Abstract
Little is known on how karst plants adapt to highly heterogeneous habitats via adjusting leaf anatomical structures. Phyllostachys glauca McClure is a dominant species that grow across different microhabitats in the limestone mountains of Jiangxi Province, China. We investigated the leaf anatomical structures, plant biomass, soil water content, soil total nitrogen (TN), and soil total phosphorus (TP) from three habitats characterized by different rock exposure, including high rock exposure (HRE), medium rock exposure (MRE) and low rock exposure (LRE), and aimed to discern the relationships between the leaf anatomical plasticity and edaphic factors. The leaves of P. glauca in different habitats showed significant anatomical plasticity in two aspects. First, the leaves adjusted cuticle thickness, papillae length, bulliform cell size and mesophyll thickness to lower water loss and then adapt to the water-deficient habitats (HRE). Second, the leaves enlarged vessels and vascular bundles (first-order and second-order parallel veins) to improve water and nutrient transportation and then enhance plant growth in nitrogen-rich habitats (HRE). Soil water and soil nutrients purely explained the total variation of leaf anatomical traits by 21.7% and 15.7%, respectively, and had a shared proportion of 15.8%. Our results indicated that the leaf anatomical variations in different habitats were associated with both soil water and soil nutrients. Moreover, we found that leaf anatomical structures were more affected by TN than TP. The present study advanced the current understanding of the strategies employed by karst plants to cope with highly heterogeneous habitats via leaf anatomical plasticity.
1. Introduction
Currently, climate change and increasing anthropogenic activities have generated a more variable environment that plants need to survive [1,2,3]. Phenotypic plasticity is a key strategy for plants to adapt to heterogeneous habitats and changing environments [4,5,6,7]. Via adjusting morphological and physiological traits, plants respond to the ambient environmental changes and enhance their fitness [5,7,8,9]. This process is usually referred to as phenotypic plasticity. Exploring the mechanism of phenotypic plasticity will help us understand future changes in species distribution and community composition, and manage the forest and crop production [10,11,12]. At present, phenotypic plasticity is a central issue of plant eco-physiological studies [13].
Leaf phenotypic plasticity is crucial for plant growth and reproduction because the leaf is responsible for many important physiological activities such as gas exchange and assimilate production. Previous studies show that leaves are more sensitive to environmental changes than other organs, and thus, their phenotypic plasticity properly reflects the adaptive strategies to environments [14,15,16,17]. Variations in leaf anatomical structure imply functional adaptations to changing environments, and it has received increasing attention in recent years [10,17,18,19]. Of the environmental factors, soil water is the most focused factor selected to explore how plants cope with water deficit or drought via anatomical adjustments.
The change in soil water causes leaf plasticity in the epidermis, mesophyll, and vascular structure. Previous studies show that soil water deficit results in increases in the thickness of leaf cuticle, epidermis, mesophyll, as well as the length and density of epidermal appendages, and decreases in the vessel size to reduce water loss and ensure hydraulic safety [10,16,18,20,21,22]. On the other hand, some experiments found that the responses of leaf anatomical plasticity were various, and the magnitude and direction of their response might depend on the extent of drought and specific species [18,23,24,25,26]. In some monocotyledons families such as Poaceae, Cyperaceae, and Juncaceae, bulliform cells are efficient structures and result in leaf curling and inhibiting water loss [18,27,28]. Under water stress, contrasting responses of bulliform cells were observed. The size of bulliform cells was enlarged in Stipa lagascae Roem. & Schult. [18] whereas it shrank in Sugarcane [28]. Therefore, discerning the variations in leaf anatomical features can interpret the adaptive strategies of plants to drought.
Besides soil water, previous findings show that soil nutrients also have a remarkable influence on anatomical structures [4,15,29,30]. Some studies confirmed that additions of nitrogen (N) or phosphorus (P) increase the size of midribs, vascular bundles and vessels, and the cross-sectional area of xylems [4,31,32,33]. In an experiment of N and P addition, researchers found that both N and P have effects on leaf anatomical traits, although the effect of N outweighs that of P [4]. However, we still know little about how leaf anatomical traits respond to changes in both soil water and soil nutrients.
Karst habitats are characterized by rocky and shallow soil, with heterogeneous soil water and nutrients [34,35,36,37]. Plants frequently suffer from water stress due to the low water-holding capacity of rocky soils [35,36,38], and soil water is regarded as the primary factor limiting plant growth in karst. On the other hand, some studies confirmed that soil nutrients also play an important role in the growth of karst plants [39,40,41,42]. However, little is known on how karst plants adjust their leaf anatomical traits to adapt to the highly heterogeneous soil water and soil nutrients. In the present study, we aimed to discern the leaf anatomical plasticity and its connections with the soil water and soil nutrients by exploring a running bamboo (Phyllostachys glauca McClure), a species dominated in limestone mountains in Jiangxi province, China. The bamboo species can occupy the rocky habitats in limestone mountains where other species find it hard to live [43,44]. We hypothesized that: (1) the leaves of P. glauca show evident plasticity in anatomical traits in different habitats to adapt to the change in soil water and soil nutrients; and (2) the leaf anatomical variations were chiefly related to soil water rather than soil nutrients because the soil water is the most important factor determining plant distribution and growth in karst.
