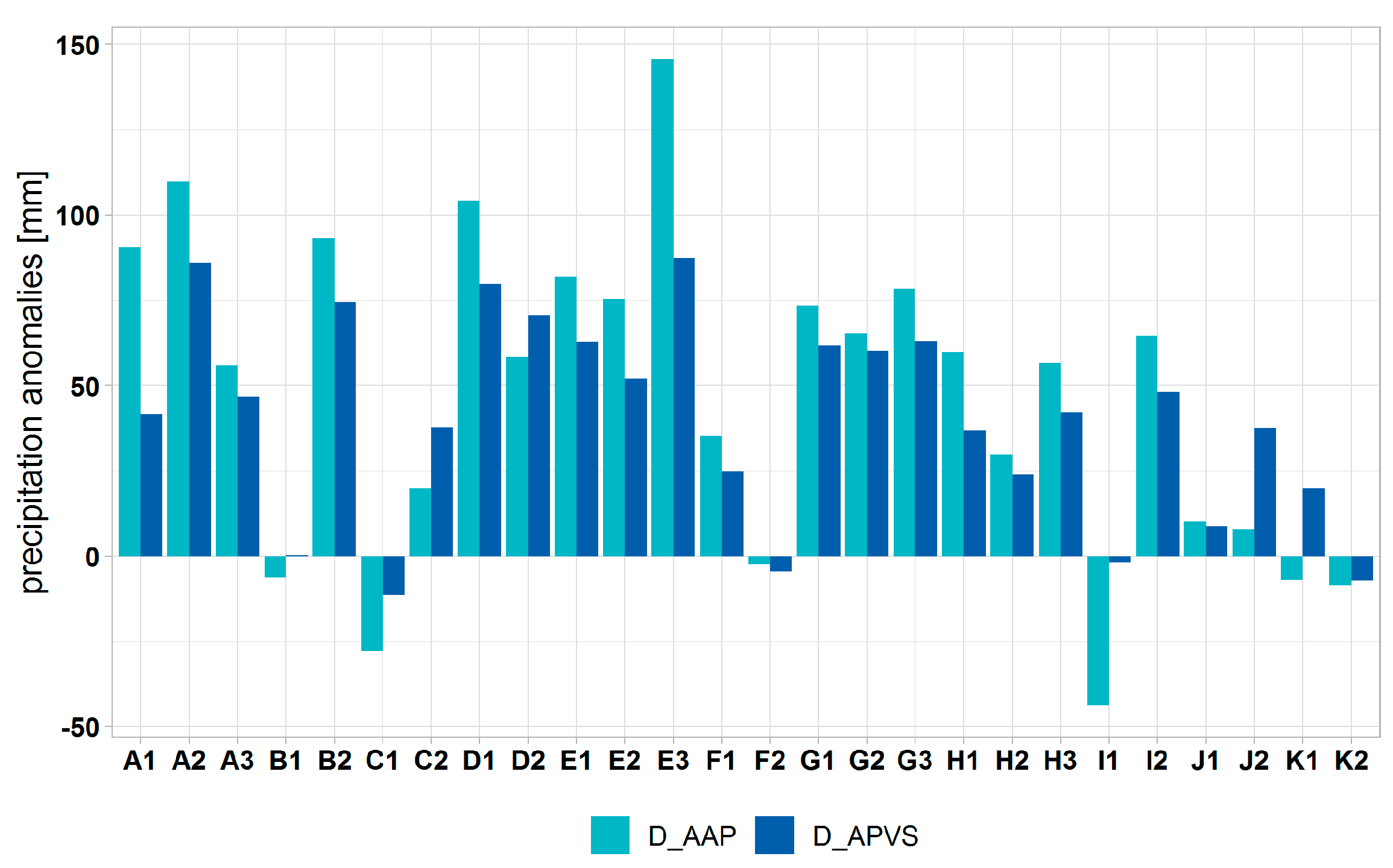
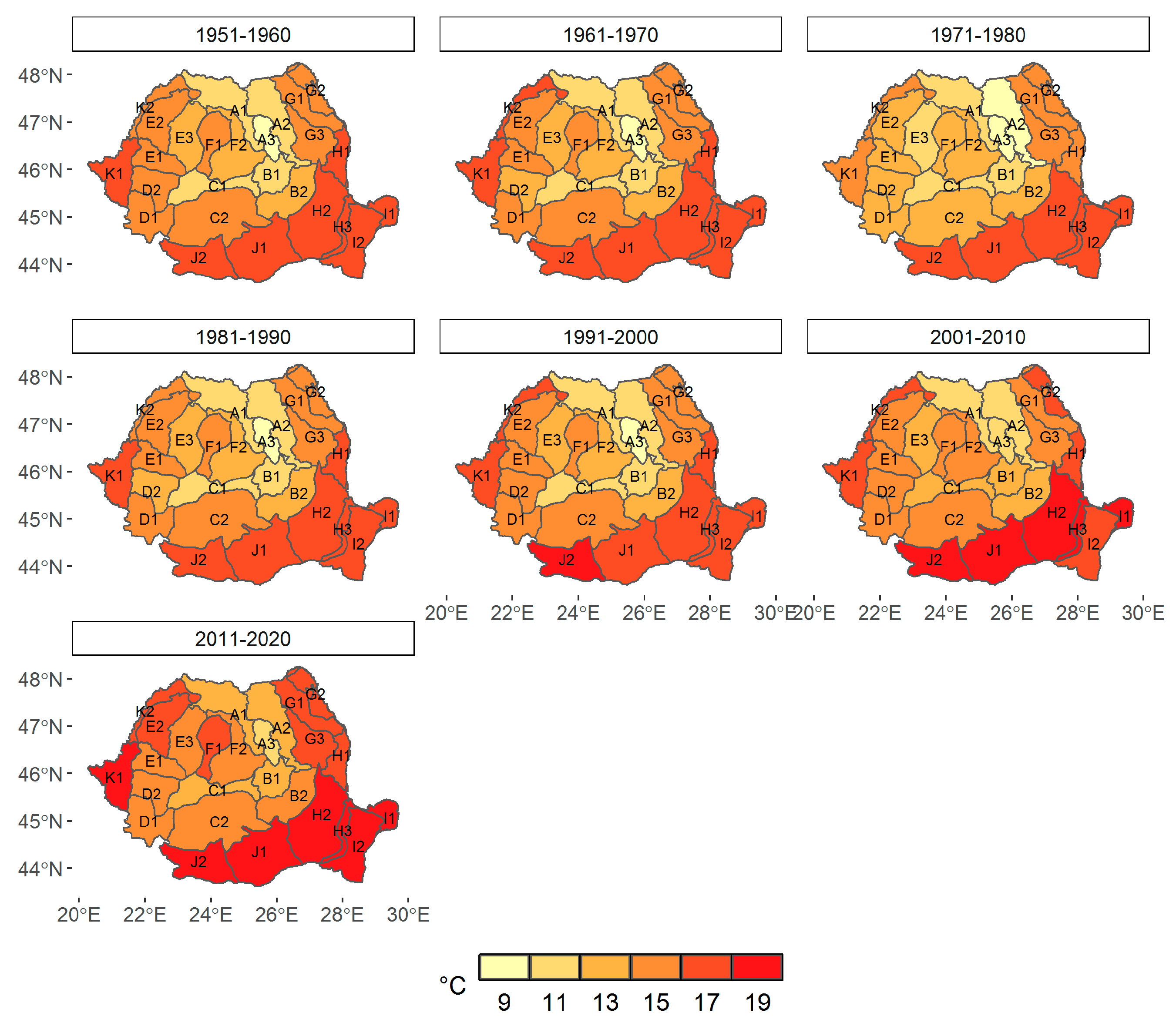
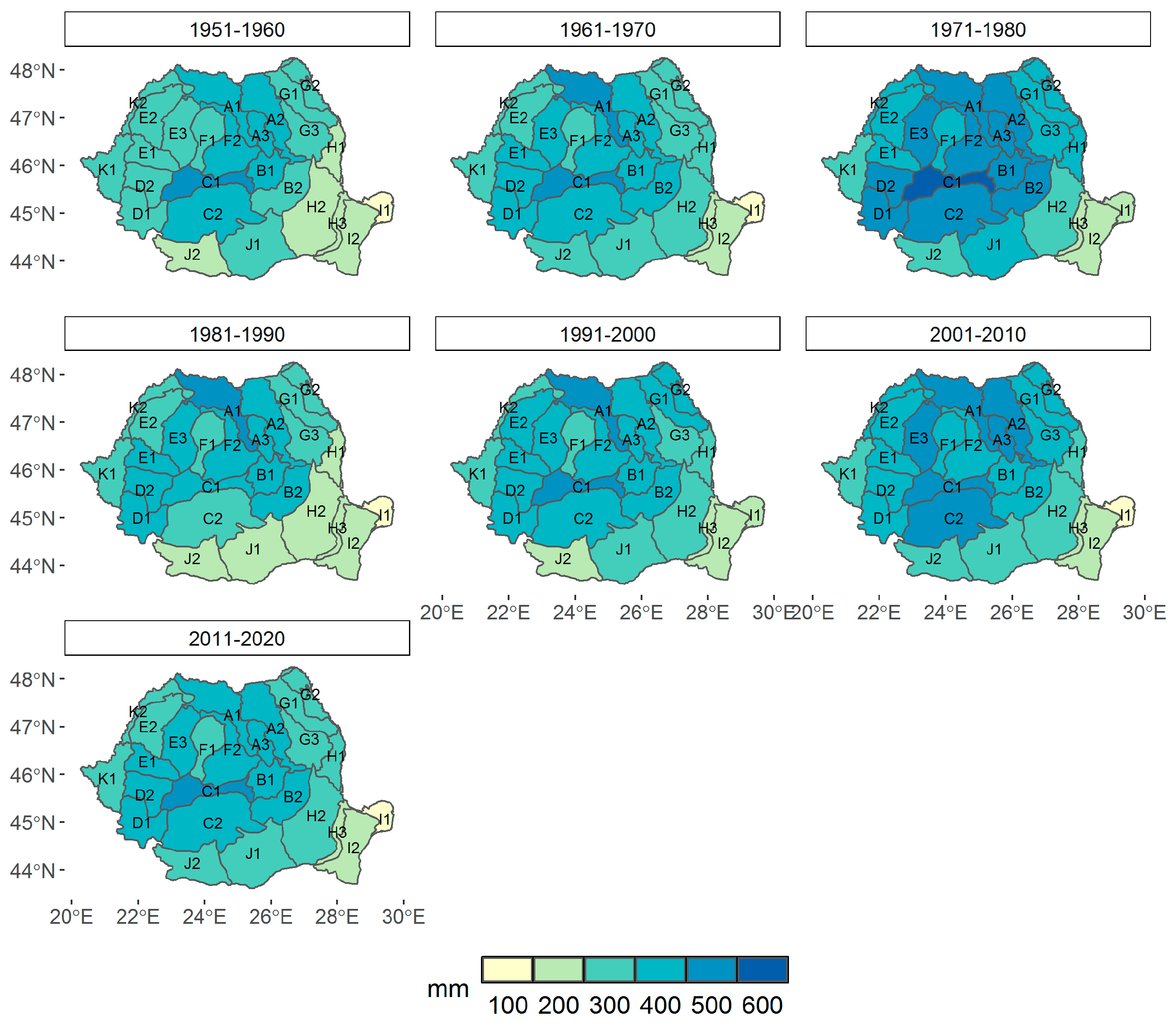
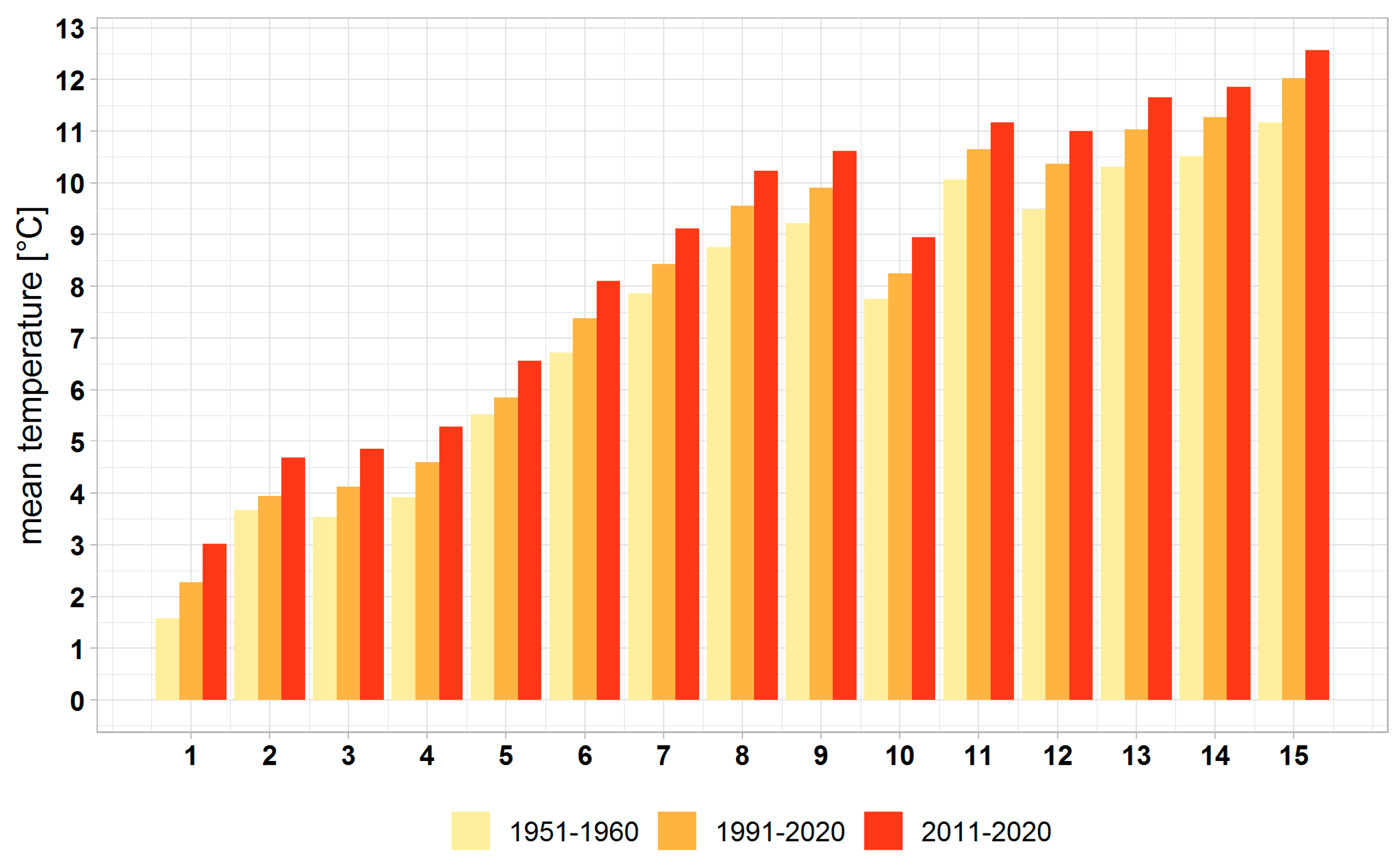
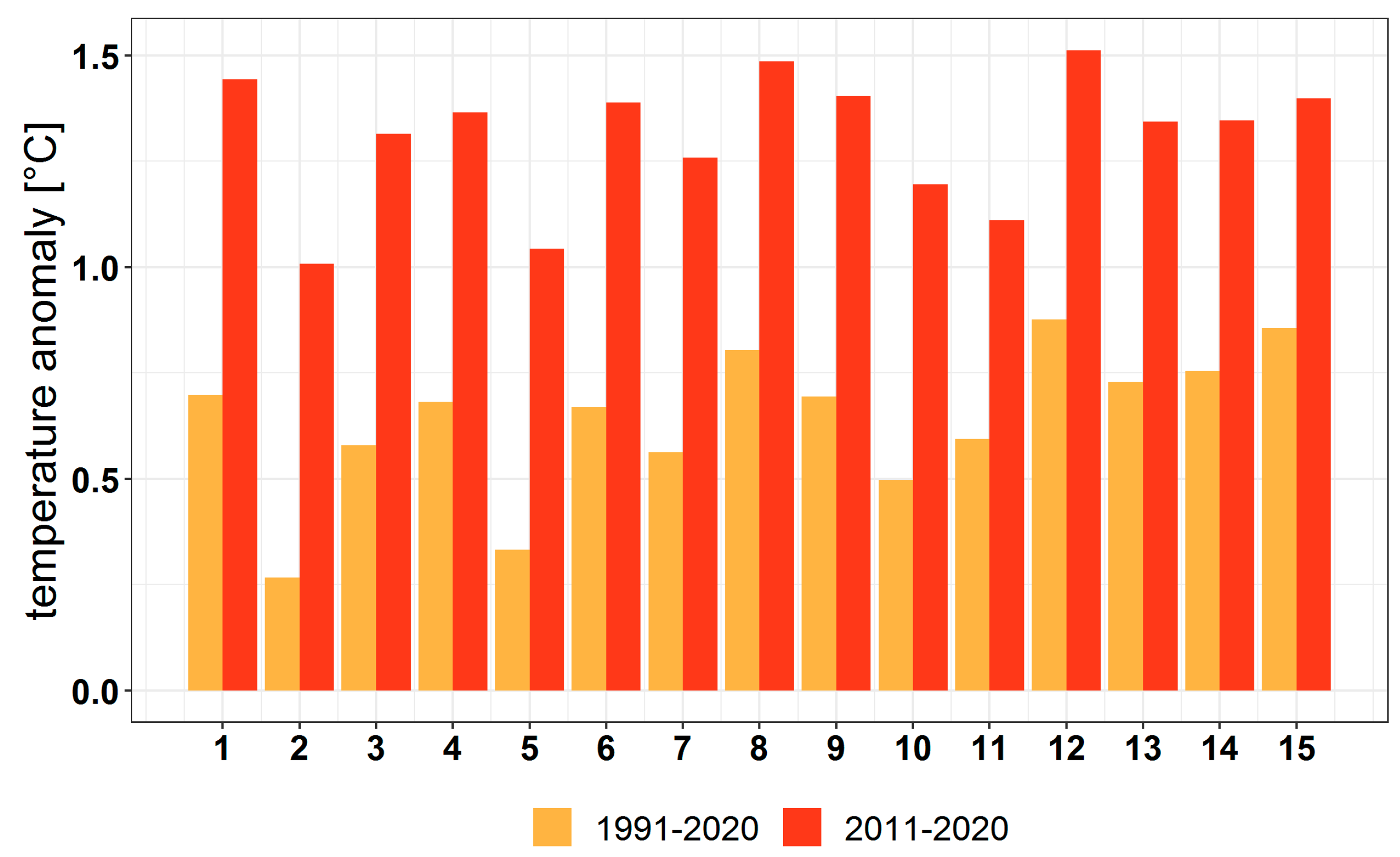
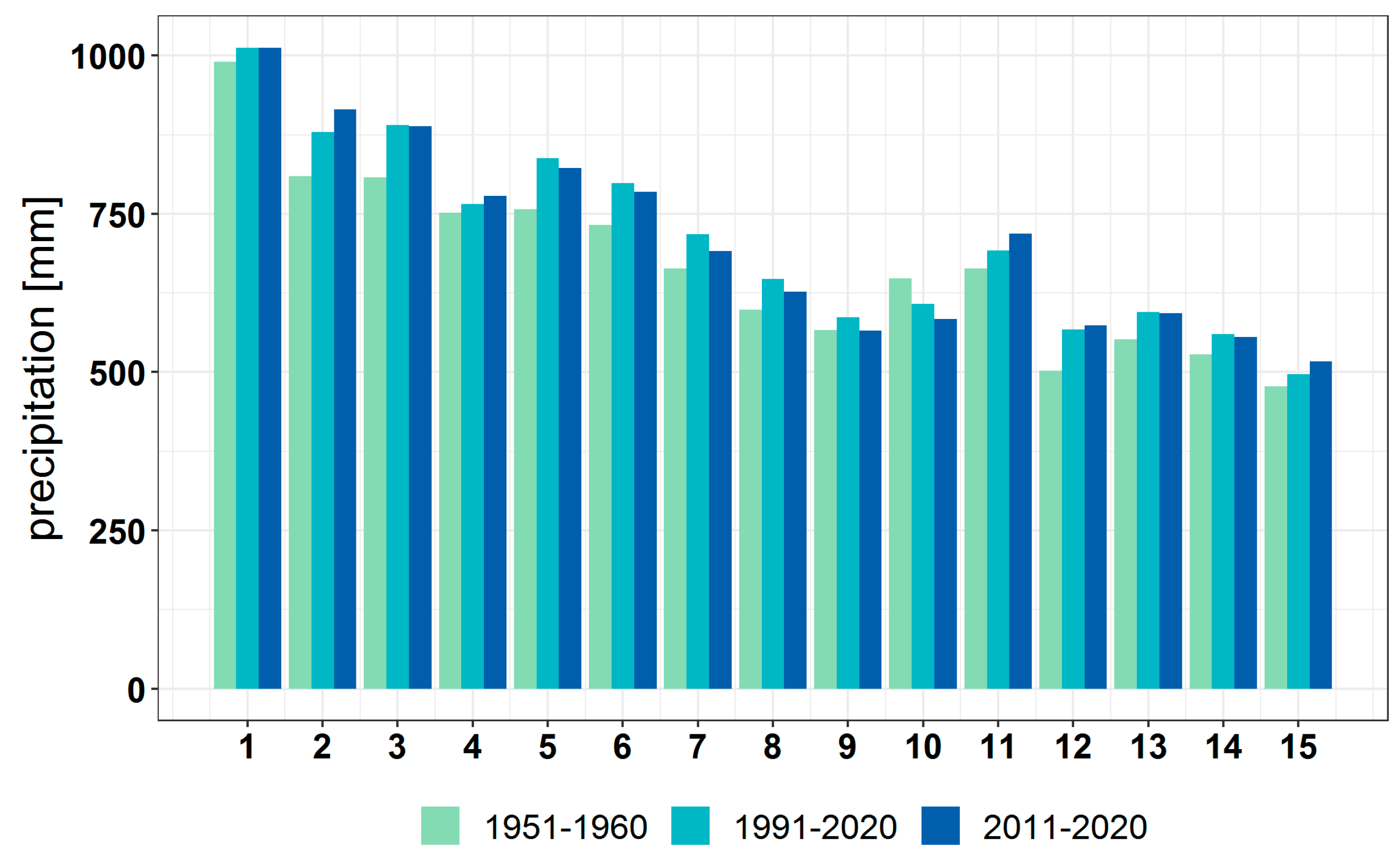
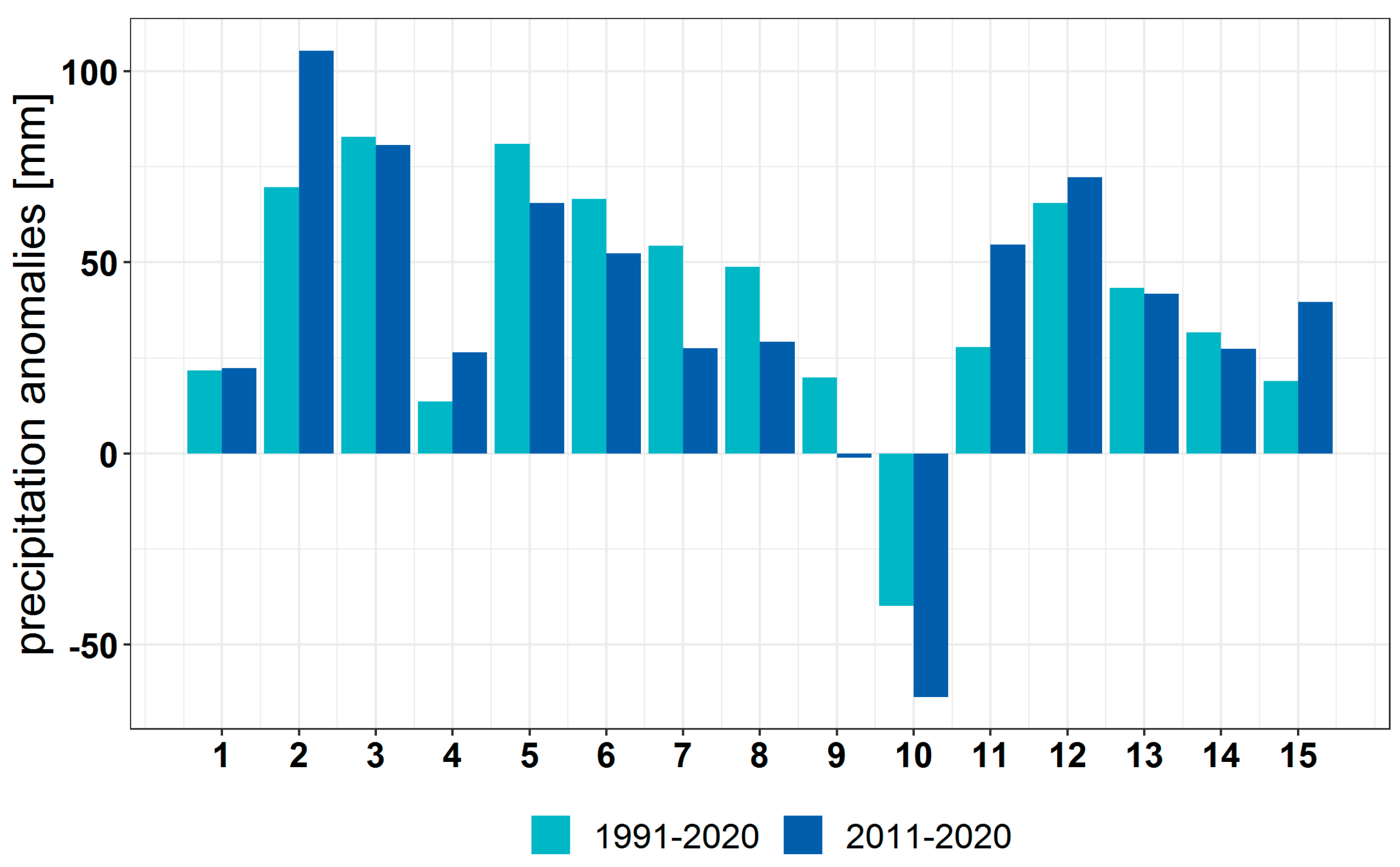
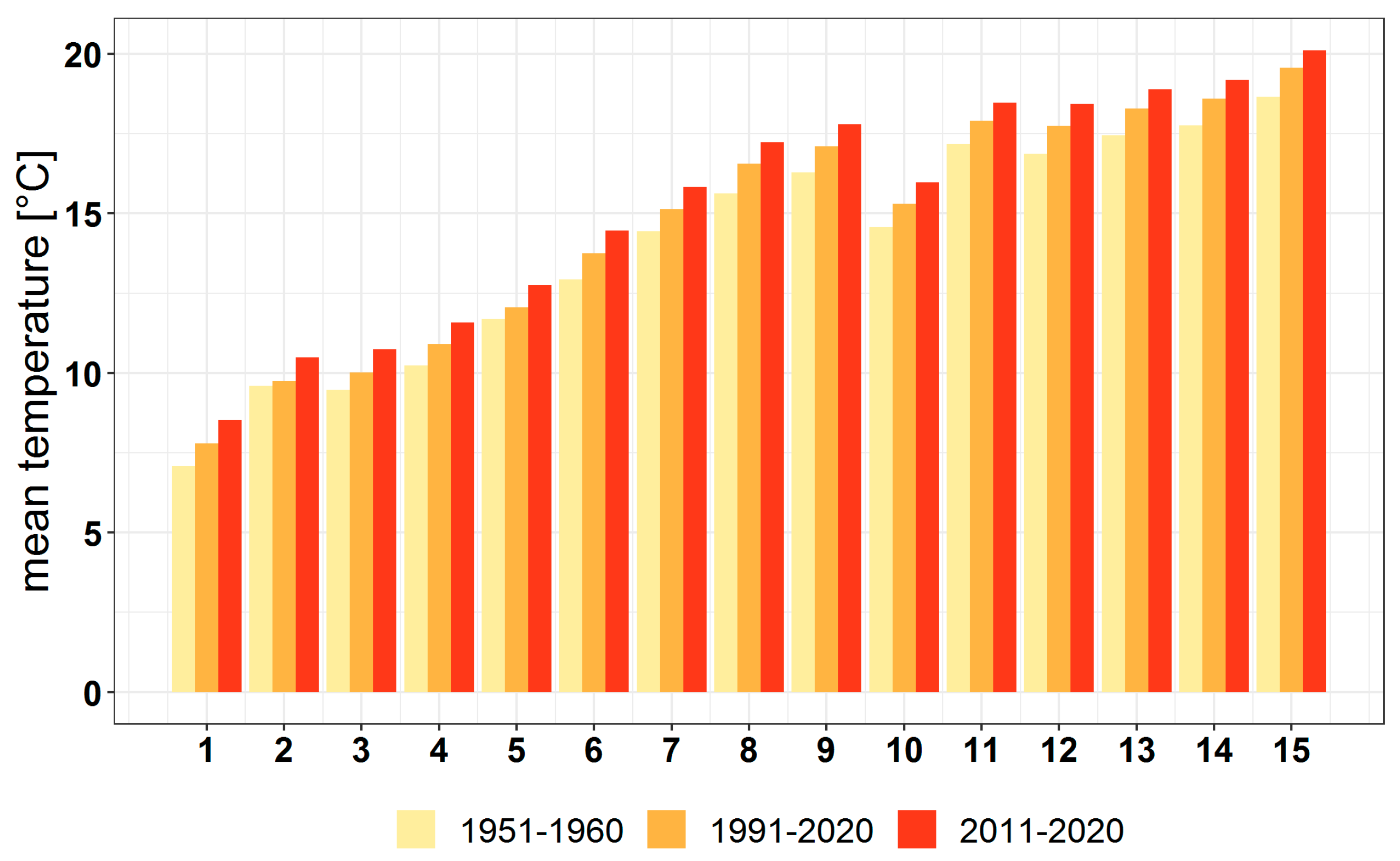
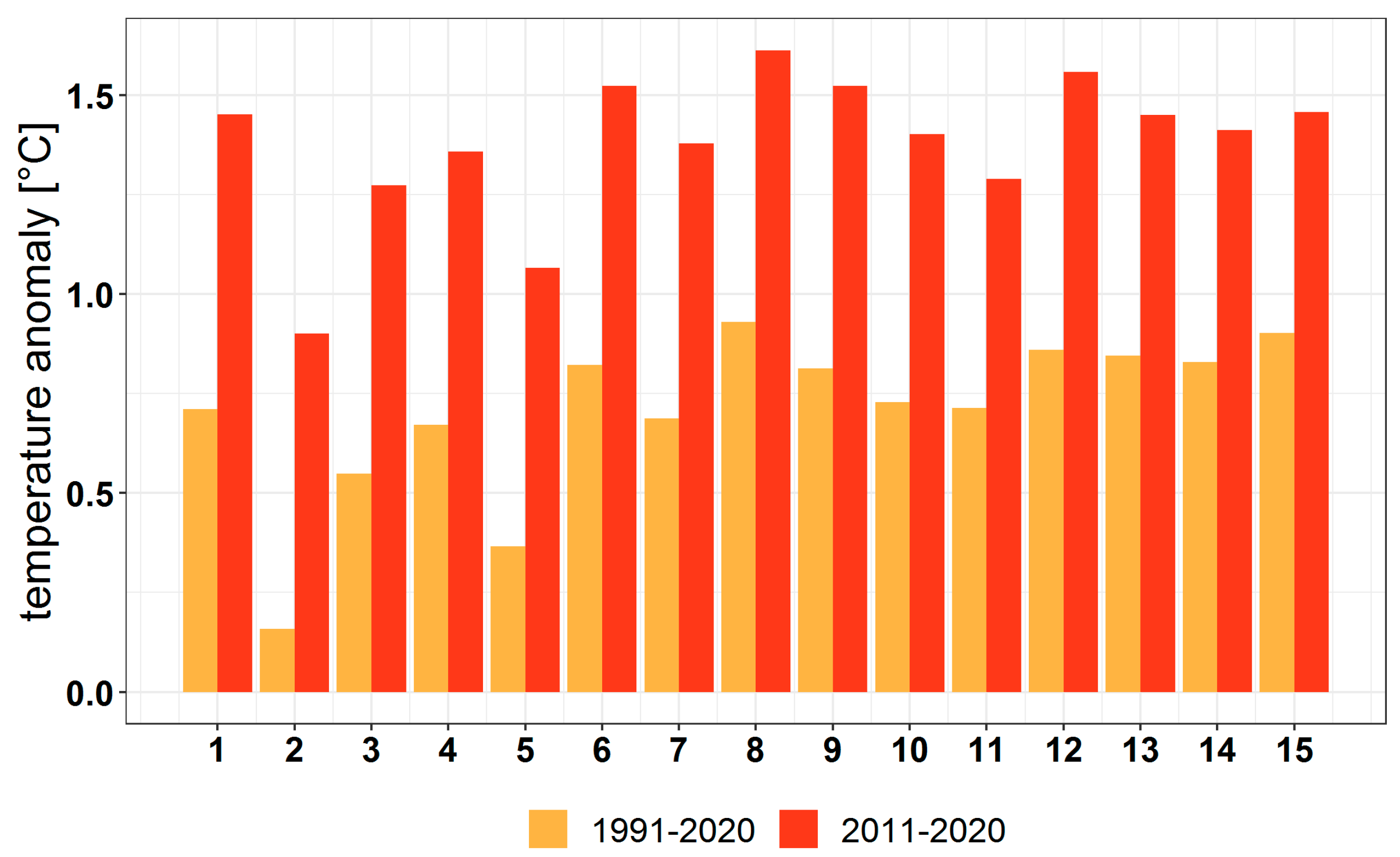
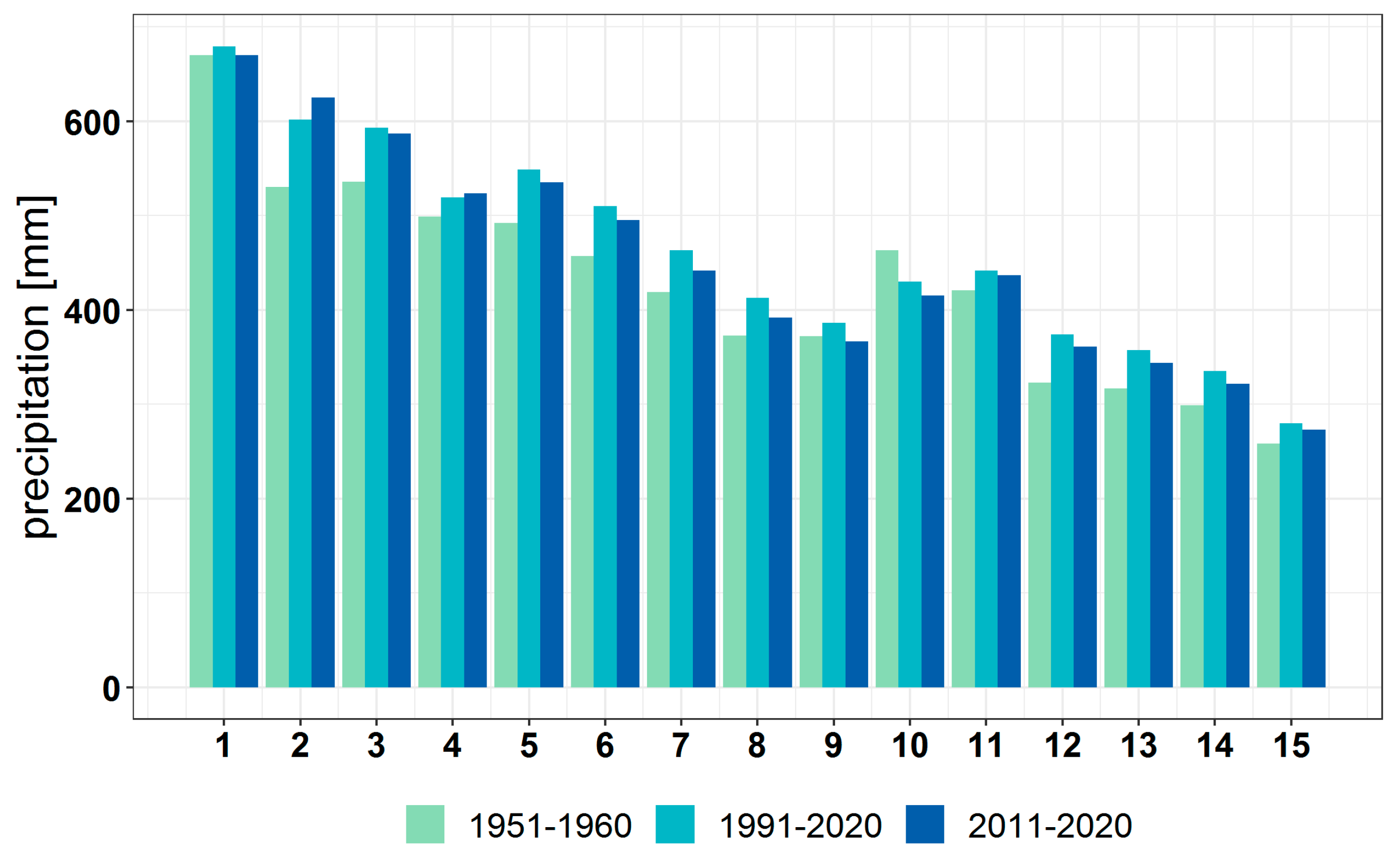
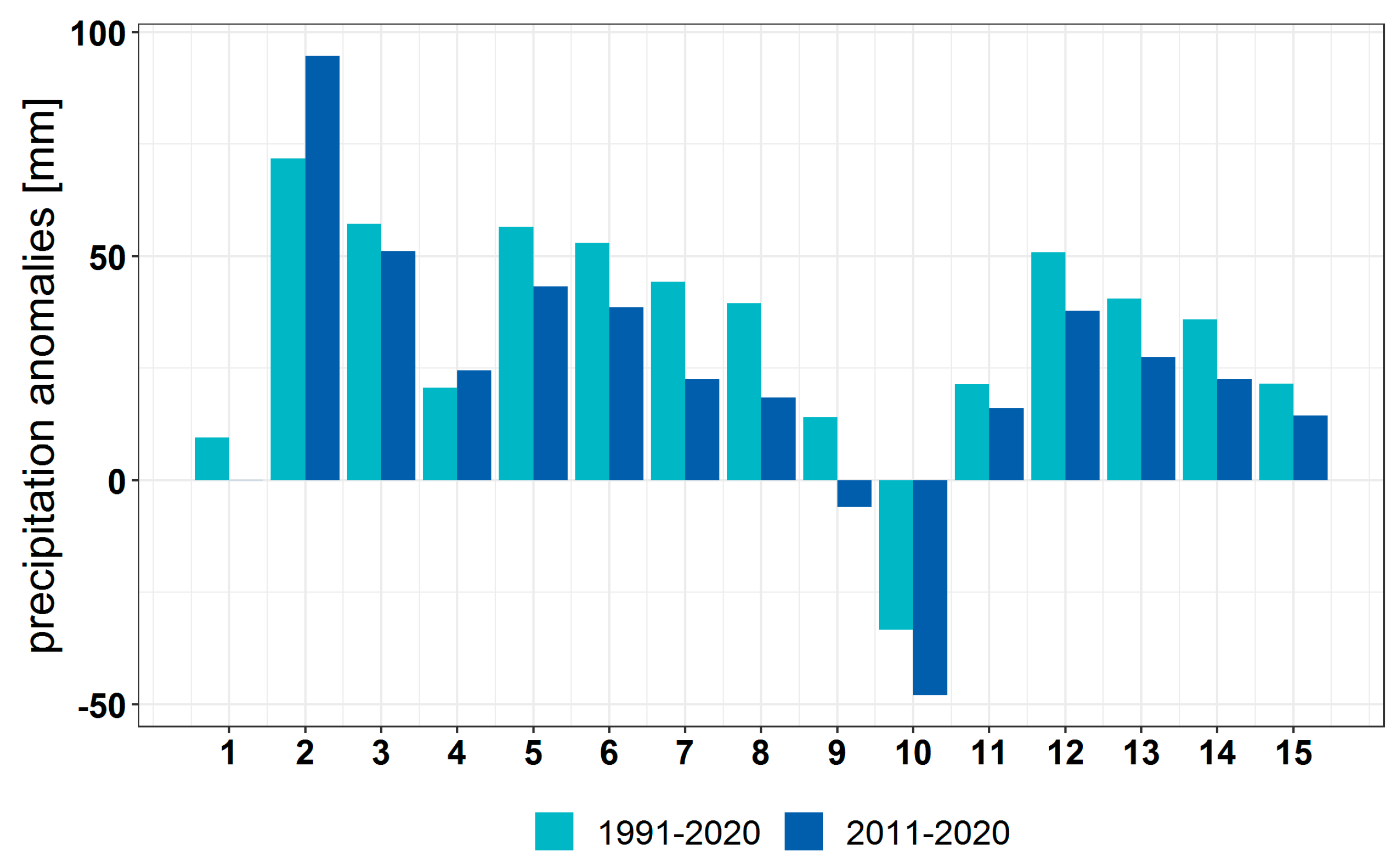
3.1. Climate Change at the Provenance Subregion Level
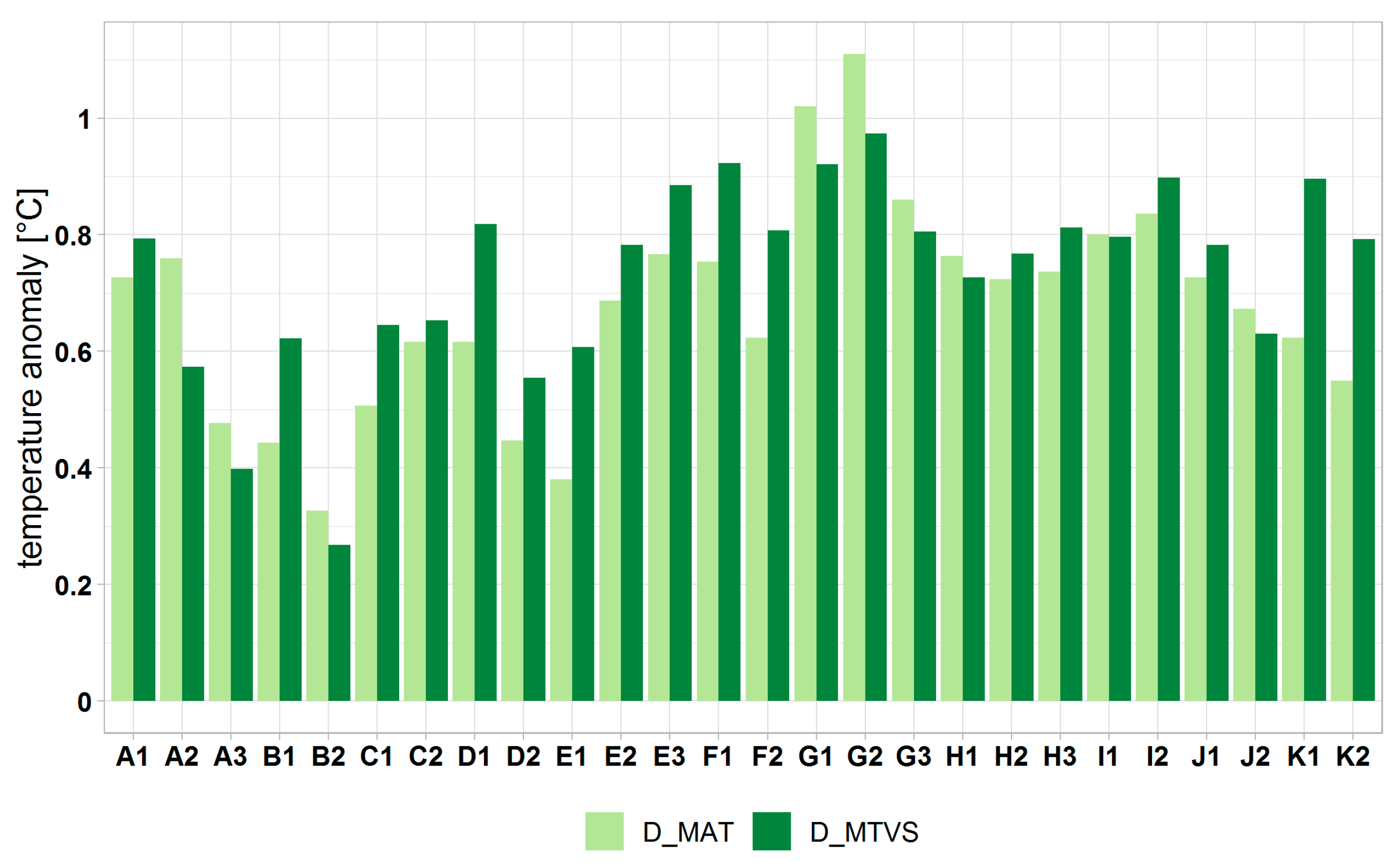
3.2. Climate Change at the Forest-Type Level under Normal Ecological Conditions
3.2.1. Variation in the Main Climatic Parameters at the Forest-Type Level for 1951–2020
3.2.2. Ecoclimatic Index Calculated for Forest Types under Normal Ecological Conditions
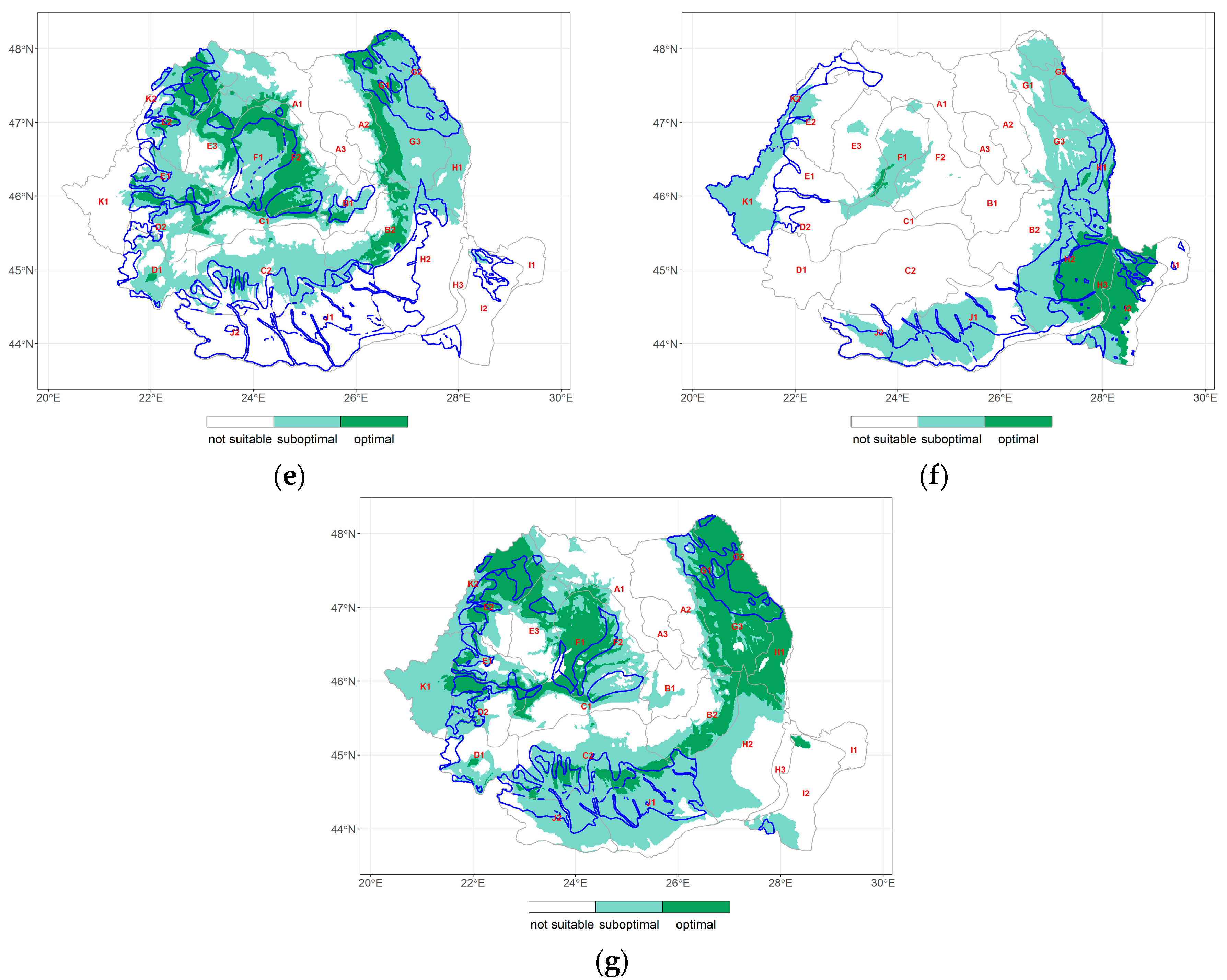
3.3. Climate Change at the Forest-Type Level under Extreme Ecological Conditions
3.3.1. Variation in the Main Climatic Parameters at the Forest-Type Level for 1951–2020
3.3.2. Ecoclimatic Index Calculated for Forest Types under Extreme Ecological Conditions
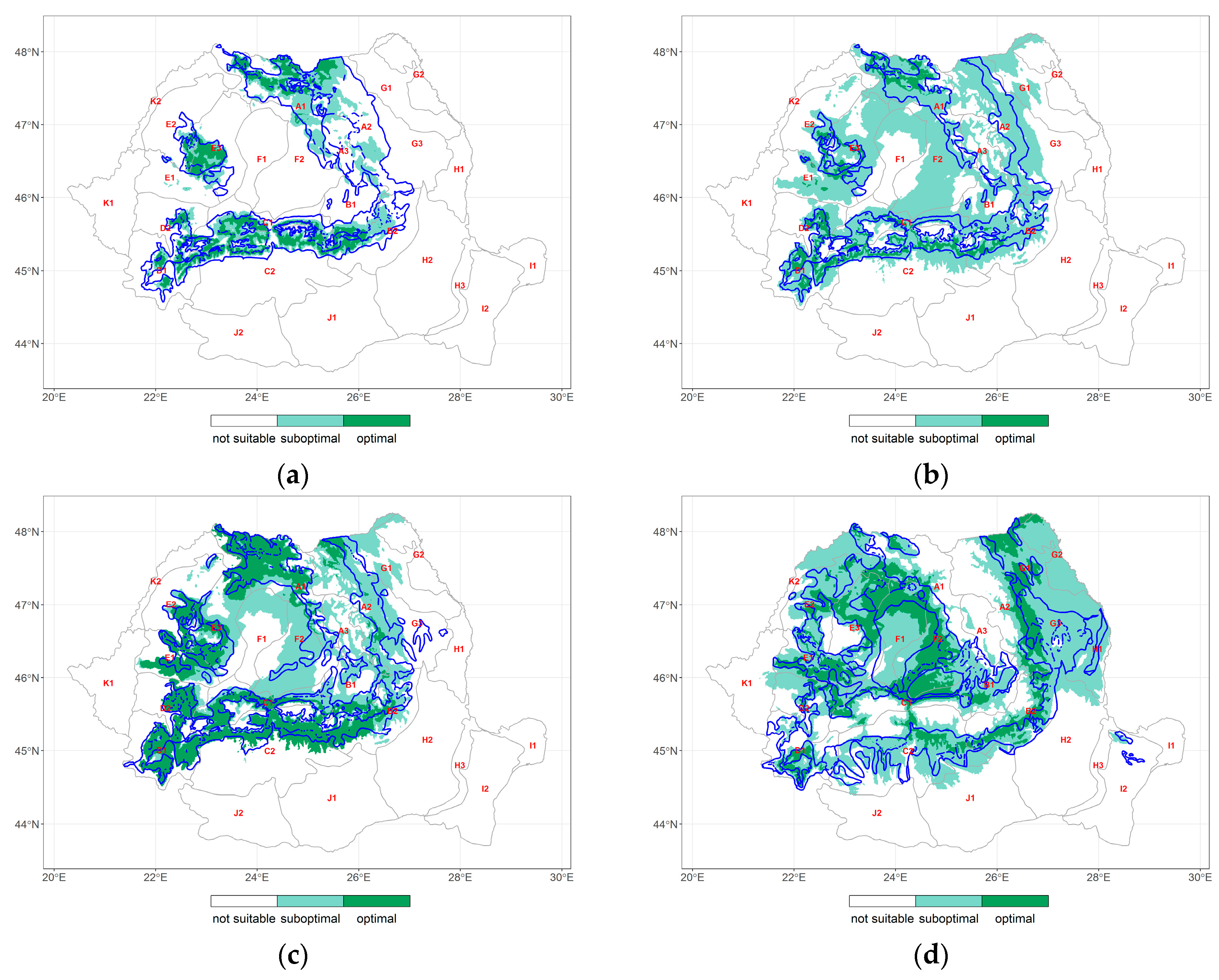
3.4. Changes in the Distribution of Climate Envelope of the Forest Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, G.A.; Aitken, S.N. Area-Based Breeding Zones to Minimize Maladaptation. Can. J. For. Res. 2004, 34, 695–704. [Google Scholar] [CrossRef]
- Ying, C.C.; Yanchuk, A.D. The Development of British Columbia’s Tree Seed Transfer Guidelines: Purpose, Concept, Methodology, and Implementation. For. Ecol. Manag. 2006, 227, 1–13. [Google Scholar] [CrossRef]
- Buiteveld, J.; de Vries, S.M.G.; Kranenborgen, K.G. Delimitations of Regions of Provenance for Forest Reproductive Material inWestern Europe; Alterra-Report; Alterra, Research Instituut voor de Groene Ruimte: Wageningen, The Netherlands, 2000; 45p. [Google Scholar]
- Ducci, F.; Vannuccini, M.; Carone, G.; Vedele, S.; Cili, S.; Apuzzo, S. Delimitations of Regions of Provenance for Forest Reproductive Material in Western Europe. Ann. C.R.A.-SEL 2008, 35, 133–142. [Google Scholar]
- Alía, R.; del Barrio, J.M.G.; Iglesias, S.; Mancha, J.A.; De Miguel, J.; Nicolás, J.; Pérez, F.; de Ron, D.S. Regiones de Procedencia de Especies Forestales En España; MARM: Madrid, Spain, 2009. [Google Scholar]
- Auñon, F.J.; del Barrio, J.M.G.; Mancha, J.A.; de Vries, S.M.G.; Alía, R. Regions of Provenance of European Beech (Fagus Sylvatica L.) in Europe. In Genetic Resources of European Beech (Fagus sylvatica L.) for Sustainable Forestry; Ministerio de Ciencia e Innovacion: Madrid, Spain, 2011; pp. 141–148. [Google Scholar]
