Culturable Endophytic Fungi in Fraxinus excelsior and Their Interactions with Hymenoscyphus fraxineus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Cultures
2.2. Study Sites and Sampling
2.3. Isolation of Fungal Endophytes
2.4. Molecular Identification of Endophytes
2.5. Dual Culture Bioassay
2.6. Field Bioassay
2.7. Data Analysis
3. Results
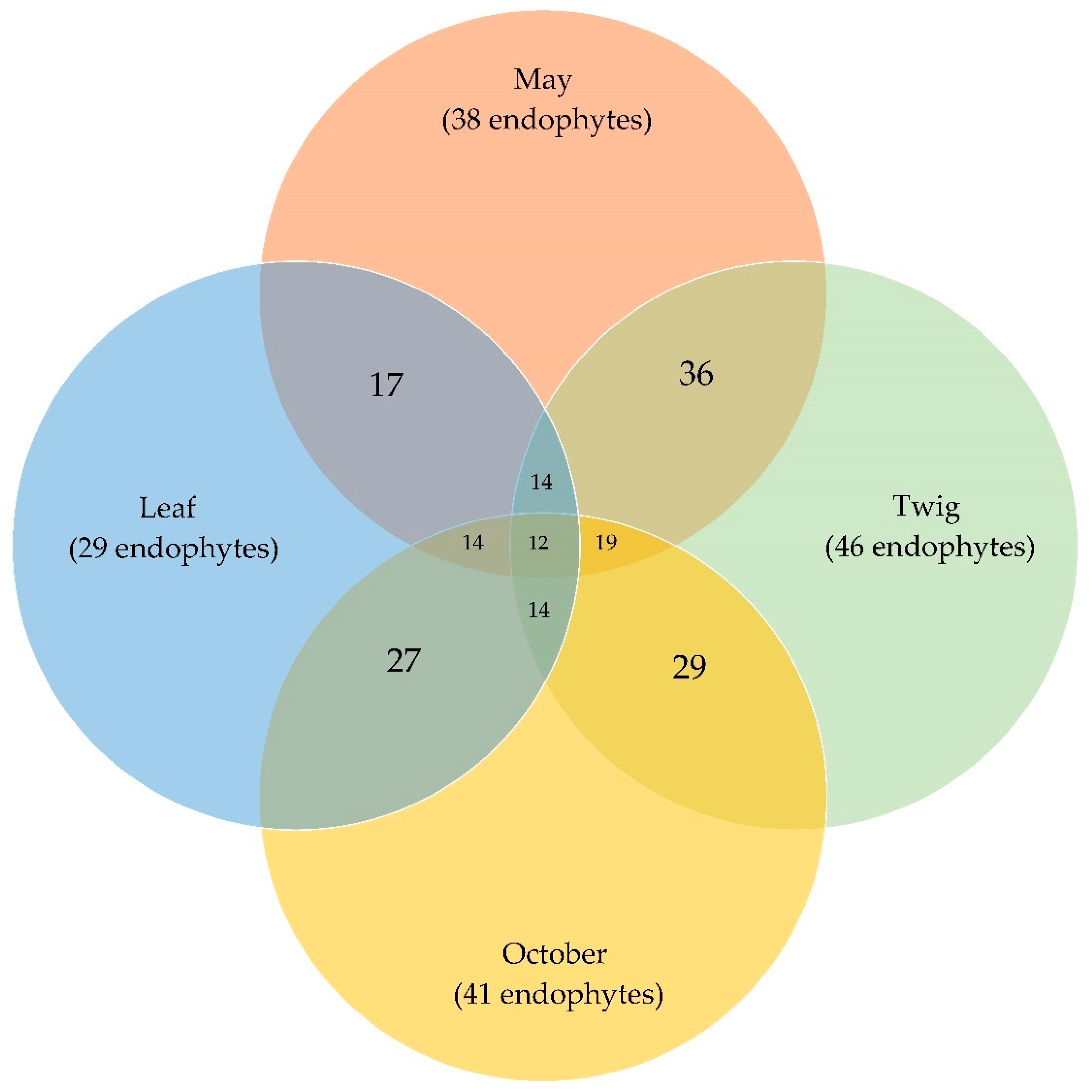
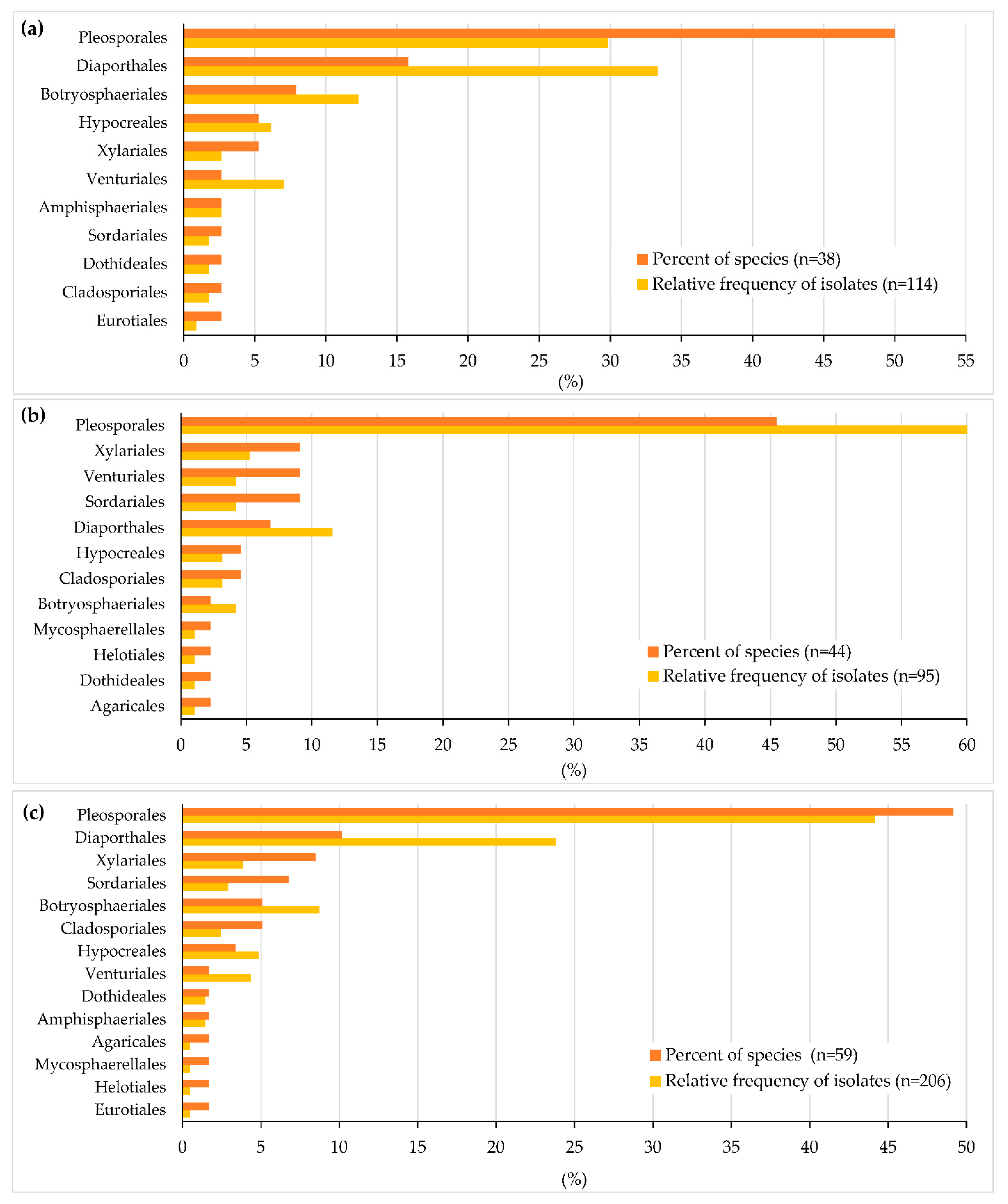
3.1. Endophytic Mycobiota in Ash Leaves and Twigs
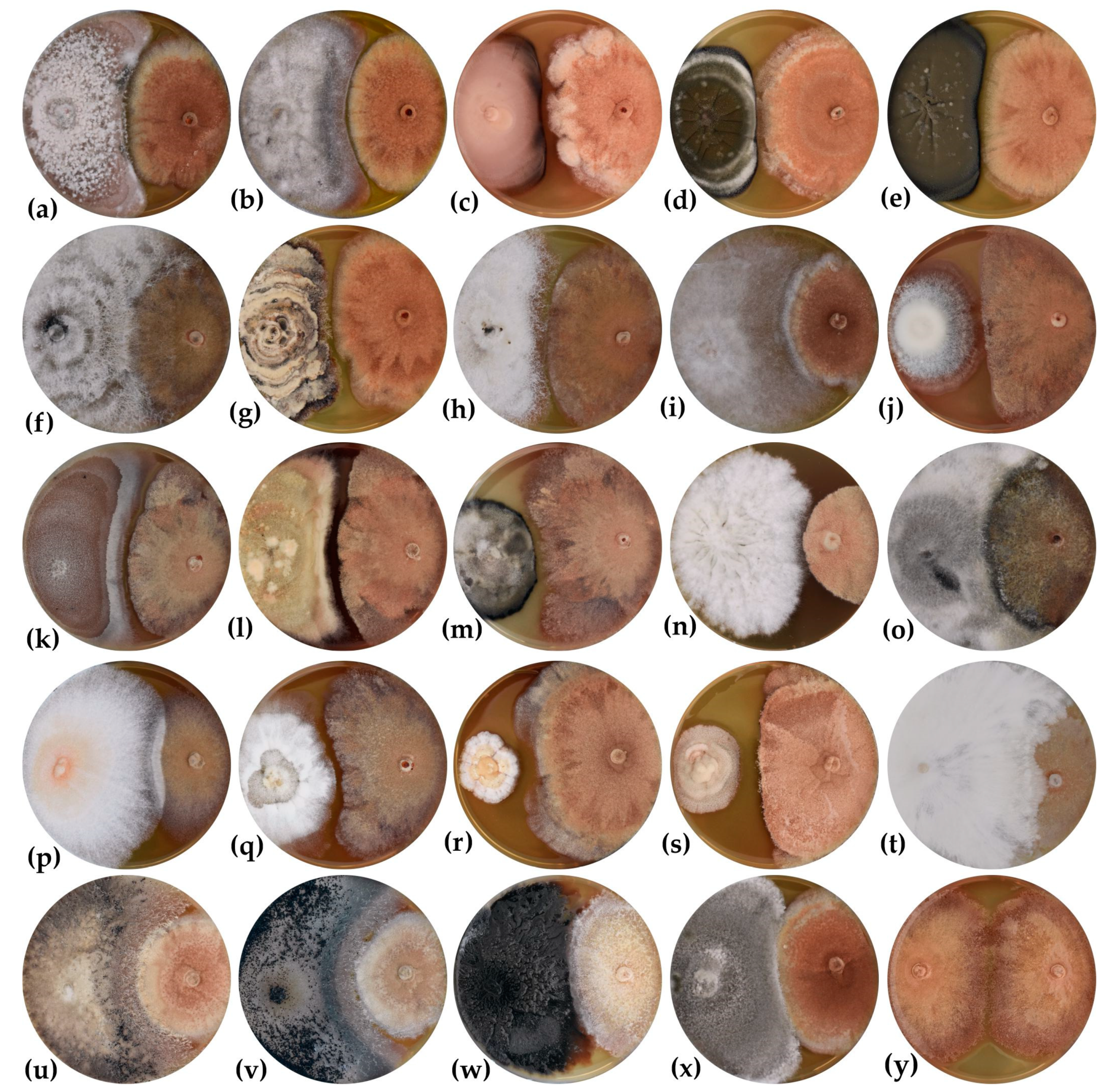
3.2. Inhibitory Effect of Endophytes against H. fraxineus on Artificial Medium
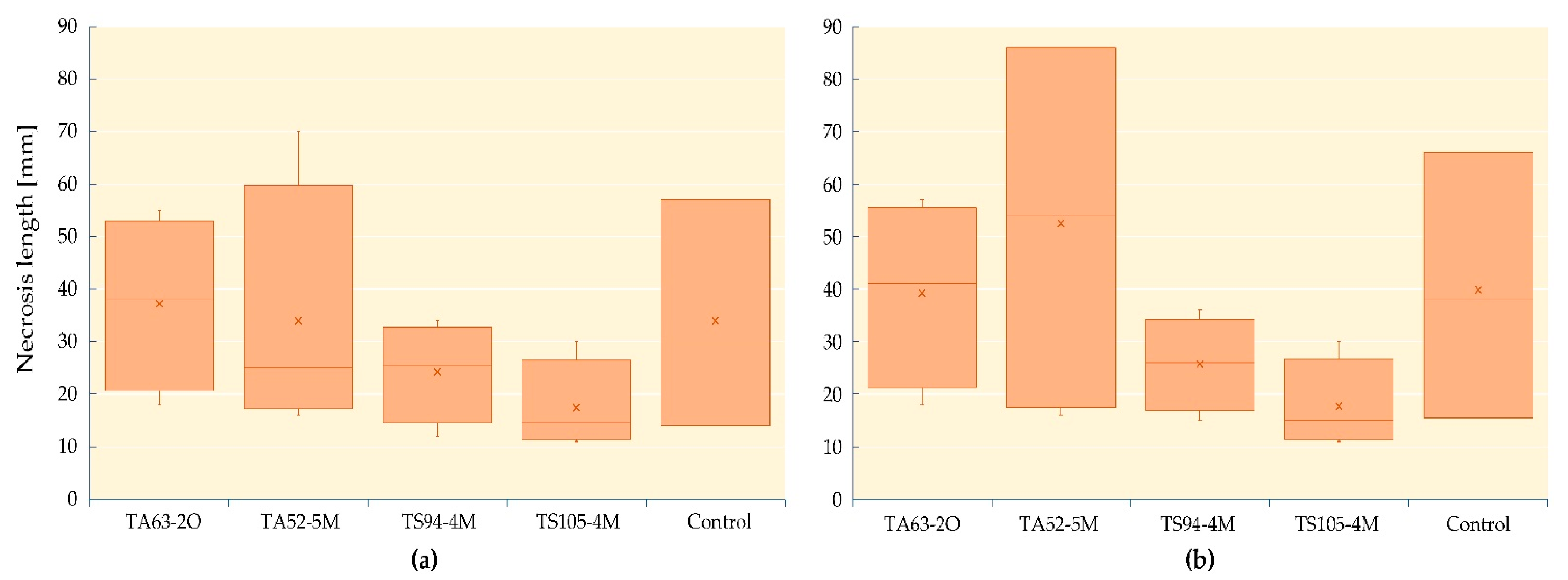
3.3. In Planta Evaluation of the Inhibitory Effect of Endophytes
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Przybyl, K. Fungi associated with necrotic apical parts of Fraxinus excelsior shoots. For. Pathol. 2002, 32, 387–394. [Google Scholar] [CrossRef]
- Bakys, R.; Vasaitis, R.; Barklund, P.; Thomsen, I.M.; Stenlid, J. Occurrence and pathogenicity of fungi in necrotic and non-symptomatic shoots of declining common ash (Fraxinus excelsior) in Sweden. Eur. J. For. Res. 2009, 128, 51–60. [Google Scholar] [CrossRef]
- Kowalski, T.; Czekaj, A. Symptomy chorobowe i grzyby na zamierających jesionach (Fraxinus excelsior L.) w drzewostanach Nadleśnictwa Staszów. Leśne Pract. Badaw. 2010, 71, 357–368. [Google Scholar] [CrossRef]
- Kowalski, T.; Kraj, W.; Bednarz, B. Fungi on stems and twigs in initial and advanced stages of dieback of European ash (Fraxinus excelsior) in Poland. Eur. J. For. Res. 2016, 135, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Cross, H.; Sønstebø, J.H.; Nagy, N.E.; Timmermann, V.; Solheim, H.; Børja, I.; Kauserud, H.; Carlsen, T.; Rzepka, B.; Wasak, K.; et al. Fungal diversity and seasonal succession in ash leaves infected by the invasive ascomycete Hymenoscyphus fraxineus. New Phytol. 2017, 213, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Cook, R.T.A. Taxonomic Manual of Erysiphales (Powdery Mildews), 1st ed.; CBS Biodiversity Series 11; CBS: Utrecht, The Netherlands, 2012; 707p. [Google Scholar]
- Sinclair, W.A.; Lyon, H.H.; Johnson, W.T. Diseases of Trees and Shrubs, 1st ed.; Cornell University Press: Ithaca, NY, USA, 1987; 575p. [Google Scholar]
- Mejía, L.C.; Castlebury, L.A.; Rossman, A.Y.; Sogonov, M.V.; White, J.F. A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Stud. Mycol. 2011, 68, 211–235. [Google Scholar] [CrossRef]
- Ryvarden, L.; Melo, I. Poroid Fungi of Europe, 2nd ed.; Synopsis Fungorum 37; Fungiflora A/S: Oslo, Norway, 2017; 430p. [Google Scholar]
- Zúbrik, M.; Kunca, A.; Vakula, J.; Galko, J.; Leontovyč, R.; Konôpka, B.; Gubka, A.; Nikolov, C.H.; Rell, S.; Longauerová, V.; et al. Hmyz a Huby: Atlas Poškodení Lesných Drevín, 2nd ed.; National Forest Centre, Forest Research Institute: Zvolen, Slovakia, 2019; 243p. [Google Scholar]
- Longauerová, V.; Maľová, M.; Kunca, A. Poznatky z hynutia jaseňov spôsobovaného hubou Hymenoscyphus pseudoalbidus (ana. Chalara fraxinea). In Aktuálne Problémy v Ochrane Lesa 2013; Kunca, A., Ed.; National Forest Centre: Zvolen, Slovakia, 2013; pp. 77–81. [Google Scholar]
