Abstract
Groundwater plays a significant role in influencing the growth and distribution of Robinia pseudoacacia L. plantations, with the largest planting area in the Yellow River Delta, by affecting the soil water–salt environment. This study aimed to clarify the mechanism of groundwater’s influence on the growth of R. pseudoacacia under different levels of groundwater mineralization (GWM) and groundwater depth (GWD). We simulated GWM of 0, 2 and 4 g L−1, and GWD of 0.8, 1.3 and 1.8 m. As GWM increased, soil relative water content (SRWC) and soil salt (dissolved salt) content (SSC) increased; sapling biomass (SB), stem mass (SM), leaf mass (LM), photosynthesis characteristics (maximum net photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (E) and water use efficiency (WUE)) decreased; root mass (RM), root mass ratio (RMR) and root–shoot ratio (RSR) first increased then decreased; stem mass ratio (SMR) first decreased then increased; and leaf mass ratio (LMR) increased. As GWD increased, SRWC decreased, but SSC first increased then decreased; SB, RM, RMR, RSR, and photosynthesis characteristics increased; SM and LM first increased then decreased; and SMR and LMR decreased. SRWC and SSC were negatively correlated with SB and photosynthesis characteristics. SRWC was negatively correlated with RMR and RSR, whereas it was positively correlated with LMR. SSC was negatively correlated with SMR, whereas it was positively correlated with LMR. The first principal component, including SB, RM, and photosynthesis characteristics, was related to sapling growth. The second principal component, including RMR, SMR, and RSR, was mainly related to biomass allocation. In conclusion, GWM and GWD affected the soil water and salt content, which were key factors influencing the photosynthesis and growth of R. pseudoacacia. Adjustments in biomass allocation and photosynthesis were the main adaptive strategies of R. pseudoacacia to salt, drought, and flooding stress.
1. Introduction
Water stored underground in the saturated and subsurface zones below the soil is an important source of water for plants in water-limited ecosystems [1]. Through the connection between the saturation zone, the aeration zone and plants in the vertical direction, groundwater leads to significant eco-environmental changes [2,3]. Groundwater mineralization (GWM) and groundwater depth (GWD) play important roles in affecting the soil water and salt environment [4,5,6]. Increase of GWM could promote the upward migration of water and salt ions to the topsoil [7]. Numerous studies showed that the soil salt (dissolved salt) content (SSC) could increase with GWM, exponentially or linearly in the plow or topsoil layer [8,9]. However, another study showed that there was no significant relation between GWM and soil salt migration [10]. In arid inland or muddy coastal saline alkali land lacking freshwater resources, affected by leaching and melting, the strong affinity of salt itself to soil moisture, and meteorological factors, the variation of GWD is also closely correlated with the soil water–salt movement [11,12]. As GWD increases, the path of groundwater reaching the topsoil becomes longer, resulting in the decrease of the moisture and salinity of the soil profile [13,14]. In the Yellow River Delta (the typical muddy coastal saline alkali land), the area with lower GWD has higher soil water content and salt content [15]. With the variation of soil texture, vegetation type, micro topography, and climatic environment, the correlation between the soil water content, the salt content, and the GWD of different soil profiles is quite different. For example, the soil water and salt did not completely change synchronously with GWD, and there existed an obvious turning point with the change of GWD [16].
The results of large-scale investigations in recent years have shown that both GWM and GWD act as key ecological factors determining the distribution, the health status, and the water use strategies of vegetation by influencing the soil water–salt characteristics in the Yellow River Delta [17,18,19]. In the Yellow River Delta, the degree of mineralization of groundwater is generally high [20]; areas with shallow GWD tended to have higher soil salinity and were suitable for the distribution of salt-tolerant plants, including herbaceous species and shrub species, while areas with deeper GWD tended to have lower soil salinity and were suitable for the growth of tree species [21]. Through its effect on changes in soil water and salt, the groundwater influences the material transport and energy balance related to the photosynthetic productivity of plants [22,23], and it further exerts a profound impact on photosynthetic processes and the growth of plants [24,25]. However, there are few studies on how the GWM and GWD affect the plant growth in this area, and the related influence mechanism remains unclear.
The Robinia pseudoacacia L. plantations planted in the 1970s and 1980s are the largest arbor plantation in the Yellow River Delta but have shown extensive shoot dieback and even patchy mortality of trees, which seriously weakened the plantations’ ecological functions. To reveal the decline mechanism of R. pseudoacacia plantations, scholars have conducted a series of studies on the soil water and salt dynamics [26], stand structure and canopy health [27], stand nutrition [28], and photosynthetic physiology [29]. In the Yellow River Delta, the GWD of the areas with a serious decline of R. pseudoacacia is usually low [15], but the influence mechanism of groundwater on the growth of R. pseudoacacia is not clear. In this study, we simulated three GWM levels (0, 2, and 4 g L−1) and three GWDs (0.8, 1.3, and 1.8 m) and determined the effects of GWM and GWD on the soil relative water content (SRWC) and SSC and on the plant biomass, biomass allocation, and photosynthesis characteristics of R. pseudoacacia, explored the correlations between soil properties and R. pseudoacacia growth, and clarified the response mechanism of R. pseudoacacia to water and salt stress driven by groundwater, to provide a theoretical basis for the efficient cultivation and sustainable management of R. pseudoacacia plantations in the Yellow River Delta.
2. Materials and Methods
2.1. Study Area
The experiment was conducted in a greenhouse of the nursery in Kenli District, Dongying City, Shandong Province (118°15′–119°19′ E, 37°24′–38°10′ N). The experiment site is located in the temperate semi-humid monsoon climate zone, with significant temperature differences between the four seasons. It is dry and windy in spring, hot and humid in summer, cool in autumn, and dry and frozen in winter. The annual average temperature is 11.9 °C, the average daily temperature is 10.7 °C, the average precipitation is 592.2 mm, the annual average evaporation is 1908.2 mm, and the evaporation–precipitation ratio is 3.22.
2.2. Experimental Design
According to previous study conducted by Xia et al. [30] in the Yellow River Delta, groundwater salinity values higher than the level of saline water (8 g L−1) severely inhibited the growth and photosynthetic efficiency of Tamarix chinensis Lour. whose salt tolerance is much higher than that of R. pseudoacacia. Besides, after the pre-test, we found that R. pseudoacacia was severely stressed when the GWM was higher than 4 g L−1. The results of a large-scale field survey on R. pseudoacacia plantations in the Yellow River Delta showed that the soil moisture, salinity, and the degree of stand dieback increase with decreasing GWD [15]. The withered shoots and death of R. pseudoacacia plantations are more serious in the area where GWD (in the low-water-level period (December), the same below) is less than 1 m, while the good-growth R. pseudoacacia plantations are mostly located in the area where GWD is not less than 1.8 m [17,31]. Thus, after fully considering the actual situation of the R. pseudoacacia planting area in the Yellow River Delta, the indoor test conditions, and the survival rate of saplings, we set three groundwater mineralization levels (0, 2, and 4 g L−1) and three representative groundwater depths (0.8, 1.3, and 1.8 m), with a total of 9 treatments with 3 replications per treatment in this study.
