Thermal Characteristics and Simulation of Enzymatic Lignin Isolated from Chinese Fir and Birch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
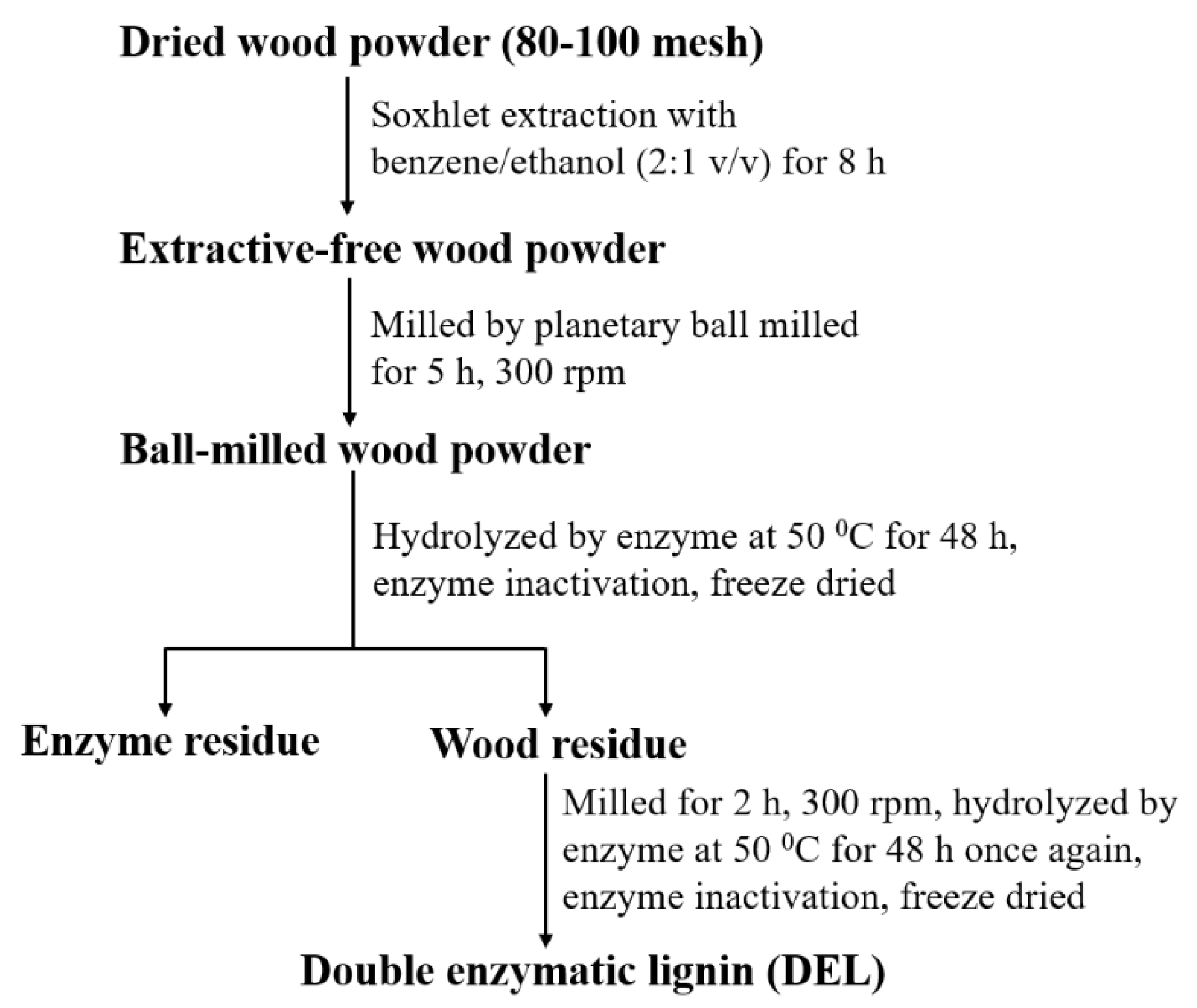
2.2. Lignin Isolation
2.3. Fourier-Transform Infrared Spectroscopy
2.4. Quantitative 2D-Heteronuclear Single Quantum Coherence (2D-HSQC) NMR Spectroscopy
2.5. Heat Flow and Specific Heat Capacity Test
2.6. Thermal Conductivity Test
2.7. Thermogravimetric Test
2.8. Calculation and Simulation of Thermal Degradation Kinetic Parameters
3. Results and Discussion
3.1. FTIR Analysis
3.2. 2D-HSQC NMR Analysis
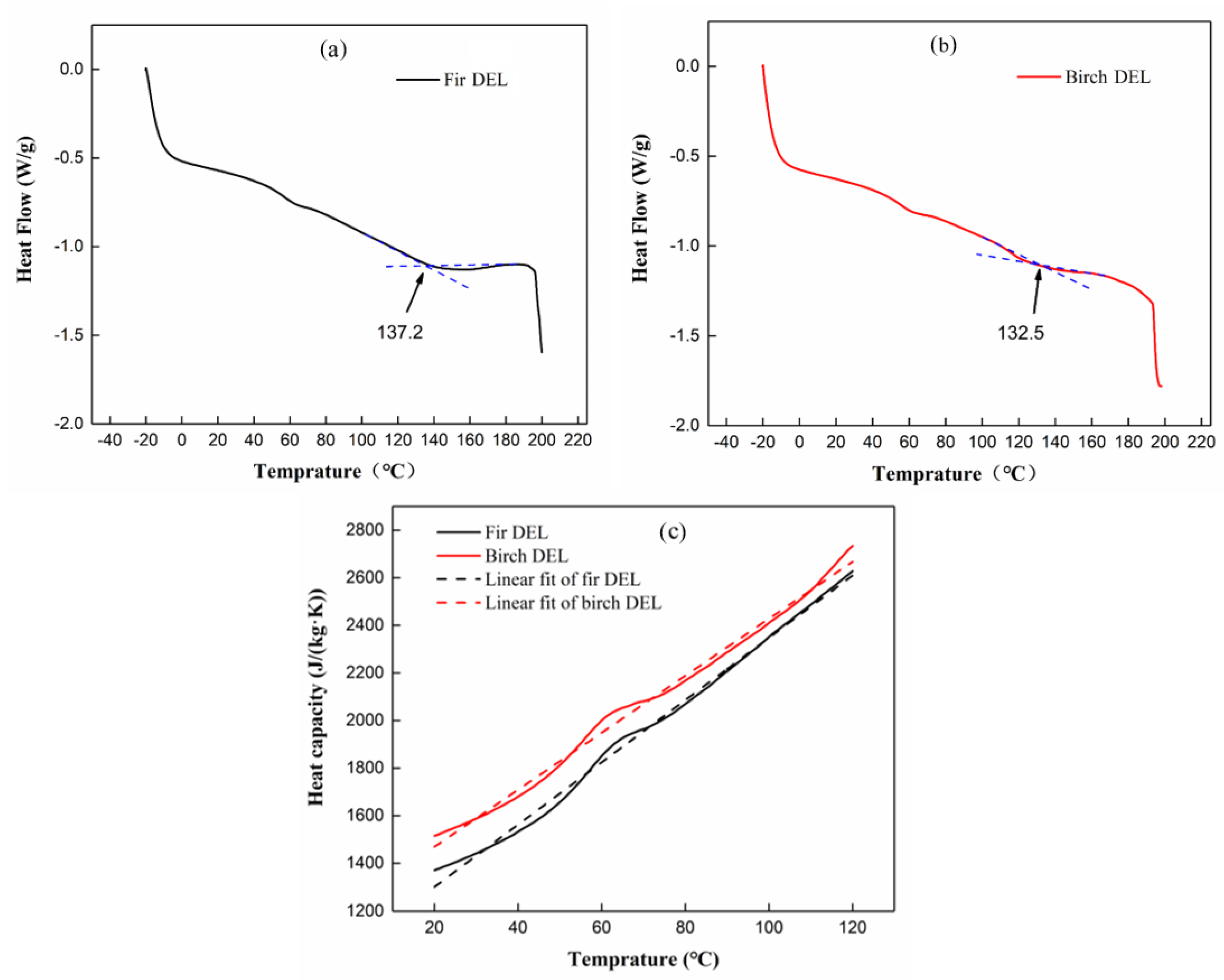
3.3. Glass Transition Temperature and Heat Capacity Analysis
R2 = 0.99
R2 = 0.99
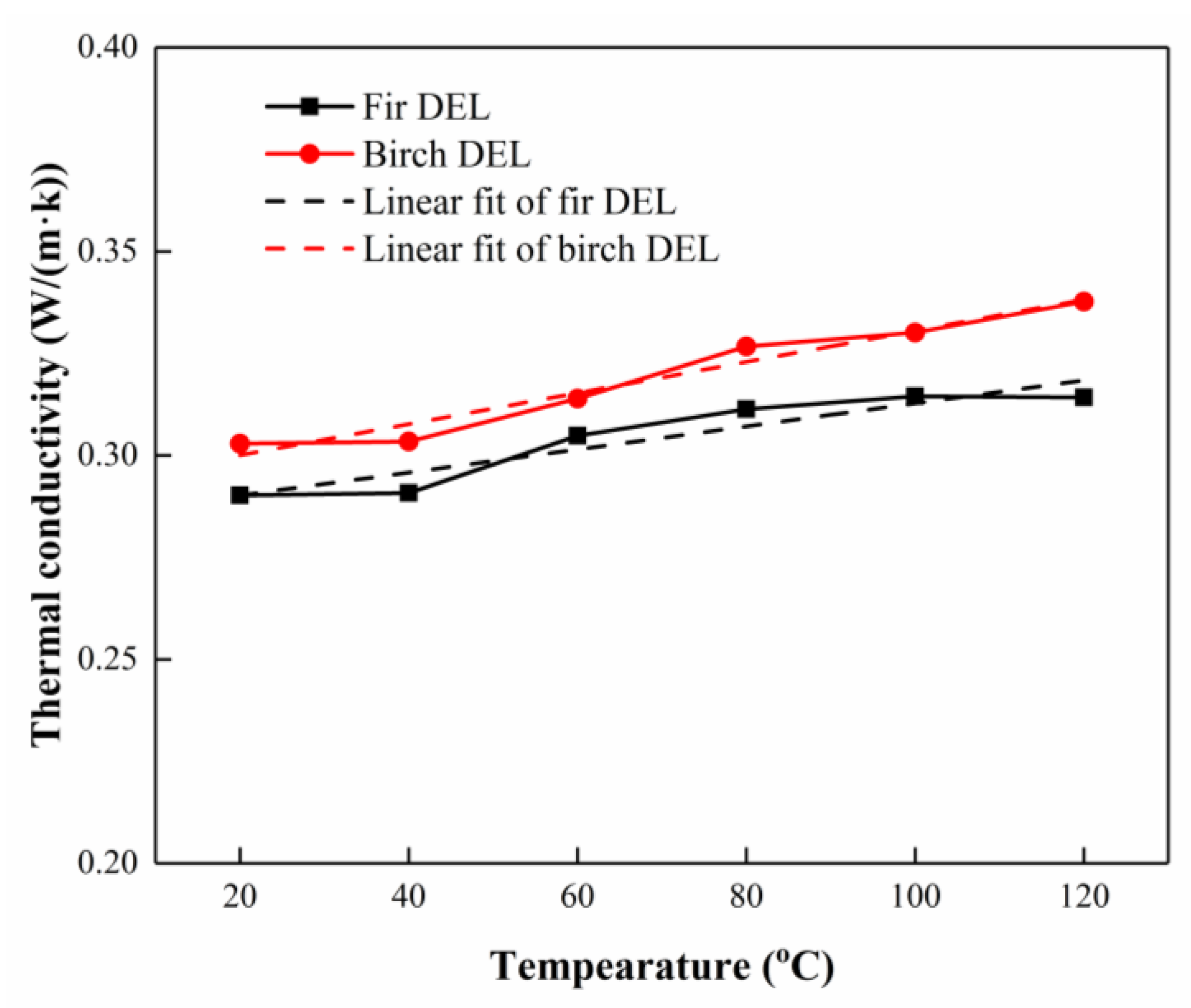
3.4. Thermal Conductivity Analysis
R2 = 0.88
R2 = 0.96
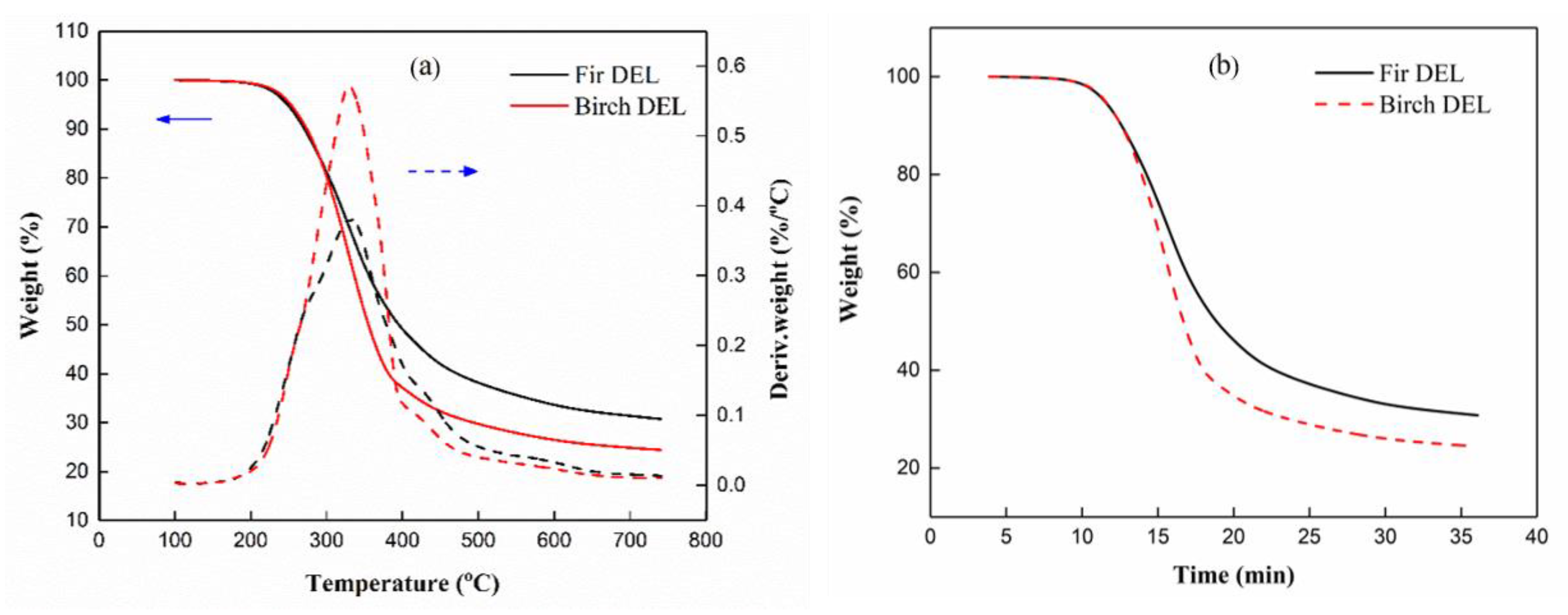
3.5. Thermal Stability Analysis
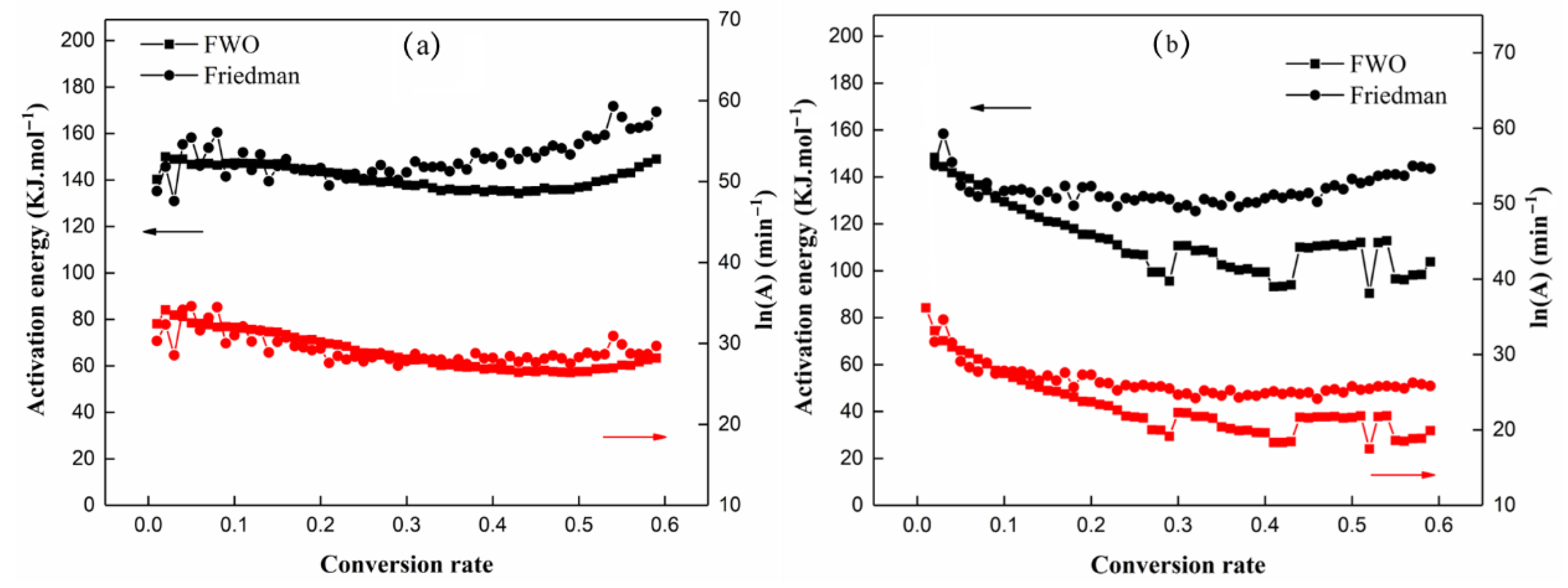
3.6. Thermal Degradation Kinetics
R2 = 0.78
R2 = 0.83
R2 = 0.80
R2 = 0.90
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Kathahira, R.; Elder, T.J.; Beckham, G.T. Chapter 1: A Brief Introduction to Lignin Structure. In Lignin Valorization: Emerging Approaches; 2018; pp. 1–20. Available online: https://www.srs.fs.usda.gov/pubs/ja/2018/ja_2018_elder_002.pdf (accessed on 20 May 2022). [CrossRef] [Green Version]