- Pastirčáková, K.; Ivanová, H.; Pastirčák, M. Species diversity of fungi on damaged branches and leaves of ashes (Fraxinus spp.) in different types of stands in Slovakia. Cent. Eur. For. J. 2018, 64, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Kádasi Horáková, M.; Barta, M.; Adamčíková, K.; Ostrovský, R.; Pastirčáková, K. Hymenoscyphus fraxineus on Fraxinus excelsior in Slovakia: Distribution and mating types. Biologia 2022. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Hosoya, T.; Baral, H.-O.; Hosaka, K.; Kakishima, M. Hymenoscyphus pseudoalbidus, the correct name for Lambertella albida reported from Japan. Mycotaxon 2012, 122, 25–41. [Google Scholar] [CrossRef]
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol. Plant Pathol. 2014, 15, 5–21. [Google Scholar] [CrossRef]
- Cleary, M.; Nguyen, D.; Marčiulynienė, D.; Berlin, A.; Vasaitis, R.; Stenlid, J. Friend or foe? Biological and ecological traits of the European ash dieback pathogen Hymenoscyphus fraxineus in its native environment. Sci. Rep. 2016, 6, 21895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D.; Soytong, K. The Fungal Endophyte Dilemma. Fungal Divers 2008, 33, 163–173. [Google Scholar]
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal Endophytes: A Continuum of Interactions with Host Plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance Generated by Plant/Fungal Symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.J.; Redman, R.S.; Henson, J.M. The Role of Fungal Symbioses in the Adaptation of Plants to High Stress Environments. Mitig. Adapt. Strateg. Glob. Chang. 2004, 9, 261–272. [Google Scholar] [CrossRef]
- Gao, F.K.; Dai, C.C.; Liu, X.Z. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010, 4, 1346–1351. [Google Scholar]
- Mengistu, A.A. Endophytes: Colonization, Behaviour, and Their Role in Defense Mechanism. Int. J. Microbiol. 2020, 2020, 6927219. [Google Scholar] [CrossRef]
- González-Teuber, M.; Vilo, C.; Guevara-Araya, M.J.; Salgado-Luarte, C.; Gianoli, E. Leaf resistance traits influence endophytic fungi colonization and community composition in a South American temperate rainforest. J. Ecol. 2020, 108, 1019–1029. [Google Scholar] [CrossRef]
- Pujade-Renaud, V.; Déon, M.; Gazis, R.; Ribeiro, S.; Dessailly, F.; Granet, F.; Chaverri, P. Endophytes from Wild Rubber Trees as Antagonists of the Pathogen Corynespora cassiicola. Phytopathology 2019, 109, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, M.; Dubach, V.; Von Buol, L.; Sieber, T.N. Effects of endophytic fungi on the ash dieback pathogen. FEMS Microbiol. Ecol. 2016, 92, fiw142. [Google Scholar] [CrossRef] [PubMed]
- Haňáčková, Z.; Havrdová, L.; Černý, L.; Zahradník, D.; Koukol, O. Fungal endophytes in ash shoots—Diversity and inhibition of Hymenoscyphus fraxineus. Balt. For. 2017, 23, 89–106. [Google Scholar]
- Kosawang, C.; Amby, D.B.; Bussaban, B.; McKinney, L.V.; Xu, J.; Kjær, E.D.; Collinge, D.B.; Nielsen, L.R. Fungal communities associated with species of Fraxinus tolerant to ash dieback, and their potential for biological control. Fungal Biol. 2018, 122, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Ulrich, K.; Behrendt, U.; Kube, M.; Ulrich, A. Analyzing Ash Leaf-Colonizing Fungal Communities for Their Biological Control of Hymenoscyphus fraxineus. Front. Microbiol. 2020, 11, 590944. [Google Scholar] [CrossRef]