Before the test, we built three 0.8-m-deep cement tanks in the greenhouse during the period 15–25 January 2020 and applied waterproof materials at the bottom of the cement tank to prevent water leakage. At the same time, PVC round pipes (inner diameter of 45 cm) with different heights were prepared to simulate different GWDs through control of the PVC pipe length. A 0.03 m interstitial layer was set at the upper part of the PVC pipe to facilitate water management, and a 0.8 m flooded depth was set at the bottom part of the PVC pipe. Three GWD treatments were simulated and set up, which included 0.8, 1.3, and 1.8 m, and the corresponding PVC pipe heights were 1.63, 2.13, and 2.63 m. In the range of simulated groundwater depth, four holes (2 cm in diameter) were drilled every 10 cm in the vertical direction around each PVC pipe as soil sampling holes and plugged tightly with plugs. In the flooded portion of the PVC pipe, 4 water inlet holes (1 cm in diameter) were drilled every 20 cm in the vertical direction. The bottom and the surrounding of the flooded part of the PVC pipe were wrapped with permeable felt to ensure that the simulated groundwater can enter the pipe from the surrounding water inlets to wet the soil and the soil in the pipe would not leak out. Nine PVC round pipes of three heights (with 3 replications) were placed into each pond, for a total of 27 PVC round pipes. All pipes were fixed with construction scaffolding.
During 1–5 February 2020, we took 20 cm as a soil layer, calculated the filling amount according to the soil bulk density, and filled the air-dried soil in the PVC pipe. The soil samples used in this study were original soil in the non-seedling area of the nursery. The soil particle size distribution was 10.13% coarse sand, 25.77% fine sand, and 64.10% silt and clay. The initial average soil salinity was 1‰, pH was 7.43, field capacity was 32.06%, and the bulk density was 1.38 g m−3. The soil field capacity was measured by the Wilcox method [32]. The groundwater in the Yellow River Delta is affected by sea salts, mainly NaCl, and the soil in the Yellow River Delta belongs to chloride saline soils. Thus, the groundwater of different mineralization for the experiment was prepared by dissolving NaCl in deionized water. After the soil was filled, 0 g L−1 (deionized water), 2 g L−1 (NaCl dissolved in deionized water), and 4 g L−1 (NaCl dissolved in deionized water) groundwater were respectively poured into three cement tanks to simulate three different GWM levels. The flooding depth was controlled to 0.8 m. After the soil column was balanced and stabilized for 7 days, three 1-year-old R. pseudoacacia saplings (9.51 mm ± 0.46 mm in ground diameter, 35 cm in height, 10 cm in root width) were planted in each PVC pipe, and 1 sapling was left after one month of normal planting management. During one month of normal planting management, for all treatments, fresh water was poured from the upper part of the PVC pipe once every 10 days, 4 L each time, a total of 12 L. After that, water supply to the aboveground part was not provided. The total dissolved solids (TDS) content of groundwater and the actual flooded level were monitored at 3-day intervals and then supplied with the corresponding groundwater throughout the experimental period to ensure the stability of GWM and flooding depth. During the experiment, there was no additional precipitation or irrigation, and the water in the concrete ponds was used as the water source. The whole experiment was finished on 21 September 2020. The simulated setup for the soil columns planted with R. pseudoacacia is shown in Figure 1.
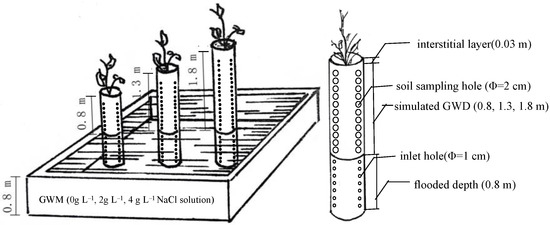
Figure 1.
The simulated setup for the soil columns planted with R. pseudoacacia.
2.3. Measurement Methods of Indices
2.3.1. Collection and Measurement of Soil Samples
During May and September 2020, soil samples were collected every 20 cm in the vertical direction from the main distribution range of the roots (0–80 cm soil layer) every two months. Three replicate samples were obtained from each soil layer. The gravitational soil water content was measured by the drying method (105 °C, 8 h). Soil relative water content (SRWC, %) was calculated as the ratio between the mass water content and the field capacity. Soil salt (dissolved salt in this study) content (SSC, ‰) was measured by the mass method (water/soil ratio of 5:1).
2.3.2. Measurement of Photosynthetic Parameters
From 18 to 20 September 2020, photosynthetic parameters were measured during 9:00–10:00 a.m., when it was sunny. The maximum net photosynthetic rate (Pn, μmol m−2 s−1), stomatal conductance (gs, mmol m−2 s−1), intercellular CO2 concentration (Ci, μmol m−2 s−1), and transpiration rate (E, mmol m−2 s−1) were measured with a Li-6800 photosynthetic measurement system (Li-COR, Lincoln, NE, USA). Before the measurement, we selected three saplings and measured three photosynthesis-light curves. When measuring light response curve, the leaves were induced for 30 min under the saturated light intensity. The photosynthetically active radiation was set as 2000, 1800, 1600, 1400, 1200, 1000, 800, 600, 400, 200, 100, 75, 50, 25, and 0 μmol m−2 s−1 for 120 s at each light intensity. According to the light response curve, the maximum light saturation light intensity of R. pseudoacacia saplings was 1200 μmol m−2 s−1, at which intensity, we measured the photosynthetic parameters. During the measurement of photosynthetic parameters, three healthy and mature pinnate compound leaves were selected from the middle of the crown of each sapling, and three leaflets were selected from each compound leaf. Water use efficiency (WUE, μmol mmol−1) was calculated as the ratio between the maximum net photosynthetic rate and transpiration rate.
2.3.3. Collection of Plant Samples and Measurement of Biomass
From 20 to 21 September 2020, the whole plants were collected for determining the biomass characteristics of R. pseudoacacia saplings. Three plants were measured for each treatment, and a total of 27 were harvested. Each sapling was divided into 3 parts: roots, stems, and leaves. Each part of the sapling was desiccated at 105 °C for 30 min and followed by 65 °C drying for constant weight for sapling biomass (SB, g), root mass (RM, g), stem mass (SM, g), and leaf mass (LM, g). The root mass ratio (RMR, g g−1) was calculated as the ratio between root mass and sapling biomass. The stem mass ratio (SMR, g g−1) was determined as the ratio between stem mass and sapling biomass. The leaf mass ratio (LMR, g g−1) was measured as the ratio between leaf mass and sapling biomass. The root–shoot ratio (RSR, g g−1) was calculated as the ratio between root mass and the sum of stem biomass and leaf biomass.