- Matyas, C. Modeling Climate Change Effects with Provenance Test Data. Tree Physiol. 1994, 14, 797–804. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Ying, C.C.; Spittlehouse, D.L.; Hamilton, D.A. Genetic Responses to Climate in Pinus Contorta: Niche Breadth, Climate Change, and Reforestation. Ecol. Monogr. 1999, 69, 375–407. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T. Potential Effects of Climate Change on Ecosystem and Tree Species Distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Wang, T.; O’Neill, G.; Aitken, S.N. Integrating Environmentaland Genetic Effects to Predict Responses of Tree Populations Toclimate. Ecol. Appl. 2010, 20, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Hamann, A.; Gylander, T.; Chen, P.Y. Developing Seed Zones and Transfer Guidelines with Multivariate Regression Trees. Tree Genet. Genomes 2011, 7, 399–408. [Google Scholar] [CrossRef]
- Davis, M.; Shaw, R. Range Shifts and Adaptive Responses to Quaternary Climate Change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. Global Controls of Forest Line Elevation in the Northern and Southern Hemispheres. Glob. Ecol. Biogeogr. 2000, 9, 253–268. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H. Diversity and Productivity in Forests: Evidence from Long-Term Experimental Plots. In Forest Diversity and Function. Ecological Studies; Scherer-Lorenzen, M., Körner, C., Schulze, E.D., Eds.; Springer: Berlin, Germany, 2005; Volume 176. [Google Scholar] [CrossRef]
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, I.; Jones, R.; Kolli, R.K.; Kwon, W.T.; Laprise, R.; et al. Regional Climate Projections. In Climate Change 2007: The Physical Science Basis. The Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S.D., Qin, M., Manning, Z., Chen, M., Marquis, K., Averyt, K., Tignor, M., Miller, H., Eds.; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Hamrick, J.L. Response of Forest Trees to Global Environmental Changes. For. Ecol. Manag. 2004, 197, 323–335. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Pulido, F.; Berthold, P. Microevolutionary Response to Climatic Change. Adv. Ecol. Res. 2004, 35, 151–183. [Google Scholar] [CrossRef]
- Kremer, A.; Ronce, O.; Robledo-Arnuncio, J.J.; Guillaume, F.; Bohrer, G.; Nathan, R.; Bridle, J.R.; Gomulkiewicz, R.; Klein, E.K.; Ritland, K.; et al. Long-Distance Gene Flow and Adaptation of Forest Trees to Rapid Climate Change. Ecol. Lett. 2012, 15, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, Migration or Extirpation: Climate Change Outcomes for Tree Populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Vranckx, G.; Jacquemyn, M.; Muys, B.; Honnay, O. Meta Analysis of Susceptibility of Woody Plants to Loss of Genetic Diversity through Habitat Fragmentation. Conservation Biology? J. Soc. Conserv. Biol. 2012, 26, 228–237. [Google Scholar] [CrossRef]
