Relationship between Canopy Structure and Community Structure of the Understory Trees in a Beech Forest in Japan
Abstract
:1. Introduction
2. Materials and Methods
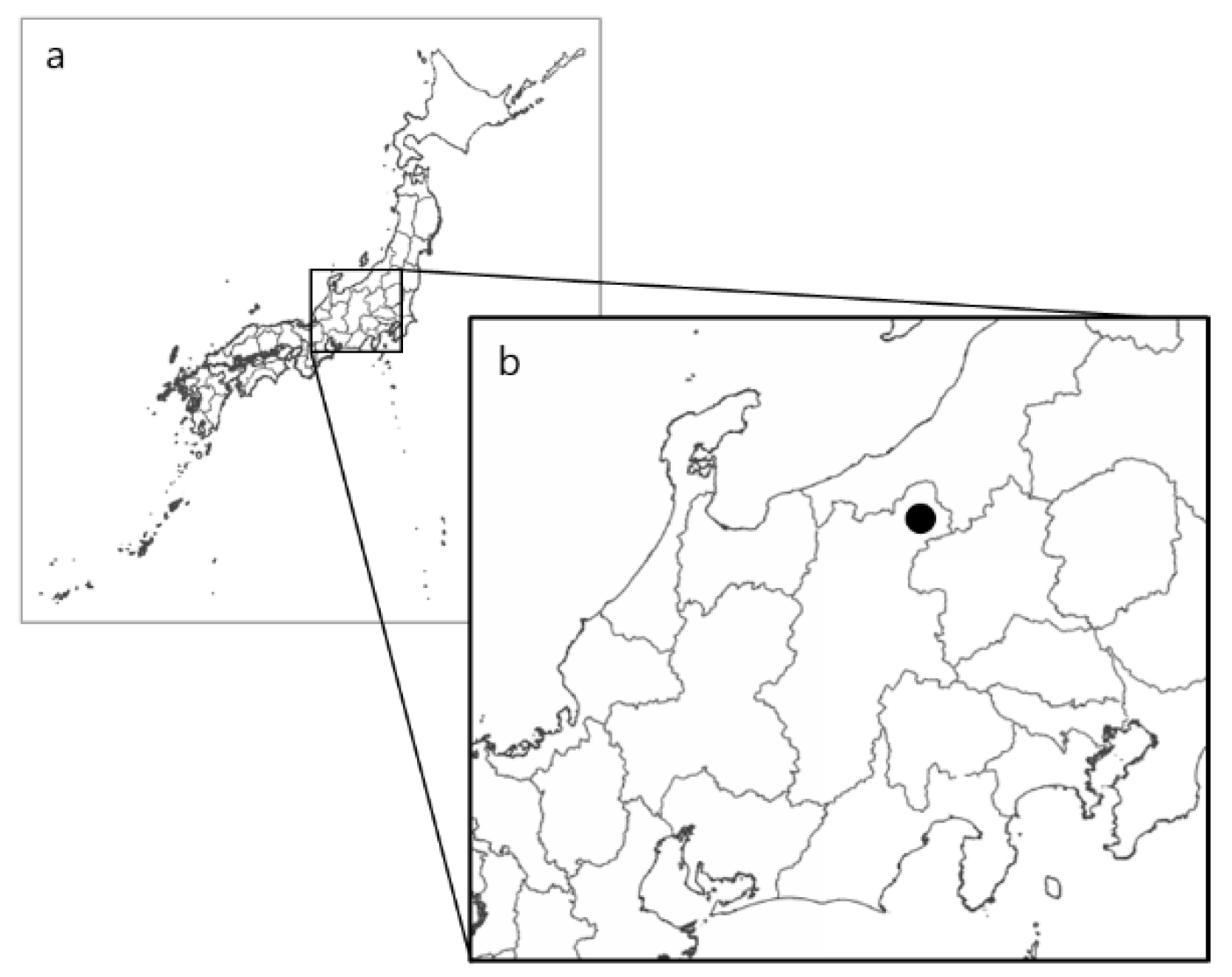
2.1. Study Area
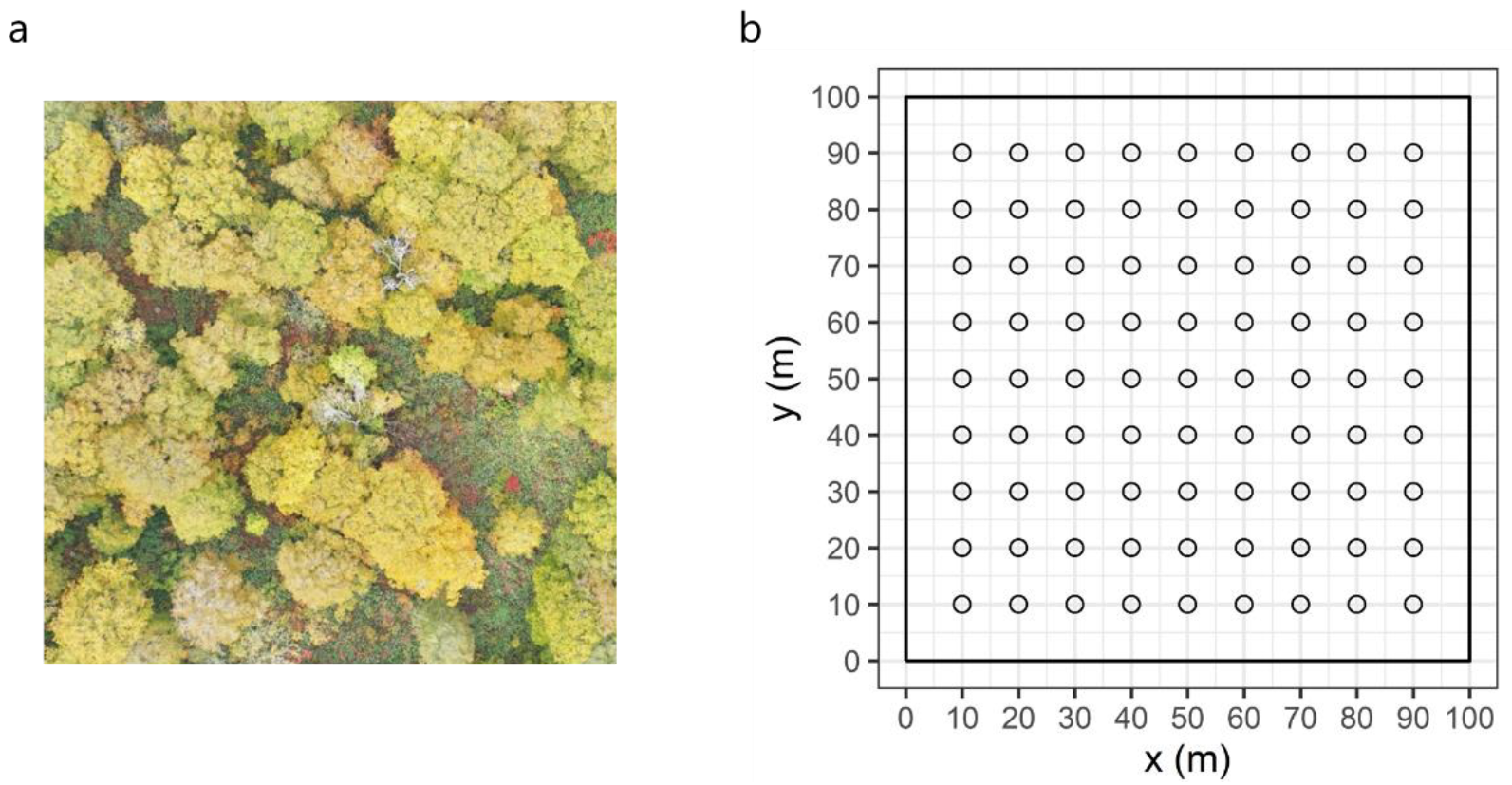
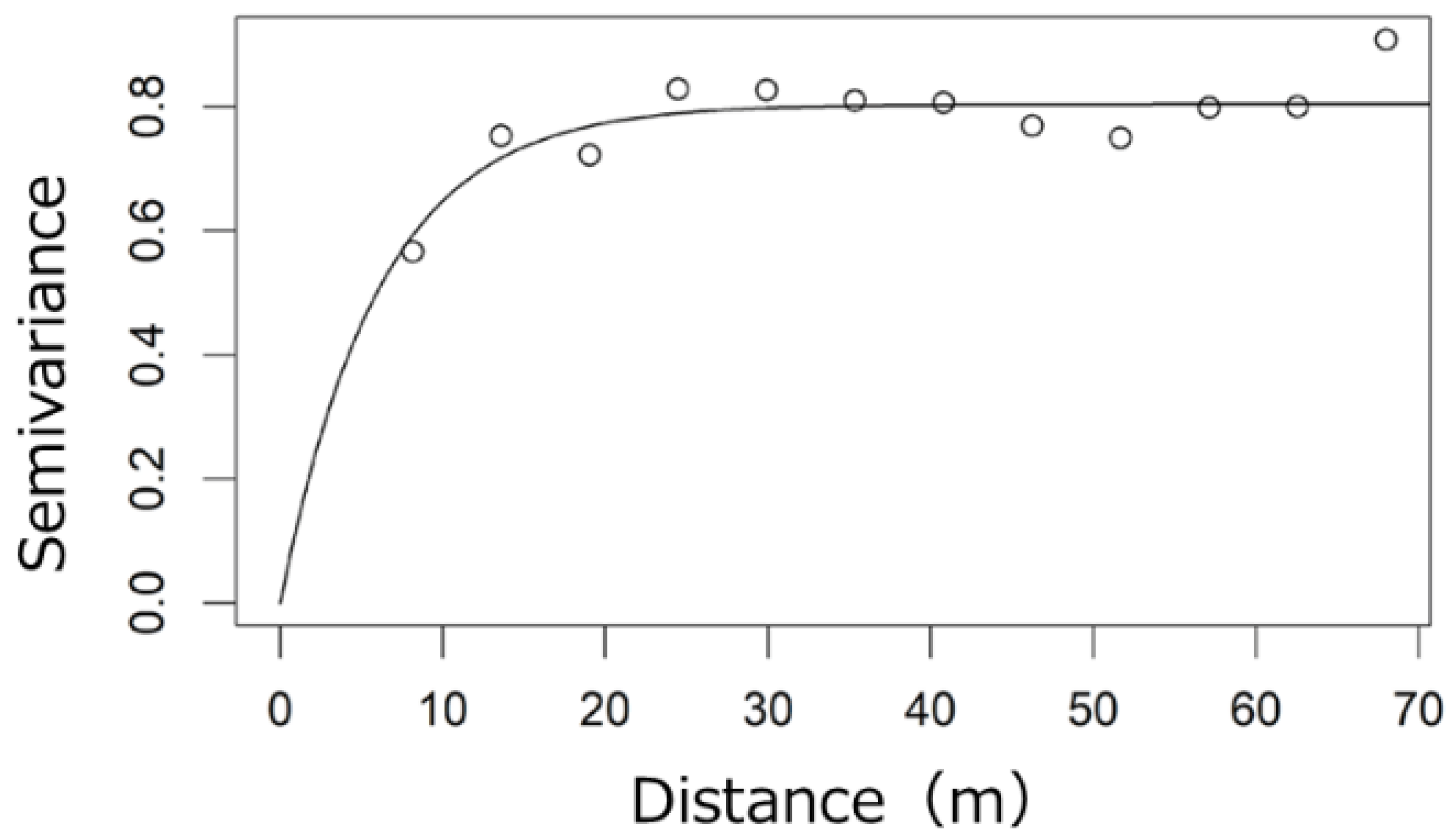
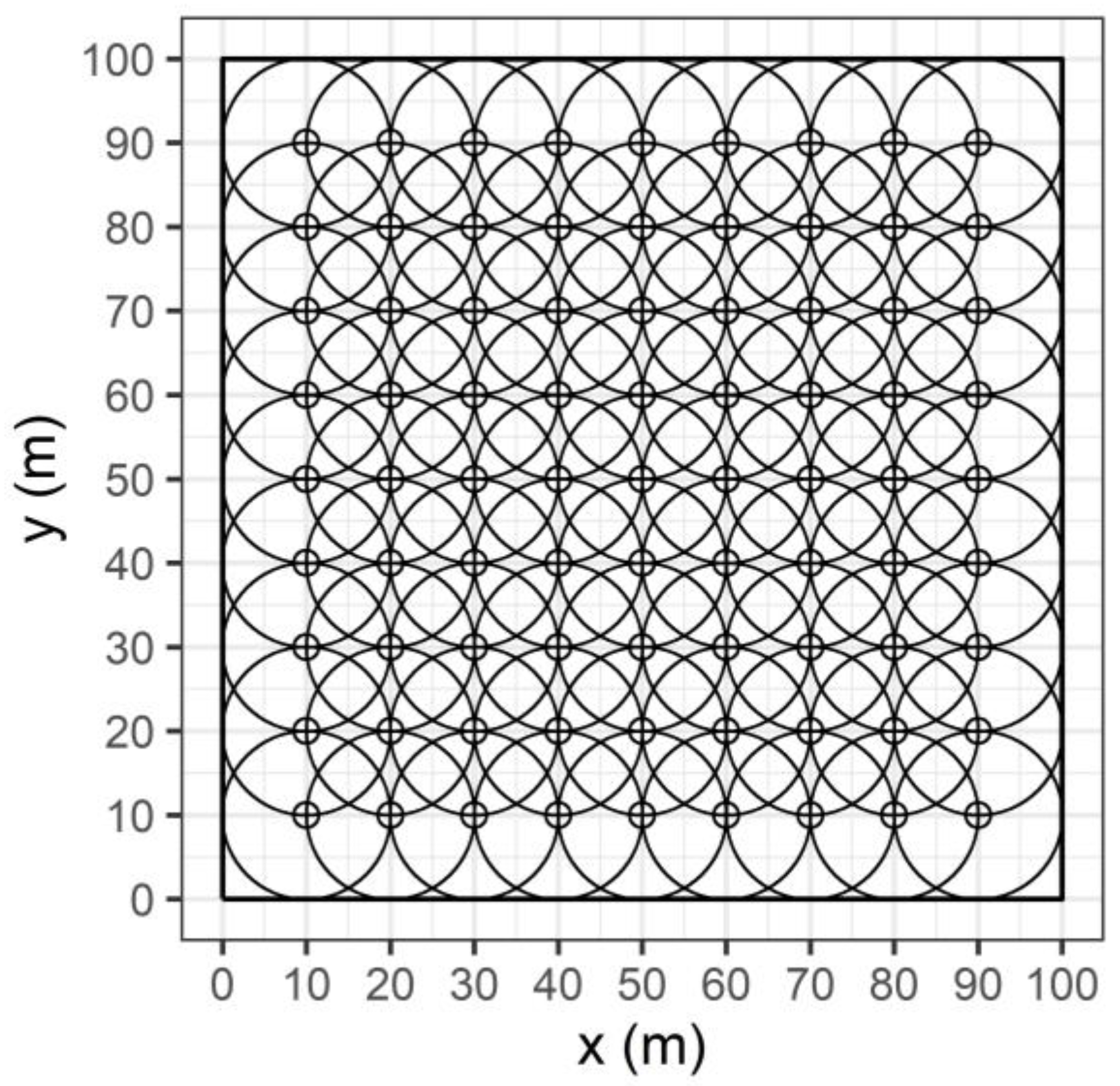
2.2. Spatial Distribution of Canopy LAI
2.3. Aboveground Net Primary Production of Wood
2.4. Data Analysis
3. Results
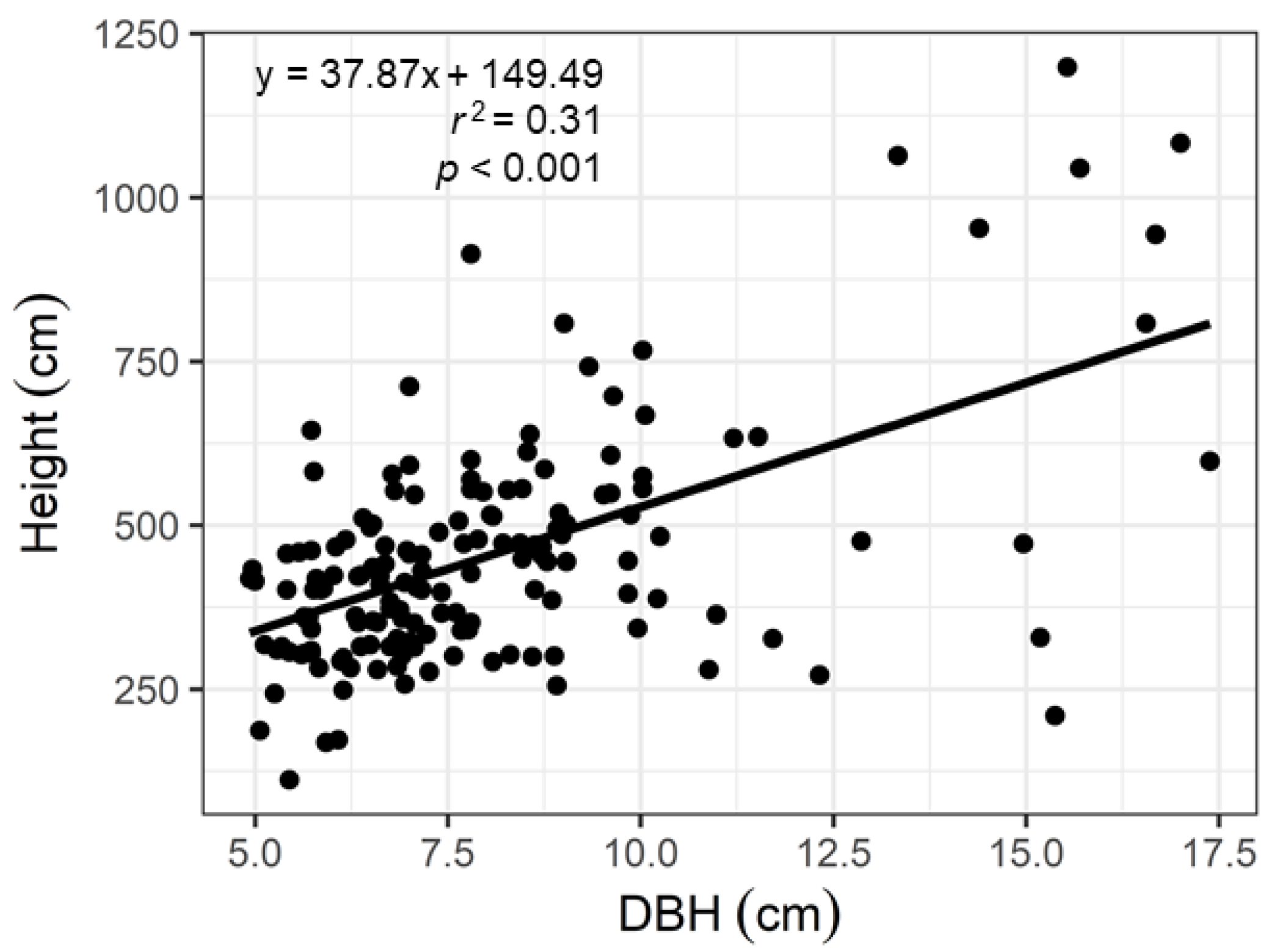
3.1. Forest Inventory Data
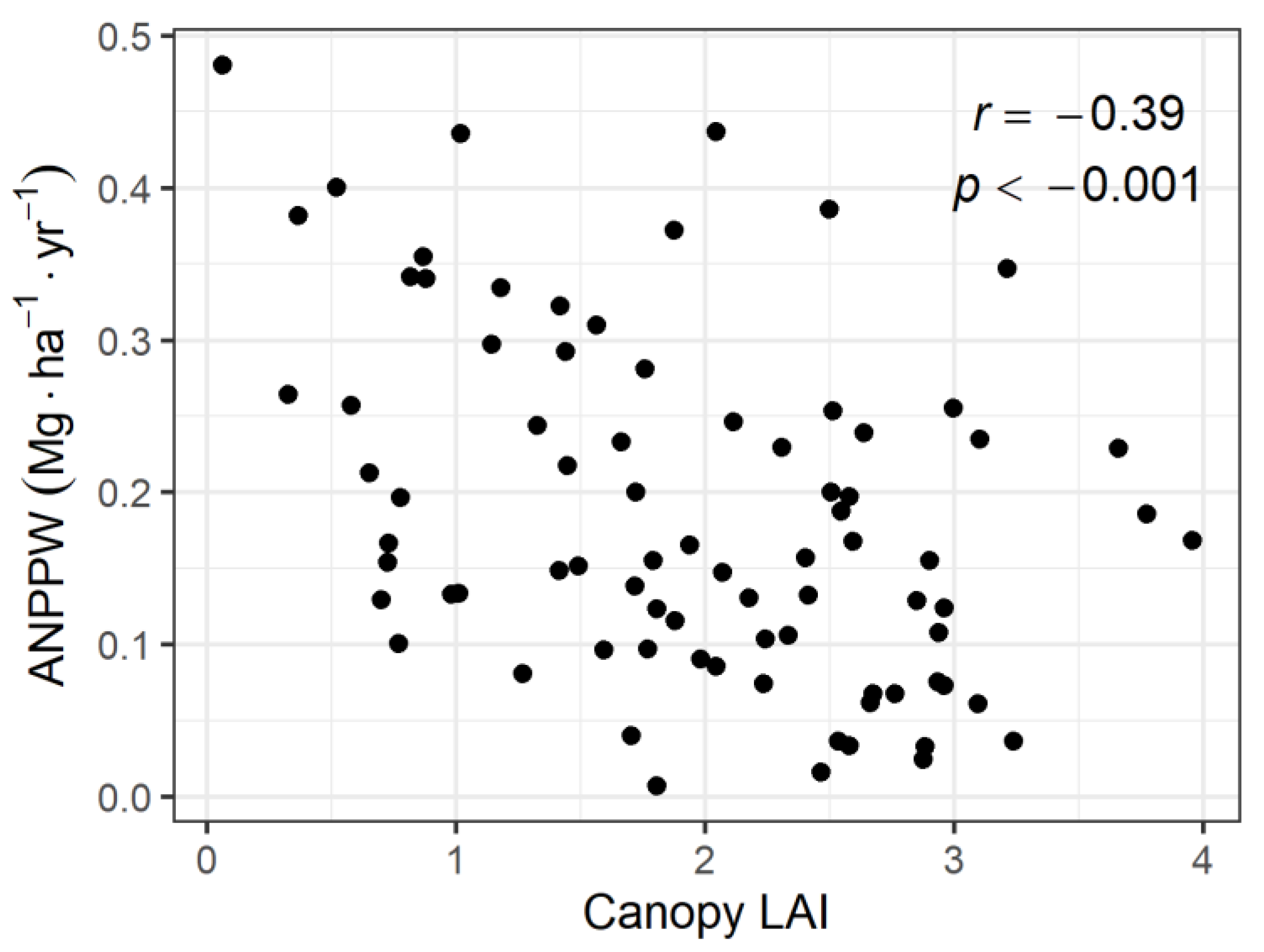
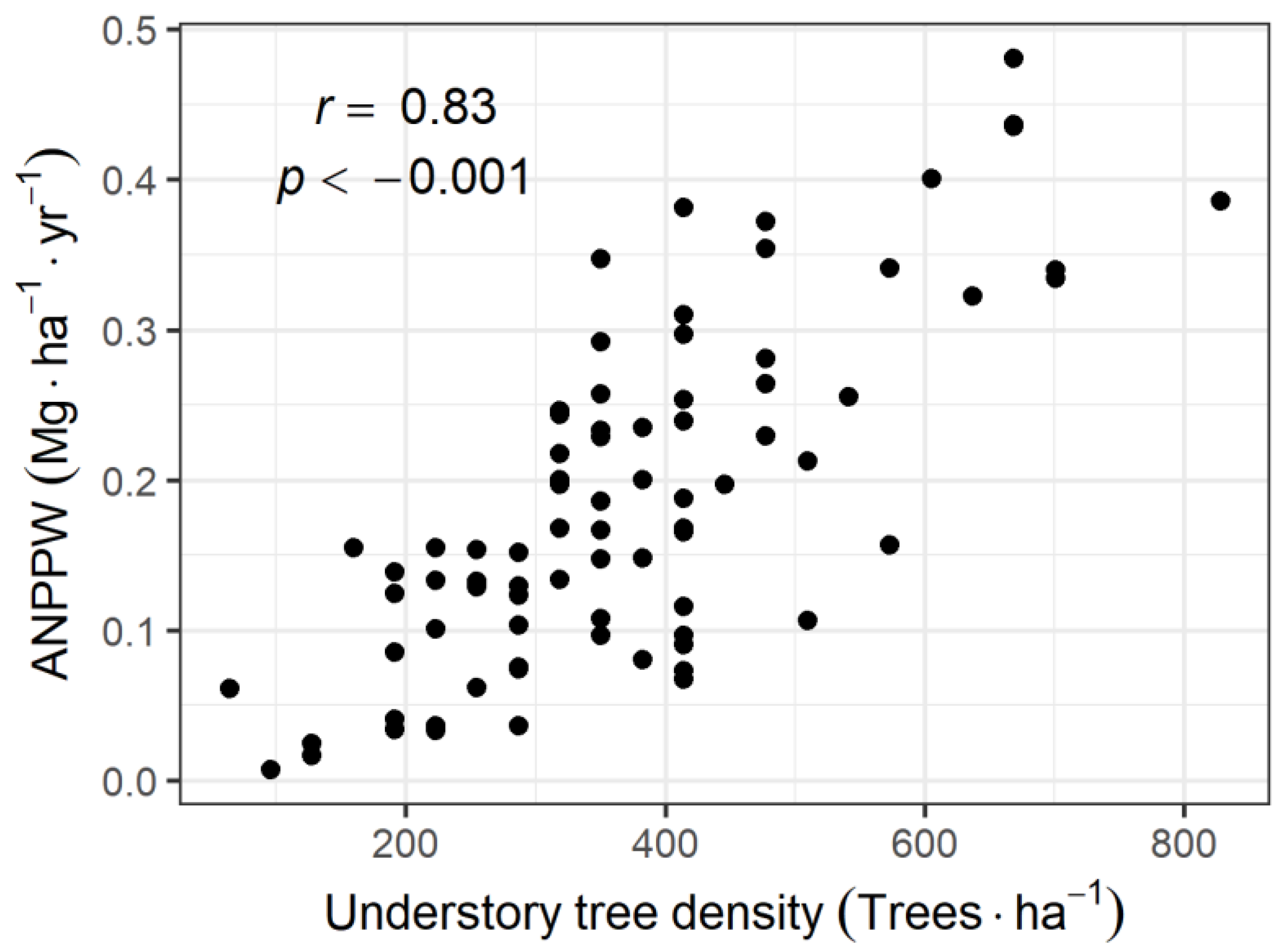
3.2. Relationship between ANPPW and Canopy LAI
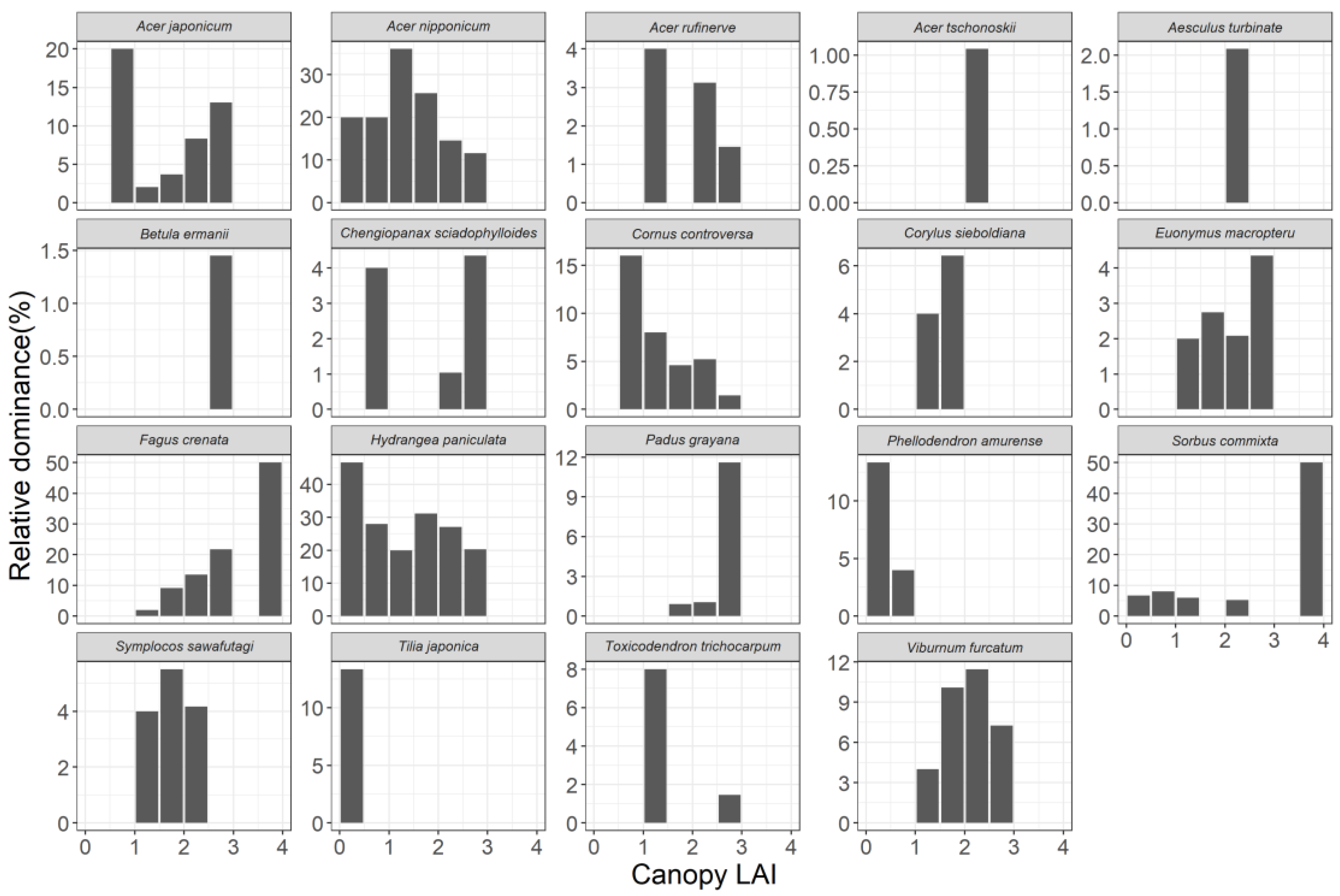
3.3. Relationship between Community Structure and Canopy LAI
4. Discussion
4.1. ANPPW of the Understory Tree Community
4.2. Relationship between Primary Production and Community Structure of the Understory Tree Community
4.3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamamoto, S.-I. Gap dynamics in climaxFagus crenata forests. Bot. Mag. Tokyo 1989, 102, 93–114. [Google Scholar] [CrossRef]
- Attiwill, P.M. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
- Diaci, J. Regeneration dynamics in a Norway spruce plantation on a silver fir-beech forest site in the Slovenian Alps. For. Ecol. Manag. 2002, 161, 27–38. [Google Scholar] [CrossRef]
- Ritter, E.; Dalsgaard, L.; Einhorn, K.S. Light, temperature and soil moisture regimes following gap formation in a semi-natural beech-dominated forest in Denmark. For. Ecol. Manag. 2005, 206, 15–33. [Google Scholar] [CrossRef]
