Abstract
Forests regulate air quality and respond to climate change by storing carbon. Assessing the driving factors of forest aboveground carbon (AGC) storage is of great importance for forest management. We assumed that different forest types would affect the relationship between species richness, stand density, individual tree size variation, and AGC. In order to test and verify it, we analyzed the inventory data of 206 fixed plots (20 m × 20 m) of Jingouling Forest Farm, taking advantage of the piecewise structural equation model (pSEM) to explore the effects of species diversity, stand structure attributes, and topography on the AGC storage in the Wangqing Forest in Jilin Province. In addition, in this study, we aimed to investigate whether the fixed factors (species diversity, stand structure attributes, and topography) influenced AGC storage more significantly than the random factor (forest type). According to the results of pSEM, the selected factors jointly explain the impact on 33% of AGC storage. The relationship between stand density and AGC is positive, and the impact of individual tree size variation on AGC storage is negative. Species richness has direct and indirect impacts on AGC storage, and the indirect impact is more significant through individual tree size variation. Both elevation and slope are significantly negatively associated with AGC storage. Forest type explains the impact on 12% of AGC storage, which means the relationship between AGC and predictors varies across forest types. The results provide a scientific basis for the protection and management decision of natural forests in northeastern China.
1. Introduction
The forest is the largest carbon pool on earth, and its importance in the carbon sink is considerable [1]. The aboveground part of the forest ecosystem carbon pool is particularly crucial, which can directly affect the carbon flux between the atmosphere and the forest ecosystem. Therefore, estimating aboveground carbon (AGC) storage accurately assists in mitigating the impact of climate change.
There are numerous driving factors of forest AGC storage, which depend on species diversity [2] as well as forest structure attributes, such as stand density [3] and individual tree size variation. Forest diversity is part of the influencing factors of aboveground biomass and AGC storage and is also affected by environmental factors [4]. The two main hypotheses explaining the positive correlation between species diversity and AGC storage are the niche complementarity hypothesis and mass ratio hypothesis [5,6]. The niche complementarity hypothesis holds that diversity can enhance AGB through maximizing resource utilization efficiency among co-occurring species or individuals through niche complementarity or facilitation. The mass ratio hypothesis predicts that AGB is mainly driven by the traits of dominant plant species [7,8]. Many researchers have considered the relationship between plant species diversity and AGC storage. Some research results are positive, which means the AGC storage of trees will increase with the increase in forest species diversity [9,10,11]. Some researchers believe that they are negatively correlated [12]. Therefore, extensive research is still necessary to explore the relationship between species diversity and AGC storage.
Individual tree size variation also affects the growth and development of forest communities [13,14,15]. Some researchers [16] concluded that the impact of species diversity on aboveground biomass in natural forests is achieved by increasing tree size inequality. Furthermore, individual tree size variation and stand density are extremely crucial to improve canopy filling and stratification. Aboveground biomass would increase by strengthening the interference of light, which can indirectly influence the AGC storage [8,17,18,19].
Abiotic factors also determine the growth of trees, directly altering the AGC storage [20,21]. Topographic factors play key roles in regulating forest structure, diversity, forest carbon storage [8,22,23]. Topography indirectly affects the growth of trees by causing heterogeneity in soil and light [24]. AGC storage varies along different elevation gradients [25], elevation changes the distribution of trees through the change of temperature, which indirectly affects the tree diversity. The slope has a certain impact on the soil, thus affecting the growth and development of trees [26,27,28,29].
Forest productivity and aboveground biomass showed significant differences among forest types in previous studies [30,31], and the difference exists in carbon storage among different forest types [32,33]. Although multiple driving factors affect AGC storage [30,34], few studies have proved whether the influence of these factors will change among different forest types. In general, more research is still required to explore the relationship between predictors and AGC storage in different types of forests.
The main purpose of this study was to examine the effects of diversity factor and stand structure attributes and topographic factors such as elevation and slope on AGC storage in different forest types. In this study, we sought to solve the following questions:
(1) How do topographic factors, diversity factors, and stand structure attributes affect AGC storage? (2) Would the driving factors of carbon storage change in different forest types? (3) Do fixed effects (topography, diversity, and stand structure attributes) contribute more to AGC storage than random effects (forest type)?