- Halecker, S.; Wennrich, J.-P.; Rodrigo, S.; Andrée, N.; Rabsch, L.; Baschien, C.; Steinert, M.; Stadler, M.; Surup, F.; Schulz, B. Fungal endophytes for biocontrol of ash dieback: The antagonistic potential of Hypoxylon rubiginosum. Fungal Ecol. 2020, 45, 100918. [Google Scholar] [CrossRef]
- Kowalski, T.; Bilański, P. Fungi Detected in the Previous Year’s Leaf Petioles of Fraxinus excelsior and Their Antagonistic Potential against Hymenoscyphus fraxineus. Forests 2021, 12, 1412. [Google Scholar] [CrossRef]
- Nawrot-Chorabik, K.; Marcol-Rumak, N.; Latowski, D. Investigation of the Biocontrol Potential of Two Ash Endophytes against Hymenoscyphus fraxineus Using In Vitro Plant–Fungus Dual Cultures. Forests 2021, 12, 1750. [Google Scholar] [CrossRef]
- Bilański, P.; Kowalski, T. Fungal endophytes in Fraxinus excelsior petioles and their in vitro antagonistic potential against the ash dieback pathogen Hymenoscyphus fraxineus. Microbiol. Res. 2022, 257, 126961. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jørgensen, H.J.L.; Latz, M.A.C.; Manzotti, A.; Ntana, F.; Rojas, E.C.; Jensen, B. Searching for novel fungal biological control agents for plant disease control among endophytes. In Endophytes for a Growing World, 1st ed.; Hodkinson, T., Doohan, F., Saunders, M., Murphy, B., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 25–51. [Google Scholar] [CrossRef] [Green Version]
- Ivanová, H. Identification and characterization of the fungus Dothiorella sarmentorum on necrotic shoots of declining ash in Slovakia. Folia Oecol. 2018, 45, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, H.; Malinicova, L.; Piknova, M.; Pristas, P. New Endophytic Fusarium spp. from Fraxinus excelsior Leaves in Slovakia. Planta Med. 2020, 86, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Adamčíková, K.; Kádasi-Horáková, M.; Jankovský, L.; Havrdová, L. Identification of Hymenoscyphus fraxineus, the causal agent of ash dieback in Slovakia. Biologia 2015, 70, 559–564. [Google Scholar] [CrossRef]
- Adamčíková, K.; Pažitný, J.; Pastirčáková, K. Individual resistance of Fraxinus angustifolia and F. excelsior clones to Hymenoscyphus fraxineus. J. Plant Protect. Res. 2018, 58, 227–233. [Google Scholar]
- Kirisits, T.; Dämpfle, L.; Kräutler, K. Hymenoscyphus albidus is not associated with an anamorphic stage and displays slower growth than Hymenoscyphus pseudoalbidus on agar media. For. Pathol. 2013, 43, 386–389. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sieber, T.N.; Schlegel, M. Communities of fungal endophytes in leaves of Fraxinus ornus are highly diverse. Fungal Ecol. 2017, 29, 10–19. [Google Scholar] [CrossRef]
- Kumar, D.S.S.; Hyde, K.D. Biodiversity and tissue-recurrence of endophytic fungi in Tripterygium wilfordii. Fungal Divers. 2004, 17, 69–90. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Kádasi-Horáková, M.; Adamčíková, K.; Pastirčáková, K.; Longauerová, V.; Maľová, M. Natural infection of Fraxinus angustifolia by Hymenoscyphus fraxineus in Slovakia. Balt. For. 2017, 23, 52–55. [Google Scholar]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Abdallah, M.F.; De Boevre, M.; Landschoot, S.; De Saeger, S.; Haesaert, G.; Audenaert, K. Fungal Endophytes Control Fusarium graminearum and Reduce Trichothecenes and Zearalenone in Maize. Toxins 2018, 10, 493. [Google Scholar] [CrossRef] [Green Version]