- Abdelwahab, M.A.; Jacob, S.; Misra, M.; Mohanty, A.K. Super-tough sustainable biobased composites from polylactide bioplastic and lignin for bio-elastomer application. Polymer 2021, 212, 123153. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.D.; Callois, J.M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Stewart, S.A.; Rendl, A.; González, Z.; Donnelly, R.F.; Larrañeta, E. Lignin and cellulose blends as pharmaceutical excipient for tablet manufacturing via direct compression. Biomolecules 2019, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Lourençon, T.V.; Alakurtti, S.; Virtanen, T.; Jääskeläinen, A.S.; Liitiä, T.; Hughes, M.; Magalhães, W.L.; Muniz, G.I.; Tamminen, T. Phenol-formaldehyde resins with suitable bonding strength synthesized from “less-reactive” hardwood lignin fractions. Holzforschung 2020, 74, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Huang, C.; Xiao, D.; Wang, P.; Luo, X.; Liu, W.; Liu, S.; Li, J.; Li, S.; Chen, Z. Melanin-inspired design: Preparing sustainable photothermal materials from lignin for energy generation. ACS Appl. Mater. Interfaces 2021, 13, 7600–7607. [Google Scholar] [CrossRef]
- Wang, L.; Ago, M.; Borghei, M.; Ishaq, A.; Papageorgiou, A.C.; Lundahl, M.; Rojas, O.J. Conductive carbon microfibers derived from wet-spun lignin/nanocellulose hydrogels. ACS Sustain. Chem. Eng. 2019, 7, 6013–6022. [Google Scholar] [CrossRef] [Green Version]
