Phenology Is Associated with Genetic and Stem Morphotype Variation in European Beech (Fagus sylvatica L.) Stands
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Measurements
2.2. Microsatellite Genotyping
2.3. Statistical Analyses
3. Results
3.1. Microsatellite Loci Statistics
3.2. Comparison among the Stands
3.3. Associations between Phenology, Stem Quality and Observed Heterozygosity
3.4. Within-Stand Genetic Structure
4. Discussion
4.1. Loci Efficiency
4.2. Strong Phenology—Morphology Associations
4.3. Genetic Background for Stochastic Phenotypic Variation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sykes, M.T.; Prentice, I.C. Climate change, tree species distributions and forest dynamics: A case study in the mixed conifer/northern hardwoods zone of northern Europe. Clim. Change 1996, 34, 161–177. [Google Scholar] [CrossRef]
- McKenney, D.W.; Pedlar, J.H.; Lawrence, K.; Campbell, K.; Hutchinson, M.F. Potential Impacts of Climate Change on the Distribution of North American Trees. BioScience 2007, 57, 939–948. [Google Scholar] [CrossRef]
- Bolte, A.; Hilbrig, L.; Grundmann, B.; Kampf, F.; Brunet, J.; Roloff, A. Climate change impacts on stand structure and competitive interactions in a southern Swedish spruce–beech forest. Eur. J. For. Res. 2010, 129, 261–276. [Google Scholar] [CrossRef]
- Kraj, W.; Sztorc, A. Genetic structure and variability of phenological forms in the European beech (Fagus sylvatica L.). Ann. For. Sci. 2009, 66, 203. [Google Scholar] [CrossRef]
- Dounavi, A.; Netzer, F.; Celepirovic, N.; Ivanković, M.; Burger, J.; Figueroa, A.; Schön, S.; Simon, J.; Cremer, E.; Fussi, B.; et al. Genetic and physiological differences of European beech provenances (F. sylvatica L.) exposed to drought stress. For. Ecol. Manag. 2016, 361, 226–236. [Google Scholar] [CrossRef]
- Ciocîrlan, E.; Sofletea, N.; Ducci, F.; Curtu, A.L. Patterns of genetic diversity in European beech (Fagus sylvatica L.) at the eastern margins of its distribution range. iForest 2017, 10, 916–922. [Google Scholar] [CrossRef]
- Merzeau, D.; Comps, B.; Thiebaut, B.; Cuguen, J.; Letouzey, J. Genetic structure of natural stands of Fagus sylvatica L. Heredity 1994, 72, 269–277. [Google Scholar] [CrossRef]
- Pyhäjärvi, T.; Kujala, S.T.; Savolainen, O. 275 years of forestry meets genomics in Pinus sylvestris. Evol. Appl. 2020, 13, 11–30. [Google Scholar] [CrossRef]
- Nagai, H.; Murata, K.; Nakano, T. Defect detection in lumber including knots using bending deflection curve: Comparison between experimental analysis and finite element modeling. J. Wood Sci. 2009, 55, 169–174. [Google Scholar] [CrossRef]