2. Materials and Methods
2.1. Site Description
The study area is located in Jingouling Forest Farm of Wangqing Forestry Bureau in Jilin Province (Figure 1), 130°05′–130°20′ E, 43°17′–43°25′ N. It belongs to the Changbai Mountain System in the eastern mountainous area of Jilin Province. The landform belongs to low mountains and hills, with an elevation of 300–1200 m and an average slope of 10°–25°. The region has a temperate continental monsoon climate, with an average annual temperature of 3.9 °C. The month with the lowest temperature in January, with an average minimum temperature of −32 °C. The temperature is the highest in July, with an average maximum temperature of 32 °C; the annual frost-free period is 138 days; the average annual precipitation is 600–700 mm, mainly in July [35]. The main soil type is dark brown soil, with an average thickness of about 40 cm.
Figure 1.
Location map of the study plots in Jingouling Forest Farm.
The main tree species are Fraxinus mandshurica Rupr., Picea jezoensis var. microsperma (Lindl.) Cheng et L. K. Fu, Betula costata Trautv., Tilia amurensis Rupr., Pinus koraiensis Sieb. et Zucc., and Acer pictum subsp. mono (Maxim.) H. Ohashi. The main shrubs are Philadelphus incanus Koehne, Spiraea pubescens Turcz., Acer ukurunduense Trautv. et Mey., Acer tegmentosum Maxim., Lonicera japonica Thunb., and Corylus mandshurica Maxim. The main herbs are Deyeuxia langsdorffii (Link) Kunth, Aegopodium alpestre Ledeb., Urtica fissa E. Pritz. and Oxalis corniculata L.
Permanent sample plots in Jingouling Forest Farm were established in 1987–1988, with the size of 20 m × 20 m; in the tree layer (DBH ≥ 5.0 cm), the tree species name, diameter at breast height (DBH, cm), coordinates (x, y) were recorded. In addition, the elevation, aspect, slope, and other data of the sample plot were also measured and recorded. The aspect 0° represents the due north and increases to 360° clockwise. Since their establishment, the sample plots have been investigated every year.
We treated the inventory data of 2017 as the original data. After eliminating the abnormal data, the data of 206 fixed plots were ultimately utilized in this study.
2.2. Quantification of Variables Used in Analyses
Aboveground carbon storage was the response variable in this study. Based on the DBH of trees in fixed sample plots, we utilized the allometric equations [36,37] (Table A1) to calculate the aboveground biomass of each tree. Then, we multiplied the carbon content of the main tree species [38] in the Changbai Mountain area (Table A2) to obtain the aboveground carbon of each tree, summed the AGC storage of all individual trees in the plots, and converted the values to Mg/ha.
We selected some biotic and abiotic variables to describe their impact on AGC storage, including topography, stand structure attributes, and species diversity. As topography attributes, elevation and slope were used as predictors to test the impact on AGC storage.
In order to characterize the diversity attributes of plants, we used tree species richness to express the species diversity, i.e., the number of species contained in the sample plots.
The stand structure attribute adopted two indices: individual tree size variation and stand density. Individual tree size variation was quantified using the coefficient of variation of DBH, which can be calculated as the ratio of the standard deviation of all DBH to average DBH in each plot [39] (Equation (1)). Stand density was expressed as the number of trees per hectare.
where represents the coefficient of variation of DBH in the sample plot, is the standard deviation of all DBH measurements in the sample plot, and is the average DBH in each plot.
2.3. Statistical Analysis
Here, we regarded forest type as a random effect. In order to find out whether fixed factors (elevation, slope, stand density, species richness, and tree DBH variation) or the random factor (forest type) can explain the change in aboveground carbon storage, we further divided the collected sample plot data into four forest types.
At first, we used the volume table [40] (Table A3) to calculate the volume of trees and then divided the plots into four different types according to the conifer–broadleaf ratio: coniferous forest, coniferous mixed forest, broad-leaved mixed forest, and coniferous and broad-leaved mixed forest. The data of the forest type classification standard are listed in Table 1.