2.4. Data Analysis
Two-way ANOVA was used to test the effects of GWM, GWD, and their interactive effects on soil properties (including SRWC and SSC) and sapling characteristics (including biomass, biomass allocation, and photosynthetic characteristics of R. pseudoacacia). When ANOVA indicated a significant overall treatment effect, a multiple comparison test (Tukey post hoc test) was carried out to compare the parameter means. Pearson correlation coefficient was used to evaluate the correlation between SRWC and SSC and sapling characteristics of R. pseudoacacia. All sapling characteristics were determined by Kaiser–Meyer–Olkin test of sampling adequacy (KMO) and Bartlett’s test of sphericity and analyzed by principal component analysis (PCA). SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) was used for ANOVA, correlation, and PCA analysis.
3. Results
3.1. Soil Water and Salt Parameters
3.1.1. Soil Relative Water Content
The results of variance analysis showed that GWM (F = 79.60, p < 0.001) and GWD (F = 955.54, p < 0.001) had significant influence on SRWC. With the increase of GWM, SRWC increased (Figure 2). As GWD increased, SRWC decreased, and the SRWC at 1.3 and 1.8 m GWD was 68.76% and 28.63% of that under 0.8 m, respectively (Figure 2). The interactions between GWM and GWD had significant effects on SRWC (F = 5.63, p = 0.004), manifested as follows: the effect of GWM on SRWC decreased as GWD increased (Figure 2).
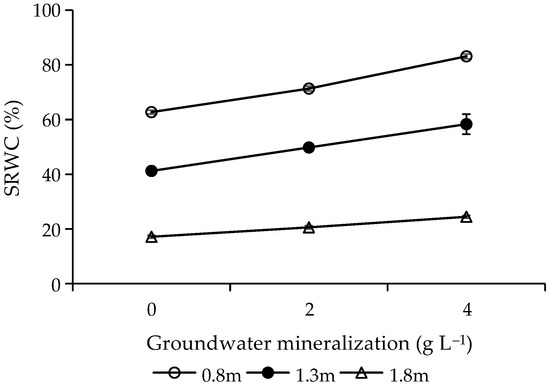
Figure 2.
Effects of groundwater mineralization and groundwater depth on soil relative water content. Error bars indicate standard errors (SEs) of the mean.
3.1.2. Soil Salt Content
According to the results of variance analysis, GWM (F = 1580.53, p < 0.001) and GWD (F = 480.83, p < 0.001) had significant influence on SSC. As GWM increased, SSC increased (Figure 3). Among different GWDs, the order of SSC was 1.3 > 0.8 > 1.8 m. The interactions between GWM and GWD had significant effects on SSC (F = 136.99, p < 0.001). With the increase of GWD, the effect of GWM on SSC decreased (Figure 3).
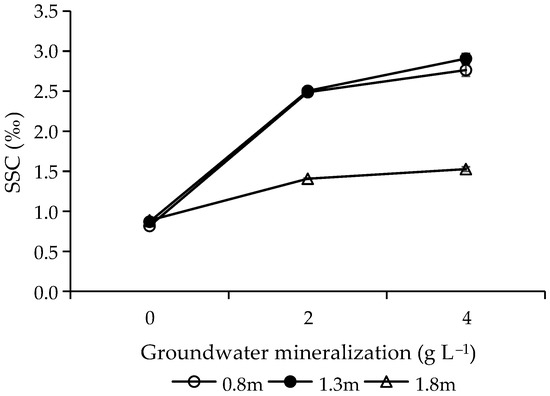
Figure 3.
Effects of groundwater mineralization and groundwater depth on soil salt content. Error bars indicate standard errors (SEs) of the mean.
3.2. Sapling Characteristics of R. pseudoacacia
3.2.1. Biomass Characteristics
The results of two-way ANOVA showed that GWM and GWD had significant effects on SB, RM, SM, and LM (Table 1). There was no significant difference in SB when the GWM was 0 and 2 g L−1 (Table A1), which was significantly larger than that at 4 g L−1. With the increase of GWM, RM first increased then decreased, and the order was 2 > 0 > 4 g L−1. SM reached the maximum at 0 g L−1, and there was no significant difference between SM when the GWM was 2 and 4 g L−1; LM significantly decreased (Figure 4). With the increase in GWD, SB and RM increased; SM reached the maximum at 1.3 m, and there was no significant difference between SM when the GWD was 0.8 and 1.8 m; LM first increased then decreased, and the order was 1.3 > 1.8 > 0.8 m (Figure 4).

Table 1.
Two-way ANOVA analyses of the effects of GWM and GWD their interaction on biomass, biomass allocation, and photosynthetic characteristics of R. pseudoacacia saplings.
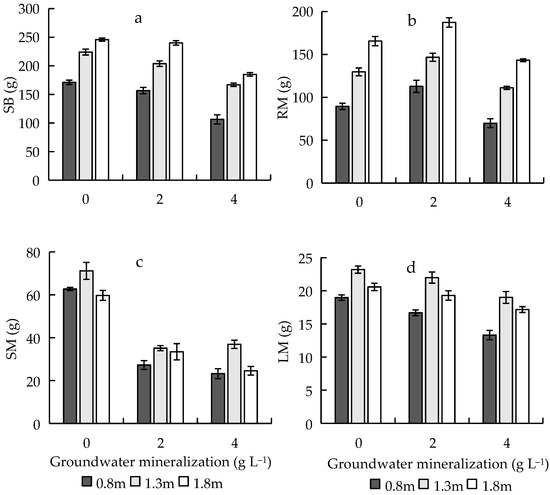
Figure 4.
Effects of groundwater mineralization and groundwater depth on biomass characteristics of R. pseudoacacia. (a) Sapling biomass; (b) root mass; (c) stem mass; (d) leaf mass. Error bars indicate standard errors (SEs) of the mean.
3.2.2. Biomass Allocation
Both GWM and GWD had significant effects on RMR, SMR, LMR, and RSR (Table 1). With the increase of GWM, RMR and RSR first increased and then decreased, and their orders were 2 > 4 > 0 g L−1; while SMR first decreased and then increased, and LMR increased (Figure 5). Among different GWDs, there was no significant difference between RMR (p = 0.125), SMR (p = 0.205), LMR (p = 0.170), and RSR (p = 0.599) when the GWDs were 0.8 and 1.3 m, RMR and RSR reached a maximum at 1.8 m GWD, SMR and LMR were minimum at 1.8 m GWD (Figure 5).