2. Materials and Methods
2.1. The Species and Study Site
Phyllostachys glauca McClure is a woody and evergreen species, 5–12 m in height and 20–50 mm in culm diameter [45], and exchanges leaves every year [46]. The bamboo is widely distributed from the Yellow River valley to the Yangtze River valley in China [45]. In our study site (Ruichang City, Jiangxi province, China; 29°23′ N~29°51′ N, 115°06′ E~115°44′ E), P. glauca dominates in the limestone mountains and forms pure bamboo forests with a total area of 9.9 km2 [44]. P. glauca grows across various microhabitats with different rock exposures. Ruichang City has a subtropical humid monsoon climate, a mean annual temperature of 16.6 °C, and a mean annual precipitation of 1394 mm, most of which falls during the growing season (from March to September).
2.2. Habitat Classification
Habitats in the limestone region are highly heterogeneous, characterized by shallow soil and high rock exposure [36,41,47]. Soils were developed from limestone, contained rock fragments, and were usually distributed discontinuously. According to previous habitat classification in karst [48,49,50], the habitats in our study site were classified into three types according to their rock exposure: high rock exposure (HRE), medium rock exposure (MRE), and low rock exposure (LRE), respectively. HRE was the habitat with a rock exposure ranging from 50% to 80%, which had shallow and discontinuously distributed soils; MRE was the habitat with a rock exposure ranging from 30% to 50%, which had a deeper soil layer than MRE; and LRE was the habitat with a rock exposure lower than 30%, which had a relatively deeper and continuously distributed soil.
2.3. Experiment Design and Field Sampling
To exclude the effect of topography on leaf anatomical traits, the randomized block design was employed in this study. In late August 2016, plots of three habitats (HRE, MRE, LRE) in the same block were established at sites with similar aspects, slopes, and elevations. The plot size was 10 m × 10 m, and there were three replicates for each habitat. In each plot, six plants of Phyllostachys glauca were randomly selected to investigate leaf anatomy and determine plant biomass.
For each plant, three healthy and mature leaves were sampled from the southern branches in the middle canopy. Leaves were cut into small pieces (about 10 mm × 5 mm) and fixed in FAA (45% alcohol, 0.25% acetic acid, and 1.85% formaldehyde). Then, leaf samples were vacuumized and taken back to laboratory for anatomical investigations. Rock exposure (%) was calculated by the proportion of the aboveground rocks to the ground surface in a plot [41]. After removing the litter layer, five soil cores (0–300 mm depth) were collected from each plot using a soil auger (46 mm internal diameter) and pooled as a single composite sample in the analyses for soil nutrients. Soil depth was assessed by coring from the soil surface to the rock matrix. In a plot, soil depth was calculated by the average of five soil corings.
2.4. Experimental Methods
2.4.1. Leaf Anatomical Investigations
After 48 h fixed in FAA, leaf specimens were put in 15% (v/v) hydrogen fluoride to de-siliconize for 38 h and dehydrated in a graded series of alcohol solutions (began at 50%). For anatomical investigations, the leaf specimens were cut into 7-μm-thick paraffin sections using a rotary microtome (Leica RM2235, Heidelberger, Germany) and stained with safranin and fast green. The sections were mounted in Canada balsam. The paraffin sections were observed and photographed using an optical microscope (Leica DM2500, Heidelberger, Germany) with a camera linked to a computer. The anatomical structures, including epidermis, cuticle, papillae, palisade and spongy tissue, bulliform cell, and vessel and vascular bundle, were investigated. QwinV3 software was used to measure those structure thickness and size, and at least 100 observations were recorded for each structure.
2.4.2. Plant Biomass Determination
After leaf anatomy samples were collected, the plants of Phyllostachys glauca were coded and harvested into five components: leaves, branches, culm, rhizomes, and roots. With careful excavation, the rhizome was cut off at the middle location between the sampling plant and the next connected plant. Roots included culm roots (roots growing on culm base) and rhizome roots (roots growing on rhizome nodes). The rhizomes and roots were washed out. Then, all component samples were dried at 70 °C for 72 h and weighed. Plant biomass was the sum of the dry mass of five components.
2.4.3. Soil Water and Nutrient Analysis
Using a soil moisture meter (HH2, Delta-T Devices, Cambridge, UK), soil water content (SWC, % vol) in each plot was measured with five replicates in the field. All SWC measurements were finished within a sunny day after five days without rainfall. After being air-dried, soil samples were cleared of roots and organic debris and ground to pass through a 1-mm sieve for the subsequent chemical analysis. Soil total nitrogen (TN) was analyzed with the Kjeldahl method (K-370, Buchi Scientific Instruments, Flawil, Switzerland). Soil total phosphorus (TP) was measured spectrophotometrically after digestion with sulfuric acid (H2SO4). The P concentrations were determined by an ultraviolet spectrophotometer (TU-1901, Beijing General Analysis Instrument, Beijing, China).