- Eriksson, G. Quercus Petrea and Quercus Robur Recent Genetic Research; Stadia Forestalia Slovenica, 146; Slovenian Forestry Institute, The Silva Slovenica Publishing Centre: Ljubliana, Slovenian, 2015; p. 96. ISSN 0353-6025. [Google Scholar]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Drénou, C.; Restrepo, D.; Slater, D. Demystifying Tree Forks: Vices and Virtues of Forks in Arboriculture. J. Bot. Res. 2020, 3, 100–113. [Google Scholar] [CrossRef]
- Ningre, F.; Colin, F. Frost damage on the terminal shoot as a risk factor of fork incidence on common beech (Fagus sylvatica L.). Ann. For. Sci. 2007, 64, 79–86. [Google Scholar] [CrossRef]
- Jennings, D.T.; Stevens, R.E. Southwestern pine tip moth. In USDA Forest Service, Forest Insect and Disease Leaflet; US Department of Agriculture, Forest Service: Washington, DC, USA, 1982; Volume 58, p. 7. [Google Scholar]
- Colin, F.; Sanjines, A.; Fortin, M.; Bontemps, J.D.; Nicolini, E. Fagus sylvatica trunk epicormics in relation to primary and secondary growth. Ann. Bot. 2012, 110, 995–1005. [Google Scholar] [CrossRef]
- Hannerz, M. Genetic and seasonal variation in hardiness and growth rhythm in boreal and temperate conifers—A review and annotated bibliography. For. Res. Inst. Swed. Rep. 1998, 2, 140. [Google Scholar]
- Levins, R. Some Demographic and Genetic Consequences of Environmental Heterogeneity for Biological Control. Bull. Entomol. Soc. Am. 1969, 15, 237–240. [Google Scholar] [CrossRef]
- Chmura, D.J.; Rożkowski, R. Variability of beech provenances in spring and autumn phenology. Silvae Genet. 2002, 51, 123–127. [Google Scholar]
- Kreyling, J.; Thiel, D.; Nagy, L.; Jentsch, A.; Huber, G.; Konnert, M.; Beierkuhnlein, C. Late frost sensitivity of juvenile Fagus sylvatica L. differs between southern Germany and Bulgaria and depends on preceding air temperature. Eur. J. For. Res. 2012, 131, 717–725. [Google Scholar] [CrossRef]
- Dittmar, C.; Elling, W. Phenological phases of common beech (Fagus sylvatica L.) and their dependence on region and altitude in Southern Germany. Eur. J. For. Res. 2006, 125, 181–188. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.Y.; Louvet, J.M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do withinspecies populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Danusevičius, D.; Jonsson, A.; Eriksson, G. Variation among open-pollinated families of Picea abies (L.) Karst. in response to simulated frost desiccation treatment. Silvae Genet. 1999, 45, 158–167. [Google Scholar]
- FAO Map Catalog. Interactive Temperature Data Maps for 10 km Grid in Europe. Available online: https://data.apps.fao.org/map/catalog/srv/eng/catalog.search#/home (accessed on 8 April 2022).