- Skidmore, A.M.; Dickinson, C.H. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Br. Mycol. Soc. 1976, 66, 57–64. [Google Scholar] [CrossRef]
- Gross, A.; Sieber, T.N. Virulence of Hymenoscyphus albidus and native and introduced Hymenoscyphus fraxineus on Fraxinus excelsior and Fraxinus pennsylvanica. Plant Pathol. 2016, 65, 655–663. [Google Scholar] [CrossRef]
- Johansson, S.B.K.; Vasaitis, R.; Ihrmark, K.; Barklund, P.; Stenlid, J. Detection of Chalara fraxinea from tissue of Fraxinus excelsior using species-specific ITS primers. For. Pathol. 2010, 40, 111–115. [Google Scholar] [CrossRef]
- Pastirčáková, K.; Adamčíková, K.; Barta, M.; Pažitný, J.; Hoťka, P.; Sarvašová, I.; Kádasi Horáková, M. Host Range of Hymenoscyphus fraxineus in Slovak Arboreta. Forests 2020, 11, 596. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Camargo, J.A. On Measuring Species Evenness and Other Associated Parameters of Community Structure. Oikos 1995, 74, 538–542. [Google Scholar] [CrossRef]
- Scholtysik, A.; Unterseher, M.; Otto, P.; Wirth, C. Spatio-temporal dynamics of endophyte diversity in the canopy of European ash (Fraxinus excelsior). Mycol. Prog. 2013, 12, 291–304. [Google Scholar] [CrossRef]
- Schlegel, M.; Queloz, V.; Sieber, T.N. The Endophytic Mycobiome of European Ash and Sycamore Maple Leaves—Geographic Patterns, Host Specificity and Influence of Ash Dieback. Front. Microbiol. 2018, 9, 2345. [Google Scholar] [CrossRef]
- Osono, T.; Mori, A. Seasonal and leaf age-dependent changes in occurrence of phyllosphere fungi of giant dogwood. Mycoscience 2005, 46, 273–279. [Google Scholar] [CrossRef]
- Guo, L.-D.; Huang, G.-R.; Wang, Y. Seasonal and Tissue Age Influences on Endophytic Fungi of Pinus tabulae formis (Pinaceae) in the Dongling Mountains, Beijing. J. Integr. Plant Biol. 2008, 50, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Juybari, H.Z.; Ghanbary, M.A.T.; Rahimian, H.; Karimi, K.; Arzanlou, M. Seasonal, tissue and age influences on frequency and biodiversity of endophytic fungi of Citrus sinensis in Iran. For. Pathol. 2019, 49, e12559. [Google Scholar] [CrossRef]
- Sadeghi, F.; Samsampour, D.; Seyahooei, M.A.; Bagheri, A.; Soltani, J. Diversity and Spatiotemporal Distribution of Fungal Endophytes Associated with Citrus reticulata cv. Siyahoo. Curr. Microbiol. 2019, 76, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Sieber, T.N. The phyllosphere mycobiome of woody plants. In Forest Microbiology, 1st ed.; Tree Microbiome: Phyllosphere, Endosphere and Rhizosphere; Asiegbu, F.O., Kovalchuk, A., Eds.; Academic Press: London, UK, 2021; Volume 1, pp. 111–132. [Google Scholar] [CrossRef]
- Pan, J.J.; May, G. Fungal-Fungal Associations Affect the Assembly of Endophyte Communities in Maize (Zea mays). Microb. Ecol. 2009, 58, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Rabiey, M.; Hailey, L.E.; Roy, S.R.; Grenz, K.; Al-Zadjali, M.A.S.; Barrett, G.A.; Jackson, R.W. Endophytes vs tree pathogens and pests: Can they be used as biological control agents to improve tree health? Eur. J. Plant Pathol. 2019, 155, 711–729. [Google Scholar] [CrossRef] [Green Version]