- Svenningsson, L.; Bengtsson, J.; Jedvert, K.; Schlemmer, W.; Theliander, H.; Evenäs, L. Disassociated molecular orientation distributions of a composite cellulose–lignin carbon fiber precursor: A study by rotor synchronized NMR spectroscopy and X-ray scattering. Carbohydr. Polym. 2021, 254, 117293. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; Jiaqiang, E.; Xue, Y. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.M.; Jameel, H. Lignin structural variation in hardwood species. J. Agric. Food Chem. 2012, 60, 4923–4930. [Google Scholar] [CrossRef] [PubMed]
- Yelle, D.J. Solution-state NMR analysis of hydroxymethylated resorcinol cured in the presence of crude milled-wood lignin from Acer saccharum. J. Appl. Polym. Sci. 2017, 134, 45398. [Google Scholar] [CrossRef]
- Björkman, A. Studies on finely divided wood. Part 1. Extraction of lignin with neutral solvents. Sven. Papp. Nord. Cellul. 1956, 59, 477–485. [Google Scholar]
- Chen, T.Y.; Wang, B.; Wu, Y.Y.; Wen, J.L.; Liu, C.F.; Yuan, T.Q.; Sun, R.C. Structural variations of lignin macromolecule from different growth years of Triploid of Populus tomentosa Carr. Int. J. Biol. Macromol. 