- Ni, X.; Berg, B.; Yang, W.; Li, H.; Liao, S.; Tan, B.; Yue, K.; Xu, Z.; Zhang, L.; Wu, F. Formation of forest gaps accelerates C, N and P release from foliar litter during 4 years of decomposition in an alpine forest. Biogeochemistry 2018, 139, 321–335. [Google Scholar] [CrossRef] [Green Version]
- Glatthorn, J.; Feldmann, E.; Pichler, V.; Hauck, M.; Leuschner, C. Biomass Stock and Productivity of Primeval and Production Beech Forests: Greater Canopy Structural Diversity Promotes Productivity. Ecosystems 2018, 21, 704–722. [Google Scholar] [CrossRef]
- Majasalmi, T.; Rautiainen, M. The impact of tree canopy structure on understory variation in a boreal forest. For. Ecol. Manag. 2020, 466, 118100. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, M.; Yamamura, Y.; Nakano, T. Large-scale canopy opening causes decreased photosynthesis in the saplings of shade-tolerant conifer, Abies veitchii. Tree Physiol. 2008, 29, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Tobita, H.; Utsugi, H.; Kitao, M.; Kayama, M.; Uemura, A.; Kitaoka, S.; Maruyama, Y. Variation in photoinhibition among Sasa senanensis, Quercus mongolica, and Acer mono in the understory of a deciduous broad-leaved forest exposed to canopy gaps caused by typhoons. Trees 2010, 24, 307–319. [Google Scholar] [CrossRef]
- Poorter, L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New Phytol. 2009, 181, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R.; Binkley, D.; Campoe, O.C.; Gspaltl, M.; Forrester, D.I.; Hendrik, P.; et al. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Osada, N.; Yasumura, Y.; Ishida, A. Leaf nitrogen distribution in relation to crown architecture in the tall canopy species, Fagus crenata. Oecologia 2014, 175, 1093–1106. [Google Scholar] [CrossRef]