Table 1.
Forest type classification standard.
Multicollinearity affects the ability of explanatory variables to explain and predict response variables. The variance inflation factor (VIF) was used to test the multicollinearity between variables. The results show that the VIFs of the predictors in this study were less than 10, so there was no multicollinearity [41,42].
Piecewise structural equation model (pSEM) was used in this study for model simulation. In addition, in order to reflect the value of pSEM, we used boxplots to evaluate the differences of fixed factors (elevation, slope, stand density, species richness, and tree DBH variation) and AGC storage in different types of forests before pSEM analysis. pSEM [43] extends the traditional SEM, which allowed us to consider the effects of mixed (random and fixed) factors on response variables. For each response variable, pSEM decomposes the complex relationship into corresponding simple or multiple linear mixed-effect regression. Each regression can be evaluated separately and then combined to produce inferences about the entire SEM.
In particular, we tested the following hypotheses (Figure 2): (1) Species richness has a positive direct impact on AGC storage and indirect impact (through tree DBH variation); (2) Tree DBH variation has a direct impact on AGC storage; (3) Stand density has a direct and indirect impact on AGC storage (through its impact on species richness and tree DBH variation); (4) Elevation has a direct and indirect impact on AGC storage (through its impact on species richness); (5) The slope has a direct impact on AGC storage.
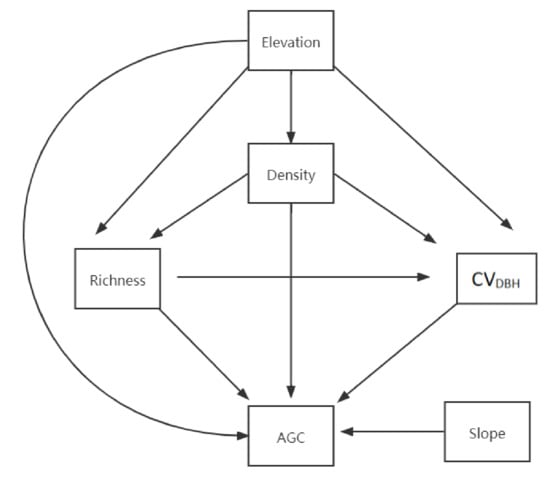
Figure 2.
A conceptual model to assume the relationship between topography, forest structure attributes, species diversity, and aboveground carbon (AGC) storage. represents the coefficient of variation of DBH in the sample plot. AGC represents the aboveground carbon.
We evaluated the overall performance of pSEM by Fisher’s C statistics when the model has Fisher’s C statistics and p > 0.05. pSEM is considered to have a sufficient fit with the data. If the fitting degree did not meet the standard after modeling with pSEM, the least p value in the significant missing path (p < 0.05) could be further incorporated into the model through directional separation, and this process would be repeated until the model fitting degree met the standard, but the supplementary path had to have a theoretical basis. When multiple models passed the Fisher’s C test, the AIC values were compared to select the optimal model. For each dependent variable in pSEM, we calculated the conditional R2 () and the marginal R2 () to calculate the variance explained by fixed () and random factors (). R v 4.0.2 software was used for all analyses. pSEM was employed by using the “piecewiseSEM” package.
3. Results
3.1. General Description
There are 206 plots with 6786 individuals, belonging to 11 families, 14 genera, and 15 species. The average DBH of the sample plot was 19.30 cm. The average AGC storage of forest was 72.44 ± 25.75 Mg/ha. Aboveground carbon storage ranged from 10.85 Mg/ha to 158.21 Mg/ha. In different types of forests, there were differences in the diameter class distribution of trees (Figure 3). The basic information of the sample plots is described in Table 2. The boxplots showing the differences amongst the variables across four types of forests are provided in Figure 4.