- Aitken, S.N.; Bemmels, J.B. Time to Get Moving: Assisted Gene Flow of Forest Trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef]
- Keenan, R.J. Climate Change Impacts and Adaptation in Forest Management: A Review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate Change and European Forests: What Do We Know, What Are the Uncertainties, and What Are the Implications for Forest Management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- Alistair, S.J.; Josep, P. Running to Stand Still: Adaptation and the Response of Plants to Rapid Climate Change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar]
- Jones, T.A. When Local Isn’t Best. Evol. Appl. 2013, 6, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- De Kort, H.; Mergeay, J.; Vander Mijnsbrugge, K.; Decocq, G.; Maccherini, S.; Kehlet Bruun, H.H.; Honnay, O.; Vandepitte, K. An Evaluation of Seed Zone Delineation Using Phenotypic and Population Genomic Data on Black Alder Alnus Glutinosa. J. Appl. Ecol. 2014, 51, 1218–1227. [Google Scholar] [CrossRef]
- Parnuta, G.; Lorent, A.; Teodoroiu, M.; Petrila, M. Regiuni de Provenienta Pentru Materialele de Baza Din Care Se Obtin Materialele Forestiere de Reproducere Din Romania (Provenance Regions for Basic Materials to Produce Forest Reproductive Material in Romania); Forest Publishing: Bucharest, Romania, 2010. [Google Scholar]
- Enescu, V.; Donita, N.; Bindiu, C.; Contescu, L. Zonele de Recoltare a Semintelor Forestiere in R.S. Romania (Provenance Zones for Harvesting Forest Seeds in Romania); Forest Research and Management Institute: Bucharest, Romania, 1988. [Google Scholar]
- Cheval, S.; Birsan, M.V.; Dumitrescu, A. Climate Variability in the Carpathian Mountains Region over 1961–2010. Glob. Planet. Change 2014, 118, 85–96. [Google Scholar] [CrossRef]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Thomson, A.; Daniel, J.S.; Kainuma, M.L.T.; Matsumoto, K.; Lamarque, J.; Raper, S.C.B.; Riahi, K.; et al. The RCP Greenhouse Gas Concentrations and Their Extensions from 1765 to 2300. Clim. Change 2011, 109, 213–241. [Google Scholar] [CrossRef]
- Spinoni, J.; Szalai, S.; Szentimrey, T.; Lakatos, M.; Bihari, Z.; Nagy, A.; Németh, Á.; Kovács, T.; Mihic, D.; Dacic, M.; et al. Climate of the Carpathian Region in the Period 1961–2010: Climatologies and Trends of 10 Variables. Int. J. Climatol. 2015, 35, 1322–1341. [Google Scholar] [CrossRef]