2017, 101, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Wang, B.; Wen, J.L.; Yuan, T.Q.; Sun, R.C. Structural Characteristics of Lignin Macromolecules from Different Eucalyptus Species. ACS Sustain. Chem. Eng. 2017, 5, 11618–11627. [Google Scholar] [CrossRef]
- Qi, C.; Hou, S.; Lu, J.; Xue, W.; Sun, K. Thermal characteristics of birch and its cellulose and hemicelluloses isolated by alkaline solution. Holzforschung 2020, 74, 1099–1112. [Google Scholar] [CrossRef]
- Yu, M.; He, D.; Zhang, Y.; He, D.; Wang, X.; Zhou, J. Characterization of lignin extracted from Acanthopanax senticosus residue using different methods on UV-resistant behavior. Int. J. Biol. Macromol. 2021, 192, 498–505. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Liu, Q.; Gu, Y.; Luo, Z.; Cen, K.; Fransson, T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009, 27, 562–567. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.; Bridgwater, T. Effect of the Temperature on the Composition of Lignin Pyrolysis Products. Energy Fuels 2010, 24, 4470–4475. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, X.D.; Liu, H.M.; Wang, D.M.; Qin, G.Y. Structural characterization of Chinese quince fruit lignin pretreated with enzymatic hydrolysis. Bioresour. Technol. 2018, 262, 212–220. