Abstract
Fire disturbance can affect the function of the boreal forest ecosystem through litter decomposition and nutrient element return. In this study, we selected the Larix gmelinii forest, a typical forest ecosystem in boreal China, to explore the effect of different years (3 years, 9 years, 28 years) after high burn severity fire disturbance on the decomposition rate (k) of leaf litter and the Carbon:Nitrogen:Phosphorus (C:N:P) stoichiometry characteristics. Our results indicated that compared with the unburned control stands, the k increased by 91–109% within 9 years after fire disturbance, but 28 years after fire disturbance the decomposition rate of the upper litter decreased by 45% compared with the unburned control stands. After fire disturbance, litter decomposition in boreal forests can be promoted in the short term (e.g., 9 years after a fire) and inhibited in the long term (e.g., 28 years after a fire). Changes in litter nutrient elements caused by the effect of fire disturbance on litter decomposition and on the C, N, and C:N of litter were the main litter stoichiometry factors for litter decomposition 28 years after fire disturbance. The findings of this research characterize the long-term dynamic change of litter decomposition in the boreal forest ecosystem, providing data and theoretical support for further exploring the relationship between fire and litter decomposition.
1. Introduction
Fire is becoming increasingly intense in the context of global climate change, which has a substantial and persistent impact on boreal forests [1,2,3], especially on the decomposition of litter and the cycling of nutrients [4,5]. Litter decomposition shows different change rules under different climate and soil conditions, so it is highly dependent on environment [6,7]. Climate factors such as temperature and humidity have a great impact on litter decomposition. Higher temperatures and humidity are conducive to the activities of decomposers in soil, thus affecting the decomposition of litter [8,9]. At the same time, the changes of soil factors have different effects on litter decomposition, mainly in two aspects: biological factors (soil animals, microorganisms, etc.) and abiotic factors (physical and chemical properties such as soil temperature, humidity, pH value, etc.) in the soil affect litter decomposition [10,11,12,13]. Therefore, the relationships between fire, litter stoichiometry, and decomposition become the key to boreal forest ecosystem carbon sequestration and fuel management after fire disturbance [14,15].
The litter decomposition microenvironment (e.g., increasing temperature and reducing water) changes after a fire, which affects the composition of the soil animal community [16] (e.g., decreasing large-scale springtails and increasing small-scale springtails). These interactions between litter fragmentation and soil organisms further promote litter decomposition and carbon (C) and nitrogen (N) mineralization [17,18,19]. In contrast, inhibition effects include decreased soil N and phosphorus (P) content [20,21]. This chemical imbalance aggravates the nutrient limitation of microbial activity [22], and the subsequent decrease in fungal biomass slows litter decomposition [1]. At the same time, the long-term effects of species invasion on vegetation and decomposers after fire will also affect the litter decomposition rate (k) and the cycling of nutrients [23,24,25,26,27,28,29].
There is no uniform regularity in the decomposition and change of litter after a fire; some studies have found that k increased after a fire [30,31,32,33,34]. In contrast, other studies found that litter k slowed after a fire [1]. In terms of stoichiometric changes, studies have shown that the decomposition of litter after a fire will cause high sensitivity to stoichiometric imbalances on local scales [22]. Under nutrient-sufficient conditions, the hydrolysis of C drives the decomposition of litter, microbial N restriction (high litter C:N or low litter N:P) and P restriction (high litter C:P or N:P) appear, and the driving effect of N and P on decomposition is enhanced [35,36,37,38]. Frequent fire disturbance reduces the coupling relationship between litter C:N:P stoichiometry, the N:P ratio of microbial biomass, and enzyme activity, and the forest tends toward an N-restricted ecosystem [39].
Although much research has been done on litter decomposition and the nutrient elements of litter [40,41,42,43], there is still no conclusion on the change in k and the nutrient return patterns of forest ecosystems in boreal China after disturbance by fire. In this experiment, we selected the Larix gmelinii forest, a typical forest ecosystem in boreal China [44], to explore the influence of high burn severity fire disturbance on the decomposition rate (k) of leaf litter and the C, N, and P stoichiometric characteristics. We hypothesized that (1) after high burn severity fire disturbance, k value will accelerate; (2) high burn severity fire disturbance will retard the return of N and P nutrients to forest litter; and (3) the main driving factors affecting litter decomposition in the boreal forest after fire were litter C, N, and C:N. In order to verify the above hypotheses, the objectives of this study were to clarify the long-term variation pattern of litter decomposition in the boreal forest of China after high burn severity fire disturbance, to explore the relationship between litter decomposition and litter C:N:P stoichiometry after fire disturbance, and ultimately to provide data support for the post-fire restoration in boreal forest ecosystems.
2. Materials and Methods
2.1. Site Description
The experimental study was located in the Great Xing’an Mountain area, China (52°09′07″ N, 125°19′55″ E to 52°23′24″ N, 125°114′48″ E; Figure 1). This area is the main forest in northern China, on the southern edge of the Siberian ecosystem. Forest fires also occur frequently [45,46]. The climate of the area is a continental monsoon climate in a cold temperate zone. The atmosphere is humid and rainy in the summer. The annual average temperature is −2.8 °C, the minimum temperature is −52.3 °C, and the frost-free period is 90–110 days. Larix gmelinii forest is the top zonal plant community. The dominant woody plants in this area are Larix gmelinii, Betula platyphylla, and Populus davidiana.
Figure 1.
Map of the study region.
To verify the role of fire (regardless of the time since fire disturbance and of both the upper and lower litter layers) in the linear relationship between litter stoichiometry and litter decomposition, we used the k value of various fields and the corresponding annual average stoichiometric value for linear fitting.
2.2. Experimental Design
We selected three sites of Larix gmelinii forest in the Great Xing’an Mountains that had burned in 1987 (Tahe), 2005 (Nanwenghe), and 2012 (Mohe) as the fire disturbance test sites (Figure 1), and we investigated the basic soil data (Table 1). All fire samples were subject to high-severity fire. We selected three replicate stands in the burned area to conduct our investigation, and we selected the nearby unburned area as the control in each burned area. A total of 18 experimental stands were selected in the burned and unburned areas for our study conducted in May 2015. The size of each stand was 400 m2 (20 m × 20 m), and the distance between stands was more than 200 m to ensure the independence of data acquisition. In each research area, an adjacent unburned forest was selected as the control stand. To show that the difference in the decomposition of litter was caused by fire disturbance rather than litter itself, we placed the same litter bag in the burned and unburned control stands in each area (Figure 2). To consider the response of litter decomposition to environmental changes after fire disturbance, we divided the litter into layers to clarify the mechanism of litter decomposition.

Table 1.
Overview of the study area (n = 3).

Figure 2.
Map of the experimental design. Note: Green: the litter arranged on the upper layer; Orange: the litter arranged on the lower layer. The shape of the litter decomposition bag used was the same for each sample taken at sites representing the same number of years since the fire occurred, indicating that the litter was homogeneous at the beginning of the layout.
2.3. Data Collection
In October 2015, we collected fallen needles in the Larix gmelinii forest from various areas and placed 10 g into 15 × 20 cm2 decomposition bags. The decomposition bags were paired: the upper decomposition bag was placed on the surface of the litter layer, and the lower decomposition bag was placed directly on the soil of forestland with the litter layer removed. A total of 432 bags were placed in the sample areas; 72 bags were placed in each sample area.
We collected nine decomposition bags per sample area per month in May, July, August, and September of 2016. We cleaned, dried, and weighed the litter from the bags. The remaining needles in each pack were crushed with a grinder and passed through a 60-mesh sieve. We digested the sample with the sulfuric acid-hydrogen peroxide method and measured the total C, N, and P contents by spectrophotometry (METASH V5000) and a Multi C/N analyzer (Multi C/N 3000, Analytik Jena, Burladingen, Germany). All litter samples were obtained from the field by the author and brought back to the laboratory for determination, but they were not kept in a publicly available herbarium.