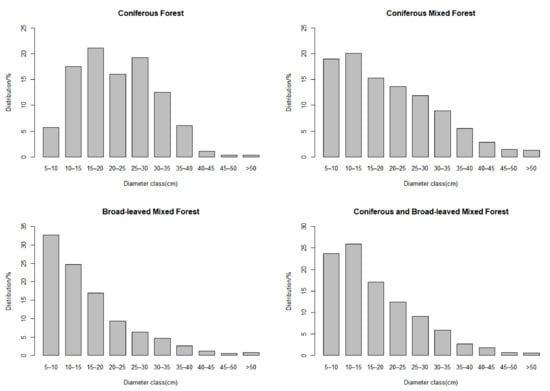
Figure 3.
Distribution of diameter classes in different types of forests.

Table 2.
Summary of the variables across forest types.
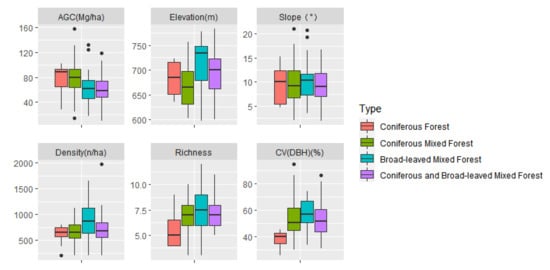
Figure 4.
Boxplots of variables used in different types of forests.
3.2. Effects of Predictors on AGC Storage
The bivariate relationships between AGC and significant variables are described in Figure 5. AGC storage increased with increasing species richness and stand density. However, AGC storage significantly decreased with the increase in elevation and slope. Figure 6 shows a pSEM with the random factor (forest type). Solid and dotted lines indicate significant and non-significant paths. The numbers of close to the variables represent the variance explained by the fixed factors and all factors in the model, respectively. We found that pSEM explained the marginal and random changes of 21% and 12% of aboveground carbon storage, respectively. The difference in AGC growth was partially dependent on forest types. AGC storage increased with the increase in stand density (= 0.21) and species richness (= 0.16). AGC storage decreased with the increase in elevation (=0.17), slope (=0.28), and DBH variation (=0.15). Stand density promoted the increase in species richness and inhibited the variation in DBH. Stand density and the variation in DBH mainly depended on the random effect (i.e., forest type), whereas species richness mainly depended on fixed effects.
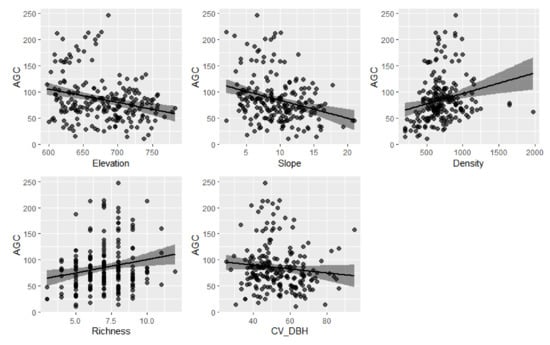
Figure 5.
Bivariate relationships between aboveground carbon (AGC) storage and five predictors (elevation, slope, density, richness, CVDBH).
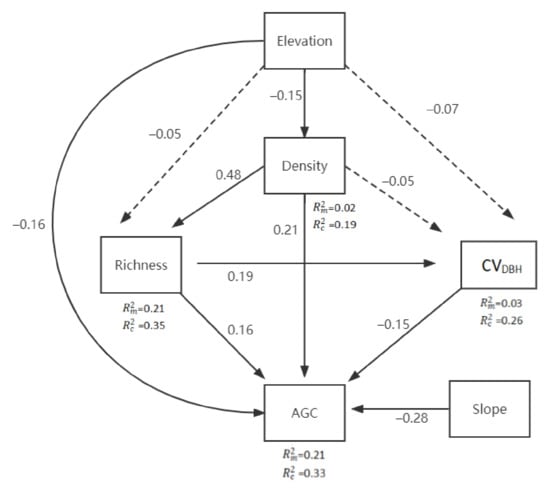
Figure 6.
The proposed piecewise structural equation model (pSEM) for testing the influences of predictors on aboveground carbon storage. Fisher’s C = 6.142, p = 0.407, AIC = 52.142; represents the coefficient of variation of DBH in the sample plot. AGC represents the aboveground carbon. Density represents stand density.