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Quantitative Structures and Thermal Properties of Birch Lignins after Ionic Liquid Pretreatment. J. Agric. Food Chem. 2012, 61, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 4323–4328. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Qi, C. Multi-Model and Visual Calculation Software of Thermal Degradation Kinetic Parameters for Biomass and Wood. 2018. Available online: http://js.editorbar.com (accessed on 17 January 2022).
- Coats, A.W.; Redfern, J. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Abd El-Wahab, M.M.M. Thermal decomposition kinetics of some new unsaturated polyesters. Thermochim. Acta 1995, 256, 271–280. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, T.; Wu, Z.; Zeng, G.; Huang, D.; Shen, Y.; He, X.; Lai, M.; He, Y. Study on biodegradation process of lignin by FTIR and DSC. Environ. Sci. Pollut. Res. 2014, 21, 14004–14013. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Liu, J.; Wu, J. FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl. Surf. Sci. 2013, 280, 799–805. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Ehara, K.; Takada, D.; Saka, S. GC-MS and IR spectroscopic analyses of the lignin-derived products from softwood and hardwood treated in supercritical water. J. Wood Sci. 2005, 51, 256–261. [Google Scholar] [CrossRef]
- Strahan, G.D.; Mullen, C.A.; Stoklosa, R.J. Application of Diffusion-Ordered NMR Spectroscopy to the Characterization of Sweet Sorghum Bagasse Lignin Isolated After Low Moisture Anhydrous Ammonia (LMAA) Pretreatment. BioEnergy Res. 2022, 1–10. Available online: https://link.springer.com/article/10.1007/s12155-021-10385-y (accessed on 20 May 2022). [CrossRef]
- Heikkinen, S.; Toikka Mm Fau-Karhunen, P.T.; Karhunen Pt Fau-Kilpeläinen, I.A.; Kilpeläinen, I.A. Quantitative 2D HSQC (Q-HSQC) via suppression of J-dependence of polarization transfer in NMR spectroscopy: Application to wood lignin. J. Am. Chem. Soc. 2003, 125, 4362–4367. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of lignin structures and lignin-carbohydrate complex (LCC) linkages by quantitative 13C and 2D HSQC NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef]
- Del Rio, J.C.; Rencoret, J.; Marques, G.; Li, J.; Gellerstedt, G.; Jimenez-Barbero, J.; Gutiérrez, A.N.A. Structural characterization of the lignin from jute (Corchorus capsularis) fibers. J. Agric. Food Chem. 2009, 57, 10271–10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejos, M.; Felissia, F.; Curvelo, A.; Zambon, M.D.; Ramos, L.; Area, M. Chemical and physico-chemical characterization of lignins obtained from ethanol-water fractionation of bagasse. BioResources 2011, 6, 1158–1171. Available online: https://hdl.handle.net/11336/43206 (accessed on 15 January 2022).