2.4. Data Analysis
Linear model analyses were performed using the statistical analysis software SPSS 16.0 (SPSS Institute, Inc., Chicago, IL, USA). One-way ANOVA was used to compare significant differences in C, N, and P nutrients and k after fire disturbance. A two-way ANOVA was used to compare the interactions between fire disturbance, year, and litter layer, followed by least significant differences tests for post hoc comparisons. To refine the relationships between C, N, and P and the decomposition rate after fire disturbance, we used the “ggplot2” package (Dixon 2003) in R4.0.5 software (R core team, 2018) for the linear fitting map. We used the “vegan” package (Dixon 2003) for redundancy analysis (RDA).
2.5. Litter Decomposition Model
The decomposition index model was [47,48]:
where X0 is the initial weight of the litter, Xt is the weight of the remaining debris after decomposition for a period of time (t), and “a” is the fitting parameter.
Xt/X0 = a∙e−kt,
The half-life of litter decomposition was calculated as:
t0.05 = ln0.5/(−k),
The entire decomposition time (95% decomposition) was calculated as follows:
where “k” is the litter decomposition coefficient (g/g∙a) and “t” is the litter decomposition time expressed in years.
t0.95 = ln0.05/(−k),
3. Results
3.1. Dynamic Change in Litter Decomposition after Fire Disturbance
After 1 year of decomposition, the residual mass of litter in the upper litter layer was 4.47% lower in the 3-year postfire stands and 7.24% lower in the 9-year postfire stands than in the paired unburned stands (p > 0.05), but in the 28-year postfire stands, it was 16.08% significantly higher than that in paired unburned stands (p < 0.05, Figure 2).
The quality of litter residue in the fire disturbance years sites was different from those without fire. For 3 years postfire stands, the residual mass of litter arranged in the lower layer was 5.99% higher than that of unburned litter (p > 0.05); for 28 years postfire stands, the residual mass of litter arranged in the lower layer was 2.21% lower than that of unburned litter (p > 0.05); but, for 9 years postfire stands, the residual mass of litter arranged in the lower layer was 10.19% lower than that of unburned litter (p < 0.05, Figure 3).
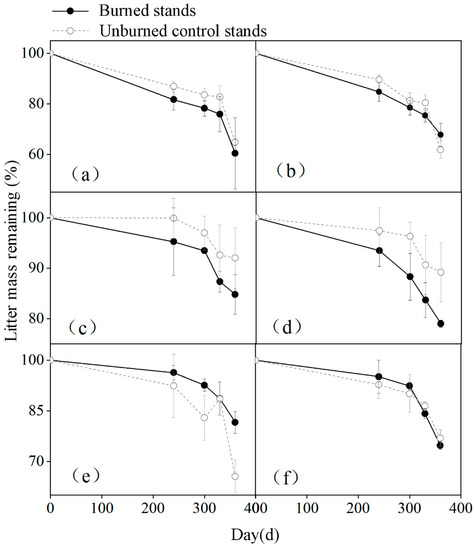
Figure 3.
Decomposition of Larix leaf litter in bags placed on the soil surface (left) and leaf litter surface (right) for one year in burned and unburned control stands at different ages after fire disturbance in a Larix gmelinii forest (n = 3). Note: The data are the mean ± standard error. (a,b) show the surpluses of litter mass after a three-year fire disturbance. (c,d) show the surplus of litter mass after a nine-year fire disturbance. (e,f) show the litter mass surplus after 28 years of fire disturbance. On the left is the upper litter layer, and on the right is the lower litter layer.
Under all treatments with different burning years, the k value of needle leaf litter was less than 0.41. In different years postfire, the effects of fire vs. no fire on the k value were different. In 9-year postfire stands, the decomposition rate of the upper litter increased by 91% (p < 0.05) compared with that of the unburned control stands, and it decreased by 45% (p < 0.05) compared with that of the 28-year postfire stands. Samples taken from the 9-years postfire stands, showed a decomposition rate of the lower litter having increased by 109% (p < 0.05) compared to that of the unburned control stands (Table 2).

Table 2.
Fitting rate of coniferous litter decomposition (n = 3).
3.2. Litter C, N, and P Contents and Stoichiometric Ratios
In the 3-year postfire stands, the average annual C content of the lower litter layer was 1.3% lower than that of the paired unburned stands (p < 0.05), and the middle yearly C:N ratio of the lower litter layer was 87.7% higher than that of the paired unburned sample areas (p < 0.05). In the 9-year postfire stands, the average annual N content of the lower litter layer was 19.6% less than that in paired unburned stands (p < 0.05). In the 28-year postfire disturbance stands, the average annual C content of the upper litter layer was significantly lower than that in the paired unburned stands (p < 0.05). All other changes in C, N, and P and their proportion in the litter were not significant (p > 0.05) (Figure 4).

Figure 4.
Carbon (C), nitrogen (N), and phosphorous (P) contents and stoichiometric ratios of leaf litter in stands of different years after fire disturbance in the Larix gmelinii forest (n = 3). Note: The data are the average annual value ± standard error. Capital letters represent differences between groups, and small letters represent differences within groups. (a) indicates the change of C concentration of litter in different layers under different burning years; (b) indicates the change of C:N of litter in different layers under different burning years; (c) indicates the change of N concentration of litter in different layers under different burning years; (d) indicates the change of C:P of litter in different layers under different burning years; (e) Indicates the change of P concentration in different layers of litter under different burning years; (f) indicates the change of N:P in different layers of litter under different burning years.
3.3. Litter Stoichiometry and Decomposition Rate
In the unburned sample stand, the effect of litter N, P, C:N ratio, C:P ratio, and k value was greater than that in the burned stands (p < 0.05, Figure 5, Table A3); at the same time, there was no significant linear relationship between C and k value, N:P ratio, and k value in the burned and unburned control stands (p > 0.05). With increasing k values, the contents of N and k decreased gradually; in contrast, the C:N ratio and the C:P ratio increased gradually with increasing k values. Based on this, we concluded that the k value of litter was higher under the condition of fire disturbance. In the case of a large k value, the lower the return efficiency of N and P; at the same time, the change rate of the C:N ratio increased. The faster the litter decomposes, the higher the change rate of the C:N ratio.
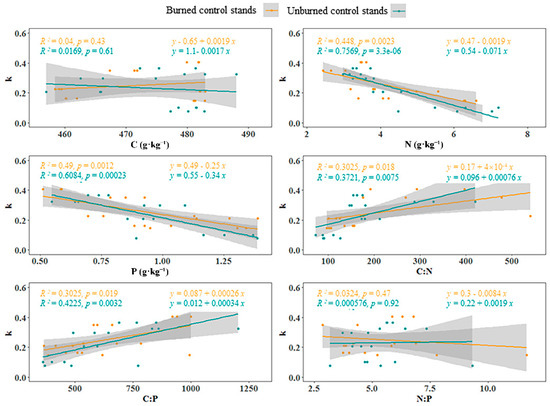
Figure 5.
Linear relationships between C, N, P, and their ratios and the litter decomposition rate (k) (n = 3). Note: The grey area is the 95% confidence interval.
The redundancy analysis (RDA) examined the multivariate relationships of C, N, and P and their ratios with the k values. In the unburned stands, the two main RDA axes (RDA1 and RDA2) accounted for 71.33% and 27.82% of the variation, respectively. In the burned stands, RDA1 and RDA2 accounted for 62.84% and 36.38%, respectively. The C:N and C:P ratios were positively associated with k, while the N and the P contents were negatively associated with k. In burned stands, the C:N ratio appeared more associated with k (Figure 6). To further clarify the relationship between fire, k, and litter elements, we built a mixed effect model. The results showed that P and N:P ratio-driven litter decomposition changed to C:N and N:P ratio-driven litter decomposition after fire disturbance (Figure 6).