In terms of the relative contribution of predictors, we found that stand density, CVDBH, and species richness accounted for more than 55% of total variations for AGC. The direct effects of elevation, density, and species richness were larger than the indirect effects. Slope and CVDBH only had direct effects on AGC storage (Figure 7).
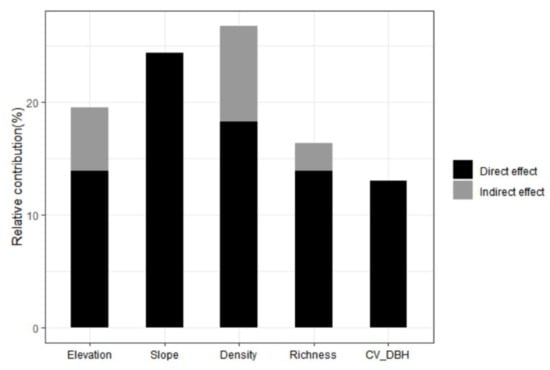
Figure 7.
Relative contributions of multiple predictors to aboveground carbon (AGC) storage.
4. Discussion
This study shows that the AGC storage in different forest types is mainly caused by fixed factors (topography, forest structure attributes, species richness), with topography as the main reason explaining the AGC change.
Topography had a direct effect on AGC storage. Specifically, differences in topographic factors may result in microenvironment heterogeneity of resource availability (light, water, and soil nutrients) [24]. Higher elevation gradients receive lower temperature and soil moisture content [44]. Therefore, elevation had a negative impact on AGC storage, resulting in lower AGC storage. The slope had a negative impact on AGC storage, which is because slope plays an important role in determining soil properties, having a negative impact on forest growth by changing soil moisture change and soil erosion [45]. In addition, topography can indirectly affect AGC storage through stand density, which is consistent with previous studies [46]. We also assumed that elevation indirectly affected AGC storage by affecting species diversity and individual tree size variation. However, the results show that the relationship between elevation and diversity was not significant, and the relationship between elevation and diversity could not be described as a linear relationship but a complicated, nonlinear relationship caused by multiple factors.
The results of pSEM in this study show that species diversity had a strong, positive impact on AGC storage, which is consistent with previous studies [9,47,48,49]. This can be explained by the niche complementarity hypothesis [50] (i.e., a higher diversity allows a community to access a larger fraction of the total resource pool). Species diversity had a direct impact on AGC storage, and species diversity can also indirectly increase AGC storage through multiple factors such as stem density [19] and individual tree size variation [51]. We assumed that individual tree size variation positively affected AGC storage. However, contrary to our expectations, the results show that individual tree size variation had a strong negative effect on AGC storage. This may be due to the fact that the degree to which individual trees are affected by competition depends on their own and adjacent individual size, quantity, characteristics of utilized resources, and spatial influence area [52]. Changing the individual tree size variation will have a certain impact on competition and will then influence biomass accumulation. With the asymmetric competition mechanism, larger trees monopolize light resources more than proportionally to their size; therefore, the intraspecific and interspecific competition for the limited resources in forests may influence individual tree size variation—large-sized trees may eliminate small-sized trees, which consequently decreases AGB [53].
In this study, stand density promoted AGC storage. High stand density can optimize resources utilization through canopy packing, finally leading to higher AGB [54]. We also found a positive correlation between stand density and species richness, which supports the species energy hypothesis, indicating that higher energy availability supports higher species richness, resulting in higher AGB [5,6,55]. What supports this finding is that the negative effect of elevation on stand density is also derived from the species energy hypothesis.
As for the random effect (forest type), AGC storage varied among different forest types. The study forests are uneven-aged forest stands, and it has been reported that faster-growing forest stands may be dominated by species with shorter lifespans [56] and consequently lower AGC. On the other hand, the differences in productivity of different forest types may reflect the feedback effect of plant species composition [57]; some studies found that even minor changes in species composition can result in variation in forest biomass and carbon density among different forest types [58]. In addition, natural disturbances such as storms [59] and insect outbreaks [60] might diminish AGC storage; therefore, an increase in AGC storage can be achieved through sustainable forest management.
