Abstract
Four evergreen broadleaf Rhododendron spp. (Rhododendrons), namely, Rhododendron aganniphum, R. nyingchiense, R. wardii, and R. triflorum, occur in harsh subalpine habitats in the southwest Qinghai-Tibet Plateau (QTP), China. Considering that the four Rhododendrons cannot escape their unique environment, they must evolve a set of adaptations to survive, but the information is lacking. To uncover their physiological adaptation characteristics, in the present study, we monitored their physiological characteristics by determination of their seasonal variation in antioxidant enzyme activity, osmotic adjustment substrates, and carbohydrate contents, and their pigment content and photosynthetic efficiency. The results showed that superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) activities and proline content of four Rhododendrons had a significant difference in autumn and were insignificant in summer. Specifically, R. aganniphum had the maximum protective enzyme activity and proline content in winter as well as chl a, b, and car contents. The values of maximal quantum yield (Fv/Fm), photochemical efficiency (ΦPSII), and non-photochemical quenching (NPQ) of four Rhododendrons were significantly higher in summer than in other seasons. The lower qP indicated the four Rhododendrons were susceptible to photoinhibition. Overall, the four Rhododendrons had similar physical characteristics in subalpine habitats. The parameters of the maximum quantum yield of photosystem II (PSII), the actual quantum yield of PSII, the non-photochemical chlorophyll fluorescence quenching, and chlorophyll a content increased in summer. Meanwhile, the protective enzyme activity and total soluble sugar content, proline content, and carotenoid content increased in spring, autumn, and winter. These results suggested that the four Rhododendrons can adapt to subalpine habitats by heat dissipation to avoid the damage of excessive radiation during the warm season while scavenging reactive oxygen and increasing the intracellular fluid concentration to avoid damage caused by chilling temperatures during the cold seasons. These findings would provide a reference for the conservation and application of these valuable ornamental evergreen broadleaf Rhododendrons, and enrich theory of plant eco-physiology in the high altitudes of the QTP.
1. Introduction
Rhododendron is a large genus of Ericaceae in woody plants that plays an important role in horticultural and landscape fields. Out of the 1215 Rhododendron spp. worldwide, 649 species are present in China [1]. China is one of the centers and the origin of wild Rhododendron, which is mainly distributed in the Yunnan-Guizhou Plateau of Southwest China, where the altitude is generally between 1000 and 2000 m with limited sunshine and with cool, foggy, and humid environments. In adapting to special climatic conditions, the distribution of Rhododendron spp. changes regionally during the speciation worldwide [1,2,3]. In the vast south of China, R. spp. range from east to west, even to the Qinghai-Tibet Plateau (QTP), where the altitude is generally between 3000 and 4000 m. Meanwhile, in contrast to Yunnan-Guizhou Plateau climate, the QTP has intense solar radiation, cold temperature, strong wind, snow melt, and low oxygen level. Sygera mountain, which is located in the southeastern QTP, has four main evergreen broadleaf Rhododendron spp. (Rhododendrons) at an altitude ranging from 3200–4500 m subalpine areas; these species are R. aganniphum, R. nyingchiense, R. wardii, and R. triflorum. These Rhododendron shrubs form populations along the slope and survive for long periods in the adverse climate of the cold subalpine region of QTP.
The multiple stresses in high mountains affect the growth of native plants. At the same time, native plants can make a series of physiological adjustments to this adverse environment during long-term adaptation. Meanwhile, photosystem I (PSI) and photosystem II (PSII) jointly regulate light energy conversion and oxygen release in green plants. For instance, R. catawbiense adapts to temperature fluctuations by adjusting leaf mineral content, pigment content, photosynthetic properties and PSII activity in Lake Waban, Eastern USA [4]. In particular, R. × hybridum and Picea omorika also presented seasonal variations in PSII efficiency, such as maximal quantum yield of PSII photochemistry (Fv/Fm) and photochemical efficiency (ΦPSII), reflecting the acclimation processes during the whole year [5]. Different R. catawbiense ecotypes adapt to cold and light by regulating their physiological and biochemical traits of leaf pigment content, net photosynthetic rate, stomatal conductance, protective enzyme activity, and gene expression in Ames, IA, USA [6]. Rhododendron decorum in the Yunnan-Guizhou Plateau of Southwest China prevented significant photoinhibition in PSI and high non-photochemical quenching (NPQ) in PSII to cope with excess light energy in winter [7]. R. anthopogon adapts to Indian, that is, the western Himalaya, climate by adjusting eco-physiological and biogeochemical traits with that of R. catawbiense [8]. Abies faxoniana adapts to the high-altitude climate by adjusting leaf mineral content and photosynthetic and growth traits [9]. Abies georgei var. smithii also adapts to the special climate of QTP by adjusting leaf photosynthesis, antioxidant enzyme activity such as superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT), and proline content [10]. Previous research also found Salix longistamina had the higher water use efficiency, biomass accumulation, photochemistry efficiency, and lowest water loss in order to survive in QTP, while Ulmus pumila had the lower transportation rate and light compensation point with the higher water potential and apparent quantum yield [11]. Four herbaceous species adapt to the QTP by adjusting photosynthetic pigment content [12]. Potentilla saundersiana adapts to the QTP by adjusting mineral content, protective enzyme activity, osmotic adjustment substrate content, and hormone content [13]. Sinopodophyllum hexandrum adapts to different light regimes in the QTP by adjusting the phenotypic traits and photosynthesis [14]. Meadow forage grasses adapt to the QTP by adjusting leaf phenotype (leaf area, thickness, stomatal parameters) and function to regulate the photosynthesis and biomass allocation [15]. These results indicated that plants living in the QTP or in adversity elsewhere have wide a range of physiological plasticity traits to cope with the diverse climatic conditions [8]. Generally, plants could self-regulate the survival traits to adapt to high altitude climate in subalpine regions; these traits mainly include the following categories: protective enzyme activity, osmotic adjustment substrate contents, photosynthesis, and fluorescence.
Considering the special geography and climate of QTP, only few studies on the adaptability of evergreen coniferous trees, deciduous broad-leaved trees, and herbs have been reported. The four evergreen broadleaf Rhododendrons mentioned above are the native shrubs in the subalpine region of QTP, but little information about their eco-physiological characteristics is known, which considerably limits the conservation and utilization of these valuable ornamental plants. Thus, two main questions were investigated: (1) How do Rhododendron plants cope with seasonal climate change by regulating physiological parameters? (2) Are there significant differences in their physiological regulation characteristics at different altitudes? Therefore, we determined their seasonal variation in antioxidant enzyme activity, osmotic adjustment substrate content, pigment content, and photosynthetic efficiency to clarify their physiological responses.