- Linderholm, H.W. Growing season changes in the lastcentury. Agric. For. Meteorol. 2006, 137, 1–14. [Google Scholar] [CrossRef]
- Heide, O.M. Daylength and thermal time responses of budburst during dormancy release in some northern deciduous trees. Physiol. Plant. 1993, 88, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, I.; Hanninen, H. Adaptation of the timing of bud burst of Norway spruce to temperate and boreal climates. Silva Fenn. 2002, 36, 695–701. [Google Scholar] [CrossRef][Green Version]
- Falusi, M.; Calamassi, R. Bud dormancy in beech (Fagus sylvatica L.). Effect of chilling and photoperiod on dormancy release of beech seedlings. Tree Physiol. 1990, 6, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.G. Concepts and terminology of apical dominance. Am. J. Bot. 1997, 84, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Dun, E.A.; Beveridge, C.A. Quick guide Apical dominance. Curr. Biol. 2017, 27, R853–R909. [Google Scholar] [CrossRef]
- Dun, E.A.; Ferguson, B.J.; Beveridge, C.A. Apical Dominance and Shoot Branching. Divergent Opinions or Divergent Mechanisms? Plant Physiol. 2006, 142, 812–819. [Google Scholar] [CrossRef]
- De Vries, D.P. Rootstock Breeding. In Encyclopedia of Rose Science; Plant Research International (PRI): Wageningen, The Netherlands, 2003; pp. 639–645. [Google Scholar] [CrossRef]
- Cline, M.G.; Harrington, C.A. Apical dominance and apical control in multiple flushing of temperate woody species. Can. J. For. Res. 2007, 37, 74–83. [Google Scholar] [CrossRef]
- Holsten, E.H.; Hennon, P.E.; Trummer, L.; Schultz, M. Insects and Diseases of Alaskan Forests. In USDA Forest Service, Alaska Region; US Department of Agriculture, Forest Service, Alaska Region, State and Private Forestry, Forest Health Protection: Washington, DC, USA, 2001; Volume R10-TP-87, p. 242. [Google Scholar]
- Heiermann, J.; Schütz, S. The effect of the tree species ratio of European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.) on polyphagous and monophagous pest species—Lymantria monacha L. and Calliteara pudibunda L. (Lepidoptera: Lymantriidae) as an example. For. Ecol. Manag. 2008, 255, 1161–1166. [Google Scholar] [CrossRef]
- van Couwenberghe, R.; Gégout, J.C.; Lacombe, E.; Collet, C. Light and competition gradients fail to explain the coexistence of shade-tolerant Fagus sylvatica and shade-intermediate Quercus petraea seedlings. Ann. Bot. 2013, 112, 1421–1430. [Google Scholar] [CrossRef]
- Pilkauskas, M.; Augustaitis, A.; Marozas, V. Growth peculiarities of European beech trees outside their natural distribution range in Lithuania. In Proceedings of the 5th International Scientific Conference “Rural Development 2011”, Kaunas, Lithuania, 24–25 November 2011; Volume 5, pp. 106–110. [Google Scholar]