Moreover, it is important to note that we did not measure wood density directly, which is one of the variables modeling stand-level carbon storage [61]. Future studies should further investigate the performance of the above factors on AGC storage spanning different forest types.
5. Conclusions
In this study, the relationship between various variables and aboveground carbon storage in different types of the forest was described by using pSEM. We found that AGC is directly affected by the changes in topography, species diversity, and forest structure attributes. Among them, topography was the most important driving factor affecting AGC storage, and stand density and species richness were positively related to AGC storage, while CVDBH was negatively related to AGC storage. Forest AGC storage varied among different forest types, which is in agreement with previous studies. In addition, consistent with the species energy hypothesis, we also found a positive correlation between stand density and species richness, indicating that higher energy availability supports stands with higher species richness. We should give more consideration to improving biodiversity, contributing to species renewal, and forest management in the future.
Author Contributions
Conceptualization, X.W.; project administration, W.G.; methodology, B.J. and J.H.; validation, B.J. and X.W.; formal analysis, B.J. and X.W.; investigation, B.J., L.C., and J.L.; data curation, B.J. and M.S.; writing—original draft preparation, B.J.; writing—review and editing, B.J. and W.G.; visualization, B.J. supervision, W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Plan (Grant No. 2017YFC050410101).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We sincerely thank Jingyuan He for his valuable comments on the manuscript. We also thank Lei Chai and Minggang Sun for their assistance in the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Allometric equations for the calculation of aboveground biomass for each tree species.
Table A1.
Allometric equations for the calculation of aboveground biomass for each tree species.
| Species | Equation | References |
|---|---|---|
| Abies holophylla | agb = 1000 × 0.0737 × (dbh)2.51264 | Chen and Zhu 1989 |
| Abies nephrolepis | agb = 1000 × 0.0737 × (dbh)2.51264 | Chen and Zhu 1989 |
| Acer pictum subsp. mono | agb = 10(1.930+2.535×log10(dbh)) | Wang et al. 2006 |
| Betula platyphylla | agb = 10(2.159+2.367×log10(dbh)) | Wang et al. 2006 |
| Betula costata | agb = 10(2.214+2.400×log10(dbh)) | Wang et al. 2006 |
| Carpinus cordata | agb = 1000 × 0.09802 × (dbh)2.2993 | Chen and Zhu 1989 |
| Fraxinus chinensis subsp. rhynchophylla | agb = 10(2.213+2.417×log10(dbh)) | Wang et al. 2006 |
| Fraxinus mandshurica | agb = 10(2.216+2.408×log10(dbh)) | Wang et al. 2006 |
| Juglans mandshurica | agb = 10(2.235+2.287×log10(dbh)) | Wang et al. 2006 |
| Larix gmelinii | agb = 10(1.997+2.451×log10(dbh)) | Wang et al. 2006 |
| Maackia amurensis | agb = 1000 × 0.0737 × (dbh)2.51264 | Chen and Zhu 1989 |
| Phellodendron amurense | agb = 10(1.942+2.232×log10(dbh)) | Wang et al. 2006 |
| Picea jezoensis var. komarovii | agb = 1000 × 0.0744 × (dbh)2.5411 | Chen and Zhu 1989 |
| Picea koraiensis | agb = 1000 × 0.0744 × (dbh)2.5411 | Chen and Zhu 1989 |
| Pinus koraiensis | agb = 10 (2.236+2.144×log10(dbh)) | Wang et al. 2006 |
| Populus davidiana | agb = 10(1.826+2.558×log10(dbh)) | Wang et al. 2006 |
| Quercus mongolica | agb = 10(2.002+2.456×log10(dbh)) | Wang et al. 2006 |
| Taxus cuspidata | agb = 1000 × 0.0737×(dbh)2.51264 | Chen and Zhu 1989 |
| Tilia amurensis | agb = 10(1.606+2.668×log10(dbh)) | Wang et al. 2006 |
| Tilia mandshurica | agb = 10(1.606+2.668×log10(dbh)) | Wang et al.2006 |
| Ulmus davidiana | agb = 1000 × 0.09802 × (dbh)2.2993 | Chen and Zhu 1989 |
| Ulmus laciniata | agb = 1000 × 0.09802 × (dbh)2.2993 | Chen and Zhu 1989 |
| Ulmus macrocarpa | agb = 1000 × 0.09802 × (dbh)2.2993 | Chen and Zhu 1989 |
| Other species | agb = 10(1.826+2.558×log10(dbh)) | Chen and Zhu 1989 |
All allometric equations of the dominant species in our study were obtained from published references. For the missing species, we used the value of the same genera from (1) Chen, C.; Zhu, J. Biomass Manual of Main Trees in Northeastern China. China Forestry Press, Beijing, China, 1989. and (2) Wang, C. Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For. Ecol. Manag. 2006, 222, 9–16.