2. Materials and Methods
2.1. Study Site
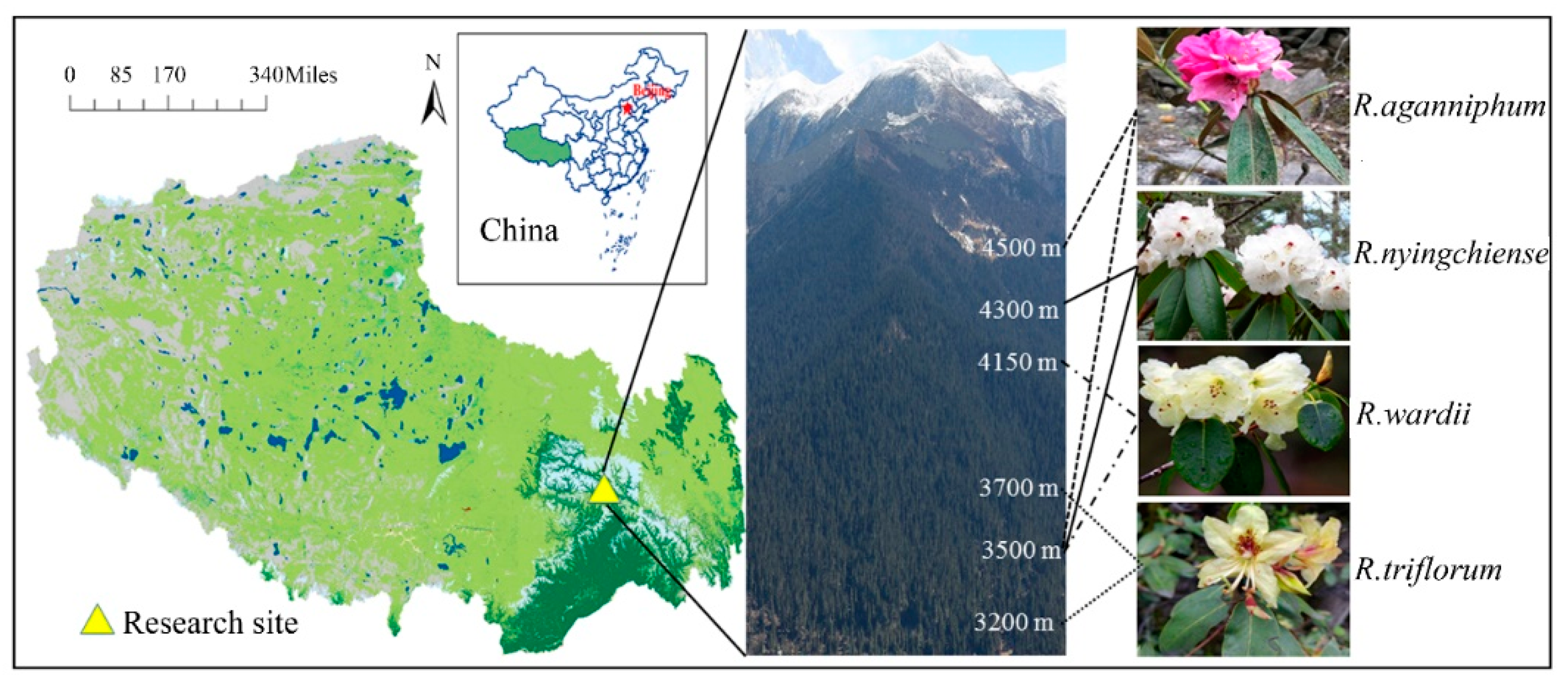
Sygera mountain is located in Nyingchi County, Tibet in Southeast QTP of China, and at the junction of Nyenchen Tanglha Mountains and the Himalayas. Sygera mountain also belongs to Nyenchen Tanglha Mountains. The location of the research area is approximately east longitude of 93°35′, north latitude 29°37′, and at an altitude of 3200–4500 m on the east slope of the mountain. The forest line is at 4600 m, and the peak of the mountain is 5300 m (Figure 1). This subalpine region has low rain and snow in spring and winter and abundant rain in summer and autumn, and warmth is in season with rain. According to the records from a weather station of the National Forest Ecosystem Observation and Research Station of China, at an altitude of 3900 m in this area of the year from 1985–2010, the annual average temperature was −0.73 °C, whereas the average highest temperature (in mid-late July to early August) and lowest temperature (in January) were approximately 8.15 and −4.06 °C, respectively. The annual precipitation and evaporation capacity were approximately 1100 and 570 mm, respectively, and 80% of the annual precipitation was obtained from June to September. The annual mean sunshine duration was 1200 h, and the soil pH was between 4 and 6.
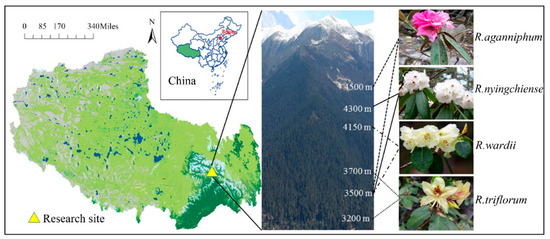
Figure 1.
Research site and distribution of four Rhododendrons on Sygera mountain. The white numbers on the mountain indicate the elevation.
2.2. Materials
Four main Rhododendrons, namely R. aganniphum, R. nyingchiense, R. wardii, and R. triflorum, are distributed on the Sygera mountain at different elevations (Figure 1). The main accompanying species are: A. georgei var. smithii, Sabina saltuaria, Salix cupularis, and Sorbus rehderiana. Their healthy 2-year-old functional leaves growing on top of the main branch were selected and marked from the concentrated distribution of each Rhododendron. Field detections were performed on 5 April, 1 August (generally the warmest time of the year), 9 November, and 13 January (generally the coldest time of the year) 2016, which represented local spring, summer, autumn, and winter, respectively. However, the climate during spring, autumn, and winter was actually cold. Previous researchers found that the growing season of native wood plant is from May to August in the whole year [16], so we designated summer as warm season (the growing season), and the three other seasons as cold season (the nongrowing season). All field detections were conducted at the same time as sunny days in different seasons. Meanwhile, leaves were taken back into the lab at the foot of the mountain, and lab detection was carried out immediately.
2.3. Field Detection of Leaf Photosynthetic Efficiency
Chlorophyll fluorescence (ChlF) parameters were determined using the equipped LI 6400XT-40 (Li-Cor Inc., Lincoln, NE, USA) according to the operation manual. The temperature of the leaf chamber was 15 ± 0.5 °C. The selected leaves were first darkened for 30 min before determination. After the initial measurement of dark-adapted minimum and maximum fluorescence (F0 and Fm) were measured in the dark, the parameters of the maximum quantum yield of PSII [Fv/Fm = (Fm − F0)/Fm]. Then, the leaves were continuously irradiated with actinic light (1500 μmol m−2 s−1) in order to measure the steady-state fluorescence (Fs) and maximum fluorescence yield (Fm’) of irradiated leaves. The actual quantum yield of PSⅡ (ΦPSII) [ΦPSII = (Fm’ − Fs)/Fm’], the photochemical quenching (qP), and NPQ were calculated according to Roháček [17].