- Abe, S.; Masaki, T.; Nakashizuka, T. Factors influencing sapling composition in canopy gaps of a temperate deciduous forest. Vegetatio 1995, 120, 21–32. [Google Scholar] [CrossRef]
- Yamamoto, S. Gap regeneration of major tree species in different forest types of Japan. Vegetatio 1996, 127, 203–213. [Google Scholar] [CrossRef]
- Tilman, D. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef] [Green Version]
- Forrester, D.I.; Kohnle, U.; Albrecht, A.T.; Bauhus, J. Complementarity in mixed-species stands of Abies alba and Picea abies varies with climate, site quality and stand density. For. Ecol. Manag. 2013, 304, 233–242. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Lu, D.; Wang, G.G.; Yu, L.; Zhang, T.; Zhu, J. Seedling survival within forest gaps: The effects of gap size, within-gap position and forest type on species of contrasting shade-tolerance in Northeast China. For. Int. J. For. Res. 2018, 91, 470–479. [Google Scholar] [CrossRef]
- Ida, H. Forest structure in a beech (Fagus crenata Blume) stand on a 1-ha permanent plot for the Monitoring Sites 1000 Project in Kayanodaira, central Japanese snowbelt. Bull. Inst. Nat. Educ. Shiga Height. Shinshu Univ. 2013, 50, 33–40. [Google Scholar]
- Cai, Y.; Tanioka, Y.; Kitawaga, T.; Ida, H.; Hirota, M. Gross primary production of dwarf bamboo, Sasa senanensis, in a mature beech forest with a substantial gap-mosaic structure. J. Plant Res. 2021, 134, 209–221. [Google Scholar] [CrossRef]
- Ishihara, M.I.; Suzuki, S.N.; Nakamura, M.; Enoki, T.; Fujiwara, A.; Hiura, T.; Hoshino, D.; Homma, K.; Hoshizaki, K.; Ida, H.; et al. Forest stand structure, composition, and dynamics in 34 sites over Japan. Ecol. Res. 2011, 26, 1007–1008. [Google Scholar] [CrossRef]