2.5. Statistics
To check the differences in soil features and leaf anatomical traits among different habitats (HRE, MRE, LRE), one-way ANOVAs were applied with post hoc LSD tests. Pearson correlation analysis was performed to detect the relationships between leaf anatomical traits and plant biomass, and interrelationships between SWC, soil depth, and rock exposure. Redundancy analysis (RDA) was used to explore the effects of edaphic factors (SWC, soil depth, rock exposure, TN, and TP) on the leaf anatomical traits. Variation partitioning was conducted to identify the pure contributions of soil water and soil nutrients to the variance of anatomical traits. To simplify the interrelationships in the RDA, the traits from the same category were combined as follows: epidermis thickness was the sum of upper epidermis thickness and lower epidermis thickness; mean vessel diameter was the averaged diameter of vessels in three veins (midrib, first-order parallel vein, and second-order parallel vein); mean diameter of vascular bundle was the averaged diameter of vascular bundles in three veins. One-way ANOVAs and Person correlation analysis were conducted in SPSS 16.0 (SPSS Inc., Chicago, IL, USA), and RDA and variation partitioning were performed using CANOCO 5.0 (Microcomputer Power, Ithaca, NY, USA). Figures were graphed with OriginPro 9.0 (Origin Lab Corporation, Northampton, MA, USA).
3. Results
3.1. Soil Features in Three Habitats
The investigated three habitats, high rock exposure (HRE), medium rock exposure (MRE), and low rock exposure (LRE), were distinct from each other in rock exposure, water content, and soil nutrients (Table 1). HRE was characterized by the highest rock exposure, TN and TP, and the lowest soil depth and water content. By contrast, LRE owned the lowest rock exposure and TN, and the highest soil depth and water content. TP in MRE was significantly lower than that in HRE and LRE.

Table 1.
Rock exposure and soil characteristics of three habitats in Phyllostachys glauca forest.
3.2. Leaf Anatomical Features in Three Habitats
The leaf anatomical features of Phyllostachys glauca were similar in the three habitats: HRE, MRE, and LRE (Figure 1). Both upper and lower epidermis were composed of a layer of cells, including epidemic cells, bulliform cells, and stomata. The outer wall of epidermic cells was suberized or silicified to form cuticles. The bulliform cells were transparent with thin cell walls and composed of 3–7 sectorial cells embedded into mesophyll. As to the location, bulliform cells were located between parallel veins. Many stomata and papillae were distributed on the lower epidermis.
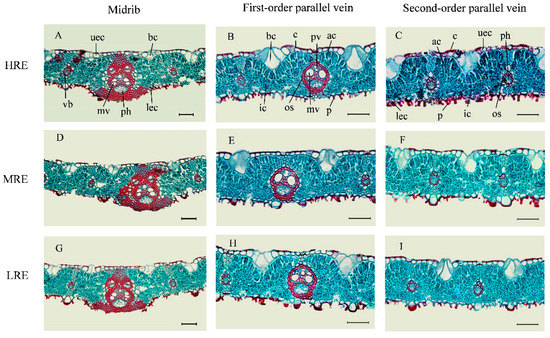
Figure 1.
Leaf cross-sections of Phyllostachys glauca from three habitats in the limestone mountain. (A–C), the anatomical features of the midrib, first-order parallel vein, and second-order parallel vein in the habitat of high rock exposure (HRE), respectively; (D–F), the anatomical features of the midrib, first-order parallel vein, and second-order parallel vein in the habitat with medium rock exposure (MRE), respectively; and (G–I), the anatomical features of the midrib, first-order parallel vein, and second-order parallel vein in the habitat of low rock exposure (LRE), respectively. Scale bars are 50 µm. vb, vascular bundle; uec, upper epidermal cell; mv, meta-xylem vessel; ph, phloem; lec, lower epidermal cell; bc, bulliform cell; ic, irregular cell; c, cuticle; os, outer-bundle sheath; pv, proto-xylem vessel; ac, arm cell; and p, papillae.
Arm cells and irregular cells composed the palisade tissue and spongy tissue, respectively. Arm cells were below the upper epidermis, whereas irregular cells were close to the lower epidermis. The midrib was a complex vascular system composed of multiple vascular bundles, with well-developed sclerenchyma. The outer-bundle sheath of the midrib was composed of 2–3 layers of cells. The vascular bundles of first-order and second-order parallel veins were close to the lower epidermis. Evident meta-xylem vessels existed in the midribs and first-order parallel veins.
3.3. Leaf Anatomical Variations in Three Habitats
In three habitats, the thickness of the upper and lower epidermis was similar (p > 0.05) (Figure 2). Conversely, the cuticle thickness significantly declined with a decrease of habitat rock exposure (p < 0.05). The papillae length of HRE and MRE were 12.52 ± 0.18 µm and 12.08 ± 0.14 µm respectively, which were significantly higher than that of LRE (p < 0.05), with the value of 11.45 ± 0.15 µm.
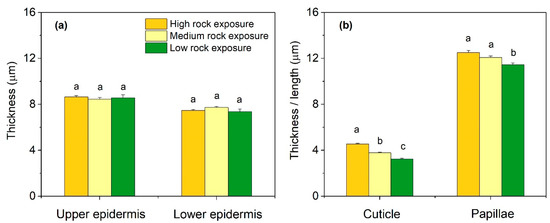
Figure 2.
Leaf epidermal traits of Phyllostachys glauca from three habitats (high rock exposure, medium rock exposure, and low rock exposure) in the limestone mountain. (a), the thickness of upper epidermis and lower epidermis; (b), the thickness of cuticle and the length of papillae. Values are means ± S.E. (n = 100). Lowercase letters that differ within an anatomical trait indicate significant (p < 0.05) differences among different habitats.
The thickness of mesophyll tissue varied in different habitats (Figure 3). With an increase of rock exposure, the thickness of palisade tissue significantly increased (p < 0.05). The thickness of spongy tissue in habitats of HRE and MRE was remarkably higher than that of LRE (p < 0.05). In the three habitats, the leaf of HRE had the greatest bulliform cells, with a mean cross-sectional area of 1986 ± 35.32 µm2.