2.4. Determination of Leaf Inclusions
The photosynthetic pigment, chlorophyll (chl) a, chl b, and carotenoid (car), contents were detected from fresh leaves by 80% acetone extraction, and the contents were calculated according to the method introduced in the literature [18]. The total soluble sugar (TSS) and proline contents were detected following the methods of Yemm and Willis [19] and Hodges [20], respectively. Each 0.5 g of leaf material was homogenized with the extraction buffer containing 50 mM phosphate buffer (pH 7.4). Then, the homogenate was centrifuged for 30 min at 12,000× g, and the supernatant was obtained for enzyme analysis. All the operations were carried out at 0–4 °C. SOD and POD activities were determined by Grellet’s modified NBT staining method [21] with CAT activity by using the method of Chance and Maehly [22].
2.5. Statistical Analysis
Data were analyzed using SPSS v18.0 (SPSS Inc., Chicago, IL, USA) software. Results were analyzed by one-way ANOVA. LSD multiple comparison tests were used to separate significant differences, and the difference was considered to be significant when p < 0.05. The graphs were generated using Sigmaplot v13.0 (Systat Software Inc., San Jose, CA, USA) software. Principal component analysis (PCA) was performed to identify the major eco-physiological variations using CANOCO v4.5 software for Windows [23].
3. Results
3.1. Protective Enzyme Activity
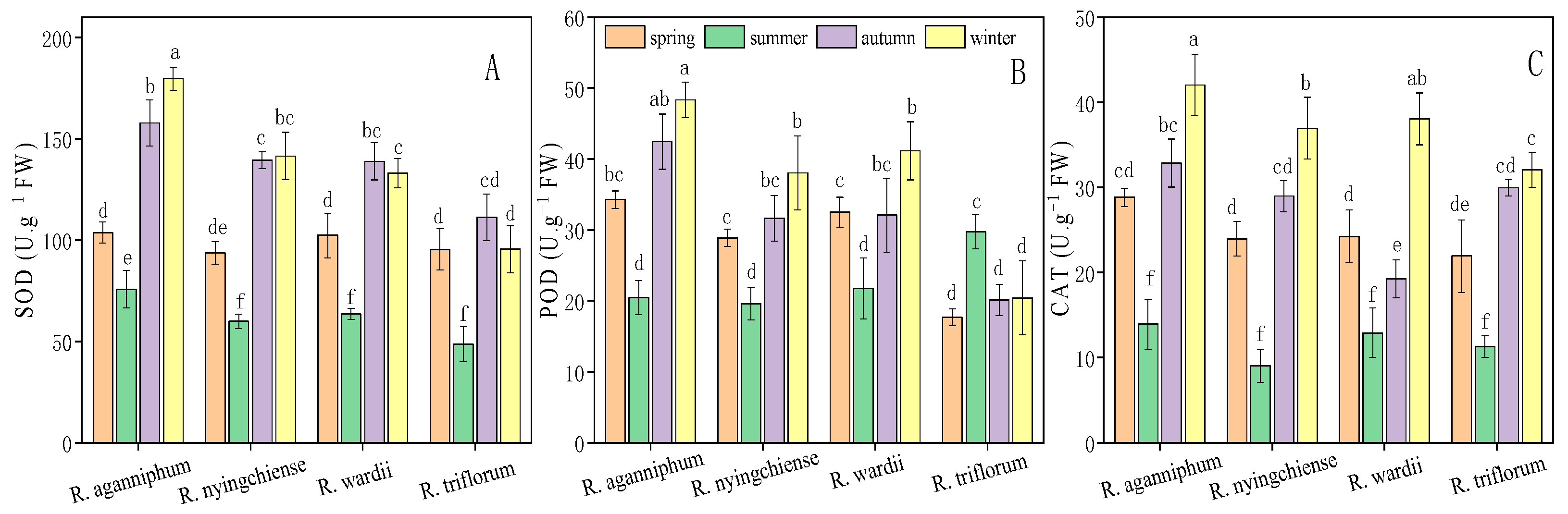
The general trend is that the activities of all enzymes were low during summer and relatively high in the three other seasons. R. aganniphum presented the highest SOD (179.57 U·g−1 FW) and POD (48.35 U·g−1 FW) activities during winter across the year (Figure 2A,B). The insignificant differences in SOD activities of R. wardii or R. triflorum was observed during autumn and winter. Meanwhile, POD activities (28.90–32.51 U·g−1 FW) of R. nyingchiense and R wardii were insignificant during spring and autumn. Contrastingly, R. triflorum showed less variable POD activity than that of the three other species (Figure 2A–C). CAT activities of four Rhododendrons presented the similar change trend along with the season change, and the maximum and minimum values of each appeared in winter and summer, respectively. Additionally, CAT activity of R. aganniphum was higher than the others in the observation period.

Figure 2.
Protective enzyme SOD (A), POD (B), and CAT (C) activity of four Rhododendrons in different seasons under subalpine habitat on Sygera mountain of the QTP. Values are expressed as mean ± SE (n = 9), and lowercase letters represent the significant differences among the four Rhododendrons in four seasons (p < 0.05).