Figure 6.
Redundancy analysis of leaf litter nutrients and stoichiometry and k values in burned and unburned control (a) and burned (b) stands of three different years postfire (n = 3).
4. Discussion
4.1. Effect of Fire Disturbance on k
Our results showed that the k value did not continue to accelerate after disturbance by fire (Figure 3, Table 2 and Table A1). This result was different from the initial increase of k value [30,31,32] and the decrease after fire disturbance [1,49,50,51,52] as well as the research results that there is no change in the early stage 15 years after fire disturbance, and it was suppressed in the later stage [53] (Table A2). For boreal forest ecosystems, sunlight and rain become important regulatory factors in the upper litter layer in the short term after fire disturbance [54]. The k value seems to increase due to the enhancement of photodegradation and the increase in black carbon [30,31,32]. With more time since the fire [8], the external water environment also affects nutrient element limitation [55]. Fire disturbance causes deeper soil water infiltration [56]. This will lead to the downward transfer of the soluble components in litter and soil, thus influencing litter decomposition by affecting microorganisms and enzymes. Warm and humid sites promote enzyme activity [9], fungal biomass, microorganism abundance, levels of chitinase, and arthropods all increase, while vegetation (notable shrub) cover increases. These changes weaken the promoting effect of fire on decomposition, and the increase in k slows until a decrease appears [1,23,57,58]. In the long term, high-severity wildfire disturbance changes the properties of litter with the germination of shrubs and grass, the accumulation of refractory substances, and the increase in decomposition pressures, resulting in slower decomposition rates [8,18,57,59]. The lower decomposition rate will further promote the occurrence of fire pairs, which means that litter decomposition driven by fire is more efficient than that driven by microorganisms [56].
However, this study also found that the upper and the lower litter layers had different responses to fire disturbance. The litter microenvironment plays a critical role in boreal forest ecosystem decomposition [60,61]. After fire disturbance, and an increase in vegetation and shrub cover, the upper litter layer decomposition rate decreases significantly [32]. Whereas after the fire, the lower litter layer decomposition increases significantly [18,33,58,62]. Fire changes the microenvironment and then affects the litter decomposition process [39,63,64,65,66,67].
The transformation of fungi and microorganisms in the microenvironment plays an important role in the decomposition of litter [6,10,68]. After fire disturbance, the potential activity of decomposers is reduced, which will reduce the decomposition of a variety of organic compounds [11]. Fungi have an impact on the activity of soil animals and microorganisms [69]. After fire disturbance, the temperature increases, the pH value and moisture content of the soil changes, and the living habitat of soil animals are affected, resulting in a reduction in the activity of soil animals and microorganisms to inhibit the decomposition of litter [70]. Additionally, extracellular enzyme activity is an essential indicator of litter decomposition in complex microenvironments after fire disturbance [33,71,72]. The decrease in extracellular enzyme activity explains why litter rate decomposition decreases after a fire [33,71].
4.2. Effect of Fire Disturbance on C, N, and P Stoichiometry
Our results showed that litter C and N returned faster in burned stands than in the unburned control stands, confirming the hypothesis that the return of litter nutrients to the boreal forest will be accelerated after severe fire disturbance (Figure 4, Table A1). Due to the decrease in soil-available N and P after fire disturbance, microorganisms have a more robust demand for litter, which further leads to the acceleration of a lower litter layer N return [63]. Fire disturbance decouples N and P cycling, and unaffected decomposers control their C:N ratio by adjusting C and P turnover time and N mineralization rates, resulting in the disproportionate return of litter nutrient elements and a short transition to an N-restricted ecosystem [39,73]. In contrast to our results, Yang [34] found that the litter N and P contents in boreal forest ecosystems increased with the time since fire; the results showed a decrease after 4 to 14 years and then a return to the unburned level 40 years after a fire. This result was probably due to the different types and amounts of microorganisms and bacteria affecting litter nutrient return. During decomposition after a fire, the upper litter layer’s coverage increases the relative abundance of mycorrhizal fungi, gram-positive bacteria, and gram-negative bacteria in the lower litter layer [32,74,75]. Fungi tend to assimilate relatively simple substrates, whereas gram-positive bacteria show a preference for more complex substrates, which means that the upper litter layer has a certain inferiority in nutrient return, especially in the process of microbial recovery after a fire [76,77,78,79].
Additionally, this study showed that the interaction between fire and the litter layer had a significant effect on the N:P ratio in the short term (Table A1), consistent with previous results. For example, the litter N:P ratio is affected by the time since burning, and it returns closer to the control level with increasing time [34]. Fire disturbance can accelerate litter P release in the upper layer, and the effect of fire disturbance on litter P is greater than that of N, especially in P-poor soil with alternating dry and wet seasons, which further affects the N:P ratio [80,81]. However, some studies have also shown that fire disturbance can weaken the return rate of litter N and P to the soil, which may be affected by fire frequency [82]. Therefore, fire disturbance generally accelerates the nutrient release of litter, and it regulates the litter N:P ratio.
4.3. The Relationship between Litter Nutrient Elements and Decomposition Rate
The “ecological stoichiometry hypothesis” believes that element migration occurs and that it is an important cause that affects decomposition [35], litter decomposition and nutrient changes co-occur, so their relationship is key to understanding the nutrient cycling processes of forest ecosystems [83]. Our results showed that litter P and N:P ratio-driven litter decomposition changed to C, N, and N:P ratio-driven litter decomposition after fire disturbance (Figure 5 and Figure 6). We do not deny that decomposers are the most vital driving force of decomposition. Using the litter element itself as a driving factor to analyze litter changes makes the decomposition problem more accessible and more intuitive [57]. However, current research shows no consistent conclusion on which element is the most critical factor driving decomposition [35,84].
Some studies believe that the demand for microorganisms in a specific environment determines the relationship between elements and the decomposition rate [85]. Due to the order of microorganisms for N, the litter decomposition of high-N leaves is faster, while a low N content will inhibit litter decomposition [39,63,86]. Fire disturbance changed the preference of microorganisms for N and P, causing litter nitrogen to replace P to drive decomposition [22]. In this regard, Saura [87] proposed that rainwater interception under drought conditions reduces the N decomposition of litter, which explains the reduction in litter N to a certain extent. Some scholars think that there is a similar “Alfred ratio” in the microbial N and P contents. When the N:P ratio is more than 16, it is limited by P; when the N:P ratio is less than 14, it is limited by N, and when the N:P ratio is between 14 and 16, it is limited by both N and P [88]. However, some scholars believe that the loss of litter quality is mainly driven by the loss of C [22] because the loss of litter quality and C content have the same pattern [41]. The soluble C content of litter is linearly correlated with microbial activity, thus affecting the k [19]. Additionally, both the C:N ratio and the C:P ratio are closely related to the litter k value [89]. Chacón [90] suggested that litter P content and C:N ratio can be used to predict litter mass surplus or loss.
Fire disturbance increases the limitations of N and P in boreal forests through soil-available N, and it makes this demand more urgent [22,28,39,91,92]. The change in litter-driving elements after a fire is consistent with the shift in dominant microbial populations [93]. The abundance of bacteria is generally higher under N restriction, while P restriction is more suitable for fungal survival [94]. P and N:P ratio-driven litter decomposition changed to C:N and N:P ratio-driven litter decomposition after fire disturbance (Figure 4). The effects of soil conditions and invertebrates on decomposition also increase after fire disturbance, which may be related to the higher C:N and lower C:P ratios at higher decomposition rates [95,96].