- Ownley, B.H.; Pereira, R.M.; Klingeman, W.E.; Quigley, N.B.; Leckie, B.M. Beauveria bassiana, a dual purpose biological control with activity against insect pests and plant pathogens. In Emerging Concepts in Plant Health Management, 1st ed.; Lartey, R.T., Caesar, A.J., Eds.; Research Signpost: Thiruvananthapuram, India, 2004; pp. 255–269. [Google Scholar]
- Anjum, R.; Afzal, M.; Baber, R.; Khan, M.A.J.; Kanwal, W.; Sajid, W.; Raheel, A. Endophytes: As Potential Biocontrol Agent—Review and Future Prospects. J. Agric. Sci. 2019, 11, 113. [Google Scholar] [CrossRef] [Green Version]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Siddiqui, J.A.; Akutse, K.S.; Ramos Aguila, L.C.; Xu, Y. General Limitations to Endophytic Entomopathogenic Fungi Use as Plant Growth Promoters, Pests and Pathogens Biocontrol Agents. Plants 2021, 10, 2119. [Google Scholar] [CrossRef]
- Ganley, R.J.; Sniezko, R.A.; Newcombe, G. Endophyte-mediated resistance against white pine blister rust in Pinus monticola. For. Ecol. Manag. 2008, 255, 2751–2760. [Google Scholar] [CrossRef]
- Berger, G.; Czarnocka, K.; Cochard, B.; Oszako, T.; Lefort, F. Biocontrol Endotherapy with Trichoderma spp. and Bacillus amyloliquefaciens against Phytophthora spp.: A Comparative Study with Phosphite Treatment on Quercus robur and Fagus sylvatica. J. Agric. Sci. Technol. 2015, 5, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J.; Baptista, P. Filamentous fungi as biocontrol agents in olive (Olea europaea L.) diseases: Mycorrhizal and endophytic fungi. Crop Prot. 2021, 146, 105672. [Google Scholar] [CrossRef]
- Marcol, N.; Pukalski, J.; NawrotChorabik, K.; Kowalski, T.; Latowski, D. Will black pigments save European ash (Fraxinus excelsior L.)?—EPR analysis of melanin production in dual-culture of endophytic and pathogenic fungi. In Proceedings of the 45th FEBS Congress, Molecules of Life: Towards New Horizons, Ljubljana, Slovenia, 3–8 July 2021. [Google Scholar]
- Tsantrizos, Y.S.; Xu, X.-J.; Sauriol, F.; Hynes, R.C. Novel quinazolinones and enniatins from Fusarium lateritium Nees. Can. J. Chem. 1993, 71, 1362–1367. [Google Scholar] [CrossRef]
- Gerlach, W.; Nirenberg, H. The Genus Fusarium, a Pictorial Atlas, 1st ed.; Mitt. Biol. Bundesanst. Land-Forstwirsch. Berlin-Dahlem; Kommissionsverlag P. Parey: Berlin, Germany, 1982; Volume 209, 406p. [Google Scholar] [CrossRef]
- Vitale, S.; Santori, A.; Wajnberg, E.; Castagnone-Sereno, P.; Luongo, L.; Belisario, A. Morphological and Molecular Analysis of Fusarium lateritium, the Cause of Gray Necrosis of Hazelnut Fruit in Italy. Phytopathology 2011, 101, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.D.; Gao, A.Q. Pathogenicity of Dothiorella gregaria to Rosaceae and other host trees. For. Pest Dis. 2004, 23, 21–23. [Google Scholar]
- Chen, Q.; Jiang, J.; Zhang, G.; Cai, L.; Crous, P. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef] [Green Version]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 15 May 2022).