- Gregorova, A. Application of differential scanning calorimetry to the characterization of biopolymers. Applications of Calorimetry in a Wide Context-Differential Scanning Calorimetry. Isothermal Titration Calorim. Microcalorim. 2013, 3–20. Available online: https://www.intechopen.com/chapters/42247 (accessed on 20 May 2022). [CrossRef] [Green Version]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Voitkevich, O.V.; Kabo, G.J.; Blokhin, A.V.; Paulechka, Y.U.; Shishonok, M.V. Thermodynamic properties of plant biomass components. Heat capacity, combustion energy, and gasification equilibria of lignin. J. Chem. Eng. Data 2012, 57, 1903–1909. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Nakamura, K.; Hatakeyama, H. Studies on heat capacity of cellulose and lignin by differential scanning calorimetry. Polymer 1982, 23, 1801–1804. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Ru, B.; Sun, W.; Wang, Y.; Luo, Z. Comparison of the pyrolysis behavior of pyrolytic lignin and milled wood lignin by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2014, 108, 78–85. [Google Scholar] [CrossRef]
- Damayanti Wu, H.S. Pyrolysis kinetic of alkaline and dealkaline lignin using catalyst. J. Polym. Res. 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. A systematic study of the kinetics of lignin pyrolysis. Thermochim. Acta 2010, 498, 61–66. [Google Scholar] [CrossRef]
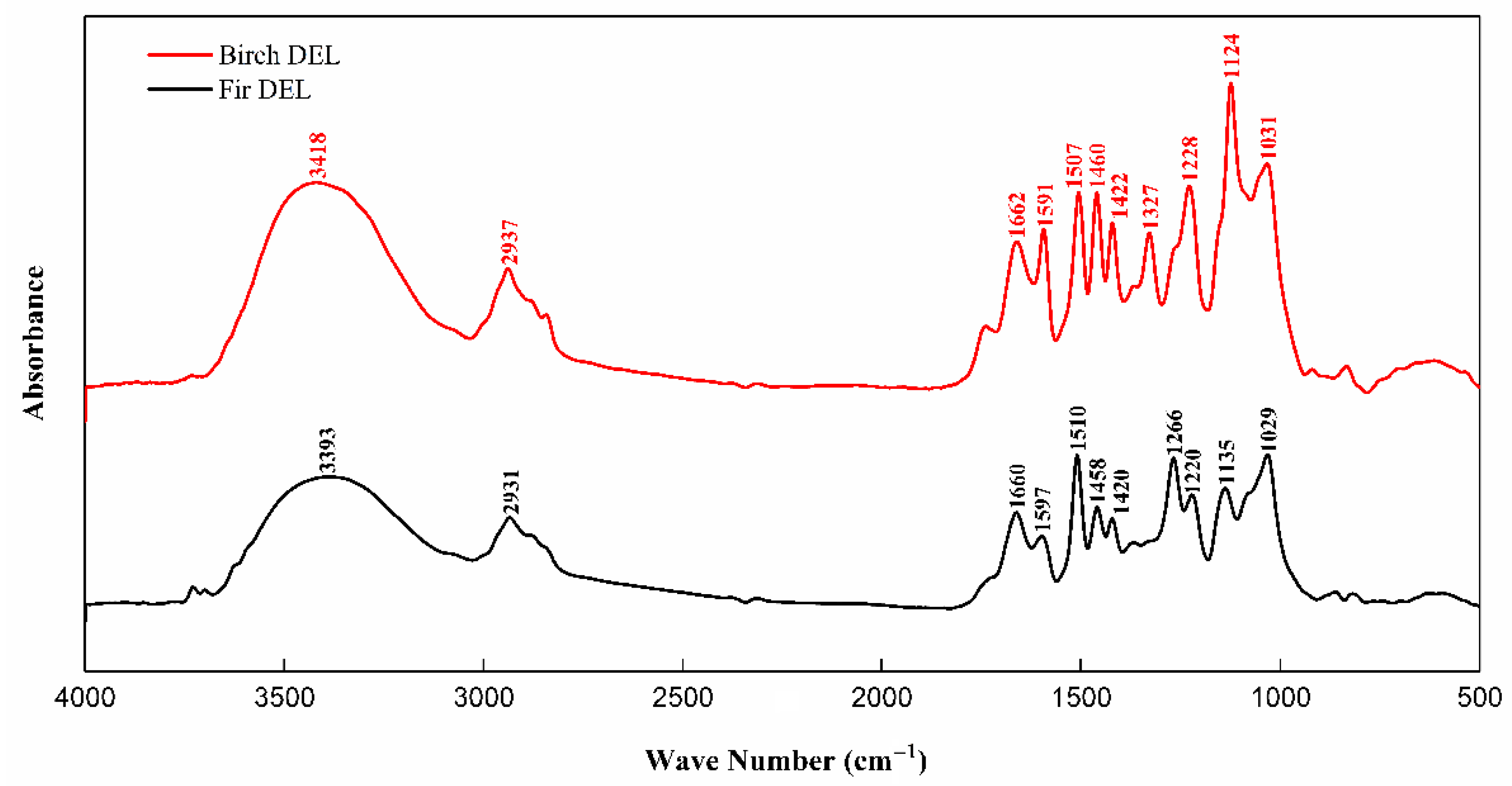


| Wave Number (cm−1) | Absorption Peak Assignment |
|---|---|
| 3393, 3418 | Hydroxyl (O-H) |
| 2931, 2937 | Methyl, Methylene, Methine (C-H) |
| 1715~1710 | Non-conjugated carbonyl (C=O), Ester group |
| 1660~1665 | Conjugated carbonyl (C=O) |
| 1600~1590 | Aromatic ring (skeletal vibration) |
| 1510–1505 | Aromatic ring (skeletal vibration) |
| 1455~1465 | C-H bending vibrations of methyl and methylene |
| 1420, 1422 | Aromatic ring (skeletal vibration) |
| 1327 | Lilac Ring (C-O) |
| 1228, 1266 | Ring of Guaiac (C-O) |
| 1124 | Lilac Ring (C-O) |