At the same time, relevant studies show that the frequency and the severity of fire interference determines its impact on ecosystem processes [8], and the litter coverage after fire is lower than that without fire [97]. High-frequency fire has an inhibitory effect on litter decomposition [96]. High-frequency fire leads to an increase in the temperature of litter itself, a decrease in water content, and a decrease in the effectiveness of soil N and P. The decomposition rate of litter produced at frequent combustion sites slows down and reduces the N cycle in the region, but the C:N ratio of litter increases with increasing fire frequency [51,96]. The implementation of reasonable prescribed burning measures is conducive to litter decomposition. Ficken [95] found that the k value of planned fires once every three years was higher than that of yearly planned fires and no fires. Prescribed burning greatly reduces the accumulation of litter, promotes litter decomposition, and reduces the frequency of fire [98].
As an important ecological factor, fire disturbance should not be ignored in understanding nutrient cycling processes in boreal forests [22]. Future research should explore how the inflection point of the k value changes with time after combustion, the impact of postfire environmental changes on litter decomposition, and the impact of postfire products such as black carbon and ash on litter decomposition. Further consideration of the vertical plant-litter-soil pathways should reveal the migration patterns of C, N, and P through the forest ecosystem and help to clarify the role of fire disturbance in the stoichiometric change in forest litter nutrients [39].
5. Conclusions
Fire disturbance can affect the function of the boreal forest ecosystem through litter decomposition and nutrient element return. Fire disturbance promotes litter decomposition and nutrient cycling in the short term. However, over longer time scales as forests recover, the promotion turns into inhibition, which is inconsistent with the first and the second hypotheses. Changes in litter nutrient elements caused by the effect of fire disturbance on litter decomposition, C, N, and C:N of litter were the main litter stoichiometry factors for litter decomposition 28 years after fire disturbance, which is consistent with our third hypothesis. Our results indicated that the role of fire disturbance on litter decomposition and nutrient cycling should not be ignored in the boreal forest of China. After twenty-eight years, fire still functions as an inhibitory effect on litter decomposition in the boreal forest. Based on this, our research provides a data supplement for the dynamic change of litter decomposition in different years after fire, which can explore the driving factors of fire disturbance on the rate of litter decomposition and the stoichiometry of C, N, and P in the boreal forest ecosystem of China, and it can characterize the long-term dynamic change of litter decomposition in the boreal forest ecosystem, providing theoretical support for further exploring the relationship between fire and litter decomposition.
Author Contributions
Conceptualization, L.S. and T.H.; methodology, F.L. and B.Z.; software, F.L and Z.S.; validation, L.S., T.H. and F.L.; formal analysis, F.L. and Z.S.; writing—original draft preparation, F.L., Z.S. and G.J.B.; writing—review and editing, L.S. and T.H.; supervision, T.H.; project administration, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research were financially supported by the National Natural Science Foundation of China (No. 31001324, No. 32071777), Youth Lift Project of China Association for Science and Technology (No. YESS20210370), and Heilongjiang Province Outstanding Youth Joint Guidance Project (No. LH2021C012).
Acknowledgments
We greatly appreciate the “Northern Forest Fire Management Key Laboratory” of the State Forestry and Grassland Bureau and the “National Innovation Alliance of Forest and Grassland Fire Prevention and Control Technology”, China, for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Stoichiometry and two-way ANOVA of C, N and P in different layers of litter under the same burning age.
Table A1.
Stoichiometry and two-way ANOVA of C, N and P in different layers of litter under the same burning age.
| Disturbance Years | Litter Layer | Control | C Content (g·kg−1) | N Content (g·kg−1) | P Content (g·kg−1) | C:N Ratio | C:P Ratio | N:P Ratio | k |
|---|---|---|---|---|---|---|---|---|---|
| 3 | Upper | Burned | 481.20 ± 0.63 | 3.52 ± 0.04 | 0.56 ± 0.02 | 294.14 ± 58.93 | 953.10 ± 23.89 | 6.31 ± 0.26 | 0.41 ± 0.14 |
| Unburned | 483.49 ± 2.48 | 3.20 ± 0.14 | 0.61 ± 0.03 | 345.68 ± 39.12 | 964.61 ± 119.56 | 6.36 ± 0.58 | 0.33 ± 0.04 | ||
| Lower | Burned | 470.17 ± 1.41 | 2.71 ± 0.18 | 0.85 ± 0.04 | 307.63 ± 89.41 | 611.93 ± 24.53 | 3.36 ± 0.50 | 0.35 ± 0.47 | |
| Unburned | 476.32 ± 2.67 | 3.44 ± 0.15 | 0.74 ± 0.02 | 163.93 ± 5.98 | 767.97 ± 46.17 | 5.71 ± 0.08 | 0.37 ± 0.03 | ||
| p value | B | 0.06558 | 0.17984 | 0.352155 | 0.443 | 0.24216 | 0.01837 * | 0.472 | |
| L | 0.00176 ** | 0.07107 | 0.000174 *** | 0.179 | 0.00366 ** | 0.00217 ** | 0.875 | ||
| L * B | 0.35831 | 0.00489 ** | 0.026756 * | 0.126 | 0.3077 | 0.02184 * | 0.267 | ||
| 9 | Upper | Burned | 481.74 ± 0.57 | 6.39 ± 0.11 | 1.20 ± 0.12 | 101.90 ± 2.62 | 664.55 ± 177.81 | 8.21 ± 1.87 | 0.15 ± 0.03 |
| Unburned | 480.06 ± 1.60 | 5.87 ± 0.74 | 1.26 ± 0.19 | 102.22 ± 12.08 | 541.92 ± 119.68 | 5.94 ± 1.79 | 0.08 ± 0.06 | ||
| Lower | Burned | 482.35 ± 0.29 | 5.09 ± 0.25 | 1.27 ± 0.07 | 114.75 ± 7.17 | 423.69 ± 35.80 | 4.48 ± 0.66 | 0.21 ± 0.01 | |
| Unburned | 480.52 ± 0.98 | 6.33 ± 0.50 | 1.26 ± 0.06 | 90.89 ± 11.36 | 405.20 ± 26.32 | 5.14 ± 0.42 | 0.10 ± 0.06 | ||
| p value | B | 0.114 | 0.4612 | 0.812 | 0.233 | 0.537 | 0.568 | 0.013 * | |
| L | 0.602 | 0.3939 | 0.785 | 0.936 | 0.123 | 0.132 | 0.138 | ||
| L * B | 0.943 | 0.0953 | 0.789 | 0.222 | 0.647 | 0.312 | 0.495 | ||
| 28 | Upper | Burned | 460.79 ± 0.61 | 3.89 ± 0.25 | 0.95 ± 0.04 | 148.42 ± 6.74 | 499.60 ± 20.30 | 4.13 ± 0.28 | 0.14 ± 0.03 |
| Unburned | 467.87 ± 3.63 | 3.55 ± 0.16 | 1.00 ± 0.05 | 159.28 ± 10.77 | 573.33 ± 44.55 | 4.21 ± 0.33 | 0.30 ± 0.05 | ||
| Lower | Burned | 459.01 ± 0.30 | 3.61 ± 0.21 | 0.86 ± 0.14 | 296.33 ± 122.06 | 666.45 ± 71.82 | 5.67 ± 0.79 | 0.23 ± 0.00 | |
| Unburned | 462.86 ± 2.96 | 4.14 ± 0.22 | 0.91 ± 0.03 | 188.32 ± 12.18 | 557.22 ± 19.46 | 4.53 ± 0.23 | 0.22 ± 0.02 | ||
| p value | B | 0.0497 * | 0.6649 | 0.51 | 0.454 | 0.701 | 0.2855 | 0.002 * | |
| L | 0.1891 | 0.4967 | 0.284 | 0.189 | 0.129 | 0.0798 | 0.833 | ||
| L * B | 0.5129 | 0.0765 | 0.999 | 0.363 | 0.074 | 0.2283 | 0.002 * |
Note: *, **, *** represents p < 0.05, p < 0.01, p < 0.001, respectively.