- Kembrytė, R.; Danusevičius, D.; Buchovska, J.; Baliuckas, V.; Kavaliauskas, D.; Fussi, B.; Kempf, M. DNA-based tracking of historical introductions of forest trees: The case of European beech (Fagus sylvatica L.) in Lithuania. Eur. J. For. Res. 2021, 140, 435–449. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; de Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change–range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Fiodorov, J.A. Forests of the Amber Land; Knyznoje izdalestvo: Kaliningrad, Russia, 1990; p. 65. (In Russian) [Google Scholar]
- Gregorius, H.R.; Kownatzki, D. Spatiogenetic characteristics of beech stands with different degrees of autochthony. BMC Ecol. 2005, 5, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kempf, M.; Konnert, M. Distribution of genetic diversity in Fagus sylvatica at the north-eastern edge of the natural range. Silva Fenn. 2016, 50, 17. [Google Scholar] [CrossRef]
- Ulaszewski, B.K. Neutralna i Adaptacyjna Zmienność Genetyczna Buka Zwyczajnego Fagus sylvatica L. na Podstawie Analiz Genomowych (Neutral and Adaptive Genetic Diversity of European Beech Fagus sylvatica L. Based on Genomic Analyses). Ph.D. Dissertation, Kazimierz the Great University, Bydgoszcz, Poland, 2018; p. 185, (In Polish with extended English summary). [Google Scholar]
- Pastorelli, R.; Smulders, M.J.M.; Van’t Westende, W.P.C.; Vosman, B.; Giannini, R.; Vettori, C.; Vendramin, G.G. Characterization of microsatellite markers in Fagus sylvatica L. and Fagus orientalis Lipsky. Mol. Ecol. Notes 2003, 3, 76–78. [Google Scholar] [CrossRef]
- Lefèvre, S.; Wagner, S.; Petit, R.J.; de Lafontaine, G. Multiplexed microsatellite markers for genetic studies of beech. Mol. Ecol. Resour. 2012, 12, 484–491. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakamura, T.; Tsumura, Y. Development and polymorphism of microsatellite markers for Fagus crenataand the closely related species, F. japonica. Theor. Appl. Genet. 1999, 99, 11–15. [Google Scholar] [CrossRef]
- Asuka, Y.; Tomaru, N.; Nisimura, N.; Tsumura, Y.; Yamamoto, S. Heterogeneous genetic structure in a Fagus crenata population in an old-growth beech forest revealed by microsatellite markers. Mol. Ecol. 2004, 13, 1241–1250. [Google Scholar] [CrossRef]
- Vornam, B.; Decarli, N.; Gailing, O. Spatial Distribution of Genetic Variation in a Natural Beech Stand (Fagus sylvatica L.) Based on Microsatellite Markers. Conserv. Genet. 2004, 5, 561–570. [Google Scholar] [CrossRef]
- Dumolin, S.; Demesure, B.; Petit, R.J. Inheritance of chloroplast and mitochondrial genomes in pedunculated oak investigated with an efficient PCR method. Theor. Appl. Genet. 1995, 91, 1253–1256. [Google Scholar] [CrossRef]