- Tanioka, Y.; Cai, Y.; Ida, H.; Hirota, M. A spatial relationship between canopy and understory leaf area index in an old-growth cool-temperate deciduous forest. Forests 2020, 11, 1037. [Google Scholar] [CrossRef]
- Komiyama, A.; Nakagawa, M.; Kato, S. Common Allometric Relationships for Estimating Tree Biomasses in Cool Temperate Forests of Japan. J. Jpn. For. Soc. 2011, 93, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Kishima, T.; Okamoto, S.; Hayashi, S. Atlas of Wood in Colour; Hoikusha: Osaka, Japan, 1962. [Google Scholar]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J.; Deborah, A.; Clark Sandra Brown David, W.; Kicklighter Jeffrey, Q.; Chambers John, R.; et al. Measuring net primary production in forests: Concepts and field methods. Ecol. Appl. 2001, 11, 356–370. [Google Scholar] [CrossRef]
- Ewers, B.E.; Pendall, E. Spatial patterns in leaf area and plant functional type cover across chronosequences of sagebrush ecosystems. Plant Ecol. 2008, 194, 67–83. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, F.; Zhu, Y.; Li, F.; Jin, G. Spatial heterogeneity of leaf area index in a temperate old-growth forest: Spatial autocorrelation dominates over biotic and abiotic factors. Sci. Total Environ. 2018, 634, 287–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H.; Taylor, A.R. Positive species diversity and above-ground biomass relationships are ubiquitous across forest strata despite interference from overstorey trees. Funct. Ecol. 2017, 31, 419–426. [Google Scholar] [CrossRef]
