Molecular Mechanisms Regulating the Columnar Tree Architecture in Apple
Abstract
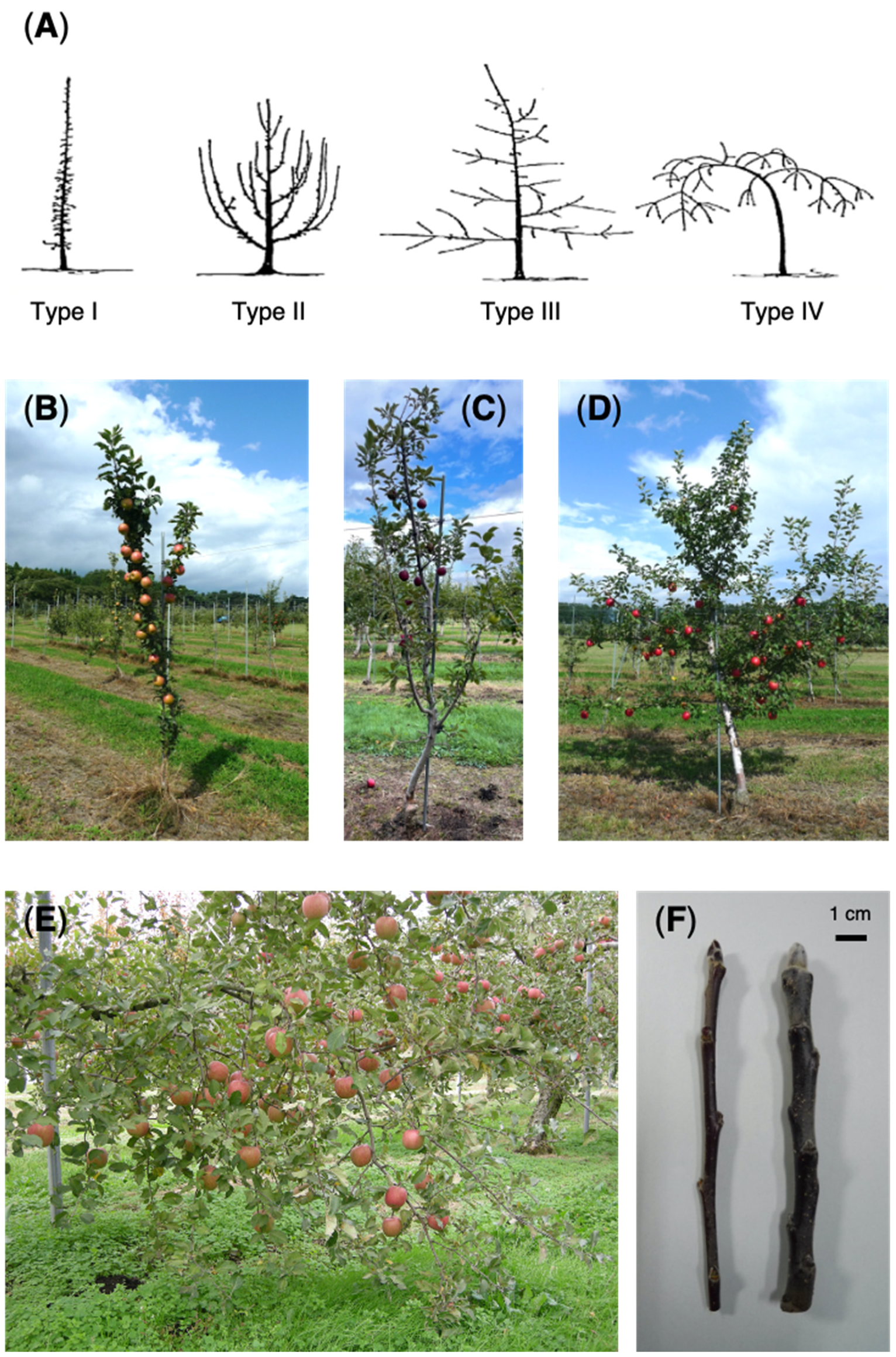
1. Introduction
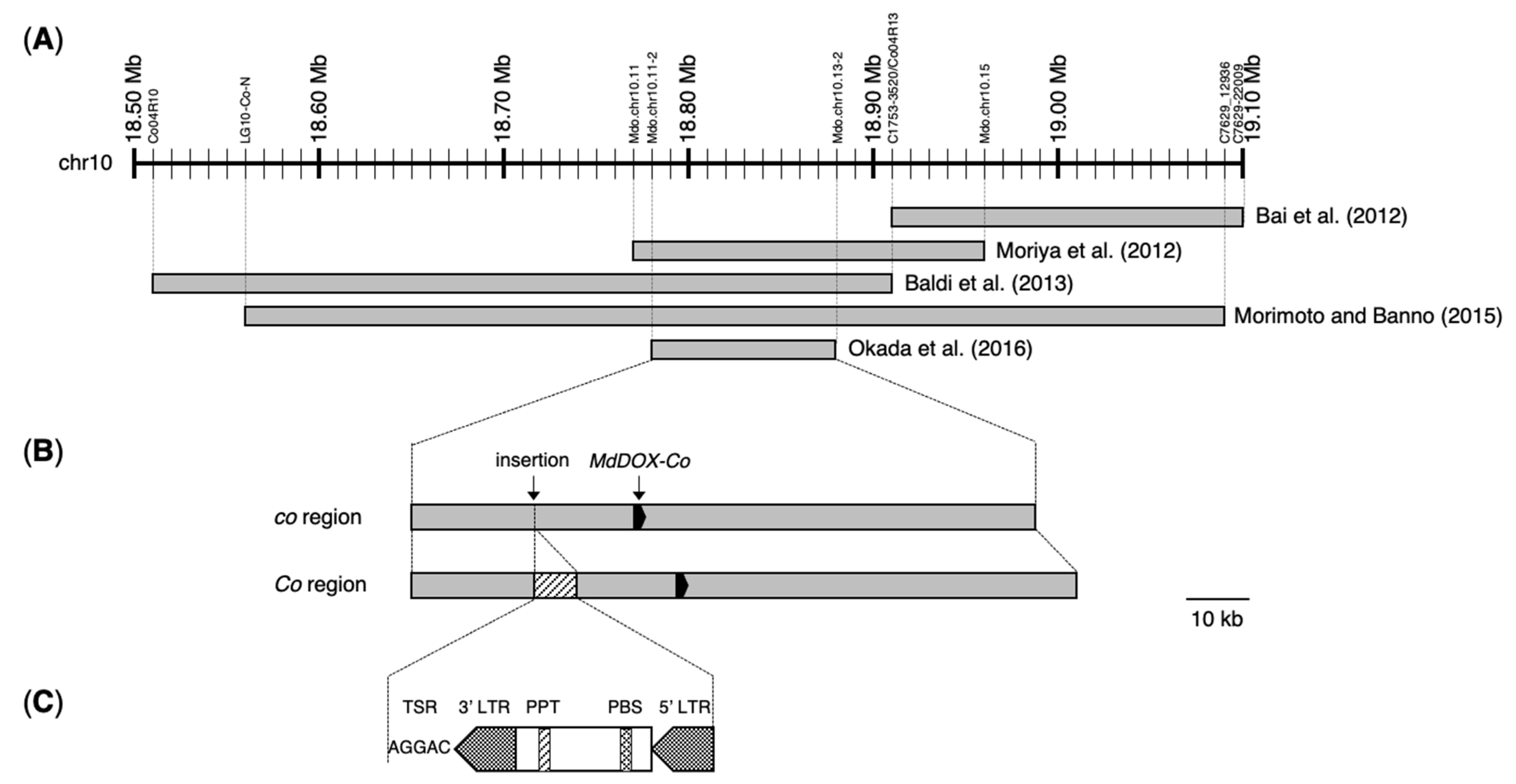
2. Fine Mapping of the Co Locus
3. Identification of Mutation in ‘McIntosh Wijcik’
4. Exploration of Co Candidate Genes
5. Expression Analysis of MdDOX-Co
6. Phenotypes of Transgenic Plants Overexpressing MdDOX-Co
7. Characterization of MdDOX-Co
8. Functions of MdDOX-Co and the Mechanisms of the Columnar Growth Phenotype
9. Modifier Genes and Other Genes Involved in the Columnar Growth Phenotype
10. Marker-Assisted Selection (MAS) Systems for Selecting Columnar Apples
11. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Costes, E.; Lauri, P.É.; Regnard, J.L. Analyzing fruit tree architecture: Implications for tree management and fruit production. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc: Oxford, UK, 2006; Volume 32, pp. 1–61. [Google Scholar]
- Petersen, R.; Krost, C. Tracing a key player in the regulation of plant architecture: The columnar growth habit of apple trees (Malus × domestica). Planta 2013, 238, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hollender, C.A.; Dardick, C. Molecular basis of angiosperm tree architecture. New Phytol. 2015, 206, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.L.; Hollender, C.A. Branching out: New insights into the genetic regulation of shoot architecture in trees. Curr. Opin. Plant Biol. 2019, 47, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, L.; Singh, R.; Brown, S.; Dardick, C.; Xu, K. Exploring DNA variant segregation types in pooled genome sequencing enables effective mapping of weeping trait in Malus. J. Exp. Bot. 2018, 69, 1499–1516. [Google Scholar] [CrossRef] [PubMed]
- Lapins, K.O. Inheritance of compact growth type in apple. J. Am. Soc. Hortic. Sci. 1976, 101, 133–135. [Google Scholar]
- Talwara, S.; Grout, B.W.W.; Toldam-Andersen, T.B. Modification of leaf morphology and anatomy as a consequence of columnar architecture in domestic apple (Malus × domestica Borkh.) trees. Sci. Hortic. 2013, 164, 310–315. [Google Scholar] [CrossRef]
- Costes, E.; Gion, J.M. Genetics and genomics of tree architecture. Adv. Bot. Res. 2015, 74, 157–200. [Google Scholar]
- Okada, K. Columnar growth phenotype in apple results from a deficiency of bioactive gibberellin. Kajitsu Nippon 2021, 76, 70–73. (In Japanese) [Google Scholar]