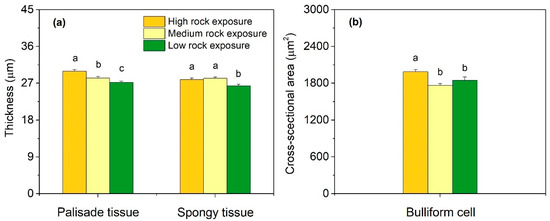
Figure 3.
Mesophyll anatomical traits of Phyllostachys glauca from three habitats (high rock exposure, medium rock exposure, and low rock exposure) in the limestone mountain. (a), the thickness of palisade and spongy tissue; (b), the cross-sectional area of bulliform cell. Values are means ± S.E. (n = 100). Lowercase letters that differ within an anatomical trait indicate significant (p < 0.05) differences among different habitats.
The leaf vessels in HRE were significantly greater in diameter than those in MRE and LRE (p < 0.05), irrespective of vessels from the midrib, first-order parallel vein or second-order parallel vein (Figure 4a). Likewise, the vascular bundle diameter of leaf first-order parallel vein and second-order parallel vein in HRE were greatest in the three habitats. As to the vascular bundle diameter of the midrib, the values of HRE and LRE were significantly higher than that of MRE (p < 0.05) (Figure 4b).
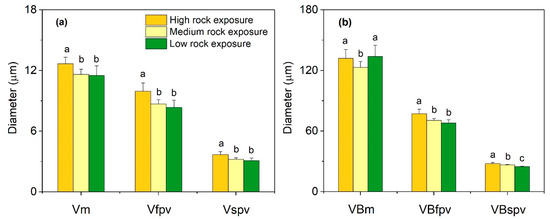
Figure 4.
Vascular anatomical traits of Phyllostachys glauca from three habitats (high rock exposure, medium rock exposure, and low rock exposure) in the limestone mountain. (a), the vessel diameters of different veins; (b), the vascular bundle diameters of different veins. Vm, vessels of midrib; Vfpv, vessels of first-order parallel vein; Vspv, vessels of second-order parallel vein; VBm, vascular bundles of midrib; VBfpv, vascular bundles of first-order parallel vein; and VBspv, vascular bundles of second-order parallel vein. Values are means ± S.E. (n = 100). Lowercase letters that differ within an anatomical trait indicate significant (p < 0.05) differences among different habitats.
3.4. Relationships between Anatomical Traits and Edaphic Factors
The first four axes of RDA explained 53.2% of the total variance of leaf anatomical traits in three habitats. Approximately 47% of the total variance was not explained by edaphic factors, including soil water content (SWC), soil depth, rock exposure, soil total nitrogen (TN), and soil total phosphorus (TP). The first two axes represented the most explained variance (35.43% for the first axis and 9.48% for the second axis), whereas the third and fourth axes only explained 5.59% and 2.70%, respectively. Based on the RDA of the first two axes (Figure 5), enhanced SWC explicitly increased the mean vascular bundle diameter and cross-sectional area of bulliform cell, and remarkably decreased the cuticle thickness, mean vessel diameter, and papillae length. Compared with other leaf anatomical traits, mean vessel diameter, cross-sectional area of bulliform cell, palisade tissue thickness, and epidermis thickness were more closely associated with soil nutrients (TN and TP).
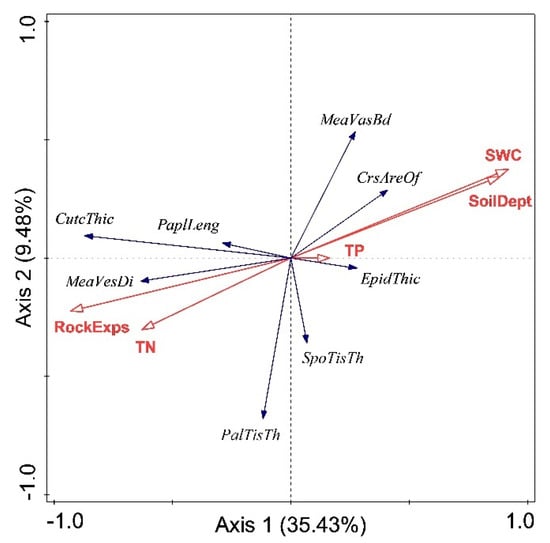
Figure 5.
Redundancy analysis (RDA) of relationships among leaf anatomical traits and edaphic factors for Phyllostachys glauca in the limestone mountain. CutcThic, cuticle thickness; PaplLeng, papillae length; MeavasBd, mean vascular bundle diameter; CrsAreOf, cross-sectional area of bulliform cell; EpidThic, epidermis thickness; SpoTisTh, spongy tissue thickness; PalTisTh, palisade tissue thickness; MeaVesDi, mean vessel diameter; SWC, soil water content; SoilDept, soil depth; TP, soil total phosphorus; TN, soil total nitrogen; and RockExps, rock exposure. Blue lines represent leaf anatomical traits, and red lines represent edaphic factors.