- Ionita, M.; Scholz, P.; Chelcea, S. Assessment of Droughts in Romania Using the Standardized Precipitation Index. Nat. Hazards 2016, 81, 1483–1498. [Google Scholar] [CrossRef]
- Birsan, M.V.; Dumitrescu, A.; Micu, D.M.; Cheval, S. Changes in Annual Temperature Extremes in the Carpathians since AD 1961. Nat. Hazards 2014, 74, 1899–1910. [Google Scholar] [CrossRef]
- Birsan, M.-V.; Micu, D.-M.; Niţă, I.-A.; Mateescu, E.; Szép, R.; Keresztesi, Á. Spatio-Temporal Changes in Annual Temperature Extremes over Romania (1961–2013). Rom. J. Phys. 2019, 64, 816. [Google Scholar]
- Busuioc, A.; Dobrinescu, A.; Birsan, M.V.; Dumitrescu, A.; Orzan, A. Spatial and Temporal Variability of Climate Extremes in Romania and Associated Large-Scale Mechanisms. Int. J. Climatol. 2015, 35, 1278–1300. [Google Scholar] [CrossRef]
- Birsan, M.V.; Dumitrescu, A. Snow Variability in Romania in Connection to Large-Scale Atmospheric Circulation. Int. J. Climatol. 2014, 34, 134–144. [Google Scholar] [CrossRef]
- Micu, D.M.; Dumitrescu, A.; Cheval, S.; Nita, I.A.; Birsan, M.V. Temperature Changes and Elevation-Warming Relationships in the Carpathian Mountains. Int. J. Climatol. 2021, 41, 2154–2172. [Google Scholar] [CrossRef]
- Birsan, M.V.; Marin, L.; Dumitrescu, A. Seasonal Changes in Wind Speed in Romania. Rom. Rep. Phys. 2013, 65, 1479–1484. [Google Scholar]
- Birsan, M.V.; Nita, I.-A.; Craciun, A.; Sfica, L.; Keresztesi, Á.; Szep, R.; Micheu, M. Observed Changes in Mean and Maximum Monthly Wind Speed over Romania since Ad 1961. Rom. Rep. Phys. 2020, 72, 702. [Google Scholar]
- Busuioc, A.; Birsan, M.V.; Carbunaru, D.; Baciu, M.; Orzan, A. Changes in the Large-Scale Thermodynamic Instability and Connection with Rain Shower Frequency over Romania: Verification of the Clausius-Clapeyron Scaling. Int. J. Climatol. 2016, 36, 2015–2034. [Google Scholar] [CrossRef]
- Manea, A.; Birsan, M.V.; Tudorache, G.; Cărbunaru, F. Changes in the Type of Precipitation and Associated Cloud Types in Eastern Romania (1961–2008). Atmos. Res. 2016, 169, 357–365. [Google Scholar] [CrossRef]
- Nita, I.A.; Sfîcă, L.; Apostol, L.; Radu, C.; Birsan, M.V.; Szep, R.; Keresztesi, Á. Changes in Cyclone Intensity over Romania According to 12 Tracking Methods. Rom. Rep. Phys. 2020, 72, 706. [Google Scholar]
- Nita, I.-A.; Apostol, L.; Patriche, C.; Sfica, L.; Bojariu, R.; Birsan, M.-V. Frequency of Atmospheric Circulation Types over Romania According to Jenkinson-Collison Method Based on Two Long-Term Reanalysis Datasets. Rom. J. Phys. 2022, 67, 812. [Google Scholar]