- Okada, K. Molecular mechanism and maker-assisted selection of columnar apples. Agric. Biotechnol. 2019, 3, 8–12. (In Japanese) [Google Scholar]
- Tobutt, K.R. Breeding columnar apples at East Malling. Acta Hortic. 1985, 159, 63–68. [Google Scholar] [CrossRef]
- Fisher, D.V. The ‘Wijcik spur McIntosh’. Fruit Var. J. 1995, 49, 212–213. [Google Scholar]
- Kelsey, D.F.; Brown, S.K. ‘McIntosh Wijcik’: A columnar mutation of ‘McIntosh’ apple providing useful in physiology and breeding research. Fruit Var. J. 1992, 46, 83–87. [Google Scholar]
- Sassa, H. Molecular mechanism of the S-RNase-based gametophytic self-incompatibility in fruit trees of Rosaceae. Breed. Sci. 2016, 66, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Inomata, Y.; Kudo, K.; Wada, M.; Masuda, T.; Bessho, H.; Suzuki, K. The influence of the training system on characteristics of tree growth, fruit productivity and dry matter production of columnar-type apple tree ‘Maypole’. Hortic. Res. 2004, 3, 387–392. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Blazek, J.; Krelinova, J. Tree growth and some other characteristics of new columnar apple cultivars bred in Holovousy, Czech Republic. Hortic. Sci. 2011, 38, 11–20. [Google Scholar] [CrossRef]
- Guitton, B.; Kelner, J.J.; Velasco, R.; Gardiner, S.E.; Chagne, D.; Costes, E. Genetic control of biennial bearing in apple. J. Exp. Bot. 2012, 63, 131–149. [Google Scholar] [CrossRef]
- Iwanami, H.; Moriya-Tanaka, Y.; Honda, C.; Hanada, T.; Wada, M. Apple thinning strategy based on a model predicting flower-bud formation. Sci. Hortic. 2019, 256, 108529. [Google Scholar] [CrossRef]
- Conner, P.J.; Brown, S.K.; Weeden, N.F. Randomly amplified polymorphic DNA-based genetic linkage maps of three apple cultivars. J. Am. Soc. Hortic. Sci. 1997, 122, 350–359. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef]
- Bai, T.; Zhu, Y.; Fernandez-Fernandez, F.; Keulemans, J.; Brown, S.; Xu, K. Fine genetic mapping of the Co locus controlling columnar growth habit in apple. Mol. Genet. Genom. 2012, 287, 437–450. [Google Scholar] [CrossRef][Green Version]
- Moriya, S.; Okada, K.; Haji, T.; Yamamoto, T.; Abe, K. Fine mapping of Co, a gene controlling columnar growth habit located on apple (Malus × domestica Borkh.) linkage group 10. Plant Breed. 2012, 131, 641–647. [Google Scholar] [CrossRef]