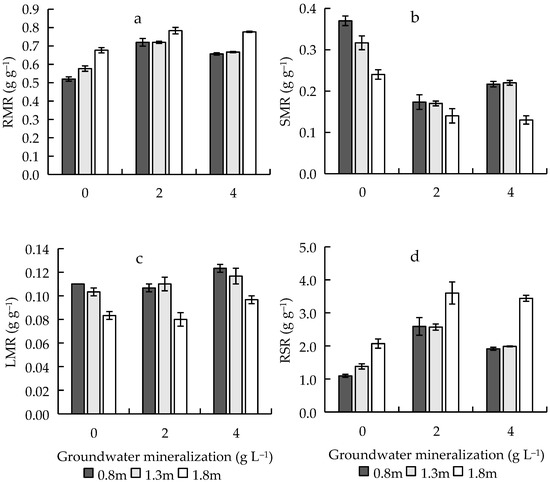
Figure 5.
Effects of groundwater mineralization and groundwater depth on biomass allocation characteristics of R. pseudoacacia. (a) Root mass ratio; (b) stem mass ratio; (c) leaf mass ratio; (d) root–shoot ratio. Error bars indicate standard errors (SEs) of the mean.
3.2.3. Photosynthetic Characteristics
In this study, GWM and GWD had significant effects on Pn, gs, Ci, E, and WUE (Table 1). Pn, gs, Ci, E, and WUE decreased with increasing GWM but increased with increasing GWD (Figure 6). The interaction between GWM and GWD had significant effects on gs and E (Table 1).
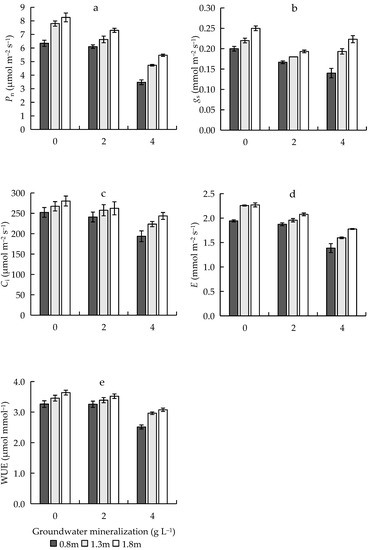
Figure 6.
Effects of groundwater mineralization and groundwater depth on the photosynthetic characteristics of R. pseudoacacia. (a) Maximum net photosynthetic rate; (b) stomatal conductance; (c) intercellular CO2 concentration; (d) transpiration rate; (e) water use efficiency. Error bars indicate standard errors (SEs) of the mean.
3.3. Correlation Analysis between Soil Water and Salt, and R. pseudoacacia Characteristics
According to Table 2, SRWC and SSC were negatively correlated with SB, Pn, gs, Ci, E, and WUE. SRWC was negatively correlated with RMR and RSR, whereas it was positively correlated with LMR. SSC was negatively correlated with SMR, whereas it was positively correlated with LMR. SB was positively correlated with RM, SM, LM, Pn, gs, Ci, E, and WUE, whereas it was negatively correlated with LMR.

Table 2.
Correlation coefficients between soil properties and R. pseudoacacia characteristics. SRWC: soil relative water content; SSC: soil salt content; SB: sapling biomass; RM: root mass; SM: stem mass; LM: leaf mass; RMR: root mass ratio; SMR: stem mass ratio; LMR: leaf mass ratio; RSR: root–shoot ratio; Pn: maximum net photosynthetic rate; gs: stomatal conductance; Ci: intercellular CO2 concentration; E: transpiration rate; WUE: water use efficiency. Significance code: ** p < 0.01, * p < 0.05.
3.4. Principal Component Analysis of R. pseudoacacia Characteristics
According to the results of PCA (Table 3), the first principal component (52.58%) included SB, Pn, E, RM, Ci, and gs, which were related to sapling growth. The second principal component (36.38%) included SMR, RMR, and RSR, which were mainly related to biomass allocation. The cumulative variance contribution rate of the two principal components was 88.96%.

Table 3.
Principal component analysis of R. pseudoacacia characteristics. SB: sapling biomass; RM: root mass; RMR: root mass ratio; SMR: stem mass ratio; RSR: root–shoot ratio; Pn: maximum net photosynthetic rate; gs: stomatal conductance; Ci: intercellular CO2 concentration; E: transpiration rate.
4. Discussion
4.1. Effects of Groundwater Mineralization and Groundwater Depth on Soil Water and Salt Parameters
In this experiment, we found that both SRWC and SSC increased with GWM, which indicated that the increase of GWM could promote the upward migration of soil water through capillary action, thus driving the upward migration of soil salt [30]. High groundwater mineralization is the premise of realizing soil salt accumulation, and the positive correlation between groundwater mineralization and soil salt content has been confirmed in many studies [33,34]. In this study, we also found that SSC increased with the increase of GWM. However, some studies found that the relationship between groundwater mineralization and soil salt transport is not significant [10,35], which may be caused by the differences between the physical and chemical properties of the soil itself and the external microclimate conditions.
As GWD increased, SRWC decreased, which was similar to previous results [13]. The GWD can affect the SWC by affecting the speed and amount of groundwater rising to the soil surface under the influence of capillarity and gravity [36]. Under the condition of high groundwater level, the salt in groundwater easily accumulates on the surface through capillary action and results in soil salinization. When the GWD increases to larger than the critical depth of evaporation, it is difficult for salt to reach the surface. Through field investigation conducted in the Yellow River Delta, Ma et al. [21] also found that when the GWD was less than 2.5 m, groundwater was involved in the process of soil salt accumulation and the salinization of surface soil decreased as the GWD increased. However, in our study, SSC reached the maximum at 1.3 m, which meant that there appeared a turning point of soil salt accumulation when the GWD was 1.3 m, which was similar to the result of 1.2 m in the simulated experiment conducted by Xia et al. [37]. This may be because when the GWD is too low (0.8 m GWD in this study), the soil salt accumulates rapidly on the surface, similar to forming a protective layer with a crust property, reducing water evaporation, resulting in a decreasing trend of soil salinity. Besides, GWM and GWM jointly affected SSC in this paper. Based on the spatial model, Fan et al. [38] also found that the intensification of soil salinization in the Yellow River Delta was related to the decrease of GWD and the increase of GWM.