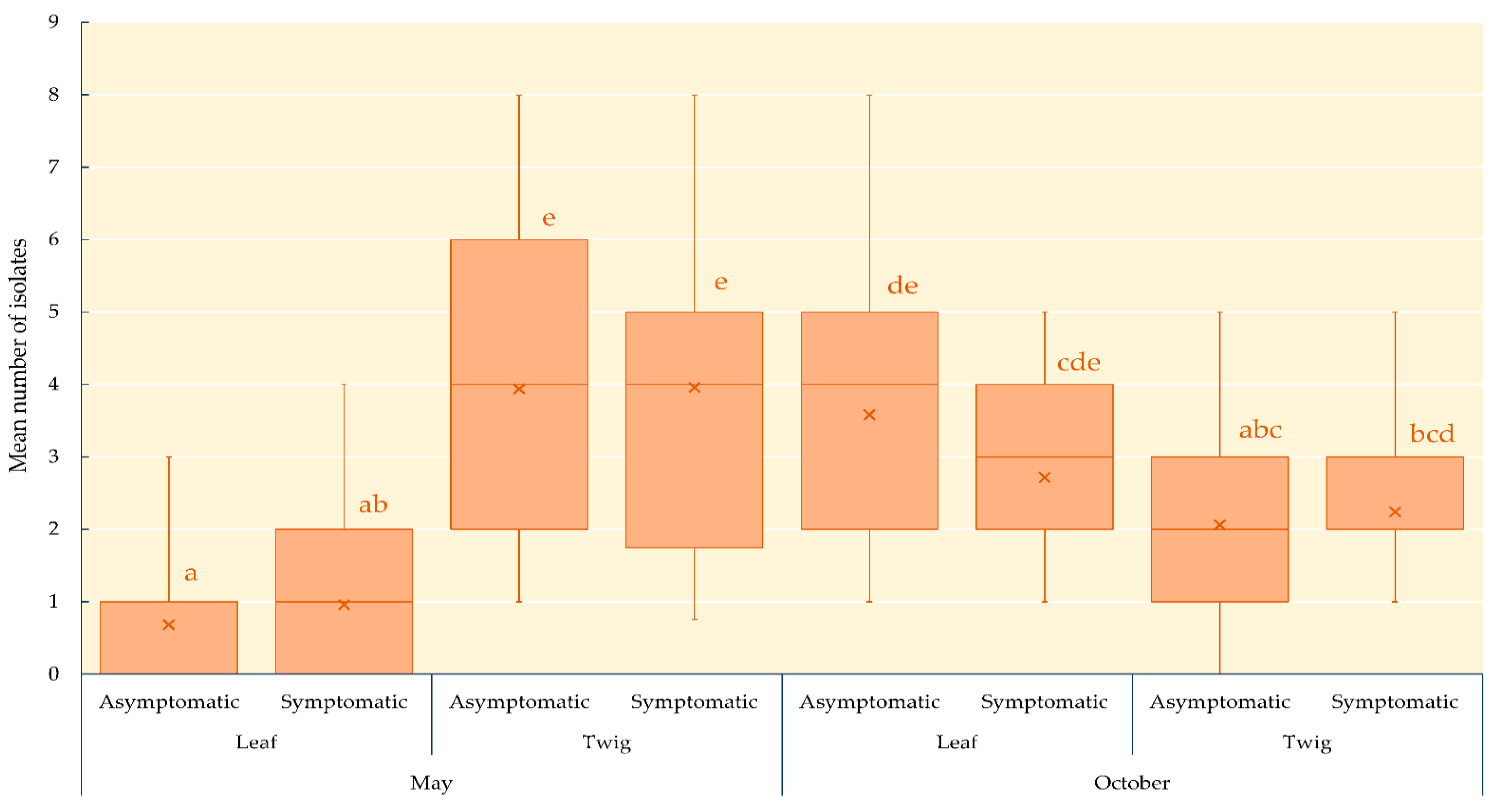

| Date of Sampling | Health Status of Trees * | Tissue Location on Trees | Mean Colonization Rate of Tissue Samples (%) ** | Total Number of Isolates | Mean Number of Isolates per Tissue Sample ** |
|---|---|---|---|---|---|
| May 2019 | Asymptomatic | Leaf | 13.6 ± 2.0 | 34 | 0.7 ± 0.1 |
| Twig | 68.4 ± 4.8 | 197 | 4.0 ± 0.3 | ||
| Symptomatic | Leaf | 19.2 ± 2.9 | 48 | 1.0 ± 0.2 | |
| Twig | 72.0 ± 4.7 | 198 | 4.0 ± 0.3 | ||
| October 2019 | Asymptomatic | Leaf | 68.0 ± 4.1 | 179 | 3.6 ± 0.3 |
| Twig | 41.2 ± 3.3 | 103 | 2.0 ± 0.2 | ||
| Symptomatic | Leaf | 54.4 ± 3.2 | 136 | 2.7 ± 0.2 | |
| Twig | 44.8 ± 2.9 | 112 | 2.2 ± 0.2 |
| Order | Taxon | Number of Fungal Isolates | GenBank Acc. No. ITS | BLASTn | Identities % | ||||
|---|---|---|---|---|---|---|---|---|---|
| AL (May/Oct) | SL (May/Oct) | AT (May/Oct) | ST (May/Oct) | Total Number (May/Oct) | |||||
| Agaricales | Hygrophorus sp. * | 0/1 | 0/1 | LT716040 | 78.49 | ||||
| Amphisphaeriales | Lepteutypa fuckelii | 2/0 | 1/0 | 3/0 | OM950730 | MZ045855 | 100 | ||
| Botryosphaeriales | Diplodia fraxini ** | 2/0 | 1/0 | 2/0 | 5/0 | OM950731 | MT587349 | 99.82 | |
| Dothiorella gregaria ** | 0/1 | 6/1 | 2/2 | 8/4 | OM950732 | MN685280 | 99.44 | ||
| Microdiplodia sp. | 1/0 | 1/0 | OM950733 | FJ228194 | 99.11 | ||||
| Cladosporiales | Cladosporium allicinum | 0/1 | 0/1 | OM950734 | MT573471 | 100 | |||
| Cladosporium cladosporioides | 1/0 | 1/0 | 2/0 | OM950735 | MT635286 | 100 | |||
| Cladosporium tenuissimum | 0/1 | 0/1 | 0/2 | OM950736 | LT603045 | 100 | |||
| Diaporthales | Cytospora pruinosa | 1/0 | 1/0 | OM950737 | MW447045 | 99.83 | |||
| Diaporthe eres ** | 0/1 | 8/0 | 17/3 | 25/4 | OM950738 | OM442980 | 100 | ||
| Diaporthe nobilis | 1/0 | 2/0 | 3/0 | OM950739 | KJ609011 | 99.65 | |||
| Diaporthe oncostoma ** | 1/2 | 1/2 | 0/1 | 0/1 | 2/6 | OM950740 | LN714541 | 99.82 | |
| Diaporthe rudis ** | 0/1 | 2/0 | 3/0 | 5/1 | OM950741 | MW032267 | 99.66 | ||
| Diaporthe vacuae | 2/0 | 2/0 | OM950742 | MZ127189 | 99.66 | ||||
| Dothideales | Aureobasidium pullulans | 1/1 | 1/0 | 2/1 | OM950743 | MW560221 | 100 | ||
| Eurotiales | Aspergillus pseudoglaucus | 1/0 | 1/0 | OM950744 | KX696361 | 100 | |||
| Helotiales | Neofabraea vagabunda | 0/1 | 0/1 | OM950745 | KT923785 | 99.63 | |||
| Hypocreales | Fusarium avenaceum | 1/1 | 1/1 | OM950746 | MW016661 | 100 | |||
| Fusarium lateritium ** | 4/1 | 2/1 | 6/2 | OM950747 | JQ693397 | 100 | |||
| Mycosphaerellales | Ramularia endophylla | 0/1 | 0/1 | OM950748 | MH859364 | 100 | |||
| Pleosporales | Alternaria alternata ** | 0/3 | 1/8 | 0/7 | 5/2 | 6/20 | OM950749 | MK183818 | 100 |
| Alternaria infectoria ** | 0/2 | 0/2 | 0/1 | 0/5 | OM950750 | MK461063 | 100 | ||
| Alternaria longipes | 0/1 | 0/1 | OM950751 | MH712187 | 99.82 | ||||
| Aposphaeria corallinolutea | 0/1 | 0/1 | OM950752 | MT177916 | 99.80 | ||||
| Ascochyta medicaginicola | 1/2 | 1/2 | OM950753 | KF293990 | 100 | ||||
| Ascochyta pisi | 0/1 | 0/1 | OM950754 | MH854853 | 99.61 | ||||
| Comoclathris incompta ** | 1/1 | 1/1 | 2/2 | OM950755 | KU973715 | 99.83 | |||
| Coniothyrium ferrarisianum * | 1/0 | 1/0 | MH860854 | 97.09 | |||||
| Didymella aliena ** | 1/1 | 2/3 | 3/4 | OM950756 | KC311486 | 99.81 | |||
| Didymella glomerata | 0/1 | 2/0 | 2/1 | OM950757 | MN075513 | 100 | |||
| Didymella macrostoma | 0/1 | 0/1 | OM950758 | MH858090 | 99.81 | ||||
| Epicoccum nigrum ** | 0/1 | 0/2 | 2/1 | 2/4 | OM950759 | KX664321 | 100 | ||
| Epicoccum thailandicum * | 1/0 | 1/0 | MG975626 | 80.79 | |||||
| Foliophoma camporesii | 1/1 | 1/1 | OM950760 | MN244200 | 99.65 | ||||
| Lophiostoma corticola | 0/1 | 1/0 | 1/1 | OM950761 | KT004559 | 99.62 | |||
| Muriphaeosphaeria viburni | 1/0 | 1/0 | OM950762 | MW446984 | 99.48 | ||||
| Neodidymelliopsis camporesii | 0/1 | 1/1 | 1/2 | OM950763 | MN244199 | 99.81 | |||