Table A2.
Carbon content for each tree species in Changbai Mountain forest area.
Table A2.
Carbon content for each tree species in Changbai Mountain forest area.
| Species | Carbon Content |
|---|---|
| Acer pictum subsp. mono | 0.4456 |
| Populus davidiana | 0.4877 |
| Tilia amurensis | 0.4944 |
| Ulmus davidiana | 0.3859 |
| Betula costata | 0.4976 |
| Betula platyphylla | 0.4508 |
| Quercus mongolica | 0.4840 |
| Fraxinus mandshurica | 0.4537 |
| Maackia amurensis | 0.4553 |
| Pinus koraiensis | 0.5339 |
| Larix gmelinii | 0.5079 |
| Other coniferous tree species | 0.5166 |
| Other broad-leaved tree species | 0.4609 |

Table A3.
Volume equations for each tree species. Volume: ; height: .
Table A3.
Volume equations for each tree species. Volume: ; height: .
| Species | Parameters | |||||
|---|---|---|---|---|---|---|
| a | b | c | h1 | h2 | h3 | |
| Larix olgensis | 8.47 × 10−5 | 1.97 | 0.75 | 34.59 | 650.53 | 18.0 |
| Pinus koraiensis | 7.62 × 10−5 | 1.90 | 0.86 | 21.84 | 309.16 | 14.0 |
| Abies nephrolepis | 5.79 × 10−5 | 1.89 | 0.99 | 46.40 | 2137.92 | 47.0 |
| Picea jezoensis var. komarovii | 5.79 × 10−5 | 1.89 | 0.99 | 46.40 | 2137.92 | 47.0 |
| Acer pictum subsp. mono | 4.88 × 10−5 | 1.84 | 1.05 | 24.82 | 402.09 | 16.3 |
| Fraxinus mandshurica | 5.33 × 10−5 | 1.88 | 1.00 | 29.44 | 468.93 | 15.7 |
| Phellodendron amurense | 5.33 × 10−5 | 1.88 | 1.00 | 29.44 | 468.93 | 15.7 |
| Betula platyphylla | 5.33 × 10−5 | 1.88 | 1.00 | 29.44 | 468.93 | 15.7 |
| Tilia amurensis | 5.33 × 10−5 | 1.88 | 1.00 | 29.44 | 468.93 | 15.7 |
| Betula costata | 5.33 × 10−5 | 1.88 | 1.00 | 29.44 | 468.93 | 15.7 |
| Populus ussuriensis | 5.33 × 10−5 | 1.88 | 1.00 | 29.44 | 468.93 | 15.7 |
| Ulmus pumila | 5.33 × 10−5 | 1.88 | 1.00 | 29.44 | 468.93 | 15.7 |
| Ulmus davidiana | 5.33 × 10−5 | 1.88 | 1.00 | 29.44 | 468.93 | 15.7 |
| Taxus cuspidata | 7.62 × 10−5 | 1.9 | 0.86 | 21.84 | 309.16 | 14 |
| Abies fabri | 7.62 × 10−5 | 1.9 | 0.86 | 21.84 | 309.16 | 14 |
| Quercus mongolica | 5.33 × 10−5 | 1.88 | 1 | 29.44 | 468.93 | 15.7 |
| Populus davidiana | 5.33 × 10−5 | 1.88 | 1 | 29.44 | 468.93 | 15.7 |
| Other coniferous tree species | 7.62 × 10−5 | 1.9 | 0.86 | 21.84 | 309.16 | 14 |
| Other broad-leaved tree species | 5.33 × 10−5 | 1.88 | 1 | 29.44 | 468.93 | 15.7 |
References
- Dixon, R.K.; Solomon, A.; Brown, S.; Houghton, R.; Trexier, M.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Tetemke, B.A.; Birhane, E.; Rannestad, M.M.; Eid, T. Species diversity and stand structural diversity of woody plants predominantly determine aboveground carbon stock of a dry Afromontane forest in Northern Ethiopia. For. Ecol. Manag. 2021, 500, 119634. [Google Scholar] [CrossRef]