- Mensah, S.; Du Toit, B.; Seifert, T. Diversity–biomass relationship across forest layers: Implications for niche complementarity and selection effects. Oecologia 2018, 187, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, W.; Zhang, C.; Zhao, X.; von Gadow, K.; Zhang, Z. Biodiversity-ecosystem functioning relationships of overstorey versus understorey trees in an old-growth temperate forest. Ann. For. Sci. 2019, 76, 64. [Google Scholar] [CrossRef]
- Kumar, P.; Chen, H.Y.H.; Searle, E.B.; Shahi, C. Dynamics of understorey biomass, production and turnover associated with long-term overstorey succession in boreal forest of Canada. For. Ecol. Manag. 2018, 427, 152–161. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, D.; Zhang, W. Effects of gaps on regeneration of woody plants: A meta-analysis. J. For. Res. 2014, 25, 501–510. [Google Scholar] [CrossRef]
- Shibano, S.; Watanabe, S. Stratification of a Fagus crenata stand in the Ohira-yama area. Trans. Meet. Hokkaido Branch Jpn. For. Soc. 1989, 37, 64–66. [Google Scholar] [CrossRef]
- Ishida, M.; Kikuzawa, K.; Asai, T.; Mizui, N.; Seiwa, K. Distribution and height growth of young trees in the canopy gap and in the closed stand. J. Jpn. For. Soc. 1991, 73, 145–150. [Google Scholar]
- Chávez, V.; Macdonald, S.E. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. For. Ecol. Manag. 2010, 259, 1067–1075. [Google Scholar] [CrossRef]
- Kanno, H.; Seiwa, K. Sexual vs. vegetative reproduction in relation to forest dynamics in the understorey shrub, Hydrangea paniculata (Saxifragaceae). Plant Ecol. Former. Veg. 2004, 170, 43–53. [Google Scholar] [CrossRef]