| Neosetophoma aseptata | 2/0 | 2/0 | OM950764 | NR164449 | 99.12 | ||||
| Nothophoma spiraeae | 2/0 | 2/0 | OM950765 | OM287410 | 99.43 | ||||
| Paracucurbitaria corni | 1/0 | 1/0 | OM950766 | MT547826 | 98.70 | ||||
| Phaeosphaeria sp. | 0/1 | 0/1 | 0/2 | OM950767 | LC171698 | 99.81 | |||
| Phoma herbarum | 0/1 | 0/1 | OM950768 | KP739881 | 99.62 | ||||
| Phoma sp. | 0/1 | 0/1 | OM950769 | MG098303 | 99.31 | ||||
| Pleosporales sp. | 2/0 | 2/0 | OM950770 | MT777338 | 100 | ||||
| Praetumpfia obducens | 1/0 | 1/0 | OM950771 | NR147688 | 98.30 | ||||
| Pseudocamarosporium brabeji | 0/1 | 0/1 | OM950772 | KR909143 | 99.32 | ||||
| Pyrenophora triseptata | 0/1 | 0/1 | OM950773 | MT548680 | 99.84 | ||||
| Sporormiella minima | 2/0 | 2/0 | OM950774 | MG098329 | 99.43 | ||||
| Stemphylium vesicarium ** | 1/2 | 0/2 | 0/1 | 1/0 | 2/5 | OM950775 | MZ452063 | 100 | |
| Sordariales | Dichotomopilus erectus | 0/1 | 0/1 | OM950776 | MN956887 | 99.64 | |||
| Chaetomium globosum * | 0/1 | 0/1 | MH858130 | 74.45 | |||||
| Sordaria fimicola | 0/1 | 1/0 | 1/0 | 2/1 | OM950777 | MN341410 | 99.82 | ||
| Sordaria lappae | 0/1 | 0/1 | OM950778 | MH858210 | 100 | ||||
| Venturiales | Fraxinicola fraxini ** | 2/1 | 6/0 | 8/1 | OM950779 | MW447009 | 99.63 | ||
| Xylariales | Anthostoma amoenum | 0/1 | 0/1 | OM950780 | KC774569 | 98.67 | |||
| Hypoxylon fragiforme | 0/1 | 0/1 | OM950781 | EF155508 | 100 | ||||
| Nemania diffusa | 0/1 | 0/1 | OM950782 | MZ078701 | 99.65 | ||||
| Nemania serpens | 1/0 | 1/0 | OM950783 | MF161316 | 99.48 | ||||
| Rosellinia corticium ** | 1/1 | 0/1 | 1/0 | 2/2 | OM950784 | KY593990 | 99.81 | ||
| Total number of isolates | 7/22 | 13/24 | 33/26 | 61/20 | 114/92 | ||||
| Species richness (S) | 6/17 | 6/14 | 14/17 | 30/14 | 38/41 | ||||
| Simpson’s index of diversity (D) | 0.816/0.934 | 0.722/0.852 | 0.872/0.895 | 0.899/0.910 | 0.925/0.929 | ||||
| Shannon’s index of diversity (H) | 1.748/2.471 | 1.525/2.179 | 2.331/2.502 | 2.931/2.528 | 3.152/3.232 | ||||
| Isolate Name | Endophytic Fungus | Interaction Type * | Mean Inhibition Index ± SE ** |
|---|---|---|---|
| TS94-4M | Fusarium lateritium | D2 (4.00) | 0.575 ± 0.017 a |
| TS105-4M | Didymella aliena | D1 | 0.326 ± 0.019 b |
| TA63-2O | Didymella macrostoma | D2 (3.33) | 0.318 ± 0.020 bc |
| TA52-5M | Dothiorella gregaria | D2 (3.33) | 0.280 ± 0.041 bcd |
| LS92-2O | Alternaria alternata | D1 | 0.267 ± 0.026 bcde |
| TA93-2O | Dothiorella gregaria | D2 (3.00) | 0.212 ± 0.010 bcdef |
| TA35-3O | Ascochyta medicaginicola | D1 | 0.202 ± 0.050 bcdef |
| LS25-3O | Didymella aliena | B1 | 0.197 ± 0.007 bcdef |
| TA31-4M | Sporormiella minima | C | 0.196 ± 0.018 bcdef |
| TA31-3O | Pyrenophora triseptata | D1 | 0.195 ± 0.001 bcdef |
| LA45-4O | Rosellinia corticium | B2 | 0.191 ± 0.043 bcdef |
| LA74-1O | Sordaria lappae | D1 | 0.189 ± 0.029 bcdef |
| TS85-2M | Diaporthe rudis | D2 (2.00) | 0.188 ± 0.038 bcdef |
| LS62-2O | Alternaria alternata | D1 | 0.176 ± 0.020 bcdef |
| TS102-1O | Diaporthe eres | B1 | 0.175 ± 0.007 bcdef |
| TA63-1O | Ascochyta medicaginicola | D1 | 0.174 ± 0.020 bcdef |
| TA34-1M | Sordaria fimicola | B1 | 0.153 ± 0.015 bcdef |
| TA23-1O | Phoma herbarum | D2 (9.33) | 0.150 ± 0.019 bcdef |
| TA102-2O | Didymella glomerata | D1 | 0.139 ± 0.007 bcdef |
| TA63-1M | Ascochyta medicaginicola | D1 | 0.134 ± 0.024 bcdef |
| TA93-1M | Microdiplodia sp. | D2 (6.00) | 0.128 ± 0.051 bcdef |
| TS31-1M | Epicoccum nigrum | B1 | 0.126 ± 0.007 bcdef |
| TS91-2M | Rosellinia corticium | D2 (5.00) | 0.119 ± 0.014 bcdef |
| TA52-2O | Hypoxylon fragiforme | B1 | 0.117 ± 0.072 bcdef |
| LS21-1O | Stemphylium vesicarium | A | 0.114 ± 0.028 bcdef |
| LA44-5O | Diaporthe eres | B1 | 0.111 ± 0.031 bcdef |
| LA25-3O | Stemphylium vesicarium | A | 0.106 ± 0.044 bcdef |
| LS31-2O | Diaporthe rudis | D1 | 0.104 ± 0.022 bcdef |