4.2. Effects of Groundwater Mineralization and Groundwater Depth on Biomass and Biomass Allocation Characteristics of R. pseudoacacia
Biomass is the most intuitive index to reflect the growth status and stress tolerance of trees. Generally, with the increase of groundwater mineralization, plant growth is inhibited more seriously [39]. In this study, the SB and LM decreased with the increase of GWM, which was related to the increasing SSC driven by increasing GWM. Differently, Xia et al. [30] found that the sapling biomass of T. chinensis increased first and then decreased as GWM increased. This difference may be caused by the higher salt tolerance of T. chinensis than that of R. pseudoacacia. However, there was no significant difference in SB between 0 and 2 g L−1 in our study, which was partly related to the significant increase of RM at 2 g L−1, indicating that the aboveground part of R. pseudoacacia was more sensitive to salt stress than the root system. The plasticity of the biomass allocation pattern is an important morphological response strategy for trees to cope with stressful environments [40]. With the increase in GWM, the RMR and RSR first increased and then decreased, and the order was 2 > 4 > 0 g L−1. The increase of RSR is a common response of plants under salt stress, which is conducive to limit the accumulation of toxic ions in the aboveground part [41]. Moreover, increasing the biomass allocation to roots under salt stress helps plants obtain more water and nutrients so as to dilute the salt in cells and enhance stress resistance, as well as to promote growth. The above results showed that R. pseudoacacia had salt tolerance to some extent, which was consistent with the research results of Mao et al. [29].
The biomass of the saplings and organs of R. pseudoacacia was the lowest under 0.8 m GWD, which was related to the highest SRWC and SSC. Waterlogging stress caused by low GWD and its corresponding salt stress inhibit the growth of trees by inhibiting the growth of roots [42]. In terms of the biomass allocation, the RMR and RSR also reached minimum when the GWD was 0.8 m. Under waterlogging stress, trees would increase the proportion of aboveground parts by reducing RMR and RSR [43] so as to ensure the normal progress of photosynthesis and provide a basis for transporting O2 from the air to the flooded part. With the increase of GWD, the decrease of SRWC and its promotion of root growth were the important reasons for the significant increase of SB. The RMR and RSR were the largest at 1.8 m. It is found that under drought stress, the photosynthetic products of trees will preferentially transfer to the underground part and promote the increase of the root–shoot ratio [44], which helps trees improve water absorption and survive droughts. Therefore, R. pseudoacacia has strong drought tolerance but poor adaptability to waterlogging. According to the results of large-scaled investigations, the broken top rate, dead shoot rate, and mortality of R. pseudoacacia plantations in the Yellow River Delta total 41.4%, and the survival rate of the declining R. pseudoacacia plantations in the whole Yellow River Delta is less than 70 % [45]. The combination of waterlogging and salt stress caused by low GWD may be a significant cause for the severe decline, such as widespread shoot dieback and tree mortality, of R. pseudoacacia plantations in the Yellow River Delta.
4.3. Effects of Groundwater Mineralization and Groundwater Depth on Photosynthetic Characteristics of R. pseudoacacia
Photosynthesis is the basic metabolic process of plants. Plant photosynthetic efficiency can characterize the health status and photosynthetic production capacity of plants in a certain environment and can be used to predict and characterize the adaptability and plasticity of plants to stress [46,47]. With the increase of GWM, the Pn, gs, Ci, E, and WUE of R. pseudoacacia decreased. Correlation analysis showed that SSC was negatively correlated with the Pn, gs, Ci, E, and WUE, which indicated that the photosynthetic characteristics of R. pseudoacacia were sensitive to salt stress. The inhibition of photosynthetic process by stress can be classified into two types: stomatal restriction and non-stomatal restriction [48,49]. In this study, the gs and Ci decreased with the increasing GWM, indicating that the decline of photosynthetic capacity of R. pseudoacacia may be inhibited by stomatal limitation.
In this study, with the increase of GWD, the Pn, gs, Ci, E, and WUE increased. Correlation analysis showed that SRWC was negatively correlated with the Pn, gs, Ci, and RM. When the GWD was 0.8 m, the Pn decreased under waterlogging stress, which was closely related to the closure of the leaf stomata. At the same time, there was a significant positive correlation between the RM and the Pn. This may because, as the main absorption organ, the root system provided water and nutrients for leaf photosynthesis, thus the decline of RM had a significant inhibitory effect on leaf photosynthesis. With the increase of GWD, SRWC decreased, and the waterlogging stress was relieved, which increased gs, Ci, and RM and thus the photosynthetic efficiency of leaves. According to previous studies, trees can adapt to drought stress by improving water use efficiency [50]. In this study, the WUE of R. pseudoacacia leaves increased with the increasing GWD, which reflected the drought resistance of R. pseudoacacia to a certain extent.
4.4. Eco-Physiological Adaptation of R. pseudoacacia Saplings to Groundwater Mineralization and Groundwater Depth
In this study, the GWM and GWD significantly affect the SRWC and SSC. SRWC and SSC were negatively correlated with the SB and photosynthetic characteristics of R. pseudoacacia and had significant roles in limiting the growth of R. pseudoacacia. The SB was positively correlated with photosynthetic characteristics, indicating that photosynthesis was the important physiological process affecting R. pseudoacacia growth. Furthermore, there existed a significant negative correlation between LMR and photosynthetic characteristics, indicating that there was a trade-off between leaf biomass distribution and photosynthesis. The first principal component (including SB, RM, and photosynthetic characteristics) mainly reflected the growth of R. pseudoacacia saplings. The regulation of photosynthesis was closely related to the biomass accumulation of R. pseudoacacia.
We also found that under the influence of the GWM and GWD, the biomass distribution of R. pseudoacacia changed significantly, especially the regulation of RM was a common process to adapt to stresses. With the increase of GWM, the increasing RMR and RSR is an important way for trees to adapt to salt stress. However, the RMR and RSR were the largest at 2 g L−1, which was related to the inhibition of root growth at 4 g L−1. Under different GWDs, the RMR and RSR were the largest at 1.8 m, which acted as the response to drought stress. The second principal component (including RMR, SMR, and RSR) mainly reflected the biomass allocation characteristics of R. pseudoacacia. The regulation of the root system was favorable for R. pseudoacacia to adapt to the variation of the GWM and GWD.
5. Conclusions
GWM and GWD affected soil water and salt content. With the increase of GWM, SRWC and SSC increased. With the increase of GWD, the SRWC increased, and the SSC first increased and then decreased. The GWD of 1.3 m was the turning-point GWD with the largest accumulation of soil salts. With the increase of GWM, the SB decreased significantly; however, with the increase of GWD, SB increased significantly. Photosynthetic activity was a critical physiological process affecting the increase of SB. SRWC and SSC were negatively correlated with biomass and photosynthesis and were the key factors limiting sapling growth. The regulation of biomass allocation was the important strategy for R. pseudoacacia adapting to the water–salt stress driven by the variations of GWM and GWD. The conclusions drawn from this study would provide essential guidelines for the transformation and sustainable management of large-scale declining R. pseudoacacia plantations in the Yellow River Delta.
Author Contributions
Conceptualization, B.C. and P.M.; formal analysis, L.G.; investigation, L.G., Y.P., W.L. and C.T.; data curation, B.J. and Z.C.; writing—original draft preparation, L.G.; writing—review and editing, P.M. and B.C.; supervision, B.C.; funding acquisition, B.C. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by China Natural Science Foundation, grant number 31770668; Major Scientific Technological Innovation Projects of Shandong Province, grant number 2017CXGC0316; Natural Science Foundation of Shandong Province, grant number ZR2020MC158; Shandong wetland park construction quality basic investigation project of Shandong forestry protection and Development Service Center, grant number SDGP370000000202202001808A_001.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Effects of groundwater mineralization and groundwater depth on soil relative water content, soil salt content, and Robinia pseudoacacia characteristics.