Table A2.
Stoichiometric characteristics and two-way ANOVA of C, N and P in different layers of litter burned and unburned under different burning years.
Table A2.
Stoichiometric characteristics and two-way ANOVA of C, N and P in different layers of litter burned and unburned under different burning years.
| Control | Litter Layer | C Content (g·kg−1) | N Content (g·kg−1) | P Content (g·kg−1) | C:N Ratio | C:P Ratio | N:P Ratio | k | |
|---|---|---|---|---|---|---|---|---|---|
| Burned | Upper | 3 | 481.20 ± 0.63 | 3.52 ± 0.04 | 0.56 ± 0.02 | 294.14 ± 58.93 | 953.10 ± 23.89 | 6.31 ± 0.26 | 0.41 ± 0.14 |
| 9 | 481.74 ± 0.57 | 6.39 ± 0.11 | 1.20 ± 0.12 | 101.90 ± 2.62 | 664.55 ± 177.81 | 8.21 ± 1.87 | 0.15 ± 0.03 | ||
| 28 | 460.79 ± 0.61 | 3.89 ± 0.25 | 0.95 ± 0.04 | 148.42 ± 6.74 | 499.60 ± 20.30 | 4.13 ± 0.28 | 0.14 ± 0.03 | ||
| Lower | 3 | 470.17 ± 1.41 | 2.71 ± 0.18 | 0.85 ± 0.04 | 307.63 ± 89.41 | 611.93 ± 24.53 | 3.36 ± 0.50 | 0.35 ± 0.47 | |
| 9 | 482.35 ± 0.29 | 5.09 ± 0.25 | 1.27 ± 0.07 | 114.75 ± 7.17 | 423.69 ± 35.80 | 4.48 ± 0.66 | 0.21 ± 0.01 | ||
| 28 | 459.01 ± 0.30 | 3.61 ± 0.21 | 0.86 ± 0.14 | 296.33 ± 122.06 | 666.45 ± 71.82 | 5.67 ± 0.79 | 0.23 ± 0.00 | ||
| p value | Y | 0.0000401 *** | 0.838 | 0.778 | 0.767 | 0.1453 | 0.6739 | 0.000 *** | |
| L | 0.163 | 0.217 | 0.505 | 0.39 | 0.0931. | 0.0522 | 0.343 | ||
| L * Y | 0.35 | 0.626 | 0.297 | 0.362 | 0.0126 * | 0.0179 * | 0.141 | ||
| Unburned | Upper | 3 | 483.49 ± 2.48 | 3.20 ± 0.14 | 0.61 ± 0.03 | 345.68 ± 39.12 | 964.61 ± 119.56 | 6.36 ± 0.58 | 0.33 ± 0.04 |
| 9 | 480.06 ± 1.60 | 5.87 ± 0.74 | 1.26 ± 0.19 | 102.22 ± 12.08 | 541.92 ± 119.68 | 5.94 ± 1.79 | 0.08 ± 0.06 | ||
| 28 | 467.87 ± 3.63 | 3.55 ± 0.16 | 1.00 ± 0.05 | 159.28 ± 10.77 | 573.33 ± 44.55 | 4.21 ± 0.33 | 0.30 ± 0.05 | ||
| Lower | 3 | 476.32 ± 2.67 | 3.44 ± 0.15 | 0.74 ± 0.02 | 163.93 ± 5.98 | 767.97 ± 46.17 | 5.71 ± 0.08 | 0.37 ± 0.03 | |
| 9 | 480.52 ± 0.98 | 6.33 ± 0.50 | 1.26 ± 0.06 | 90.89 ± 11.36 | 405.20 ± 26.32 | 5.14 ± 0.42 | 0.10 ± 0.06 | ||
| 28 | 462.86 ± 2.96 | 4.14 ± 0.22 | 0.91 ± 0.03 | 188.32 ± 12.18 | 557.22 ± 19.46 | 4.53 ± 0.23 | 0.22 ± 0.02 | ||
| p value | Y | 0.0000429 *** | 0.71 | 0.496 | 0.435 | 0.0987. | 0.0391 * | 0.000 *** | |
| L | 0.112 | 0.551 | 0.936 | 0.184 | 0.2448 | 0.5468 | 0.968 | ||
| L * Y | 0.983 | 0.856 | 0.581 | 0.081 | 0.4492 | 0.4554 | 0.098 | ||
Note: *, *** represents p < 0.05, p < 0.001, respectively.

Table A3.
Linear Fit of Decomposition Rate k and Litter CNP.
Table A3.
Linear Fit of Decomposition Rate k and Litter CNP.
| Fitting Element | Control | Picture Number | Linear Equation | R Square | p Value |
|---|---|---|---|---|---|
| C | Burned | a | y = 467.37 + 20.67x | 0.03937 | 0.430 |
| Unburned | b | y = 477.42 − 9.74x | 0.01695 | 0.607 | |
| N | Burned | c | y = 6.39 − 8.72x | 0.45138 | 0.002 |
| Unburned | d | y = 6.85 − 10.62x | 0.75055 | 0.000 | |
| P | Burned | e | y = 1.43 − 1.94x | 0.49177 | 0.001 |
| Unburned | f | y = 1.42 − 1.99x | 0.64738 | 0.000 | |
| C:Nratio | Burned | g | y = 21.79 + 753.95x | 0.30394 | 0.018 |
| Unburned | h | y = 63.97 + 485.78x | 0.36926 | 0.007 | |
| C:Pratio | Burned | i | y = 345.12 + 1164.22x | 0.29938 | 0.019 |
| Unburned | j | y = 348.82 + 1251.7x | 0.42756 | 0.003 | |
| N:Pratio | Burned | k | y = 6.34 − 3.92x | 0.03296 | 0.471 |
| Unburned | l | y = 5.25 + 0.3x | 0.00058 | 0.924 |
References
- Kong, J.J.; Yang, J. A Review of effects of fire disturbance on soil environment in boreal coniferous forests. Chin. J. Soil. Sci. 2014, 45, 291–296. [Google Scholar] [CrossRef]
- Hu, T.X.; Hu, H.Q.; Li, F.; Zhao, B.Q.; Wu, S.; Zhu, G.Y.; Sun, L. Long-term effects of post-fire restoration types on nitrogen mineralisation in a Dahurian larch (Larix gmelinii) forest in boreal China. Sci. Total. Environ. 2019, 679, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Heon, J.; Arseneault, D.; Parisien, M.A. Resistance of the boreal forest to high burn rates. Proc. Natl. Acad. Sci. USA 2014, 111, 13888–13893. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O. Fire-derived charcoal causes loss of forest humus. Science 2008, 320, 629. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.A.M.; Arneth, A.; Kautz, M.; Poulter, B.; Smith, B. Important role of forest disturbances in the global biomass turnover and carbon sinks. Nat. Geosci. 2019, 12, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.E.; Vesterdal, L. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Certini, G.; Moya, D.; Lucas-Borja, M.E.; Mastrolonardo, G. The impact of fire on soil-dwelling biota: A review. For. Ecol. Manag. 2021, 488, 118989. [Google Scholar] [CrossRef]
- Petraglia, A.; Cacciatori, C.; Chelli, S.; Fenu, G.; Calderisi, G.; Gargano, D.; Abeli, T.; Orsenigo, S.; Carbognani, M. Litter decomposition: Effects of temperature driven by soil moisture and vegetation type. Plant Soil 2019, 435, 187–200. [Google Scholar] [CrossRef]
- Hopkins, J.R.; Semenova-Nelsen, T.; Sikes, B.A. Fungal community structure and seasonal trajectories respond similarly to fire across pyrophilic ecosystems. Fems Microbiol. Ecol. 2021, 97, fiaa219. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Caprio, A.C.; Georgiou, K.; Finnegan, C.; Hobbie, S.E.; Hatten, J.A.; Jackson, R.B. Low-intensity frequent fires in coniferous forests transform soil organic matter in ways that may offset ecosystem carbon losses. Glob. Chang. Biol. 2021, 27, 3810–3823. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Y.; Guan, L.L.; Wei, X.H.; Tang, X.L.; Liu, S.G.; Liu, J.X.; Zhang, D.Q.; Yan, J.H. Factors influencing leaf litter decomposition: An intersite decomposition experiment across China. Plant Soil 2008, 311, 61–72. [Google Scholar] [CrossRef]
- Kuruppuarachchi, K.; Seneviratne, G.; Madurapperuma, B.D. Carbon Sequestration in Tropical Forest Stands: Its Control by Plant, Soil and Climatic Factors. Open J. For. 2016, 6, 59–71. [Google Scholar] [CrossRef]
- Hatten, S.S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition interrestrial ecosystems. Ann. Rev. Ecol. Evo. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Harguindeguy, N.P.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Yin, R.; Eisenhauer, N.; Schmidt, A.; Gruss, I.; Purahong, W.; Siebert, J.; Schadler, M. Climate change does not alter land-use effects on soil fauna communities. Appl. Soil. Ecol. 2019, 140, 1–10. [Google Scholar] [CrossRef]