- Oosterhout, C.V.; Weetman, D.; Hutchinson, W.F. Estimation and adjustment of microsatellite null alleles in nonequilibrium populations. Mol. Ecol. Notes 2006, 6, 255–256. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Jones, O.R.; Wang, J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 2010, 3, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Ritland, K. Estimation of pairwise relatedness with molecular markers. Genetics 1999, 152, 1753–1766. [Google Scholar] [CrossRef]
- Hurme, P.; Repo, T.; Savolainen, O.; Pääkkönen, T. Climatic adaptation of bud set and frost hardiness in Scots pine (Pinus sylvestris). Can. J. For. Res. 1997, 27, 716–723. [Google Scholar] [CrossRef]
- Hansen, J.K.; Jørgensen, B.B.; Stoltze, P. Variation of quality and predicted economic returns between european beech (Fagus sylvatica L.) provenances. Silvae Genet. 2003, 5, 185–197. [Google Scholar]
- Soularue, J.P.; Kremer, A. Evolutionary responses of tree phenology to the combined effects of assortative mating, gene flow and divergent selection. Heredity 2014, 113, 485–494. [Google Scholar] [CrossRef]
- Shim, D.; Ko, J.H.; Kim, W.C.; Wang, Q.J.; Keathley, D.E.; Han, K.H. A molecular framework for seasonal growth-dormancy regulation in perennial plants. Hortic. Res.-Engl. 2014, 1, 14059. [Google Scholar] [CrossRef]
- Mijnsbrugge, K.V.; Moreels, S. Varying Levels of Genetic Control and Phenotypic Plasticity in Timing of Bud Burst, Flower Opening, Leaf Senescence and Leaf Fall in Two Common Gardens of Prunus padus L. Forests 2020, 11, 1070. [Google Scholar] [CrossRef]
- Jordan, C.Y.; Ally, D.; Hodgins, K.A. When can stress facilitate divergence by altering time to flowering? Ecol. Evol. 2015, 5, S962–S973. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Helm, R.; Zang, C. Patterns of late spring frost leaf damage and recovery in a European beech (Fagus sylvatica L.) stand in south-eastern Germany based on repeated digital photographs. Front. Plant Sci. 2015, 6, 110. [Google Scholar] [CrossRef]
- Gömöry, D.; Paule, L. Trade-off between height growth and spring flushing in common beech (Fagus sylvatica L.). Ann. For. Sci. 2011, 68, 975–984. [Google Scholar] [CrossRef]
- Wühlisch, G.V.; Krusche, D.; Muhs, H.J. Variation in temperature sum requirement for flushing of beech provenances. Silvae Genet. 1995, 44, 343–346. [Google Scholar]
- Ogris, N.; Brglez, A.; Piškur, B. Pseudodidymella fagi in Slovenia: First Report and Expansion of Host Range. Forests 2019, 10, 718. [Google Scholar] [CrossRef]
- Elzinga, J.A.; Atlan, A.; Biere, A.; Gigord, L.; Weis, A.E.; Bernasconi, G. Time after time: Flowering phenology and biotic interactions. Trends Ecol. Evol. 2007, 22, 432–439. [Google Scholar] [CrossRef]