Of those edaphic factors, SWC was positively and significantly correlated with soil depth (r = 0.977, p < 0.001), and negatively and significantly correlated with rock exposure (r = −0.964, p < 0.001). Therefore, SWC, soil depth, and rock exposure were classified as soil water factors, and TN and TP were considered as soil nutrients. According to the variation partitioning results, soil water and soil nutrients purely explained the variation of anatomical traits by 21.7% and 15.7%, respectively, and they shared 15.8% of the total variation.
3.5. Relationships between Anatomical Traits and Plant Biomass
The relationships between thirteen anatomical traits and plant biomass were checked (Table 2). The vessel diameters of first-order and second-order parallel veins closely correlated with plant biomass at the 0.05 significance level (r = 0.761 and r = 0.762, respectively), whereas the vascular bundle diameters of first-order and second-order parallel veins tightly correlated with plant biomass at the 0.01 significance level, with correlation coefficients of 0.842 and 0.900, respectively. Moreover, the correlation between the cross-sectional area of bulliform cell and plant biomass was marginally significant (r = 0.761, p = 0.053). Other anatomical traits did not show significant relationships with plant biomass (p > 0.05).

Table 2.
Correlations between plant biomass and anatomical traits of Phyllostachys glauca in the limestone mountain.
4. Discussion
4.1. The Response of Leaf Anatomical Plasticity to Habitat Heterogeneity
Our first hypothesis that the leaves of Phyllostachys glauca show evident plasticity in anatomical structure in different habitats to adapt to the change in soil water and soil nutrients was supported. In the present study, the leaves in a high rock exposure habitat had significantly higher values than those in low rock exposure habitats in the following anatomical traits: the thickness of cuticle, palisade tissue, spongy tissue, length of papillae, cross-sectional area of bulliform cell, vessel sizes, and vascular bundle sizes of first-order parallel veins and second-order parallel veins (Figure 1, Figure 2, Figure 3 and Figure 4). Increases in the thickness of the cuticle and mesophyll reduce mesophyll water loss, and the long epidermal appendage enhances leaf boundary layer resistance [15,16,18,21]. Those structural adjustments are acknowledged as adaptive features to aridity. HRE was the driest habitat of the three habitats (Table 1), and the changes in the sizes of the cuticle, palisade tissue and papillae to allow the leaves to adapt to water deficit are consistent with those found in previous studies [15,18,21].
Bulliform cells function in curling leaves, which is acknowledged as a protective mechanism against water loss under severe drought stress [18,27,28]. In this work, the size of bulliform cells in HRE was significantly greater than that in MRE and LRE (Figure 3b). Our results are in agreement with the bulliform cell plasticity of Stipa lagascae Roem. & Schult. under drought stress [18]. HRE is a severer water-shortage habitat than MRE and LRE, and the greater bulliform cells in HRE confer a more efficient leaf curling by losing their turgor [27,51,52].
In arid environments, plants tend to reduce their vessel size to avoid wall collapse and cavitation and then acclimate to drought [10,17,20,53]. By contrast, some studies reported that the vessel and vascular bundle size were increased under the additions of N or P, and inferred that the plastic responses were adjusted to enhance hydraulic conductivity and nutrient transportation efficiency [4,19,31,32]. In the present study, the soil water content (SWC) decreased with an increase in rock exposure, whereas TN increased with an increase in rock exposure (Figure 5). From the perspective of arid acclimation, the leaf vessels in HRE were supposed to be smaller than those in MRE and LRE because the SWC in HRE was the lowest in the three habitats. Contrary to expectation, the vessels were enlarged from LRE to MRE, and to HRE (Figure 4). The variation trend of vessels size in the three habitats is in accordance with the anatomical response to increasing soil nutrient contents [4,19,31]. Likewise, the increasing vascular bundle size of first-order and second-order parallel veins was likely a plastic response to the TN increase from LRE to MRE, and to HRE. Furthermore, the positive and strong correlation between the sizes of vessels and vascular bundles and plant biomass implied that the anatomical structures were adjusted to improve water and nutrient transportation and then meet the need for plant growth (Table 2).
4.2. The Effect of Soil Water and Soil Nutrients on Leaf Anatomical Traits
Our second hypothesis that the leaf anatomical variations were chiefly related to soil water rather than soil nutrients because soil water is the most important factor determining plant distribution and growth in karst was partly supported. Although the rainfall is abundant in karst regions of China, the shallow and highly permeable soils cannot afford sufficient water for plant persistent utilizing, and plants in karst habitats are often subject to water deficit [35,54,55]. Thus soil water, the limiting factor for plant growth, plays a vital role in leaf anatomical acclimation [10,20,22]. Consistent with those studies, soil water imposed a remarkable influence on the leaf anatomical traits of Phyllostachys glauca, especially the mean vascular bundle diameter, mean vessel diameter, cross-sectional area of bulliform cell, cuticle thickness and papillae length (Figure 5). According to the results of variation partitioning, soil water occupied the highest proportion of purely explained variance with the value of 21.7%.
Contrary to our expectation, soil nutrients also have a prominent effect on the mean vessel diameter, cross-sectional area of bulliform cell, palisade tissue thickness, and epidermis thickness, and have a purely explained variance of 15.7% plus a part of 15.8% shared with soil water (Figure 5). Our findings are consistent with the previous studies, which confirmed that TN and TP significantly affected the sizes of phloem, vessels, leaf midribs, and their vascular bundles [4,31,32]. On the other hand, the effects of soil water and nutrients on plants are often coupled [56,57,58,59,60]. We also found that the interactions of soil water and nutrients determine the responses of leaf anatomical structure, with a shared effect of 15.8%. Therefore, the effect of soil nutrients on the leaf anatomy of Phyllostachys glauca in limestone mountains was remarkable and significant.