- Masaki, T.; Tanaka, H.; Tanouchi, H.; Sakai, T.; Nakashizuka, T. Structure, dynamics and disturbance regime of temperate broad-leaved forests in Japan. J. Veg. Sci. 1999, 10, 805–814. [Google Scholar] [CrossRef]
- Masaki, T.; Nakashizuka, T. Seedling demography of Swida controversa: Effect of light and distance to conspecifics. Ecology 2002, 83, 3497–3507. [Google Scholar] [CrossRef]
- Wagner, S.; Collet, C.; Madsen, P.; Nakashizuka, T.; Nyland, R.D.; Sagheb-Talebi, K. Beech regeneration research: From ecological to silvicultural aspects. For. Ecol. Manag. 2010, 259, 2172–2182. [Google Scholar] [CrossRef]
- Feldmann, E.; Drößler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415–416, 38–46. [Google Scholar] [CrossRef]
- Fujita, N. One-sided distribution of the component species of the Japanese beech forest along either the Pacific or the Japan Sea. Acta Phytotaxon. Geobot. 1987, 38, 311–329. [Google Scholar]
- Abe, M.; Miguchi, H.; Nakashizuka, T. An interactive effect of simultaneous death of dwarf bamboo, canopy gap, and predatory rodents on beech regeneration. Oecologia 2001, 127, 281–286. [Google Scholar] [CrossRef]
- Komiyama, A. Tree age composition and the regeneration process of a secondary deciduous broad-leaved forest. J. Jpn. For. Soc. 1989, 71, 374–379. [Google Scholar]
- Kato, S.; Komiyama, A. Distribution patterns of understory trees and diffuse light under the canopy of a beech forest. Jpn. J. Ecol. 1999, 49, 10. [Google Scholar]
- Duguid, M.C.; Ashton, M.S. A meta-analysis of the effect of forest management for timber on understory plant species diversity in temperate forests. For. Ecol. Manag. 2013, 303, 81–90. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y.H. Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 2010, 91, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