3.2. Osmotic Adjustment Substance and Carbohydrate Contents
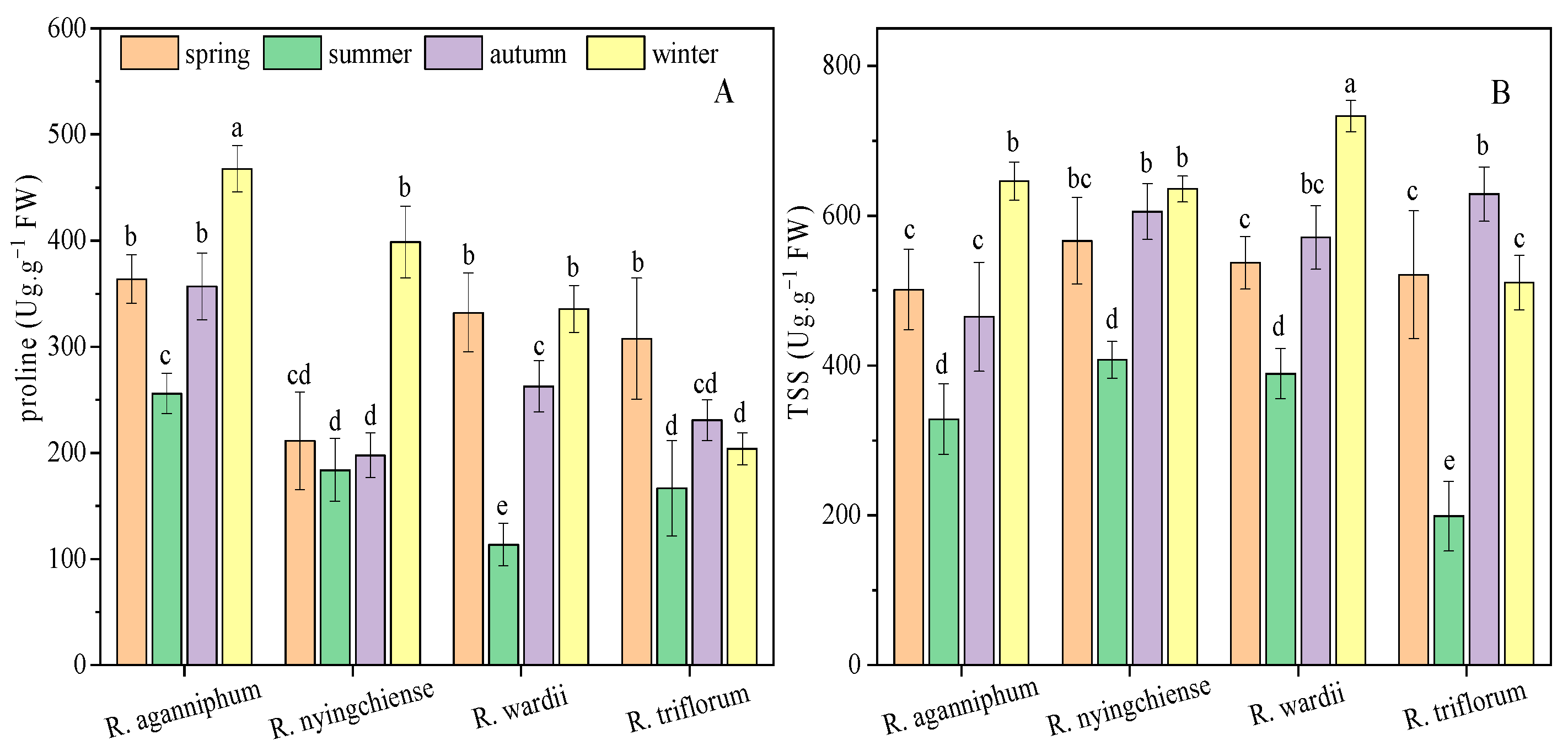
Plants often adapt to osmotic stresses, such as low temperature in high altitudes, by increasing the contents of osmotic regulatory substance and carbohydrate, such as proline and TSS. In this study, the proline content R. aganniphum decreased to the lowest value (255.75 Ug·g−1 FW) in summer and increased to the maximum value (467.66 Ug·g−1 FW) in winter (Figure 3). The proline contents of R. nyingchiense were not significantly different among spring, summer, and autumn, but the highest proline content (398.64 Ug·g−1 FW) was observed in winter. R. wardii presented the minimum proline content in summer, and the maximum ones in spring and winter. However, R. triflorum showed the maximum content (307.67 Ug·g−1 FW) during spring and remained relatively low in the three other seasons. The TSS content of all four species decreased to the lowest value during summer, and the maximum content was observed during winter in three species, namely, R. aganniphum, R. nyingchiense, and R. wardii, whereas TSS reached the maximum of R. triflorum in autumn (Figure 3). These results suggested that the osmotic regulation of the four species was diverse in different seasons under subalpine conditions.
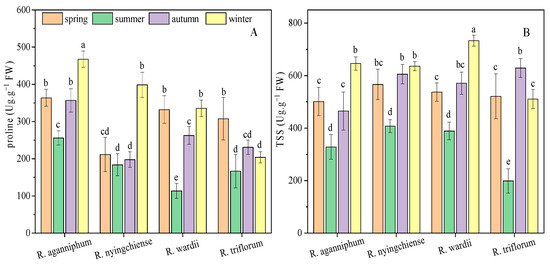
Figure 3.
Osmotic adjustment substrates proline (A) and TSS (B) content of four Rhododendrons in different seasons under subalpine habitat on Sygera mountain of the QTP. Values are expressed as mean ± SE (n = 9), and lowercase letters represent the significant differences among the four Rhododendrons in four seasons (p < 0.05).
3.3. Pigment Content and Photosynthetic Efficiency Variation
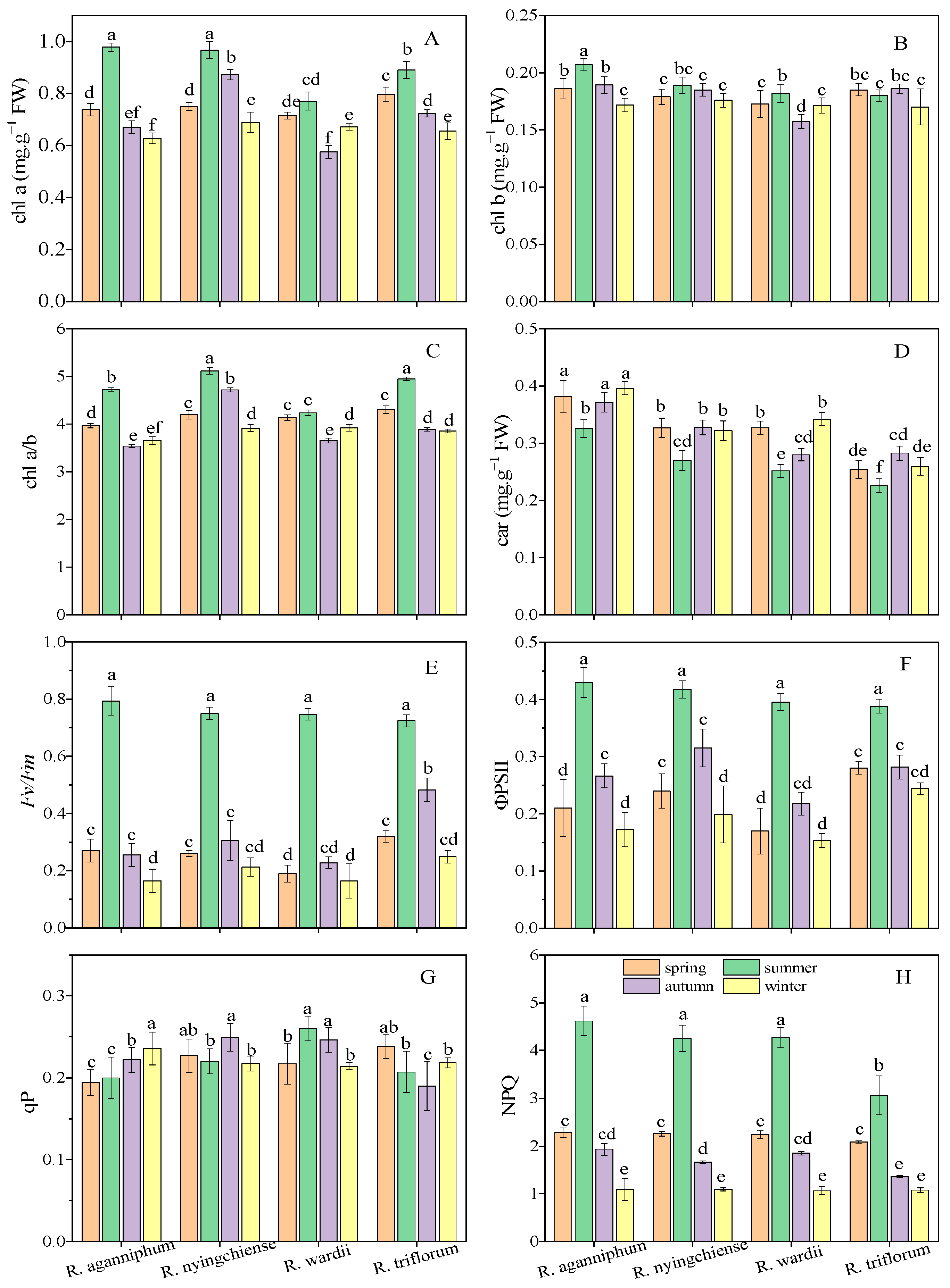
Photosynthetic pigments, including chl a, chl b, and car, play an important role in the process of photosynthesis. Different photosynthetic pigments perform specific functions under the stress of adversity. In this study, the chl a content of four Rhododendrons all increased to the peak in summer, but their chl b content did not change significantly in four seasons (Figure 4A,B). Chl a/b values of three Rhododendrons all reached the highest in summer except R. wardii. Among the four Rhododendrons, the chl a or b content was significant higher (0.98 mg·g−1 FW and 0.21 mg·g−1 FW, respectively) in R. aganniphum than that (0.89 mg·g−1 FW and 0.18 mg·g−1 FW, respectively) in R. triflorum in summer (Figure 4C). The car content in the four Rhododendrons was low in summer but remained relatively high in the three other seasons (Figure 4D).