In a recent experiment that combined N and P addition, Cai et al. (2017) found that the leaf and stem anatomical traits of Arabidopsis thaliana were affected by N addition, whereas fewer by P addition, and claimed that the anatomical responses induced by N probably satisfied hydraulic conductivity and plant growth [4]. Similarly, other than TN, TP had a limited effect on the leaf anatomical traits of P. glauca (Figure 5). In our work, the different limestone habitats had similar concentrations of TP (Table 1). Thus, the relatively homogeneous TP might be responsible for a negligible influence on leaf anatomical plasticity.
5. Conclusions
The present study has advanced the current understanding of the strategies employed by karst plants to cope with highly heterogeneous habitats via leaf anatomical plasticity. Our results show that the leaves of Phyllostachys glauca in different limestone habitats exhibited evident anatomical plasticity. The leaves adjusted their cuticle thickness, papillae length, bulliform cell size, and mesophyll thickness to lower water loss and then adapt to the water-deficient habitats. Moreover, the leaves enlarged their vessels and vascular bundles to improve water and nutrient transportation and then enhance plant growth in nitrogen-rich habitats. Although soil water is the primary limiting factor for the growth of karst plants, the leaf anatomical variations in different habitats were associated with both soil water and soil nutrients in our case. Furthermore, we found that TP imposed far less influence on leaf anatomical plasticity than TN. Our findings shed light on an adaptive mechanism of bamboo species to acclimate heterogeneous habitats from the perspective of anatomy.
Author Contributions
Conceptualization J.S., Y.F. and F.Y.; formal analysis H.W., J.S., Z.S., Z.Z., S.H. and Y.C.; investigation Y.F., J.S. and F.Y.; writing—original draft H.W. and J.S.; writing—review and editing H.W., Q.S. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 32060380, 31660198), Forestry Science and Technology Innovation Project of Jiangxi Province (No. 2021-03), and Forestry Science and Technology Extension and Demonstration Project of Central Financial Fund (No. JXTG2022-02).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Klein, T.; Yakir, D.; Buchmann, N.; Grünzweig, J.M. Towards an advanced assessment of the hydrological vulnerability of forests to climate change-induced drought. New Phytol. 2014, 201, 712–716. [Google Scholar] [CrossRef]
- Findell, K.L.; Berg, A.; Gentine, P.; Krasting, J.P.; Lintner, B.R.; Malyshev, S.; Santanello, J.A.; Shevliakova, E. The impact of anthropogenic land use and land cover change on regional climate extremes. Nat. Commun. 2017, 8, 989. [Google Scholar] [CrossRef]
- Cai, Q.; Ji, C.; Yan, Z.; Jiang, X.; Fang, J. Anatomical responses of leaf and stem of Arabidopsis thaliana to nitrogen and phosphorus addition. J. Plant Res. 2017, 130, 1035–1045. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.; Valladares, F. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Fischer, M. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 2005, 166, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Phenotypic plasticity and plant adaptation. Acta Bot. Neerl. 1995, 44, 363–383. [Google Scholar] [CrossRef]
- Dziedek, C.; Fichtner, A.; Calvo, L.; Marcos, E.; Jansen, K.; Kunz, M.; Walmsley, D.; Von Oheimb, G.; Härdtle, W. Phenotypic Plasticity Explains Response Patterns of European Beech (Fagus sylvatica L.) Saplings to Nitrogen Fertilization and Drought Events. Forests 2017, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Roiloa, S.R.; Retuerto, R. Small-scale heterogeneity in soil quality influences photosynthetic efficiency and habitat selection in a clonal plant. Ann. Bot. 2006, 98, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Bresta, P.; Nikolopoulos, D.; Stavroulaki, V.; Vahamidis, P.; Economou, G.; Karabourniotis, G. How does long-term drought acclimation modify structure-function relationships? A quantitative approach to leaf phenotypic plasticity of barley. Funct. Plant Biol. 2018, 45, 1181–1194. [Google Scholar] [CrossRef]
- Baird, A.S.; Anderegg, L.D.L.; Lacey, M.E.; HilleRisLambers, J.; Van Volkenburgh, E. Comparative leaf growth strategies in response to low-water and low-light availability: Variation in leaf physiology underlies variation in leaf mass per area in Populus tremuloides. Tree Physiol. 2017, 37, 1140–1150. [Google Scholar] [CrossRef] [Green Version]
- Fey, S.B.; Kremer, C.T.; Layden, T.J.; Vasseur, D.A. Resolving the consequences of gradual phenotypic plasticity for populations in variable environments. Ecol. Monogr. 2021, 91, e01478. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Lan, Z.; Xu, H.; Liu, W.; Wang, B.; Biswas, D.K.; Jiang, G. Leaf-level plasticity of Salix gordejevii in fixed dunes compared with lowlands in Hunshandake Sandland, North China. J. Plant Res. 2009, 122, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Yu, G.; He, N.; Hou, J. Leaf morphological and anatomical traits from tropical to temperate coniferous forests: Mechanisms and influencing factors. Sci. Rep. 2016, 6, 19703. [Google Scholar] [CrossRef] [Green Version]