| Species Name | Trees (Trees/ha) | Mean DBH 1 (cm) | Maximum DBH (cm) | Basal Area 2 (m2/ha) | Relative Dominance 3 (%) |
|---|---|---|---|---|---|
| Fagus crenata | 217 | 29.95 | 101.25 | 27.03 | 82.17 |
| Betula ermanii | 5 | 48.19 | 76.55 | 1.17 | 3.57 |
| Aesculus turbinata | 17 | 22.50 | 50.17 | 0.95 | 2.89 |
| Acer nipponicum | 190 | 7.54 | 19.32 | 0.93 | 2.84 |
| Hydrangea paniculata | 135 | 6.98 | 12.32 | 0.54 | 1.63 |
| Chengiopanax sciadophylloides | 22 | 15.31 | 38.61 | 0.52 | 1.57 |
| Acer japonicum | 75 | 8.20 | 18.56 | 0.44 | 1.35 |
| Viburnum furcatum | 85 | 6.18 | 8.85 | 0.26 | 0.79 |
| Phellodendron amurense | 10 | 14.09 | 26.07 | 0.19 | 0.59 |
| Sorbus commixta | 34 | 7.82 | 14.17 | 0.18 | 0.54 |
| Cornus controversa | 52 | 6.21 | 9.17 | 0.16 | 0.49 |
| Padus grayana | 21 | 7.72 | 10.98 | 0.10 | 0.31 |
| Acer pictum | 1 | 34.70 | 34.70 | 0.10 | 0.29 |
| Acer rufinerve | 11 | 8.94 | 11.84 | 0.07 | 0.22 |
| Euonymus macropterus | 18 | 6.74 | 8.50 | 0.07 | 0.20 |
| Symplocos sawafutagi | 22 | 5.76 | 7.16 | 0.06 | 0.18 |
| Tilia japonica | 7 | 9.62 | 16.23 | 0.06 | 0.17 |
| Corylus sieboldiana | 20 | 5.56 | 6.69 | 0.05 | 0.15 |
| Toxicodendron trichocarpum | 5 | 5.70 | 6.40 | 0.01 | 0.04 |
| Acer tschonoskii | 2 | 7.42 | 8.09 | 0.01 | 0.03 |
| Species Name | Density, All Trees (Trees/ha) | Density, Understory Trees (Trees/ha) | Understory Trees as a Percentage of Total (%) |
|---|---|---|---|
| Fagus crenata | 217 | 66 | 30.42 |
| Betula ermanii | 5 | 1 | 20.00 |
| Aesculus turbinata | 17 | 4 | 23.53 |