Figure 4.
Pigment content (A–D) and photosynthetic efficiency (E–H) of four Rhododendrons in different seasons in subalpine habitat on Sygera mountain of the QTP. Values are expressed as mean ± SE (n = 9), and lowercase letters represent the significant differences among the four Rhododendrons in four seasons (p < 0.05).
ChlF parameters reflect the effects of environmental factors on plant photosynthesis, especially under stressful condition. The Fv/Fm values of the four Rhododendrons reached their maximum value in summer and were kept between 0.7 and 0.8 (Figure 4E). The ΦPSII values of these species all reached their maximum values in summer, in which, R. aganniphum and R. nyingchiense showed the highest (0.43 and 0.42, respectively), followed by R. wardii (0.40) and then R. triflorum (0.39) (Figure 4F). The NPQ of the four species reached their maximum in summer, with the maximum values (4.62) presented in R. aganniphum (Figure 4H). However, the qP value did not change significantly among the four seasons (Figure 4F). Generally, the maximum variation in ChlF parameters occurred in the summer of the four species except qP.
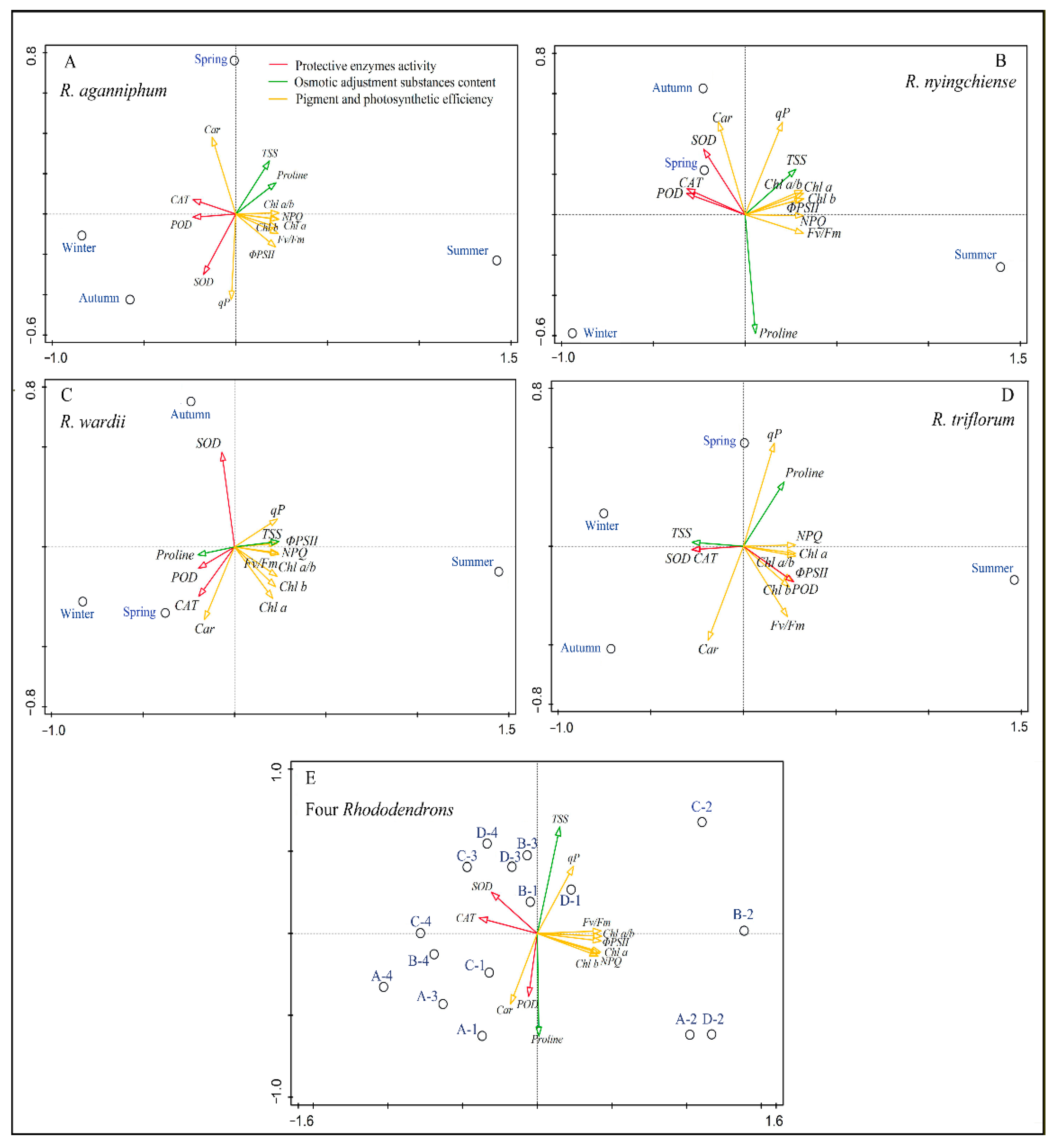
3.4. Associations among All Physiological Traits
The PCA results show that the first two PC explained up to 73.07% of the variance of the 13 physiological indicators of four Rhododendrons in different seasons under subalpine habitats (Figure 5). In the PCA outputs for each species, the first principal components (PC1) contribute up to 76.57% of variance in R. aganniphum, 73.13% of variance in R. nyingchiense, 74.42% of variance in R. wardii, 80.51% of variance in R. triflorum, and 54.69% of variance in all Rhododendrons. Additionally, the PC1 loading scores for the eco-physiological indicators of the studied Rhododendrons are listed in Table S1; the loading scores clearly suggested that variations in Fv/Fm, ΦPSII, NPQ, chl a, POD, and CAT contributed more than others in the adaptation process (Supplementary Table S1). Generally, Fv/Fm, ΦPSII, NPQ, and chl a, together with POD and CAT activities, contribute the most among all eco-physiological variations in the four Rhododendrons (Figure 5). Besides, in most cases in all PC outputs, the eco-physiological regulation trends among the four Rhododendrons were slightly different, but the overall trends were similar. In the PCA outputs, the right PC1 clearly showed ChlF parameters and chl a and b contents; those eco-physiological indicators significantly changed in the warm summer. Meanwhile, the left PC1 clearly showed car content and protective enzyme activity; those eco-physiological indicators significantly changed in the cold season of the four species (Figure 5), suggesting that there is different eco-physiological regulation in different seasons to adapt to the subalpine habitat.