- Baldi, P.; Wolters, P.J.; Komjanc, M.; Viola, R.; Velasco, R.; Salvi, S. Genetic and physical characterisation of the locus controlling columnar habit in apple (Malus × domestica Borkh.). Mol. Breed. 2013, 31, 429–440. [Google Scholar] [CrossRef]
- Morimoto, T.; Banno, K. Genetic and physical mapping of Co, a gene controlling the columnar trait of apple. Tree Genet. Genomes 2015, 11, 807. [Google Scholar] [CrossRef]
- Okada, K.; Wada, M.; Moriya, S.; Katayose, Y.; Fujisawa, H.; Wu, J.; Kanamori, H.; Kurita, K.; Sasaki, H.; Fujii, H.; et al. Expression of a putative dioxygenase gene adjacent to an insertion mutation is involved in the short internodes of columnar apples (Malus × domestica). J. Plant Res. 2016, 129, 1109–1126. [Google Scholar] [CrossRef]
- Wolters, P.J.; Schouten, H.J.; Velasco, R.; Si-Ammour, A.; Baldi, P. Evidence for regulation of columnar habit in apple by a putative 2OG-Fe(II) oxygenase. New Phytol. 2013, 200, 993–999. [Google Scholar] [CrossRef]
- Wolters, P.J. Evidence for regulation of columnar habit in apple by a putative 2OG-Fe (II) oxygenase (vol 200, pg 993, 2013). New Phytol. 2015, 207, 928. [Google Scholar]
- Otto, D.; Petersen, R.; Brauksiepe, B.; Braun, P.; Schmidt, E.R. The columnar mutation (“Co gene”) of apple (Malus × domestica) is associated with an integration of a Gypsy-like retrotransposon. Mol. Breed. 2014, 33, 863–880. [Google Scholar] [CrossRef]
- Petersen, R.; Djozgic, H.; Rieger, B.; Rapp, S.; Schmidt, E.R. Columnar apple primary roots share some features of the columnar-specific gene expression profile of aerial plant parts as evidenced by RNA-Seq analysis. BMC Plant Biol. 2015, 15, 34. [Google Scholar] [CrossRef]
- Okada, K.; Wada, M.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; Nakayasu, M.; Mizutani, M.; Nakajima, M.; Moriya, S.; Shimizu, T.; et al. Columnar growth phenotype in apple results from gibberellin deficiency by ectopic expression of a dioxygenase gene. Tree Physiol. 2020, 40, 1205–1216. [Google Scholar] [CrossRef]
- Wada, M.; Iwanami, H.; Moriya, S.; Hanada, T.; Moriya-Tanaka, Y.; Honda, C.; Shimizu, T.; Abe, K.; Okada, K. A root-localized gene in normal apples is ectopically expressed in aerial parts of columnar apples. Plant Growth Regul. 2018, 85, 389–398. [Google Scholar] [CrossRef]