- Figliuolo, G. Addressing Biodiversity Conservation Methods with Fagus sylvatica Genetic Indicators. Open J. Genet. 2014, 4, 166–174. [Google Scholar] [CrossRef][Green Version]
- Starke, R.; Ziehe, M.; Muller-Strack, G. Viability selection in juvenile populations of European beech (Fagus sylvatica L.). For. Genet. 1996, 3, 217–225. [Google Scholar]
- Gregorius, H.; Krauhausen, J.; Müller-Starck, G. Spatial and temporal genetic differentiation among the seed in a stand of Fagus sylvatica L. Heredity 1986, 57, 255–262. [Google Scholar] [CrossRef]
- Jump, A.S.; Rico, L.; Coll, M.; Peñuelas, J. Wide variation in spatial genetic structure between natural populations of the European beech (Fagus sylvatica L.) and its implications for SGS comparability. Heredity 2012, 108, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Marguardt, P.E.; Epperson, B.K. Spatial and population genetic structure of microsatellites in white pine. Mol. Ecol. 2004, 13, 3305–3315. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Adams, W.; Neale, D. Forest Genetics; CABI Publishing: Wallingford, UK, 2008; 704p, ISBN 10 0851993486. [Google Scholar]
- Soularue, J.P.; Kremer, A. Assortative mating and gene flow generate clinal phenological variation in trees. BMC Evol. Biol. 2012, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.; Czajkowski, T.; Kompa, T.; Birks, H.J.B.; Willis, K.J. The north-eastern distribution range of European beech a review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Koski, V. A study of pollen dispersal as a mechanism of gene flow in conifers. Commun. Inst. For. Fenn. 1970, 70, 78. [Google Scholar]
- Sjölund, M.J.; González-Díaz, P.; Moreno-Villena, J.J.; Jump, A.S. Gene flow at the leading range edge: The long-term consequences of isolation in European Beech (Fagus sylvatica L. Kuhn.). J. Biogeogr. 2019, 46, 2787–2799. [Google Scholar] [CrossRef]
- Jump, A.S.; Penuelas, J. Genetic effects of chronic habitat fragmentation in a wind-pollinated tree. Proc. Natl. Acad. Sci. USA 2006, 103, 8096–8100. [Google Scholar] [CrossRef]


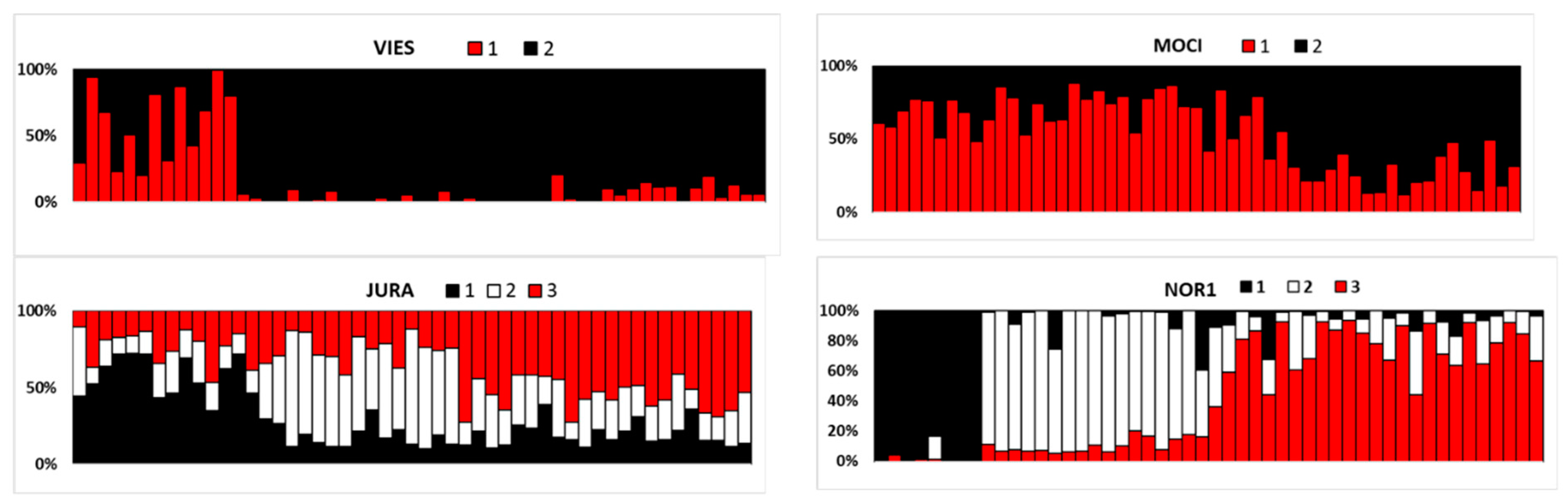

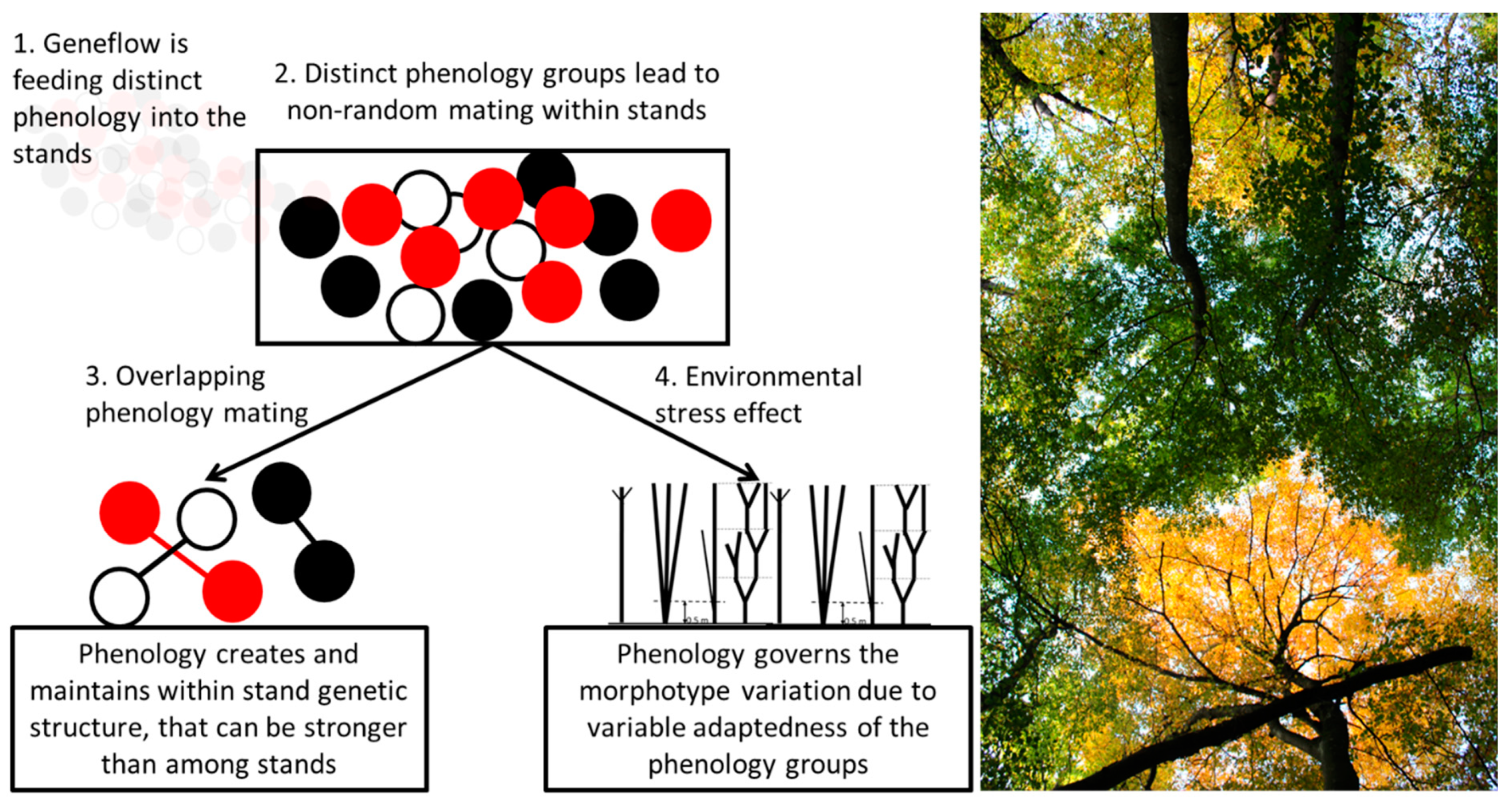
| Stand ID | Forest District | Species Composition | Area, ha | Stocking Level/Age Class | Sample Size | Lat. | Long. | Alt. m a.s.l. |
|---|---|---|---|---|---|---|---|---|
| VIES a | Viesvile | 70% beech, 30% Sc. pine | 5.9 | 0.8/100 | 55 | 55°4′52.37″ | 22°24′9.72″ | 42 |
| MOCI a | Mociskes | 80% beech, 20% Sc. pine | 3.5 | 0.9/80 | 53 | 55°6′19.98″ | 22°14′51.54″ | 30 |
| JURA a | Jurava | 90% beech, 10 oak | 1.8 | 0.6/120 | 50 | 55°8′6.15″ | 22°18′39.19″ | 39 |