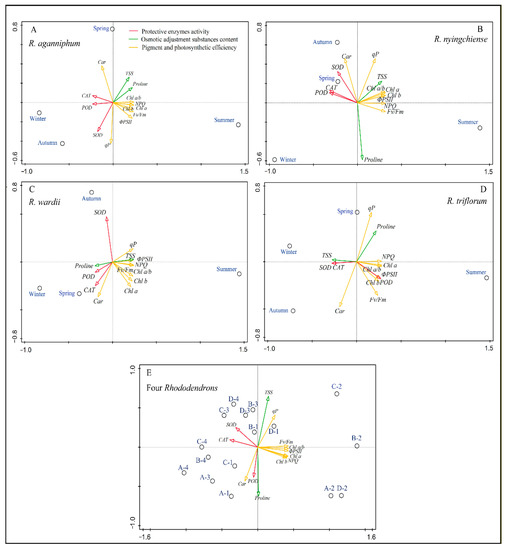
Figure 5.
Principal component analysis (PCA) on the correlation matrix of 13 eco-physiological indicators (SOD, POD, CAT, proline, TSS, chl a, chl b, chl a/b, car, Fv/Fm, ΦPSII, qP, NPQ) in spring (A), summer (B), autumn (C), and winter (D) in subalpine habitat, respectively. The hollow circles represent each species in each season. (E) represents the PCA output on the basis of the 13 eco-physiological indicators of all Rhododendrons that were studied in four seasons. Letters A, B, C, and D in front of the short dash indicate R. aganniphum, R. nyingchiense, R. wardii, and R. triflorum, respectively, while numbers 1, 2, 3, and 4 after the short dash indicate spring, summer, autumn, and winter, respectively.
4. Discussion
The QTP is the largest and highest plateau in China and has been referred to as the “roof of the world” and “third pole”. Considering its high elevation, the QTP climate is cold throughout the year and has intense solar radiation, strong wind effects, rarefied air, and snow. The Sygera mountain in the Southeast QTP is relatively humid due to the influence of warm and moist currents from the Indian Ocean rising along the Yarlung Zangbo River Valley, but it still has intense radiation and year-round low temperatures. Thus, the native plants have a very limited time to grow and a long time for dormancy. At the same time, the native plants need to constantly resist adverse environmental factors, so the plants must form unique physiological adaptation characteristics in this particular alpine environment, including the four native evergreen Rhododendrons that are different from most other evergreen Rhododendrons as they grow in moist, foggy and cool areas with weak radiation.
4.1. Biochemical Regulation of Four Rhododendron Species
Plants produce the ROS, such as 1O2, O2− and H2O2, under the environmental stress conditions (i.e., drought, cold), which can damage proteins, membrane lipids, DNA, and other cellular components [24]. Simultaneously, green plants have evolved the related adaptation mechanisms, which could produce antioxidant and secondary metabolites for protecting the plant for detoxifying ROS [25]. Therefore, the antioxidant enzymes (SOD, POD, and CAT) and osmotic adjustment (proline) play the irreplaceable roles to improve stress resistance, which can remove the ROS and regulate osmotic potential of the cell, respectively [26,27]. For instance, two evergreen Rhododendrons, i.e., R. catawbiense and R. ponticum, showed significant upregulated activities of antioxidant protective enzymes POD and CAT in a cold acclimation season [5]. The SOD, POD, and CAT activities in all olive cultivars significantly increase during the cold acclimation stage [28]. Similarly, POD and CAT activities in Asphodelus aestivus is upregulated in winter compared with in autumn [29]. The SOD and POD activities reach their highest in the cold season in six groundcover plants, namely, Vinca minor L. ‘Variegatum’, Vinca minor L. ‘Green’, Oxalis brasiliensis G. Lodd, Trifolium repens L., Phyla nodiflora L., and Frankenia thymifolia Desf [30]. Meanwhile, stress conditions exert a considerable influence on osmotic adjustment substances together with protective enzymes, among which TSS and proline are the most important. Osmotic substance accumulation can effectively reduce the freezing temperature of cells and protect plants from freezing damage. As reported in previous literature, the accumulation of soluble sugars in R. anthopogon on high mountains can aid in coping with cold temperatures, which may be the key factor in the persistence of populations of this evergreen species [8]. Most of the 16 Thellungiella accessions strongly accumulate TSS and proline in leaves during cold acclimation [31], and Arundo donax showed significantly increased TSS and proline contents during cold acclimation [32]. A similar pattern was also observed in three grapevine cultivars and in tea plants under low temperature stress [33,34]. Similar to the reports above, in the present study, in most cases, TSS and proline contents significantly increased in cold seasons, especially during winter, compared with that in summer in the four Rhododendrons. These results, combined with the increased protective enzyme activity in the cold season, suggested that efficient ROS-scavenging capacity and osmotic regulation help the four Rhododendrons to survive in the cold environment of the subalpine region in the QTP.