- Țîmpu, S.; Sfîcă, L.; Dobri, R.-V.; Cazacu, M.-M.; Nita, A.-I.; Birsan, M.-V. Tropospheric Dust and Associated Atmospheric Circulations over the Mediterranean Region with Focus on Romania’s Territory. Atmosphere 2020, 11, 349. [Google Scholar] [CrossRef]
- Cheval, S.; Busuioc, A.; Dumitrescu, A.; Birsan, M.V. Spatiotemporal Variability of Meteorological Drought in Romania Using the Standardized Precipitation Index (SPI). Clim. Res. 2014, 60, 235–248. [Google Scholar] [CrossRef]
- Cheval, S.; Dumitrescu, A.; Birsan, M.V. Variability of the Aridity in the South-Eastern Europe over 1961–2050. Catena 2017, 151, 74–86. [Google Scholar] [CrossRef]
- Birsan, M.V. Trends in Monthly Natural Streamflow in Romania and Linkages to Atmospheric Circulation in the North Atlantic. Water Resour. Manag. 2015, 29, 3305–3313. [Google Scholar] [CrossRef]
- Micheu, M.M.; Birsan, M.V.; Szép, R.; Keresztesi, Á.; Nita, I.A. From Air Pollution to Cardiovascular Diseases: The Emerging Role of Epigenetics. Mol. Biol. Rep. 2020, 47, 5559–5567. [Google Scholar] [CrossRef]
- Micheu, M.M.; Birsan, M.V.; Nita, I.A.; Andrei, M.D.; Nebunu, D.; Acatrinei, C.; Sfîcă, L.; Szép, R.; Keresztesi, Á.; Hernáez, P.F.D.A.; et al. Influence of Meteorological Variables on People with Cardiovascular Diseases in Bucharest, Romania (2011–2012). Rom. Rep. Phys. 2021, 73, 707. [Google Scholar]
- Mihai, G.; Birsan, M.V.; Dumitrescu, A.; Alexandru, A.; Mirancea, I.; Ivanov, P.; Stuparu, E.; Teodosiu, M.; Daia, M. Adaptive Genetic Potential of European Silver Fir in Romania in the Context of Climate Change. Ann. For. Res. 2018, 61, 95–108. [Google Scholar] [CrossRef]
- Mihai, G.; Alexandru, A.M.; Stoica, E.; Birsan, M.V. Intraspecific Growth Response to Drought of Abies Alba in the Southeastern Carpathians. Forests 2021, 12, 387. [Google Scholar] [CrossRef]
- Donita, N. Elaborarea Hartii Forestiere a Romaniei La Scara 1:500,000 (Development of the Romanian Forest Map at 1:500,000 Scale); Forest Research and Management Institute: Bucharest, Romania, 1996. [Google Scholar]
- Dumitrescu, A.; Birsan, M.V. ROCADA: A Gridded Daily Climatic Dataset over Romania (1961–2013) for Nine Meteorological Variables. Nat. Hazards 2015, 78, 1045–1063. [Google Scholar] [CrossRef]
- Vlăduţ, A.Ş.; Nikolova, N.; Licurici, M. Influence of Climatic Conditions on the Territorial Distribution of the Main Vegetation Zones within Oltenia Region, Romania. Olten. Stud. Comun. Stiintele Nat. 2017, 33, 154–164. [Google Scholar]