Table A1.
Effects of groundwater mineralization and groundwater depth on soil relative water content, soil salt content, and Robinia pseudoacacia characteristics.
| Indices and Units | GWD = 0.8 m | GWD = 1.3 m | GWD = 1.8 m | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 g L−1 | 2 g L−1 | 4 g L−1 | 0 g L−1 | 2 g L−1 | 4 g L−1 | 0 g L−1 | 2 g L−1 | 4 g L−1 | |
| SRWC (%) | 62.72 ± 1.22 cA | 71.31 ± 1.50 bA | 83.12 ± 1.50 aA | 41.21 ± 2.26 bB | 49.79 ± 1.59 abB | 58.31 ± 6.38 aB | 17.15 ± 0.91 cC | 20.58 ± 1.02 bC | 24.45 ± 0.83 aC |
| SSC (‰) | 0.82 ± 0.02 cB | 2.49 ± 0.03 bA | 2.76 ± 0.13 aA | 0.87 ± 0.02 cA | 2.50 ± 0.03 bA | 2.91 ± 0.11 aA | 0.89 ± 0.02 cA | 1.41 ± 0.01 bB | 1.53 ± 0.05 aB |
| SB (g) | 171.15 ± 6.56 aC | 156.75 ± 9.61 aC | 104.28 ± 18.78 bB | 224.08 ± 8.99 aB | 203.81 ± 8.23 abB | 176.53 ± 18.03 bA | 245.88 ± 4.59 aA | 240.12 ± 6.81 aA | 186.76 ± 26.87 bA |
| RM (g) | 89.45 ± 6.48 abC | 112.83 ± 12.29 aC | 67.73 ± 15.77 bB | 129.70 ± 7.84 aB | 146.69 ± 8.26 aB | 120.63 ± 18.66 aA | 165.57 ± 9.23 aA | 187.38 ± 9.48 aA | 145.02 ± 27.06 aA |
| SM (g) | 62.73 ± 1.32 aA | 27.24 ± 3.52 bA | 23.24 ± 4.02 bB | 71.17 ± 6.87 aA | 35.13 ± 2.07 bA | 36.90 ± 3.29 bA | 59.73 ± 4.00 aA | 33.45 ± 6.56 bA | 24.58 ± 3.46 bB |
| LM (g) | 18.97 ± 0.70 aB | 16.68 ± 0.74 bB | 13.32 ± 1.23 cB | 23.20 ± 0.94 aA | 21.99 ± 1.46 abA | 19.00 ± 1.55 bA | 20.58 ± 0.95 aB | 19.29 ± 1.23 abB | 17.17 ± 0.76 bA |
| RMR (g g−1) | 0.52 ± 0.02 bC | 0.72 ± 0.03 aA | 0.65 ± 0.05 aB | 0.58 ± 0.03 bB | 0.72 ± 0.01 aA | 0.68 ± 0.04 aAB | 0.67 ± 0.03 bA | 0.78 ± 0.03 aA | 0.77 ± 0.03 aA |
| SMR (g g−1) | 0.37 ± 0.02 aA | 0.18 ± 0.03 bA | 0.22 ± 0.03 bA | 0.32 ± 0.02 aB | 0.17 ± 0.01 bA | 0.21 ± 0.03 bAB | 0.24 ± 0.02 aC | 0.14 ± 0.03 bA | 0.13 ± 0.03 bB |
| LMR (g g−1) | 0.11 ± 0.00 aA | 0.11 ± 0.00 aA | 0.13 ± 0.02 aA | 0.10 ± 0.01 aA | 0.11 ± 0.01 aA | 0.11 ± 0.01 aAB | 0.08 ± 0.01 aB | 0.08± 0.01 aB | 0.09 ± 0.01 aB |
| RSR (g g−1) | 1.10 ± 0.08 bB | 2.59 ± 0.46 aA | 1.86 ± 0.42 abB | 1.38 ± 0.14 bB | 2.57 ± 0.16 aA | 2.16 ± 0.38 aB | 2.07 ± 0.24 bA | 3.60 ± 0.58 aA | 3.49 ± 0.73 aA |
| Pn (μmol m−2 s−1) | 6.35 ± 0.39 aB | 6.10 ± 0.23 aB | 3.48 ± 0.30 bC | 7.80 ± 0.33 aA | 6.63 ± 0.43 bAB | 4.73± 0.11 cB | 8.25 ± 0.57 aA | 7.30 ± 0.26 bA | 5.47 ± 0.14 cA |
| gs (mmol m−2 s−1) | 0.20 ± 0.01 aB | 0.17± 0.01 bC | 0.14 ± 0.02 bB | 0.22 ± 0.01 aB | 0.18 ± 0.00 bB | 0.19 ± 0.01 bA | 0.25 ± 0.01 aA | 0.19 ± 0.01 cA | 0.22 ± 0.02 bA |
| Ci (μmol m−2 s−1) | 252.31 ± 20.74 aA | 241.06 ± 20.99 abA | 193.70 ± 23.05 bB | 267.35 ± 19.90 aA | 257.57 ± 23.59 aA | 223.60 ± 11.06 aAB | 280.18 ± 21.29 aA | 262.41 ± 27.79 aA | 243.63 ± 14.71 aA |
| E (mmol m−2 s−1) | 1.94 ± 0.04 aB | 1.87 ± 0.05 aB | 1.39 ± 0.16 bB | 2.26 ± 0.02 aA | 1.96± 0.06 bAB | 1.60 ± 0.03 cAB | 2.27 ± 0.07 aA | 2.08 ± 0.05 bA | 1.78 ± 0.02 cA |
| WUE (μmol mmol−1) | 3.27 ± 0.19 aA | 3.26 ± 0.18 aA | 2.51 ± 0.11 bB | 3.46 ± 0.17 aA | 3.39 ± 0.15 aA | 2.96 ± 0.07 bA | 3.63 ± 0.14 aA | 3.52±0.13 aA | 3.08 ± 0.10 bA |
Different lower letters indicate that there is a significant difference between different groundwater mineralization levels under the same groundwater depth (p < 0.05), and different capital letters indicate that there is a significant difference between different groundwater depths under the same groundwater mineralization (p < 0.05). Data presented as mean ± SD.