| NOR1 b | Norkaiciai | 70% beech, 30% Sc. pine | 5.5 | 0.8/120 | 50 | 55°27′1.63″ | 21°32′22.49″ | 38 |
| Stand id/Stocking Level | Nobs | D (cm) | H (m) | H to First Fork | Stem Quality Index 1 | Stem Straightness, 5 Scores, 5 Is Very Curvy | Na | Ho | uHe | Fis |
|---|---|---|---|---|---|---|---|---|---|---|
| VIES/ | 55 | 36.3 | 31.4 | 15.6 | 3.85 | 2.53 | 9.40 | 0.68 | 0.72 | 0.05 |
| 0.8 | s.e. | 1.0 | 0.7 | 1.3 | 0.57 | 0.15 | 1.17 | 0.04 | 0.04 | 0.04 |
| MOCI/ | 53 | 33.9 | 28.5 | 21.2 | 3.37 | 2.58 | 9.07 | 0.64 | 0.73 | 0.10 |
| 0.9 | s.e. | 8.0 | 2.8 | 7.5 | 2.77 | 1.23 | 1.07 | 0.03 | 0.03 | 0.04 |
| JURA/ | 50 | 43.8 | 24.5 | 18.0 | 3.87 | 2.55 | 6.27 | 0.61 | 0.67 | 0.05 |
| 0.6 | s.e. | 13.6 | 5.9 | 7.7 | 4.13 | 1.08 | 0.43 | 0.03 | 0.03 | 0.02 |
| NOR1/ | 50 | 37.5 | 30.2 | 15.8 | 6.02 | 2.50 | 7.87 | 0.65 | 0.70 | 0.06 |
| 0.8 | s.e. | 1.37 | 0.72 | 1.21 | 1.03 | 0.20 | 1.01 | 0.04 | 0.04 | 0.02 |
| Trait | Diameter | Height | Budburst 3 Scores, 3 = Early | Leaf Senescence 4 Scores, 4 = Early | Ho |
|---|---|---|---|---|---|
| VIES (Bavaria, Age 100, n = 55, High Stocking) | |||||
| Height to fork | 0.09 | 0.36 ** | −0.21 | −0.04 | 0.00 |
| SQINDEX 1 | −0.04 | −0.18 | 0.22 | −0.10 | 0.02 |
| SQINDEX | −0.06 | −0.33 * | 0.32 * | 0.00 | 0.10 |
| STRAIGHT | −0.16 | −0.48 *** | 0.39 ** | 0.04 | 0.19 |
| JURA (Bavaria, Age 120, n = 51, Low Stocking) | |||||
| Height to fork | 0.00 | 0.75 *** | −0.17 | −0.10 | 0.10 |
| SQINDEX 1 | 0.29 * | −0.18 | 0.20 | −0.16 | −0.19 |
| SQINDEX | 0.13 | −0.30 ** | 0.27 * | −0.15 | −0.17 |
| STRAIGHT | −0.07 | −0.27 * | 0.21 | 0.00 | −0.11 |
| MOCI (Bavaria, Age 80, n = 55, Very High Stocking) | |||||
| Height to fork | −0.48 *** | −0.15 | 0.17 | 0.10 | 0.27 * |
| SQINDEX 1 | 0.44 *** | 0.19 | 0.01 | −0.02 | −0.16 |
| SQINDEX | 0.52 *** | 0.20 | 0.06 | −0.12 | −0.14 |
| STRAIGHT | 0.07 | −0.02 | 0.00 | −0.08 | 0.16 |
| NOR1 (Carpathians, Age 80, n = 50, High Stocking) | |||||
| Height to fork | 0.00 | 0.27 * | na | −0.07 | −0.25 * |
| SQINDEX 1 | 0.05 | −0.26 * | na | 0.16 | 0.13 |
| SQINDEX | −0.03 | −0.27 * | na | 0.10 | 0.09 |
| STRAIGHT | −0.09 | −0.27 * | na | −0.06 | −0.02 |
| Trait | D | H | HFORK | BUDB | LEAF | SQINDEX | STRAIGHT |
|---|---|---|---|---|---|---|---|
| Ho in VIES | 0.18 | −0.13 | 0.00 | 0.25 | 0.08 | 0.10 | 0.19 |
| n = 55 | 0.1902 | 0.3346 | 0.9757 | 0.0645 | 0.5627 | 0.4636 | 0.1624 |
| Ho in JURA | −0.01 | 0.11 | 0.10 | −0.20 | −0.22 | −0.17 | −0.11 |
| n = 51 | 0.9308 | 0.4555 | 0.4827 | 0.1513 | 0.1296 | 0.2231 | 0.4303 |
| Ho in MOCI | 0.00 | 0.04 | 0.27 | 0.18 | 0.36 | −0.14 | 0.16 |
| n = 55 | 0.973 | 0.7992 | 0.0502 | 0.1941 | 0.0082 | 0.3286 | 0.2435 |