- Sun, X.; Wen, C.; Hou, H.; Huo, H.; Zhu, J.; Dai, H.; Zhang, Y. Genes involved in strigolactone biosyntheses and their expression analyses in columnar apple and standard apple. Biol. Plant. 2020, 64, 68–76. [Google Scholar] [CrossRef]
- Sun, X.; Wen, C.; Xu, J.; Wang, Y.; Zhu, J.; Zhang, Y. The apple columnar gene candidate MdCoL and the AP2/ERF factor MdDREB2 positively regulate ABA biosynthesis by activating the expression of MdNCED6/9. Tree Physiol. 2021, 41, 1065–1076. [Google Scholar] [CrossRef]
- Wang, L.; Yu, B.; Zhao, Y.; Li, Y.; Guo, J.; Zhu, Y. A putative 2OG-Fe(II) oxygenase’s response to gibberellin deficiency is related to the internodal growth of columnar apples. Acta Physiol. Plant. 2021, 43, 70. [Google Scholar] [CrossRef]
- Watanabe, D.; Takahashi, I.; Jaroensanti-Tanaka, N.; Miyazaki, S.; Jiang, K.; Nakayasu, M.; Wada, M.; Asami, T.; Mizutani, M.; Okada, K.; et al. The apple gene responsible for columnar tree shape reduces the abundance of biologically active gibberellin. Plant J. 2021, 105, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Ono, E.; Mizutani, M. Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J. 2014, 78, 328–343. [Google Scholar] [CrossRef]
- Matsuda, J.; Okabe, S.; Hashimoto, T.; Yamada, Y. Molecular cloning of Hyoscyamine 6-beta-hydroxylase, a 2-oxoglutarate- dependent dioxygenase, from cultured roots of Hyoscyamus niger. J. Biol. Chem. 1991, 266, 9460–9464. [Google Scholar] [CrossRef]
- Nakanishi, H.; Yamaguchi, H.; Sasakuma, T.; Nishizawa, N.K.; Mori, S. Two dioxygenase genes, Ids3 and Ids2, from Hordeum vulgare are involved in the biosynthesis of mugineic acid family phytosiderophores. Plant Mol. Biol. 2000, 44, 199–207. [Google Scholar] [CrossRef]
- Spartz, A.K.; Gray, W.M. Plant hormone receptors: New perceptions. Genes Dev. 2008, 22, 2139–2148. [Google Scholar] [CrossRef]
- Bedini, A.; Mercy, L.; Schneider, C.; Franken, P.; Lucic-Mercy, E. Unraveling the initial plant hormone signaling, metabolic mechanisms and plant defense triggering the endomycorrhizal symbiosis behavior. Front. Plant Sci. 2018, 9, 1800. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Sami, F.; Siddiqui, H.; Yusuf, M.; Gruszka, D.; Hayat, S. Role of strigolactones: Signalling and crosstalk with other phytohormones. Open Life Sci. 2020, 15, 217–228. [Google Scholar] [CrossRef]