| TS43-1M | Didymella aliena | D2 (4.00) | 0.101 ± 0.035 bcdef |
| LS102-1M | Didymella aliena | D2 (3.33) | 0.100 ± 0.020 bcdef |
| LA94-1M | Rosellinia corticium | B2 | 0.100 ± 0.023 bcdef |
| LS61-2O | Neodidymelliopsis camporesii | D1 | 0.100 ± 0.028 bcdef |
| TS41-1M | Diaporthe eres | D2 (3.33) | 0.098 ± 0.027 cdef |
| LA13-3O | Alternaria alternata | B1 | 0.086 ± 0.055 def |
| LS73-3O | Sordaria fimicola | B1 | 0.083 ± 0.032 def |
| LA52-1O | Fraxinicola fraxini | D1 | 0.077 ± 0.140 def |
| TS64-1O | Cladosporium tenuissimum | D1 | 0.077 ± 0.050 def |
| LA72-1O | Anthostoma amoenum | A | 0.074 ± 0.017 def |
| LS22-1M | Diplodia fraxini | D1 | 0.072 ± 0.028 def |
| TA61-1M | Dothiorella gregaria | B1 | 0.071 ± 0.024 def |
| TS35-2O | Diaporthe oncostoma | D1 | 0.071 ± 0.021 def |
| LS103-3O | Alternaria infectoria | D1 | 0.070 ± 0.012 def |
| LA25-4O | Nemania diffusa | B2 | 0.068 ± 0.021 def |
| TS64-4O | Epicoccum nigrum | D2 (6.67) | 0.057 ± 0.093 def |
| TA82-1O | Stemphylium vesicarium | D1 | 0.052 ± 0.011 ef |
| TA94-1O | Phaeosphaeria sp. | D2 (8.67) | 0.052 ± 0.054 ef |
| TA91-3O | Ascochyta pisi | D2 (3.00) | 0.052 ± 0.026 ef |
| TA55-5M | Lepteutypa fuckelii | B2 | 0.042 ± 0.022 ef |
| TA75-3M | Diaporthe nobilis | B1 | 0.041 ± 0.017 ef |
| LS44-1O | Cladosporium tenuissimum | D1 | 0.035 ± 0.006 f |
| TS52-2M | Diaporthe eres | B1 | 0.031 ± 0.044 f |
| TS43-1O | Comoclathris incompta | D2 (2.33) | 0.030 ± 0.030 f |
| TS35-3O | Foliophoma camporesii | D1 | 0.018 ± 0.054 f |
| TS12-3M | Diaporthe eres | B1 | 0.017 ± 0.030 f |
| TA35-3M | Neosetophoma aseptata | B1 | 0.012 ± 0.022 f |
| TA55-2M | Comoclathris incompta | B2 | 0.010 ± 0.072 f |
| LA65-2O | Aposphaeria corallinolutea | D1 | 0.009 ± 0.034 f |
| TS62-1O | Neodidymelliopsis camporesii | D2 (4.33) | −0.005 ± 0.045 |
| LS15-1O | Diaporthe oncostoma | D1 | −0.006 ± 0.015 |
| TS12-1O | Phoma sp. | D2 (8.00) | −0.015 ± 0.029 |
| TS101-2O | Fusarium lateritium | D2 (3.33) | −0.017 ± 0.045 |
| LA21-5O | Ramularia endophylla | D2 (6.00) | −0.019 ± 0.028 |
| TS31-5M | Diaporthe rudis | D1 | −0.022 ± 0.016 |
| LS72-2O | Rosellinia corticium | B2 | −0.027 ± 0.024 |
| TA63-2M | Dothiorella gregaria | D2 (3.33) | −0.027 ± 0.024 |
| LA51-2O | Aureobasidium pullulans | D2 (3.00) | −0.038 ± 0.008 |
| TS24-1M | Diaporthe eres | B1 | −0.039 ± 0.079 |
| LS11-1O | Neofabraea vagabunda | D2 (8.00) | −0.042 ± 0.040 |
| TA92-1O | Lophiostoma corticola | A | −0.053 ± 0.038 |
| LS25-1M | Fraxinicola fraxini | D2 (3.33) | −0.102 ± 0.029 |
| LS42-3M | Fraxinicola fraxini | D1 | −0.106 ± 0.007 |
| TA71-6M | Diaporthe rudis | D1 | −0.109 ± 0.030 |
| LA93-1O | Cladosporium allicinum | A | −0.111 ± 0.045 |
| TS44-2M | Diaporthe eres | D1 | −0.140 ± 0.009 |
| LS85-2M | Diaporthe oncostoma | D2 (5.33) | −0.201 ± 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barta, M.; Pastirčáková, K.; Ostrovský, R.; Kobza, M.; Kádasi Horáková, M. Culturable Endophytic Fungi in Fraxinus excelsior and Their Interactions with Hymenoscyphus fraxineus. Forests 2022, 13, 1098. https://doi.org/10.3390/f13071098
Barta M, Pastirčáková K, Ostrovský R, Kobza M, Kádasi Horáková M. Culturable Endophytic Fungi in Fraxinus excelsior and Their Interactions with Hymenoscyphus fraxineus. Forests. 2022; 13(7):1098. https://doi.org/10.3390/f13071098
Chicago/Turabian StyleBarta, Marek, Katarína Pastirčáková, Radovan Ostrovský, Marek Kobza, and Miriam Kádasi Horáková. 2022. "Culturable Endophytic Fungi in Fraxinus excelsior and Their Interactions with Hymenoscyphus fraxineus" Forests 13, no. 7: 1098. https://doi.org/10.3390/f13071098
APA StyleBarta, M., Pastirčáková, K., Ostrovský, R., Kobza, M., & Kádasi Horáková, M. (2022). Culturable Endophytic Fungi in Fraxinus excelsior and Their Interactions with Hymenoscyphus fraxineus. Forests, 13(7), 1098. https://doi.org/10.3390/f13071098