- Yang, X.D.; Zhao, Y.; Warren, M.W.; Chen, J. Mechanical fragmentation enhances the contribution of Collembola to leaf litter decomposition. Eur. J. Soil. Biol. 2012, 53, 23–31. [Google Scholar] [CrossRef]
- Ludwig, S.M.; Alexander, H.D.; Kielland, K.; Mann, P.J.; Natali, S.M.; Ruess, R.W. Fire severity effects on soil carbon and nutrients and microbial processes in a Siberian larch forest. Glob. Chang. Biol. 2018, 24, 5841–5852. [Google Scholar] [CrossRef]
- Stirling, E.; Macdonald, L.M.; Smernik, R.J.; Cavagnaro, T.R. Post fire litters are richer in water soluble carbon and lead to increased microbial activity. Appl. Soil. Ecol. 2019, 136, 101–105. [Google Scholar] [CrossRef]
- Hulugalle, N.R.; Strong, C.; Mcpherson, K.; Nachimuthu, G. Carbon, nitrogen and phosphorus stoichiometric ratios under cotton cropping systems in Australian Vertisols: A meta-analysis of seven experiments. Nutr. Cycl. Agroecosystems 2017, 107, 357–367. [Google Scholar] [CrossRef]
- Schafer, J.L.; Mack, M.C. Nutrient limitation of plant productivity in scrubby flatwoods: Does fire shift nitrogen versus phosphorus limitation? Plant Ecol. 2018, 219, 1063–1079. [Google Scholar] [CrossRef]
- Butler, O.M.; Lewis, T.; Rashti, R.M.; Maunsell, S.C.; Elser, J.J.; Chen, C.R. The stoichiometric legacy of fire regime regulates the roles of micro-organisms and invertebrates in decomposition. Ecology 2019, 100, e02732. [Google Scholar] [CrossRef] [PubMed]
- De, L.J.R.; Dorrepaal, E.; Kardol, P.; Nilsson, M.C.; Teuber, L.M.; Wardle, D.A. Understory plant functional groups and litter species identity are stronger drivers of litter decomposition than warming along a boreal forest post-fire disturbance successional gradient. Soil Biol. Biochem. 2016, 98, 159–170. [Google Scholar] [CrossRef]
- Rodrigo, A.; Arnan, X.; Retana, J. Relevance of soil seed bank and seed rain to immediate seed supply after a large wildfire. Int. J. Wildland Fire 2012, 21, 449–458. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wang, C.; Sardans, J.; Zeng, C.S.; Josep, P.; Tong, C. Plant invasive success associated with higher N-use efficiency and stoichiometric shifts in the soil–plant system in the Minjiang River tidal estuarine wetlands of China. Wetl. Ecol. Manag. 2015, 23, 865–880. [Google Scholar] [CrossRef]
- Osburn, E.D.; Elliottt, K.J.; Knoepp, J.D.; Miniat, C.F.; Barrett, J.E. Soil microbial response to Rhododendron understory removal in southern Appalachian forests: Effects on extracellular enzymes. Soil. Biol. Biochem. 2018, 127, 50–59. [Google Scholar] [CrossRef]
- Jones, G.L.; Tomlinson, M.; Owen, R.; Scullion, J.; Winters, A.; Jenkins, T.; Ratcliffe, J.; Jones, D.J. Shrub establishment favoured and grass dominance reduced in acid heath grassland systems cleared of invasive Rhododendron ponticum. Sci. Rep. 2019, 9, 2239. [Google Scholar] [CrossRef]
- Hernandez, E.; Questad, E.J.; Meyer, W.M.; Suding, K.N. The effects of nitrogen deposition and invasion on litter fuel quality and decomposition in a Stipa pulchra grassland. J. Arid. Environ. 2019, 162, 35–44. [Google Scholar] [CrossRef]
- Duran, J.; Rodriguez, A.; Mendez, J.; Morales, G.; Palacios, J.M.F.; Gallardo, A. Wildfire decrease the local-scale ecosystem spatial variability of Pinus canariensis forests during the first two decades post fire. Int. J. Wildland Fire 2019, 28, 288–294. [Google Scholar] [CrossRef]
- Kurunthachaiam, S.K.; Sajwan, K.S.; Kumar, A.A.; Manian, S. Effects of surface fire on litter decomposition and occurrence of microfungi in a Cymbopogon polyneuros dominated grassland. Arch. Agron. Soil. Sci. 2007, 53, 205–219. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Grootemaat, S.; Verheijen, L.M.; Cornwell, W.K.; Bodegom, P.M.V.; Wal, R.V.D.; Aerts, R. Are litter decomposition and fire linked through plant species traits? New Phytol. 2017, 216, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Throop, H.L.; Salem, M.A.; Whitford, W.G. Fire enhances litter decomposition and reduces vegetation cover influences on decomposition in a dry woodland. Plant Ecol. 2017, 218, 799–811. [Google Scholar] [CrossRef]
- Holden, S.R.; Gutierrez, A.; Treseder, K.K. Changes in soil fungal communities, extracellular enzyme activities, and litter decomposition across a fire chronosequence in Alaskan Boreal Forests. Ecosystems 2013, 16, 34–46. [Google Scholar] [CrossRef]
- Yang, X.F.; Bao, X.L.; Hu, G.Q.; Shao, S.; Zhou, F.; Ye, J.S.; Xie, H.T.; Liang, C. C:N:P stoichiometry characteristics of litter and soil of forests in Great Xing’an Mountains with different fire years. Chin. J. Appl. Ecol. 2016, 27, 1359–1367. [Google Scholar] [CrossRef]
- Wei, M.Y.; Zhong, Y.Q.W.; Zhu, G.Y.; Liu, W.Z.; Shangguan, Z.P. Nutrient limitation of litter decomposition with long-term secondary succession: Evidence from controlled laboratory experiments. J. Soils Sediments 2020, 20, 1858–1868. [Google Scholar] [CrossRef]
- Butler, O.M.; Lewis, T.; Chen, C.R. Fire alters soil labile stoichiometry and litter nutrients in Australian eucalypt forests. Int. J. Wildland Fire 2017, 26, 783–788. [Google Scholar] [CrossRef]
- Santin, C.; Otero, X.L.; Doerr, S.H.; Chafer, C.J. Impact of a moderate/high-severity prescribed eucalypt forest fire on soil phosphorous stocks and partitioning. Sci. Total Environ. 2018, 621, 1103–1114. [Google Scholar] [CrossRef]
- Hume, A.; Chen, H.Y.H.; Taylor, A.R.; Kayahara, G.J.; Man, R.Z. Soil C:N:P dynamics during secondary succession following fire in the boreal forest of central Canada. For. Ecol. Manag. 2016, 369, 1–9. [Google Scholar] [CrossRef]
- Toberman, H.; Chen, C.R.; Lewis, T.; Elser, J.J. High-frequency fire alters C:N:P stoichiometry in forest litter. Glob. Chang. Biol. 2014, 20, 2321–2331. [Google Scholar] [CrossRef]
- Morrison, I.K. Decomposition and element release from confined jack pine needle litter on and in the feathermoss layer. Can. J. For. Res. 2003, 33, 16–22. [Google Scholar] [CrossRef]
- Hilli, S.; Stark, S.; Derome, J. Litter decomposition rates in relation to litter stocks in boreal coniferous forests along climatic and soil fertility gradients. Appl. Soil Ecol. 2010, 46, 200–208. [Google Scholar] [CrossRef]