- WMO/GWP Integrated Drought Management Programme (IDMP). Handbook of Drought Indicators and Indices; WMO-No. 1173; World Meteorological Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Ellenberg, H. Vegetation Mitteleuropas Mit Den Alpen; Eugen Ulmer: Stuttgart, Germany, 1963. [Google Scholar]
- Sofletea, N.; Curtu, L. Dendrologie; Editura Pentru Viata: Brasov, Romania, 2001. [Google Scholar]
- IPCC. Climate Change 2001: Mitigation. Contribution of Working Group III to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Dumitrescu, A.; Bojariu, R.; Birsan, M.V.; Marin, L.; Manea, A. Recent Climatic Changes in Romania from Observational Data (1961–2013). Theor. Appl. Climatol. 2015, 122, 111–119. [Google Scholar] [CrossRef]
- Savolainen, O.; Pyhäjärvi, T.; Knürr, T. Gene Flow and Local Adaptation in Trees. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Pluess, A.R.; Frank, A.; Heiri, C.; Lalagüe, H.; Vendramin, G.G.; Oddou-Muratorio, S. Genome-Environment Association Study Suggests Local Adaptation to Climate at the Regional Scale in Fagus Sylvatica. New Phytol. 2016, 210, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Mihai, G. Surse de Seminţe Testate Pentru Principalele Specii de Arbori Forestieri Din România [Tested Seed Sources for the Main Forest Tree Species from Romania]; Editura Silvică: Bucharest, Romania, 2009. [Google Scholar]
- Şofletea, N.; Curtu, A.L.; Daia, M.L.; Budeanu, M. The Dynamics and Variability of Radial Growth in Provenance Trials of Norway Spruce (Picea Abies (L.) Karst.) within and beyond the Hot Margins of Its Natural Range. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 265–271. [Google Scholar] [CrossRef][Green Version]
- Mihai, G.; Mirancea, I.; Birsan, M.V.; Dumitrescu, A. Patterns of Genetic Variation in Bud Flushing of Abies Alba Populations. IForest 2018, 11, 284–290. [Google Scholar] [CrossRef]
- Mihai, G.; Teodosiu, M.; Birsan, M.V.; Alexandru, A.M.; Mirancea, I.; Apostol, E.N.; Garbacea, P.; Ionita, L. Impact of Climate Change and Adaptive Genetic Potential of Norway Spruce at the South–Eastern Range of Species Distribution. Agric. For. Meteorol. 2020, 291, 108040. [Google Scholar] [CrossRef]
- Worrell, R. A Comparison between European Continental and British Provenances of Some British Native Trees: Growth, Survival and Stem Form. Forestry 1992, 65, 253–280. [Google Scholar] [CrossRef]
- Pâques, L.E. Forest Tree Breeding in Europe; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Whittet, R.; Cavers, S.; Ennos, R.; Cottrell, J. Genetic Considerations for Provenance Choice of Native Trees under Climate Change in England; Forestry Commission: Edinburgh, UK, 2019; pp. 1–56. [Google Scholar]