4.2. Photosynthetic Regulation and Pigment Content
Photosynthesis is significantly affected by environmental temperature, especially in cold and frozen regions, and the detection of ChlF parameters generally shows decreased ΦPSII. Physiological drought is frequent for plants because of the frozen subalpine habitat, so plants have to alter their physiological processes. For example, they reduce the stomatal conductance to control the water use efficiency, and subsequently the net photosynthetic rate [24,35]. Meanwhile, low temperatures in cold seasons also caused a response of ChlF parameters in R. catawbiense in Lake Waban, Eastern USA [4], three Picea species in the subalpine region of northeastern QTP [36], Ammopiptanthus mongolicus in northwestern China [37], and Vaccinium vitis-idaea in central Finland [38]. Similarly, in the present study, the ΦPSII and Fv/Fm values of the four Rhododendrons reached their maximum values in the warm season, then decreased with seasonal changes, and fell to the minimum during the cold season. Thus, temperature is one of the most important adverse environmental factors for the four subalpine Rhododendrons. Low temperatures during the cold season decreased ΦPSII and Fv/Fm values of species, thereby indicating that these species can only accumulate photosynthetic products for growth in the warm summer in the subalpine region. However, the relatively favorable temperature in the warm season is beneficial for plant photosynthesis, and radiation in this season is intense in the subalpine region, which may lead to interruption of photosynthetic electron transport and damage of PSII. Synchronously, qP reflects the open degree of PSII. In this study, qP of four Rhododendrons kept the range of 0.19–0.26, which indicated the low openness of PSII reaction centers. That is to say that photoinhibition appeared, which was also proved by the reduction in Fv/Fm. Usually, intense radiation absorbed by plant leaves undergoes one of three possible pathways, as follows: it can be used in photochemistry, emitted as ChlF (represented by qP), or dissipated as heat (represented by NPQ) [15,39,40]. Thus, plants have evolved effective strategies to remove excessive energy to avoid photosynthetic apparatus damage in high altitudes where radiation is extremely strong. In this study, the NPQ of the four Rhododendrons peaked in summer and decreased in cold seasons with the relatively stable qP. These suggest that the effective protection mechanism from damage of excessive radiation was mainly realized through heat dissipation in the four Rhododendrons in the subalpine habitats. Chl content and their ratio indicate the adaptation difference of plants to high and low solar radiation. Generally, sun-loving plants have better chl a and chl b contents with the higher value of chl a/b [41]. In this study, the chl a content in the four Rhododendrons increased significantly during the warm season, while the chl b content did not. The increase in the chl a content during the warm season may be due to plant chl a having stronger light absorption capacity, whereas chl b mainly absorbs long-wave light. In the subalpine area, the four Rhododendrons possibly increase chl a content under intense radiation conditions in the warm season to improve the absorption of light energy. A higher chl a or b content was observed in R. aganniphum than that in R. triflorum in summer, which may be due to their special sun-loving characteristic. In addition, R. aganniphum are distributed at relatively higher altitudes than R. triflorum; thus, it will be exposed to the intense radiation for the longer period. Car had more ability to dissipate excess excitation energy to avoid damage from strong radiation for plants in the cold season in the subalpine area. This phenomenon has also been observed under low temperatures in R. anthopogon in western Himalaya [8]; V. vitis-idaea, which is another species belonging to Ericaceae in high latitudes [38]; and model species Arabidopsis under excess radiation at chilling temperatures [42].
4.3. Associations among All Physiological Traits of Four Rhododendrons
Efficient physiological adjustments are vital for the survival of high-altitude plants, having their seasonal variations determined by environmental or ontogenetic factors [40]. In this study, although the physiological adjustment trends among the four Rhododendrons were not completely the same in different seasons, the overall trend still remained similar. From the PCA outputs, the left and right PC1 showed the similar eco-physiological traits, respectively, which is exactly corresponding to eco-physiological variation in the unique environment in warm and cold seasons, respectively, indicating that the four Rhododendrons had similar adaptive characteristics to warm and cold seasonal adversity in subalpine regions, because the stress in the warm season should be mainly caused by strong radiation, while stress in cold seasons should be mainly caused by chilling temperatures for most of the year. What is inconsistent with our results is that three woody species, Populus cathayana, Salix longistamina, and Ulmus pumila, can adapt to the QTP conditions by different physiological regulation [11]; this may result from the fact that the three species in the previous study are of different families and genera, and are far more closely related to each other, while the four species in this study are all of Ericoideae and Rhododendron, and are closely related to each other, so similar adaptation characteristics may be evolved.
5. Conclusions
The physiological regulation trends among the four Rhododendrons were slightly different, but the general trends were similar. Contrastingly, the antioxidant enzyme activities (POD, CAT) of R. aganniphum were higher than those of the others in winter, while proline and car contents of four Rhododendrons reduced as the elevation gradient decreased in the same seasons. In summer, the ΦPSII values of R. aganniphum and R. nyingchiense were higher than others in summer. This also indicates that Rhododendrons species in high altitude habitats have higher stress resistance and light energy utilization efficiency. Similarly, the Fv/Fm, ΦPSII, NPQ, and chl a content increased during the warm summer, whereas protective enzyme activity and car content increased during the cold season. In addition, the resistance to low temperature of four Rhododendrons was enhanced along with the increasing elevation. This indicated that the four Rhododendrons can adapt to the subalpine habitats by heat dissipation to avoid the damage of excessive radiation and rapidly grow in the limited time during the warm season. These findings would provide a reference for the conservation and application of these valuable ornamental evergreen broadleaf Rhododendrons in the high altitudes of QTP.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f13050653/s1, Table S1: The first principal component loading scores for the eco-physiological indicators of four Rhododendrons in four seasons.
Author Contributions
H.L. designed experiments, performed data analysis, and wrote the paper; Q.G. performed data analysis and field detection; L.Y. performed data analysis and revised the paper; H.Q. designed experiments, performed data analysis, and performed the lab detection of enzyme activity, osmotic regulating substances, and pigment content. S.W. performed species identification and photo taking. All authors have read and agreed to the published version of the manuscript.
Funding
This work was founded by the Scientific Research Foundation for Advanced Talent of Guizhou University, China ((2016) 43), the Tibetan Linzhi National Forest Ecological Research Station, China (2018-LYPT-DW-090), and the National Science Foundation of China (31660215).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- MacKay, M.; Gardiner, S. Geographic analysis of Red List Rhododendron (Ericaceae) taxa by country of origin identifies priorities for ex situ conservation. Blumea 2017, 62, 103–120. [Google Scholar] [CrossRef][Green Version]