| 1029, 1031 | Secondary alcohols, Ethers (C-O bending vibrations) |
| 830 | Aromatic rings (C-H bending vibrations) |
| Sample | Parameters | Conversation Rates | ||||
|---|---|---|---|---|---|---|
| 0.02 | 0.02–0.30 | 0.31–0.45 | 0.46–0.60 | 0.02–0.60 | ||
| Chinese fir lignin | ƒ(a) | |||||
| average | 150.0 | 147.5 | 135.8 | 140.1 | 141.2 | |
| fitting equation | −39.7a + 150.9 R2 = 0.91 | 239.6a2 − 200.3a + 176.9 R2 = 0.74 | 103.1a + 86.3 R2 = 0.91 | 127.1a2 − 95.1a + 154.9 R2 = 0.78 | ||
| ln(A) average | 34.1 | 32.3 | 27.1 | 26.97 | 29.0 | |
| ln(A) fitting equation | −19.5a + 34.0 R2 = 0.98 | −10.5a + 31.1 R2 = 0.91 | 12.9a + 20.2 R2 = 0.83 | −12.3a + 32.7 R2 = 0.83 | ||
| Birch lignin | ƒ(a) | |||||
| average | 148.4 | 133.4 | 106.5 | 105.32 | 112.7 | |
| fitting equation | −163.0a + 147.5 R2 = 0.96 | −148.7a + 156.6 R2 = 0.93 | / | 258.7a2 − 224.2a + 149.75 R2 = 0.84 | ||
| ln(A) average | 33.2 | 28.6 | 21.4 | 20.4 | 22.9 | |
| ln(A) fitting equation | −43.2a + 32.4 R2 = 0.96 | −33.8a + 32.7 R2 = 0.94 | / | 64.9a2 − 58.6a + 33.0 R2 = 0.90 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hou, S.; Shen, Z.; Wen, J.; Qi, C. Thermal Characteristics and Simulation of Enzymatic Lignin Isolated from Chinese Fir and Birch. Forests 2022, 13, 914. https://doi.org/10.3390/f13060914
Wang J, Hou S, Shen Z, Wen J, Qi C. Thermal Characteristics and Simulation of Enzymatic Lignin Isolated from Chinese Fir and Birch. Forests. 2022; 13(6):914. https://doi.org/10.3390/f13060914
Chicago/Turabian StyleWang, Jinyue, Suyun Hou, Ziling Shen, Jialong Wen, and Chusheng Qi. 2022. "Thermal Characteristics and Simulation of Enzymatic Lignin Isolated from Chinese Fir and Birch" Forests 13, no. 6: 914. https://doi.org/10.3390/f13060914
APA StyleWang, J., Hou, S., Shen, Z., Wen, J., & Qi, C. (2022). Thermal Characteristics and Simulation of Enzymatic Lignin Isolated from Chinese Fir and Birch. Forests, 13(6), 914. https://doi.org/10.3390/f13060914