| Acer nipponicum | 190 | 162 | 85.26 |
| Hydrangea paniculata | 135 | 127 | 94.07 |
| Chengiopanax sciadophylloides | 22 | 7 | 31.82 |
| Acer japonicum | 75 | 57 | 76.00 |
| Viburnum furcatum | 85 | 85 | 100.00 |
| Phellodendron amurense | 10 | 3 | 30.00 |
| Sorbus commixta | 34 | 28 | 82.35 |
| Cornus controversa | 52 | 52 | 100.00 |
| Padus grayana | 21 | 17 | 80.95 |
| Acer pictum | 1 | 0 | 0.00 |
| Acer rufinerve | 11 | 8 | 72.73 |
| Euonymus macropterus | 18 | 18 | 100.00 |
| Symplocos sawafutagi | 22 | 22 | 100.00 |
| Tilia japonica | 7 | 3 | 42.86 |
| Corylus sieboldiana | 20 | 20 | 100.00 |
| Toxicodendron trichocarpum | 5 | 5 | 100.00 |
| Acer tschonoskii | 2 | 2 | 100.00 |
| Total | 949 | 687 | 72.392 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanioka, Y.; Ida, H.; Hirota, M. Relationship between Canopy Structure and Community Structure of the Understory Trees in a Beech Forest in Japan. Forests 2022, 13, 494. https://doi.org/10.3390/f13040494
Tanioka Y, Ida H, Hirota M. Relationship between Canopy Structure and Community Structure of the Understory Trees in a Beech Forest in Japan. Forests. 2022; 13(4):494. https://doi.org/10.3390/f13040494
Chicago/Turabian StyleTanioka, Yosuke, Hideyuki Ida, and Mitsuru Hirota. 2022. "Relationship between Canopy Structure and Community Structure of the Understory Trees in a Beech Forest in Japan" Forests 13, no. 4: 494. https://doi.org/10.3390/f13040494
APA StyleTanioka, Y., Ida, H., & Hirota, M. (2022). Relationship between Canopy Structure and Community Structure of the Understory Trees in a Beech Forest in Japan. Forests, 13(4), 494. https://doi.org/10.3390/f13040494