- Matyas, C. Guidelines for the Choice of Forest Reproductive Material in the Face of Climate Change; FORGER Guidelines; Bioversity International: Rome, Italy, 2016; 8p. [Google Scholar]
- Reed, D.H.; Frankham, R. Correlation between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Leimu, R.; Mutikainen, P.; Koricheva, J.; Fischer, M. How General Are Positive Relationships between Plant Population Size, Fitness and Genetic Variation? J. Ecol. 2006, 94, 942–952. [Google Scholar] [CrossRef]
- Young, A.; Boyle, T.; Brown, T. The Population Genetic Consequences of Habitat Fragmentation for Plants. Trends Ecol. Evol. 1996, 11, 413–418. [Google Scholar] [CrossRef]
- O’Neill, G.A.; Hamann, A.; Wang, T. Accounting for Population Variation Improves Estimates of the Impact of Climate Change on Species’ Growth and Distribution. J. Appl. Ecol. 2008, 45, 1040–1049. [Google Scholar] [CrossRef]
- Pedlar, J.H.; McKenney, D.W.; Aubin, I.; Beardmore, T.; Beaulieu, J.; Iverson, L.; O’Neill, G.A.; Winder, R.S.; Ste-Marie, C. Placing Forestry in the Assisted Migration Debate. Bioscience 2012, 62, 835–842. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for Climate Change: Forestry and Assisted Migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Spittlehouse, D.L.; Stewart, R.B. Adaptation to Climate Change in Forest Management. BC J. Ecosyst. Manag. 2003, 4, 1–7. [Google Scholar]
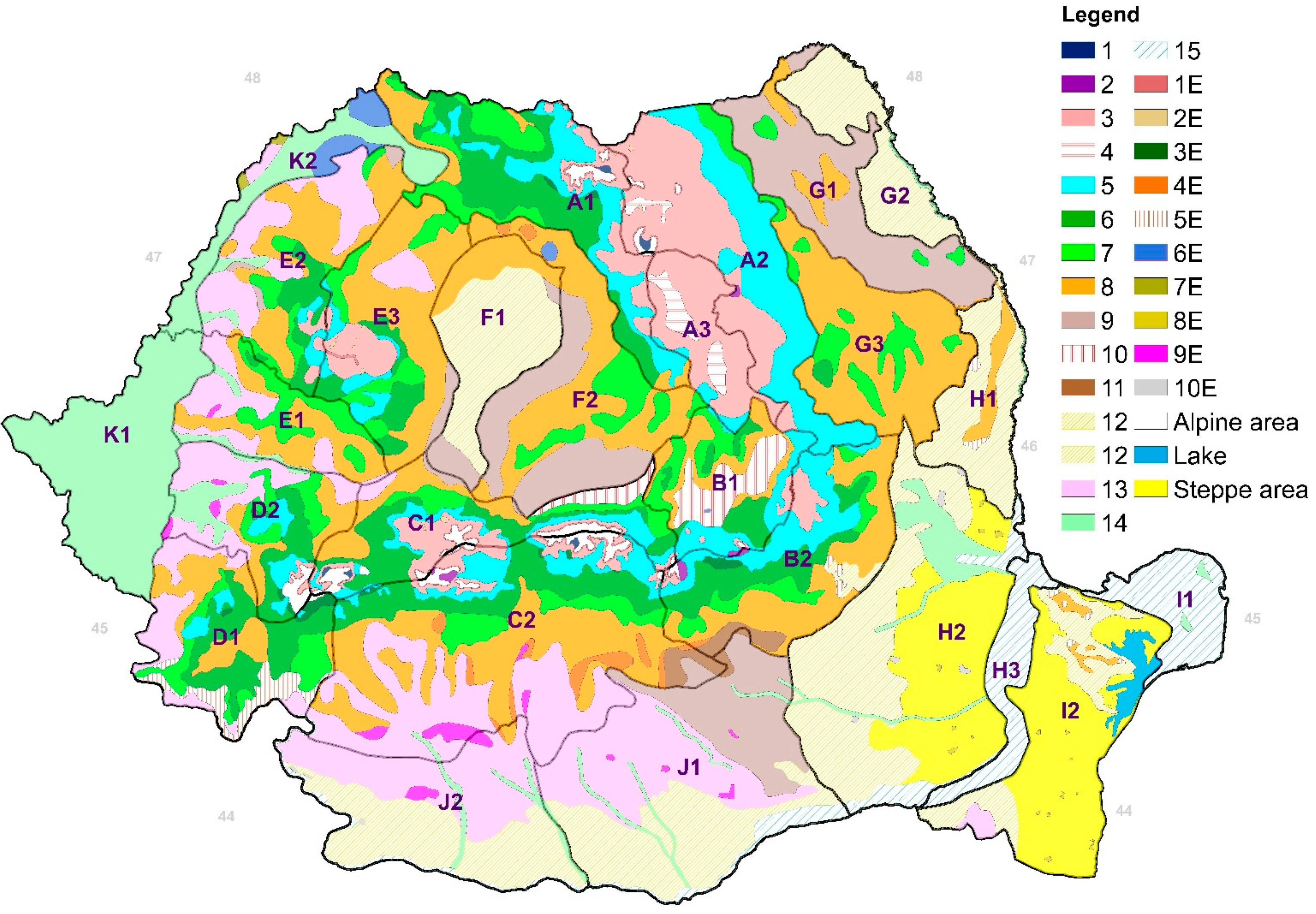
| Values of DMAI | Type of Climate | Vegetation |
|---|---|---|
| 5–10 | Arid | Desert |
| 11–25 | Semiarid | Steppe |
| 26–30 | Moderate arid | Steppe |
| 31–35 | Semihumid | Forest steppe |
| 36–40 | Moderate humid | Oak forests |
| 41–45 | Humid | Beech forests |
| 46–50 | Coniferous forests | |
| 51–55 | Very humid | Subalpine |
| 56–60 | Alpine | |
| >60 | Extremely humid |
| Values of LRI | Type of Climate |
|---|---|
| >160 | Wet |
| 160–100 | Temperate wet |
| 100–60 | Temperate warm |
| 60–40 | Semiarid |
| 40–20 | Arid |
| 20–0 | Desert |
| Code | Subregion | Code | Subregion |
|---|---|---|---|
| A1 | Eastern Carpathians, western cline | F2 | Transylvania Plateau |
| A2 | Eastern Carpathians, eastern cline | G1 | Suceva/Siret/Iasi Hills |
| A3 | Giurgeu–Ciuc depression | G2 | Jijia Plain |
| B1 | Brasov depression | G3 | Barlad Plateau |
| B2 | Curvature Carpathians, outer cline | H1 | Covur Plateau |
| C1 | Southern Carpathians, northern cline | H2 | Siret and Baragan Plains |
| C2 | Southern Carpathians, southern cline | H3 | Danube water holes |
| D1 | Mehedinti/Cerna/Semenic Mountains | I1 | Danube Delta |