- Jackson, B.G.; Nilsson, M.C.; Wardle, D.A. The effects of the moss layer on the decomposition of intercepted vascular plant litter across a post-fire boreal forest chronosequence. Plant Soil 2013, 367, 199–214. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zheng, J.Q.; Xu, Z.H.; Abdullah, K.M.; Zhou, Q.X. Effects of changed litter inputs on soil labile carbon and nitrogen pools in a eucalyptus-dominated forest of southeast Queensland, Australia. J. Soils Sediments 2019, 19, 1661–1671. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, H.Q.; Qu, Z.L.; Hu, T.X. Diurnal variation models for fine fuel moisture content in boreal forests in China. J. For. Res. 2020, 32, 1177–1187. [Google Scholar] [CrossRef]
- Hu, T.X.; Sun, L.; Hu, H.Q.; Guo, F.T. Effects of fire disturbance on soil respiration in the non-growing season in a Larix gmelinii forest in the Daxing’an Mountains, China. PLoS ONE 2017, 12, e0180214. [Google Scholar] [CrossRef]
- Li, S.; Wu, Z.W.; Yu, L.; He, H.S. The temporal and spatial clustering characteristics of forest fire in the Great Xing’an Mountains. Chin. J. Ecol. 2017, 36, 198–204. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Hui, D.F.; Luo, Y.Q.; Zhou, G.Y. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Dearden, F.M.; Dehlin, H.; Wardle, D.; Nilsson, M.C. Changes in the ratio of twig to foliage in litterfall with species composition, and consequences for decomposition across a long term chronosequence. Oikos 2006, 115, 453–462. [Google Scholar] [CrossRef]
- Wardle, D.A.; Hornberg, G.; Zackrisson, O.; Kalela-Brundin, M.; Coomes, D.A. Long-term effects of wildfire on ecosystem properties across an island area gradient. Science 2003, 300, 972–975. [Google Scholar] [CrossRef]
- Hernandez, D.L.; Hobbie, S.E. Effects of fire frequency on oak litter decomposition and nitrogen dynamics. Oecologia 2008, 158, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.D.; Mankowski, J.; Hobbie, S.E. Long-term burning interacts with herbivory to slow decomposition. Ecology 2008, 89, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Bryanin, S.; Kondratova, A.; Abramova, E. Litter decomposition and nutrient dynamics in fire-affected larch forests in the Russian far east. Forests 2020, 11, 882. [Google Scholar] [CrossRef]
- Yan, W.; Shangguan, Z.; Zhong, Y. Responses of mass loss and nutrient release in litter decomposition to ultraviolet radiation. J. Soils Sediments 2021, 21, 698–704. [Google Scholar] [CrossRef]
- Talhelm, A.F.; Smith, A.M.S. Litter moisture adsorption is tied to tissue structure, chemistry, and energy concentration. Ecosphere 2018, 9, 18. [Google Scholar] [CrossRef]
- Musetta-Lambert, J.; Muto, E.; Kreutzweiser, D.; Sibley, P. Wildfire in boreal forest catchments influences leaf litter subsidies and consumer communities in streams: Implications for riparian management strategies. For. Ecol. Manag. 2017, 391, 29–41. [Google Scholar] [CrossRef]
- Köster, K.; Berninger, F.; Heinonsalo, J.; Linden, A.; Köster, E.; Ilvesniemi, H.; Pumpanen, J. The long-term impact of low-intensity surface fires on litter decomposition and enzyme activities in boreal coniferous forests. Int. J. Wildland Fire 2016, 25, 213–223. [Google Scholar] [CrossRef]
- Springett, J.A. Effects of a single hot summer fire on soil fauna and on litter decomposition in jarrah (Eucalyptus marginata) forest in Western Australia. Aust. J. Ecol. 2010, 4, 279–291. [Google Scholar] [CrossRef]
- Jurskis, V.; Turner, J.; Lambert, M.; Bi, H.Q. Fire and N cycling: Getting the perspective right. Appl. Veg. Sci. 2011, 14, 433–434. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liu, Y.; Zhang, J.; Yang, W.Q.; He, R.L.; Deng, C.C. Microclimate exerts greater control over litter decomposition and enzyme activity than litter quality in an alpine forest-tundra ecotone. Sci. Rep. 2018, 8, 14998. [Google Scholar] [CrossRef]
- Gao, L.W.; Zhang, M.Q.; Liu, Y.; Vicente, M.L. Litter cover promotes biocrust decomposition and surface soil functions in sandy ecosystem. Geoderma 2020, 374, 114429. [Google Scholar] [CrossRef]
- Bogorodskaya, A.V.; Ivanova, G.A.; Tarasov, P.A. Post-fire transformation of the microbial complexes in soils of larch forests in the lower Angara River region. Eurasian Soil Sci. 2011, 44, 49–55. [Google Scholar] [CrossRef]
- Ondřej, V.; Kalwij, J.M.; Hédl, R. Effects of simulated historical tree litter raking on the understorey vegetation in a central European forest. Appl. Veg. Sci. 2015, 18, 569–578. [Google Scholar] [CrossRef]
- Weber, M.G. Decomposition, litter fall, and forest floor nutrient dynamics in relation to fire disturbance in eastern Ontario jack pine ecosystems. Can. J. For. Res. 1987, 17, 1496–1506. [Google Scholar] [CrossRef]
- Davies, A.B.; Rensburg, B.J.V.; Eggleton, P.; Parr, C.L. Interactive Effects of Fire disturbance, Rainfall, and Litter Quality on Decomposition in Savannas: Frequent Fire Leads to Contrasting Effects. Ecosystems 2013, 16, 866–880. [Google Scholar] [CrossRef]
- Garcia, P.P.; Prieto, I.; Ourcival, J.M.; Hatten, S.S. Disentangling the litter quality and soil microbial contribution to leaf and fine root litter decomposition responses to reduced rainfall. Ecosystems 2016, 19, 490–503. [Google Scholar] [CrossRef]
- Urgenson, L.S.; Ryan, C.M.; Halpern, C.B.; Bakker, J.D.; Belote, R.T.; Franklin, J.F.; Haugo, R.D.; Nelson, C.R.; Waltz, A.E.M. Erratum to: Visions of restoration in fire-adapted forest landscapes: Lessons from the collaborative forest landscape Restoration Program. Environ. Manag. 2017, 59, 354–355. [Google Scholar] [CrossRef]
- Hoeber, S.; Fransson, P.; Weih, M.; Stefano, M. Leaf litter quality coupled to Salix variety drives litter decomposition more than stand diversity or climate. Plant Soil 2020, 453, 313–328. [Google Scholar] [CrossRef]
- Coleman, T.W.; Rieske, L.K. Arthropod response to prescription burning at the soil-litter interface in oak-pine forests. For. Ecol. Managt. 2006, 233, 52–60. [Google Scholar] [CrossRef]
- Hartshorn, J. A Review of Forest Management Effects on Terrestrial Leaf Litter Inhabiting Arthropods. Forests 2021, 12, 23. [Google Scholar] [CrossRef]
- Gartner, T.B.; Treseder, K.K.; Malcolm, G.M.; Sinsabaugh, R.L. Extracellular enzyme activity in the mycorrhizospheres of a boreal fire chronosequence. Pedobiologia 2012, 55, 121–127. [Google Scholar] [CrossRef]