- Guan, Z.; Zhang, S.; Guan, K.; Li, S.; Hu, H. Leaf anatomical structures of Paphiopedilum and Cypripedium and their adaptive significance. J. Plant Res. 2011, 124, 289–298. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Cha?Bi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Haffani, S.; Mezni, M.; Nasri, M.B.; Chaibi, W. Comparative leaf water relations and anatomical responses of three vetch species (Vicia narbonensis L., V. sativa L. and V. villosa Roth.) to cope with water stress. Crop Pasture Sci. 2017, 68, 691–702. [Google Scholar] [CrossRef]
- Façal, B.; Raoudha, A.; Zied, H.; Mohammed, N. Anatomical adaptations of the desert species Stipa lagascae against drought stress. Biologia 2015, 70, 1042–1052. [Google Scholar]
- Wang, Y.; Donovan, L.A.; Temme, A.A. Plasticity and the role of mass-scaling in allocation, morphology, and anatomical trait responses to above- and belowground resource limitation in cultivated sunflower (Helianthus annuus L.). Plant Direct 2020, 4, e00274. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X. Some observations of the adaptations of sandy shrubs to the arid environment in the Mu Us Sandland: Leaf water relations and anatomic features. J. Arid Environ. 2001, 48, 41–48. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, G. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Binks, O.; Meir, P.; Rowland, L.; da Costa, A.C.L.; Vasconcelos, S.S.; de Oliveira, A.A.R.; Ferreira, L.; Mencuccini, M. Limited acclimation in leaf anatomy to experimental drought in tropical rainforest trees. Tree Physiol. 2016, 36, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Nawazish, S.; Hameed, M.; Naurin, S. Leaf anatomical adaptations of Cenchrus ciliaris L. from the salt range, Pakistan against drought stress. Pak. J. Bot. 2006, 38, 1723–1730. [Google Scholar]
- Burnett, S.E.; Pennisi, S.V.; Thomas, P.A.; Van Iersel, M.W. Controlled drought affects morphology and anatomy of Salvia splendens. J. Am. Soc. Hortic. Sci. 2005, 130, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, M.; Schneider, B.; Raveh, E.; Tel-Zur, N. Leaf anatomical characteristics and physiological responses to short-term drought in Ziziphus mauritiana (Lamk.). Sci. Hortic. 2010, 124, 316–322. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Patakas, A.; Kofidis, G.; Bosabalidis, A.; Nastou, A. Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Sci. Hortic. 2002, 95, 39–50. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Ecological implications of bulliform cells on halophytes, in salt and water stress natural conditions. Studia Universitatis Vasile Goldis Arad, Seria Stiintele Vietii 2011, 21, 785–792. [Google Scholar]
- Zhang, F.; Zhang, K.; Du, C.; Li, J.; Xing, Y.; Yang, L.; Li, Y. Effect of drought stress on anatomical structure and chloroplast ultrastructure in leaves of sugarcane. Sugar Tech 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Dörken, V.M.; Parsons, R.F. Morpho-anatomical studies on the change in the foliage of two imbricate-leaved New Zealand podocarps: Dacrycarpus dacrydioides and Dacrydium cupressinum. Plant Syst. Evol. 2016, 302, 41–54. [Google Scholar] [CrossRef]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2017, 214, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, Y.; Hoch, G.; Wang, Z.; Gu, J. Linkage of root morphology to anatomy with increasing nitrogen availability in six temperate tree species. Plant Soil 2018, 425, 189–200. [Google Scholar] [CrossRef]
- Hacke, U.G.; Plavcová, L.; Almeida-Rodriguez, A.; King-Jones, S.; Zhou, W.; Cooke, J.E.K. Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol. 2010, 30, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Plavcová, L.; Hacke, U.G. Phenotypic and developmental plasticity of xylem in hybrid poplar saplings subjected to experimental drought, nitrogen fertilization, and shading. J. Exp. Bot. 2012, 63, 6481–6491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Liu, Y.; Guo, K.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Comparative ecophysiological responses to drought of two shrub and four tree species from karst habitats of southwestern China. Trees 2011, 25, 537–549. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, Y.; Guo, K.; Fan, D.; Yu, L.; Yang, R. Exploitation of patchy soil water resources by the clonal vine Ficus tikoua in karst habitats of southwestern China. Acta Physiol. Plant. 2011, 33, 93–102. [Google Scholar]
- Geekiyanage, N.; Goodale, U.M.; Cao, K.; Kitajima, K. Plant ecology of tropical and subtropical Karst ecosystems. Biotropica 2019, 51, 626–640. [Google Scholar] [CrossRef]
- Hofmeister, J.; Mihaljevič, M.; Hošek, J.; Sádlo, J. Eutrophication of deciduous forests in the Bohemian Karst (Czech Republic): The role of nitrogen and phosphorus. For. Ecol. Manag. 2002, 169, 213–230. [Google Scholar] [CrossRef]
- Nardini, A.; Battistuzzo, M.; Savi, T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol. 2013, 200, 322–329. [Google Scholar] [CrossRef]