| D2 | Tarcu/Poiana Rusca Mountains | I2 | Dobrogea Plateau |
| E1 | Zarand/Metaliferi Mountains | J1 | Bucharest Plain |
| E2 | Western Apuseni Mountains | J2 | Oltenia Plain |
| E3 | Eastern Apuseni Mountains | K1 | Timis and Arad Plain |
| F1 | Transylvania Plain | K2 | Cris/Carei/Somes Plain |
| Type Code | Extreme Ecological Conditions | Type Code | Normal Ecological Conditions |
|---|---|---|---|
| 1E | Norway spruce or mixed with other species at upper altitudinal limit | 1 | Swiss pine and mixed with Norway spruce |
| 2E | Norway spruce on marshland | 2 | Larch and mixed larch and Norway spruce |
| 3E | Beech at upper altitudinal limit | 3 | Norway spruce |
| 4E | Sessile and pedunculate oak forests on pseudo-gleyic soils | 4 | Norway spruce in mountain depression |
| 5E | Thermophile sessile oak and mixed with other broadleaf species | 5 | Mixed beech and coniferous species |
| 6E | Pedunculate oak on marshland | 6 | Mountain beech |
| 7E | Thermophile pedunculate oak on sand | 7 | Hilly pure beech and mixed beech with broadleaf species |
| 8E | Quercus pedunculiflora on sand | 8 | Pure sessile oak and mixed with broadleaf species |
| 9E | Turkey oak and Hungarian oak forests on pseudo-gleyic soils | 9 | Pure pedunculate oak and mixed with other oaks species |
| 10E | Steppe island forests | 10 | Pedunculate oak in depression |
| 11 | Pedunculate oak on piedmont | ||
| 12 | Thermophile oak species | ||
| 13 | Turkey oak and Hungarian oak forests | ||
| 14 | Meadow forests (alder, ash, oak) | ||
| 15 | Poplar and willow forest |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, G.; Alexandru, A.-M.; Nita, I.-A.; Birsan, M.-V. Climate Change in the Provenance Regions of Romania over the Last 70 Years: Implications for Forest Management. Forests 2022, 13, 1203. https://doi.org/10.3390/f13081203
Mihai G, Alexandru A-M, Nita I-A, Birsan M-V. Climate Change in the Provenance Regions of Romania over the Last 70 Years: Implications for Forest Management. Forests. 2022; 13(8):1203. https://doi.org/10.3390/f13081203
Chicago/Turabian StyleMihai, Georgeta, Alin-Madalin Alexandru, Ion-Andrei Nita, and Marius-Victor Birsan. 2022. "Climate Change in the Provenance Regions of Romania over the Last 70 Years: Implications for Forest Management" Forests 13, no. 8: 1203. https://doi.org/10.3390/f13081203
APA StyleMihai, G., Alexandru, A.-M., Nita, I.-A., & Birsan, M.-V. (2022). Climate Change in the Provenance Regions of Romania over the Last 70 Years: Implications for Forest Management. Forests, 13(8), 1203. https://doi.org/10.3390/f13081203