| Ho in NOR1 | −0.02 | −0.22 | −0.25 | na | 0.10 | 0.09 | −0.02 |
| n = 50 | 0.8804 | 0.1181 | 0.0753 | 0.5087 | 0.5284 | 0.8944 |
| Genetic Group 1 | N | Na | Ne | Ho | He | Fis |
|---|---|---|---|---|---|---|
| VIES | ||||||
| 1 (early) | 13 | 6.73 (0.67) | 4.13 (0.48) | 0.73 (0.04) | 0.74 (0.04) | −0.045 (0.040) |
| 2 (late) | 42 | 8.33 (1.08) | 4.14 (0.61) | 0.66 (0.05) | 0.70 (0.04) | 0.057 (0.045) |
| MOCI | ||||||
| 1 (late) | 27 | 7.73 (0.97) | 4.02 (0.44) | 0.61 (0.03) | 0.72 (0.03) | 0.113 (0.051) |
| 2 (early) | 26 | 7.87 (0.82) | 4.37 (0.54) | 0.67 (0.03) | 0.74 (0.04) | 0.052 (0.039) |
| JURA | ||||||
| 1 (late) | 14 | 5.53 (0.68) | 3.64 (0.48) | 0.60 (0.06) | 0.66 (0.06) | 0.060 (0.039) |
| 2 (early) | 15 | 5.60 (0.72) | 3.32 (0.53) | 0.56 (0.05) | 0.60 (0.06) | −0.006 (0.042) |
| 3 (early) | 22 | 7.67 (0.73) | 4.54 (0.48) | 0.67 (0.04) | 0.75 (0.04) | 0.079 (0.044) |
| NOR1 | ||||||
| 1 (late) | 8 | 4.60 (0.45) | 2.98 (0.36) | 0.68 (0.05) | 0.64 (0.04) | −0.135 (0.053) |
| 2 (early) | 18 | 6.20 (0.60) | 3.70 (0.51) | 0.64 (0.05) | 0.68 (0.04) | 0.041 (0.036) |
| 3 (early) | 24 | 6.53 (0.65) | 3.54 (0.31) | 0.65 (0.04) | 0.69 (0.04) | 0.036 (0.034) |
| Stand id | Nobs | Number of Female Parents 1 | Freq. of Selfed Trees | Number of Full Sib Families | Number of Families with 3 or More Members | Largest Family Size 2 | Nef | Number of Clusters | Size of 3 Largest Clusters |
|---|---|---|---|---|---|---|---|---|---|
| VIES | 55 | 35 | 0.05 | 0 | 3 | 3 | 83 | 9 | 23/14/12 |
| MOCI | 53 | 39 | 0.21 | 0 | 2 | 4 | 87 | 17 | 21/10/4 |
| JURA | 51 | 40 | 0.20 | 0 | 2 | 3 | 103 | 20 | 15/10/5 |
| NOR1 | 50 | 34 | 0.02 | 2 | 3 | 3 | 68 | 8 | 25/9/5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kembrytė, R.; Danusevičius, D.; Baliuckas, V.; Buchovska, J. Phenology Is Associated with Genetic and Stem Morphotype Variation in European Beech (Fagus sylvatica L.) Stands. Forests 2022, 13, 664. https://doi.org/10.3390/f13050664
Kembrytė R, Danusevičius D, Baliuckas V, Buchovska J. Phenology Is Associated with Genetic and Stem Morphotype Variation in European Beech (Fagus sylvatica L.) Stands. Forests. 2022; 13(5):664. https://doi.org/10.3390/f13050664
Chicago/Turabian StyleKembrytė, Rūta, Darius Danusevičius, Virgilijus Baliuckas, and Jurata Buchovska. 2022. "Phenology Is Associated with Genetic and Stem Morphotype Variation in European Beech (Fagus sylvatica L.) Stands" Forests 13, no. 5: 664. https://doi.org/10.3390/f13050664
APA StyleKembrytė, R., Danusevičius, D., Baliuckas, V., & Buchovska, J. (2022). Phenology Is Associated with Genetic and Stem Morphotype Variation in European Beech (Fagus sylvatica L.) Stands. Forests, 13(5), 664. https://doi.org/10.3390/f13050664