- Knelman, J.E.; Graham, E.B.; Ferrenberg, S.; Lecoeuvre, A.; Labrado, A.; Darcy, J.L.; Nemergut, D.R.; Schmidt, S.K. Rapid shifts in soil nutrients and decomposition enzyme activity in early succession following forest fire. Forests 2017, 8, 347. [Google Scholar] [CrossRef]
- Auclerc, A.; Le Moine, J.M.; Hatton, P.J.; Bird, J.A.; Nadelhoffer, K.J. Decadal post-fire succession of soil invertebrate communities is dependent on the soil surface properties in a boreal temperate forest. Sci. Total. Environ. 2019, 647, 1058–1068. [Google Scholar] [CrossRef]
- Gui, H.; Hyde, K.; Xu, J.C.; Mortimer, P. Arbuscular mycorrhiza enhance the rate of litter decomposition while inhibiting soil microbial community development. Sci. Rep. 2017, 7, 42184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Gutknecht, J.; Nadrowski, K.; Geißler, C.; Kühn, P.; Scholten, T.; Both, S.; Erfmeier, A.; Böhnke, M.; Bruelheide, H.; et al. Relationships between soil microorganisms, plant communities, and soil characteristics in Chinese subtropical forests. Ecosystems 2012, 15, 624–636. [Google Scholar] [CrossRef]
- Zhang, P.; Li, B.; Wu, J.H.; Hu, S.J. Invasive plants differentially affect soil biota through litter and rhizosphere pathways: A meta-analysis. Ecol. Lett. 2019, 22, 200–210. [Google Scholar] [CrossRef]
- Hicks, L.C.; Meir, P.; Nottingham, A.T.; Reay, D.S.; Stott, A.W.; Salinas, N.; Whitaker, J. Carbon and nitrogen inputs differentially affect priming of soil organic matter in tropical lowland and montane soils. Soil Biol. Biochem. 2019, 129, 212–222. [Google Scholar] [CrossRef]
- Mayor, A.G.; Goiran, S.B.; Vallejo, V.R.; Bautista, S. Variation in soil enzyme activity as a function of vegetation amount, type, and spatial structure in fire-prone Mediterranean shrublands. Sci. Total. Environ. 2016, 573, 1209–1216. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.Y.; Cheng, Y.; Cai, Z.C.; Muller, C.; Zhang, J.B. Composition of soil recalcitrant C regulates nitrification rates in acidic soils. Geoderma 2019, 337, 965–972. [Google Scholar] [CrossRef]
- Grigal, D.F.; Mccoll, J.G. Litter decomposition following forest fire in Northeastern Minnesota. J. Appl. Ecol. 1977, 14, 531–538. [Google Scholar] [CrossRef]
- Brödlin, D.; Kaiser, K.; Kessler, A.; Hagedorn, F. Drying and rewetting foster phosphorus depletion of forest soils. Soil Biol. Biochem. 2019, 128, 22–34. [Google Scholar] [CrossRef]
- Wang, X.G.; Lu, X.T.; Han, X.G. Responses of nutrient concentrations and stoichiometry of senesced leaves in dominant plants to nitrogen addition and prescribed burning in a temperate steppe. Ecol. Eng. 2014, 70, 154–161. [Google Scholar] [CrossRef]
- Smith, S.W.; Speed, J.D.M.; Bukombe, J.; Hassan, S.N.; Lyamuya, R.D.; Mtweve, P.J.; Sundsdal, A.; Graae, B.J. Litter type and termites regulate root decomposition across contrasting savanna land-uses. Oikos 2019, 128, 596–607. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Mamuti, M.; Liu, H.M.; Shu, Y.Q.; Hu, S.J.; Wang, X.H.; Li, B.B.; Lin, L.; Li, X. Effects of nutrient additions on litter decomposition regulated by phosphorus-induced changes in litter chemistry in a subtropical forest, China. For. Ecol. Manag. 2017, 400, 123–128. [Google Scholar] [CrossRef]
- Jiang, T.H.; Lu, X.Y.; Ma, X.X.; Wang, X.D. Five-year study on the effects of warming and plant litter quality on litter decomposition rate in a Tibetan alpine grassland. Sci. Total Environ. 2021, 750, 142306. [Google Scholar] [CrossRef]
- Xiao, W.Y.; Chen, H.Y.H.; Kumar, P.; Chen, C.; Guan, W.Q. Multiple interactions between tree composition and diversity and microbial diversity underly litter decomposition. Geoderma 2019, 341, 161–171. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Estiarte, M.; Penuelas, J.; Lloret, F. Effects of climate change on leaf litter decomposition across post-fire plant regenerative groups. Environ. Exp. Bot. 2012, 77, 274–282. [Google Scholar] [CrossRef]
- Lin, C.F.; Peng, J.Q.; Hong, H.B.; Yang, Z.J.; Yang, Y.S. Effect of nitrogen and phosphorus availability on forest litter decomposition. Acta Ecol. Sin. 2017, 37, 54–62. [Google Scholar] [CrossRef][Green Version]
- Bengtsson, J.; Janion, C.; Chown, S.L.; Leinaas, H.P. Litter decomposition in fynbos vegetation, South Africa. Soil Biol. Biochem. 2012, 47, 100–105. [Google Scholar] [CrossRef]
- Chacón, N.; Dezzeo, N. Litter decomposition in primary forest and adjacent fire -disturbed forests in the Gran Sabana, southern Venezuela. Biol. Fertil. Soils 2007, 43, 815–821. [Google Scholar] [CrossRef]
- Jabiol, J.; Lecerf, A.; Lamothe, S.; Gessner, M.O.; Chauvet, E. Litter quality modulates effects of dissolved Nitrogen on leaf decomposition by stream microbial communities. Microb. Ecol. 2019, 77, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Taylor, Q.A.; Midgley, M.G. Prescription side effects: Long-term, high-frequency controlled burning enhances nitrogen availability in an Illinois oak-dominated forest. For. Ecol. Manag. 2018, 411, 82–89. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Pumpanen, J.; Sietio, O.M.; Heinonsalo, J.; Koster, K.; Berninger, F. The impact of wildfire on microbial C:N P stoichiometry and the fungal-to-bacterial ratio in permafrost soil. Biogeochemistry 2019, 142, 1–17. [Google Scholar] [CrossRef]
- Gusewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Ficken, C.; Wright, J.P. Effects of fire frequency on litter decomposition as mediated by changes to litter chemistry and soil environmental conditions. PLoS ONE 2017, 12, e0186292. [Google Scholar] [CrossRef]
- Brennan, K.E.C.; Christie, F.J.; York, A. Global climate change and litter decomposition: More frequent fire slows decomposition and increases the functional importance of invertebrates. Glob. Chang. Biol. 2009, 15, 2958–2971. [Google Scholar] [CrossRef]
- Heim, R.J.; Heim, W.; Darman, G.F.; Thilo, H.; Sergei, M.S.; Norbert, H. Litter removal through fire—A key process for wetland vegetation and ecosystem dynamics. Sci. Total Environ. 2021, 755, 142659. [Google Scholar] [CrossRef]
- Johnston, J.D.; Olszewski, J.H.; Miller, B.; ASchmidt, M.R.; Vernon, M.J.; Ellsworth, L.M. Mechanical thinning without prescribed fire moderates wildfire behavior in an Eastern Oregon, USA ponderosa pine forest. For. Ecol. Manag. 2021, 501, 119674. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).