References
- Barbeta, A.; Peñuelas, J. Relative contribution of groundwater to plant transpiration estimated with stable isotopes. Sci. Rep.-UK 2017, 7, 10580. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chen, Y.N.; Xu, C.C.; Li, W.H. Groundwater depth affects the daily course of gas exchange parameters of Populus euphratica in arid areas. Environ. Earth Sci. 2012, 66, 433–440. [Google Scholar] [CrossRef]
- Gou, S.; Gonzales, S.; Miller, G.R. Mapping potential groundwater-dependent ecosystems for sustainable management. Groundwater 2015, 53, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, D.; Michalska–Hejduk, D.; Krogulec, E. The relationship between vegetation and groundwater levels as an indicator of spontaneous wetland restoration. Ecol. Eng. 2013, 57, 242–251. [Google Scholar] [CrossRef]
- Fu, B.H.; Burgher, I. Riparian vegetation ndvi dynamics and its relationship with climate, surface water and groundwater. J. Arid Environ. 2015, 113, 59–68. [Google Scholar] [CrossRef]
- Abliz, A.; Tiyip, T.; Ghulam, A.; Halik, Ü.; Ding, J.L.; Sawut, M.; Zhang, F.; Nurmemet, I.; Abliz, A. Effects of shallow groundwater table and salinity on soil salt dynamics in the Keriya Oasis, Northwestern China. Environ. Earth Sci. 2016, 75, 260. [Google Scholar] [CrossRef]
- Ceuppens, J.; Wopereis, M.C.S. Impact of non–drained irrigated rice cropping on soil salinization in the Senegal River Delta. Geoderma 1992, 92, 125–140. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhang, G.H.; Yan, M.J.; Li, Y.; Zhou, Z.M. Analysis of soil salinity distribution and influencing factors in area around Bohai Sea. J. Arid Land Resour. Environ. 2012, 26, 104–109. [Google Scholar] [CrossRef]
- Chen, Y.B.; Hu, S.J.; Luo, Y.; Tian, C.Y.; Yin, C.H. Relationship between salt accumulation in topsoil of deserted land and groundwater in areas with shallow groundwater table in Kashi, Xinjiang. Acta Pedol. Sincta 2014, 51, 75–81. [Google Scholar]
- Liu, X.Z.; Yue, W.F.; Jia, S.H.; Chen, A.P. Variation of soil salinity in the Yichang irrigation district Inner Mongolia. J. Beijing Normal Univ. 2014, 50, 503–507. [Google Scholar]
- Li, X.Q.; Xia, J.B.; Zhao, X.M.; Chen, Y.P. Effects of planting Tamarix chinensis on shallow soil water and salt content under different groundwater depths in the Yellow River Delta. Geoderma 2019, 35, 104–111. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, C.Y.; Shi, F.Z.; Schneider, M.; Lv, G.H.; Li, Y. Impact of groundwater depth and soil salinity on riparian plant diversity and distribution in an arid area of China. Sci. Rep.-UK 2020, 10, 7272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Guan, T.Y.; Zhou, J.H.; Cai, W.T.; Gao, N.N.; Du, H.; Jiang, L.H.; Lai, L.M.; Zheng, Y.R. Groundwater depth and soil properties are associated with variation in vegetation of a desert riparian ecosystem in an arid area of China. Forests 2018, 9, 34. [Google Scholar] [CrossRef]
- Cui, G.Q.; Lu, Y.D.; Zheng, C.; Liu, Z.H.; Sai, J.M. Relationship between soil salinization and groundwater hydration in Yaoba Oasis, Northwest China. Water 2019, 11, 175. [Google Scholar] [CrossRef]
- Song, Y.; Wang, H.; Lu, K.Y.; Zhong, Y. Study on soil properties of Robinia pseudoacacia forest under different health conditions in Yellow River Delta based on canonical correspondence analysis. Acta Agric. Jiangxi. 2017, 29, 48–53. [Google Scholar] [CrossRef]
- Xia, J.B.; Lang, Y.; Zhao, Q.K.; Liu, P.; Su, L. Photosynthetic characteristics of Tamarix chinensis under different groundwater depths in freshwater habitats. Sci. Total Environ. 2020, 761, 143221. [Google Scholar] [CrossRef]
- Yao, L.; Liu, G.H.; Liu, Q.S.; Fei, L.F. Remote sensing monitoring the health of artificial Robinia pseudoacacia forest. Geomat. Inform. Sci. Wuhan Univ. 2010, 35, 863–867. [Google Scholar] [CrossRef]
- Lu, C.X.; Zhao, C.; Liu, J.; Li, K.L.; Wang, B.S.; Chen, M. Increased salinity and groundwater levels lead to degradation of the Robinia pseudoacacia forest in the Yellow River Delta. J. Forest. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Zhu, J.F.; Liu, J.T.; Lu, Z.H.; Li, J.S.; Sun, J.K. Water-use strategies of coexisting shrub species in the Yellow River Delta, China. Can. J. Forest. Res. 2018, 48, 1099–1107. [Google Scholar] [CrossRef]
- Guo, B.; Zang, W.Q.; Luo, W.; Wen, Y.; Yang, F.; Han, B.M.; Fan, Y.W.; Chen, X.; Qi, Z.; Wang, Z.; et al. Detection model of soil salinization information in the Yellow River Delta based on feature space models with typical surface parameters derived from Landsat8 OLI image. Geomat. Nat. Hazard Risk 2020, 11, 288–300. [Google Scholar] [CrossRef]
- Ma, Y.L.; Wand, D.; Liu, J.M.; Wen, X.H.; Gao, M.; Shao, H.B. Relationships between typical vegetations, soil salinity, and groundwater depth in the Yellow River Delta of China. Chin. J. Appl. Ecol. 2013, 24, 2423–2430. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Long–term effect of salinity on plant quality, water relations, photosynthetic parameters and ion distribution in Callistemon citrinus. Plant Biol. 2014, 16, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.B.; Ren, J.Y.; Zhao, X.M.; Zhao, F.J.; Yang, H.J.; Liu, J.H. Threshold effect of the groundwater depth on the photosynthetic efficiency of Tamarix chinensis in the Yellow River Delta. Plant Soil 2018, 433, 157–171. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, X.J.; Li, Y.; Xu, G.Q. Varying responses of two Haloxylon species to extreme drought and groundwater depth. Environ. Exp. Bot. 2019, 158, 63–72. [Google Scholar] [CrossRef]
- Qi, Z.W.; Xiao, C.L.; Wang, G.; Liang, X.J. Study on ecological threshold of groundwater in typical salinization area of Qian’an County. Water 2021, 13, 856. [Google Scholar] [CrossRef]
- Mao, P.L.; Mu, H.X.; Cao, B.H.; Qin, Y.J.; Shao, H.B.; Wang, S.M.; Tai, X.G. Dynamic characteristics of soil properties in a Robinia pseudoacacia vegetation and coastal eco–restoration. Ecol. Eng. 2016, 92, 132–137. [Google Scholar] [CrossRef]
- Meng, P.Y.; Wang, H.; Qin, S.H.; Li, X.N.; Song, Z.L.; Wang, Y.C.; Yang, Y.; Gao, J. Health assessment of plantations based on LiDAR canopy spatial structure parameters. Int. J. Digit. Earth 2022, 15, 712–729. [Google Scholar] [CrossRef]
- Jiao, S.Y.; Li, J.R.; Li, Y.Q.; Jia, J.W.; Xu, Z.Y. Soil C, N, and P distribution as affected by plant communities in the Yellow River Delta, China. PLoS ONE 2019, 14, e0226887. [Google Scholar] [CrossRef]
- Mao, P.L.; Zhang, Y.J.; Cao, B.H.; Guo, L.M.; Shao, H.B.; Cao, Z.Y.; Jiang, Q.K.; Wang, X. Effects of salt stress on eco–physiological characteristics in Robinia pseudoacacia based on salt–soil rhizosphere. Sci. Total Environ. 2016, 568, 118–123. [Google Scholar] [CrossRef]
- Xia, J.B.; Zhao, X.M.; Ren, J.Y.; Ying, L.; Qu, F.Z.; Xu, H. Photosynthetic and water physiological characteristics of Tamarix chinensis under different groundwater mineralization conditions. Environ. Exp. Bot. 2017, 138, 173–183. [Google Scholar] [CrossRef]
- Yao, L. Remote Sensing for Monitoring the Health Condition of Artifitical Robinia pseudoacacia Forests and Analysis of Their Dieback or Death in the Yellow River Delta. Ph.D. Dissertation, Wuhan University, Wuhan, China, 2013. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2018&filename=1013209372.nh (accessed on 1 May 2013).