- Du, Y.; Pan, G.; Li, L.; Hu, Z.; Wang, X. Leaf N/P ratio and nutrient reuse between dominant species and stands: Predicting phosphorus deficiencies in Karst ecosystems, southwestern China. Environ. Earth Sci. 2011, 64, 299–309. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Qiao, X.; Zhao, H.; Wang, S.; Zhang, L.; Cai, X. Effects of nitrogen, phosphorus and potassium addition on the productivity of a karst grassland: Plant functional group and community perspectives. Ecol. Eng. 2018, 117, 84–95. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Wang, S.; Yang, Y. Concentrations and resorption patterns of 13 nutrients in different plant functional types in the karst region of south-western China. Ann. Bot. 2014, 113, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Fan, Y.; Bu, W.; Wang, L.; Shi, J. Phenotypic plasticity of a dominant bamboo species (Phyllostachys glauca) in limestone mountain in northwest of Jiangxi province. Acta Agric. Univ. Jiangxiensis 2017, 39, 1178–1186. (In Chinese) [Google Scholar]
- Shi, J.; Mao, S.; Wang, L.; Ye, X.; Wu, J.; Wang, G.; Chen, F.; Yang, Q. Clonal integration driven by source-sink relationships is constrained by rhizome branching architecture in a running bamboo species (Phyllostachys glauca): A 15N assessment in the field. For. Ecol. Manag. 2021, 481, 118754. [Google Scholar] [CrossRef]
- Chen, S.; Li, D.; Zhu, G.; Wu, Z.; Lu, S.; Liu, L.; Wang, Z.; Sun, B.; Zhu, Z.; Xia, N.; et al. Poaceae (Gramineae). In Flora of China; Wu, C.-Y., Raven, P.H., Hong, D.-Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St Louis, MI, USA, 2006; Volume 22, pp. 1–653. [Google Scholar]
- Xiao, J. Bamboo Forest Management in China; Science Press: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Kang, M.; Tao, J.; Wang, J.; Ren, C.; Qi, Q.; Xiang, Q.-Y.; Huang, H. Adaptive and nonadaptive genome size evolution in Karst endemic flora of China. New Phytol. 2014, 202, 1371–1381. [Google Scholar] [CrossRef]
- Lawless, P.J.; Baskin, J.M.; Baskin, C.C. Scale-dependent classification of xeric limestone prairies: Annual or perennial grasslands? Ann. Mo. Bot. Gard. 2006, 93, 455–464. [Google Scholar] [CrossRef]
- Zhou, Y.; He, X.; Xie, Y.; Wang, M.; Wu, M.; Wu, D. Type Classification for Vegetation Restoration of Karst Mountains in Bijie. Sci. Silvae Sin. 2008, 44, 123–128. (In Chinese) [Google Scholar]
- Gao, H. Study on site classification system of steep cultivated land for quitting in Guizhou Province. Res. Soil Water Conserv. 2003, 10, 76–79. (In Chinese) [Google Scholar]
- Price, A.H.; Young, E.M.; Tomos, A.D. Quantitative trait loci associated with stomatal conductance, leaf rolling and heading date mapped in upland rice (Oryza sativa). New Phytol. 1997, 137, 83–91. [Google Scholar] [CrossRef]
- Balsamo, R.A.; Willigen, C.V.; Bauer, A.M.; Farrant, J. Drought tolerance of selected eragrostis species correlates with leaf tensile properties. Ann. Bot. 2006, 97, 985–991. [Google Scholar] [CrossRef] [Green Version]
- Bresta, P.; Nikolopoulos, D.; Economou, G.; Vahamidis, P.; Lyra, D.; Karamanos, A.; Karabourniotis, G. Modification of water entry (xylem vessels) and water exit (stomata) orchestrates long term drought acclimation of wheat leaves. Plant Soil 2011, 347, 179–193. [Google Scholar] [CrossRef]
- Fan, D.; Jie, S.; Liu, C.; Zhang, X.; Xu, X.; Zhang, S.; Xie, Z. The trade-off between safety and efficiency in hydraulic architecture in 31 woody species in a karst area. Tree Physiol. 2011, 31, 865–877. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Chen, H.; Nie, Y.; Wang, K. Dynamic variations in profile soil water on karst hillslopes in Southwest China. Catena 2019, 172, 655–663. [Google Scholar] [CrossRef]
- Tang, B.; Yin, C.; Yang, H.; Sun, Y.; Liu, Q. The coupling effects of water deficit and nitrogen supply on photosynthesis, WUE, and stable isotope composition in Picea asperata. Acta Physiol. Plant. 2017, 39, 148. [Google Scholar] [CrossRef]
- Lenka, S.; Singh, A.K.; Lenka, N.K. Water and nitrogen interaction on soil profile water extraction and ET in maize–wheat cropping system. Agric. Water Manag. 2009, 96, 195–207. [Google Scholar] [CrossRef]
- Sun, L.; Qi, Y.; Dong, Y.; He, Y.; Peng, Q.; Liu, X.; Jia, J.; Guo, S.; Cao, C. Interactions of water and nitrogen addition on soil microbial community composition and functional diversity depending on the inter-annual precipitation in a Chinese steppe. J. Integr. Agric. 2015, 14, 788–799. [Google Scholar] [CrossRef]
- Lenka, S.; Singh, A.K.; Lenka, N.K. Soil water and nitrogen interaction effect on residual soil nitrate and crop nitrogen recovery under maize–wheat cropping system in the semi-arid region of northern India. Agric. Ecosyst. Environ. 2013, 179, 108–115. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Malhi, S.S. Interactions of nitrogen with other nutrients and water: Effect on crop yield and quality, nutrient use efficiency, carbon sequestration, and environmental pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).