- Ma, Y.; Nielsen, J.; Chamberlain, D.F.; Li, X.; Sun, W. The conservation of Rhododendrons is of greater urgency than has been previously acknowledged in China. Biodivers. Conserv. 2014, 23, 3149–3154. [Google Scholar] [CrossRef]
- Wang, J.H.; Cai, Y.F.; Zhang, L.; Xu, C.K.; Zhang, S.B. Species richness of the family Ericaceae along an elevational gradient in Yunnan, China. Forests 2018, 9, 511. [Google Scholar] [CrossRef]
- Harris, G.C.; Antoine, V.; Chan, M.; Nevidomskyte, D.; Königer, M. Seasonal changes in photosynthesis, protein composition and mineral content in Rhododendron leaves. Plant Sci. 2006, 170, 314–325. [Google Scholar] [CrossRef]
- Soukupová, J.; Cséfalvay, L.; Urban, O.; Košvancová, M.; Marek, M.; Rascher, U.; Nedbal, L. Annual variation of the steady-state chlorophyll fluorescence emission of evergreen plants in temperate zone. Funct. Plant Bio. 2008, 35, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xia, Y.-P.; Krebs, S.L.; Medeiros, J.; Arora, R. Seasonal responses to cold and light stresses by two elevational ecotypes of Rhododendron catawbiense: A comparative study of overwintering strategies. Environ. Exp. Bot. 2019, 163, 86–96. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.-J.; Hu, H.; Zhang, S.B. Seasonal variations in photosystem I compared with photosystem II of three alpine evergreen broad-leaf tree species. J. Photochem. Photobiol. B Biol. 2016, 165, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Rathore, N.; Thakur, D.; Chawla, A. Seasonal variations coupled with elevation gradient drives significant changes in eco-physiological and biogeochemical traits of a high altitude evergreen broadleaf shrub, Rhododendron anthopogon. Plant Physiol. Biochem. 2018, 132, 708–719. [Google Scholar] [CrossRef]
- Ran, F.; Zhang, X.; Zhang, Y.; Korpelainen, H.; Li, C. Altitudinal variation in growth, photosynthetic capacity and water use efficiency of Abies faxoniana Rehd. et Wils. seedlings as revealed by reciprocal transplantations. Trees 2013, 27, 1405–1416. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Li, H.E.; Zhang, W.H. Variations in leaf functional traits and physiological characteristics of Abies georgei var. smithii along the altitude gradient in the Southeastern Tibetan Plateau. J. Mt. Sci. 2016, 13, 1818–1828. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Z.; Zhang, C.; Zhang, J.; Ran, A. Ecophysiological responses of three tree species to a high-altitude environment in the southeastern Tibetan plateau. Forests 2018, 9, 48. [Google Scholar] [CrossRef]
- Li, X.; Ke, X.; Zhou, H.; Tang, Y. Contrasting altitudinal patterns of leaf UV reflectance and absorbance in four herbaceous species on the Qinghai-Tibetan Plateau. J. Plant Ecol. 2018, 12, 245–254. [Google Scholar] [CrossRef]
- Ma, L.; Sun, X.; Kong, X.; Galvan, J.V.; Li, X.; Yang, S.; Yang, Y.; Yang, Y.; Hu, X. Physiological, biochemical and proteomics analysis reveals the adaptation strategies of the alpine plant Potentilla saundersiana at altitude gradient of the Northwestern Tibetan Plateau. J. Proteom. 2015, 112, 63–82. [Google Scholar] [CrossRef]
- Guo, Q.; Li, H.; Gao, C.; Yang, R. Leaf traits and photosynthetic characteristics of endangered Sinopodophyllum hexandrum (Royle) Ying under different light regimes in Southeastern Tibet Plateau. Photosynthetica 2019, 57, 548–555. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.W.; Deng, X.G.; Tian, Z.H.; Zou, L.J.; Li, M.Q.; Tang, X.Y.; Li, D.X.; Zhang, C.B.; Yan, J.J. Various adaptations of meadow forage grasses in response to temperature changes on the Qinghai-Tibet Plateau, China. Plant Growth Regul. 2019, 88, 181–193. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Zhang, W.H. Sap flow of Abies georgei var. smithii and its relationship with the environment factors in the Tibetan subalpine region, China. J. Mt. Sci. 2015, 12, 1373–1382. [Google Scholar]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Grellet Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Meth. Enzymol. 1955, 2, 764–775. [Google Scholar]
- Ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5). 2002. Available online: www.canoco.com (accessed on 6 March 2020).
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Hashempour, A.; Ghasemnezhad, M.; Fotouhi Ghazvini, R.; Sohani, M.M. Olive (Olea europaea L.) freezing tolerance related to antioxidant enzymes activity during cold acclimation and non acclimation. Acta Physiol. Plant 2014, 36, 3231–3241. [Google Scholar] [CrossRef]
- Morsy, A.A.; Hassanein, R.A.; El-Din, N.M.N.; Kawy, A.H.A. Adaptive Mechanisms of Asphodelus aestivus Brot. to withstand drought stress: Metabolic constituents and activity of antioxidant enzymes. Egypt. J. Bot. 2016, 56, 225–241. [Google Scholar]
- Esmaeili, S.; Salehi, H.; Khosh-Khui, M. Seasonal changes in some physiological and biochemical responses of six groundcover plants. Int. J. Hortic. Sci. Technol. 2017, 4, 105–116. [Google Scholar]
- Lee, Y.P.; Babakov, A.; de Boer, B.; Zuther, E.; Hincha, D.K. Comparison of freezing tolerance, compatible solutes and polyamines in geographically diverse collections of Thellungiella sp. and Arabidopsis thaliana accessions. BMC Plant Biol. 2012, 12, 131. [Google Scholar] [CrossRef]
- Pompeiano, A.; Vita, F.; Miele, S.; Guglielminetti, L. Freeze tolerance and physiological changes during cold acclimation of giant reed [Arundo donax (L.)]. Grass Forage Sci. 2015, 70, 168–175. [Google Scholar] [CrossRef]
- Rooy, S.S.B.; Salekdeh, G.H.; Ghabooli, M.; Gholami, M.; Karimi, R. Cold-induced physiological and biochemical responses of three grapevine cultivars differing in cold tolerance. Acta Physiol. Plant 2017, 39, 264. [Google Scholar] [CrossRef]
- Ban, Q.; Wang, X.; Pan, C.; Wang, Y.; Kong, L.; Jiang, H.; Xu, Y.; Wang, W.; Pan, Y.; Li, Y. Comparative analysis of the response and gene regulation in cold resistant and susceptible tea plants. PLoS ONE 2017, 12, e0188514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Zhang, X.; Sun, S.; Zhang, A.; Chen, N.; Zhao, C. Enhanced cell dehydration tolerance and photosystem stability facilitate the occupation of cold alpine habitats by a homoploid hybrid species, Picea purpurea. Aob. Plants 2018, 10, ply053. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Kim, H.S.; Han, S.G.; Yoon, T.M. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Rubert-Nason, K.F.; Yang, Q.; Fu, Q.; Feng, J.; Shi, S. Photosynthetic acclimation of an evergreen broadleaved shrub (Ammopiptanthus mongolicus) to seasonal climate extremes on the Alxa Plateau, a cold desert ecosystem. Trees 2018, 32, 603–614. [Google Scholar] [CrossRef]
- Solanki, T.; Aphalo, P.J.; Neimane, S.; Hartikainen, S.M.; Pieristè, M.; Shapiguzov, A.; Porcar-Castell, A.; Atherton, J.; Heikkilä, A.; Robson, T.M. UV-screening and springtime recovery of photosynthetic capacity in leaves of Vaccinium vitis-idaea above and below the snow pack. Plant Physiol. Biochem. 2019, 134, 40–52. [Google Scholar] [CrossRef]
- Buchner, O.; Stoll, M.; Karadar, M.; Kranner, I.; Neuner, G. Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic performance in alpine plants. Plant Cell Environ. 2015, 38, 812–826. [Google Scholar] [CrossRef]
- Vilfan, N.; van der Tol, C.; Verhoef, W. Estimating photosynthetic capacity from leaf reflectance and Chl fluorescence by coupling radiative transfer to a model for photosynthesis. New Phytol. 2019, 223, 487–500. [Google Scholar] [CrossRef]
- Magaña Ugarte, R.; Escudero, A.; Gavilán, R.G. Metabolic and physiological responses of Mediterranean high-mountain and alpine plants to combined abiotic stresses. Physiol. Plant 2019, 165, 403–412. [Google Scholar] [CrossRef]
- Lavinsky, A.O.; Gomes, F.P.; Mielke, M.S.; Franca, S. Photosynthetic acclimation in shade-developed leaves of Euterpe edulis Mart (arecaceae) after long-term exposure to high light. Photosynthetica 2014, 52, 351–357. [Google Scholar] [CrossRef]
- Ren, J.; Dai, W.; Yang, C.; Ma, X.; Zou, C.B. Physiological regulation of poplar species to experimental warming differs between species with contrasting elevation ranges. New For. 2018, 49, 329–340. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).