- Pidgeon, J.D. The measurement and prediction of available water capacity of ferrallitic soils in Uganda. J. Soil Sci. 1972, 23, 431–441. [Google Scholar] [CrossRef]
- Salehin, M.; Chowdhury, M.; Arefin, M.; Clarke, D.; Mondal, S.; Nowreen, S.; Jahiruddin, M.; Haque, A. Mechanisms and drivers of soil salinity in coastal Bangladesh. In Ecosystem Services for Well–Being in Deltas; Palgrave Macmillan: Cham, Switzerland, 2018; pp. 333–347. [Google Scholar] [CrossRef]
- Xie, W.P.; Yang, J.S.; Yao, R.J.; Wang, X.P. Impact study of impoundment of the three Gorges Reservoir on salt–water dynamics and soil salinity in the Yangtze River Estuary. J. Environ. Inform. 2020, 36, 11–23. [Google Scholar] [CrossRef]
- Thiam, S.; Villamor, G.B.; Kyei–Baffour, N.; Matty, F. Soil salinity assessment and coping strategies in the coastal agricultural landscape in Djilor district, Senegal. Land Use Policy 2019, 88, 104191. [Google Scholar] [CrossRef]
- Jeevarathinam, C.; Rajasekar, S.; Sanjuan, M.A.F. Vibrational resonance in groundwater–dependent plant ecosystems. Ecol. Complex. 2013, 15, 33–42. [Google Scholar] [CrossRef]
- Xia, J.B.; Zhao, X.M.; Zhao, Z.G.; Chen, Y.P.; Liu, J.H. Migration characteristics of soil water and salt and their interaction under different groundwater levels. Trans. Chin. Soc. Agric. Eng. 2015, 31, 93–100. [Google Scholar] [CrossRef]
- Fan, X.; Pedroli, B.; Liu, G.; Liu, Q.; Liu, H.; Shu, L. Soil salinity development in the Yellow River Delta in relation to groundwater dynamics. Land Degrad. Dev. 2012, 23, 175–189. [Google Scholar] [CrossRef]
- Liang, S.M.; Yan, H.L.; Zhang, X.M.; Xie, T.T.; Zhu, J.T.; Zhang, Z.W. Physiological response of natural C. taklimakanensis BR Pan et GM Shen to unconfined groundwater in the hinterland of the Taklimakan Desert. Chin. Sci. Bull. 2008, 53, 112–118. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Ji, D.F.; Turgeon, R.; Chen, J.E.; Lin, T.B.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.G.; Lv, Z.Q. Physiological and proteomic responses of Mulberry Trees (Morus alba. L.) to combined salt and drought stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Su, L.; Dong, B.T.; Sun, J. Effect of groundwater depth on root growth of Tamarix chinensis in the Yellow River Delta. Acta Ecol. Sin. 2021, 41, 3794–3804. [Google Scholar] [CrossRef]
- Gu, X.B.; Xue, L.; Lu, L.H.; Xiao, J.P.; Song, G.H.; Xie, M.; Zhang, H.Q. Melatonin Enhances the waterlogging tolerance of prunus persica by modulating antioxidant metabolism and anaerobic respiration. J. Plant Growth Regul. 2021, 40, 2178–2190. [Google Scholar] [CrossRef]
- Deligoz, A.; Bayar, E. Drought stress responses of seedlings of two oak species (Quercus cerris and Quercus robur). Turk. J. Agric. For. 2018, 42, 114–123. [Google Scholar] [CrossRef]
- Yang, Q.G.; Fan, L.R.; Feng, W.J.; Lou, Y.H.; Pan, H.; Wang, H.; Chao, Y.; Zhuge, Y.P. Causes and Countermeasures of Forest Decline in Saline-alkali Land of the Yellow River Delta. World Forest. Res. 2021, 2, 80–84. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Xia, J.B.; Zhang, G.C.; Zhao, Z.G.; Zhao, Y.Y.; Shao, H.B.; Sun, J.K.; Shao, C.Y.; Liu, Q. Threshold effects of photosynthetic efficiency parameters of wild jujube in response to soil moisture variation on shell beach ridges, Shandong, China. Plant Biosyst. 2014, 148, 140–149. [Google Scholar] [CrossRef]
- Sánchez, E.; Scordia, D.; Lino, G.; Arias, C.; Cosentino, S.L.; Nogués, S. Salinity and water stress effects on biomass production in different Arundo donax L. clones. Bioenergy Res. 2015, 8, 1461–1479. [Google Scholar] [CrossRef]
- Pilon, C.; Snider, J.L.; Sobolev, V.; Chastain, D.R.; Sorensen, R.B.; Meeks, C.D.; Massa, A.N.; Walk, T.; Singh, B.; Earl, H.J. Assessing stomatal and non–stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). J. Plant Physiol. 2018, 231, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non–stomatal limitations are responsible in down–regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Zhao, S.; Mao, K.; Dong, Q.L.; Liang, B.W.; Li, C.; Wei, Z.W.; Li, M.J.; Ma, F.W. Mapping QTLs for water–use efficiency reveals the potential candidate genes involved in regulating the trait in apple under drought stress. BMC Plant Biol. 2018, 18, 136. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).