- Sun, X.; Wen, C.; Zhu, J.; Dai, H.; Zhang, Y. Apple columnar gene MdDMR6 increases the salt stress tolerance in transgenic tobacco seedling and apple calli. J. Plant Growth Regul. 2021, 40, 187–196. [Google Scholar] [CrossRef]
- Dougherty, L.; Bai, T.; Brown, S.; Xu, K. Exploring DNA variant segregation types enables mapping loci for recessive phenotypic suppression of columnar growth in apple. Front. Plant Sci. 2020, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, J.; Dai, H. Characterization of transcriptional differences between columnar and standard apple trees using RNA-Seq. Plant Mol. Biol. Report. 2012, 30, 957–965. [Google Scholar] [CrossRef]
- Krost, C.; Petersen, R.; Schmidt, E.R. The transcriptomes of columnar and standard type apple trees (Malus × domestica)—A comparative study. Gene 2012, 498, 223–230. [Google Scholar] [CrossRef]
- Krost, C.; Petersen, R.; Lokan, S.; Brauksiepe, B.; Braun, P.; Schmidt, E.R. Evaluation of the hormonal state of columnar apple trees (Malus × domestica) based on high throughput gene expression studies. Plant Mol. Biol. 2013, 81, 211–220. [Google Scholar] [CrossRef]
- Tobutt, K.R. Breeding columnar apples at East Malling. Sci. Hortic. 1984, 35, 72–77. [Google Scholar] [CrossRef]
- Cmejlova, J.; Vavra, R.; Cmejla, R. A rapid real-time PCR assay for detecting columnar growth in apples (Malus × domestica). Plant Breed. 2020, 139, 1244–1250. [Google Scholar] [CrossRef]
- Claeys, H.; De Bodt, S.; Inze, D. Gibberellins and DELLAs: Central nodes in growth regulatory networks. Trends Plant Sci. 2014, 19, 231–239. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, K.; Honda, C. Molecular Mechanisms Regulating the Columnar Tree Architecture in Apple. Forests 2022, 13, 1084. https://doi.org/10.3390/f13071084
Okada K, Honda C. Molecular Mechanisms Regulating the Columnar Tree Architecture in Apple. Forests. 2022; 13(7):1084. https://doi.org/10.3390/f13071084
Chicago/Turabian StyleOkada, Kazuma, and Chikako Honda. 2022. "Molecular Mechanisms Regulating the Columnar Tree Architecture in Apple" Forests 13, no. 7: 1084. https://doi.org/10.3390/f13071084
APA StyleOkada, K., & Honda, C. (2022). Molecular Mechanisms Regulating the Columnar Tree Architecture in Apple. Forests, 13(7), 1084. https://doi.org/10.3390/f13071084