- Khan, M.N.I.; Shil, M.C.; Azad, M.S.; Sadath, M.N.; Feroz, S.; Mollick, A.S. Allometric relationships of stem volume and stand level carbon stocks at varying stand density in Swietenia macrophylla King plantations, Bangladesh. For. Ecol. Manag. 2018, 430, 639–648. [Google Scholar] [CrossRef]
- Fortuny, X.; Chauchard, S.; Carcaillet, C. Confounding legacies of land uses and land-form pattern on the regional vegetation structure and diversity of Mediterranean montane forests. For. Ecol. Manag. 2017, 384, 268–278. [Google Scholar] [CrossRef]
- Wright, D.H. Species-energy theory: An extension of species-area theory. Oikos 1983, 41, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Yan, E.-R. Relationships between biodiversity and carbon stocks in forest ecosystems: A systematic literature review. Trop. Ecol. 2017, 58, 1–14. [Google Scholar]
- Chun, J.-H.; Ali, A.; Lee, C.-B. Topography and forest diversity facets regulate overstory and understory aboveground biomass in a temperate forest of South Korea. Sci. Total Environ. 2020, 744, 140783. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Mensah, S.; Veldtman, R.; Assogbadjo, A.E.; Glèlè Kakaï, R.; Seifert, T. Tree species diversity promotes aboveground carbon storage through functional diversity and functional dominance. Ecol. Evol. 2016, 6, 7546–7557. [Google Scholar] [CrossRef]
- Amara, E.; Heiskanen, J.; Aynekulu, E.; Pellikka, P.K. Relationship between carbon stocks and tree species diversity in a humid Guinean savanna landscape in northern Sierra Leone. South. For. J. For. Sci. 2019, 81, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.M.; Baduni, N.P.; Gairola, S.; Ghildiyal, S.K.; Suyal, S. Tree diversity and carbon stocks of some major forest types of Garhwal Himalaya, India. For. Ecol. Manag. 2010, 260, 2170–2179. [Google Scholar] [CrossRef]
- Merlin, M.; Perot, T.; Perret, S.; Korboulewsky, N.; Vallet, P. Effects of stand composition and tree size on resistance and resilience to drought in sessile oak and Scots pine. For. Ecol. Manag. 2015, 339, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.R.; Finley, A.O.; D’Amato, A.W.; Bradford, J.B.; Banerjee, S. Predicting tree biomass growth in the temperate–boreal ecotone: Is tree size, age, competition, or climate response most important? Glob. Chang. Biol. 2016, 22, 2138–2151. [Google Scholar] [CrossRef]
- De Groote, S.R.; Vanhellemont, M.; Baeten, L.; Van den Bulcke, J.; Martel, A.; Bonte, D.; Lens, L.; Verheyen, K. Competition, tree age and size drive the productivity of mixed forests of pedunculate oak, beech and red oak. For. Ecol. Manag. 2018, 430, 609–617. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y. Individual size inequality links forest diversity and above-ground biomass. J. Ecol. 2015, 103, 1245–1252. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Does complementary resource use enhance ecosystem functioning? A model of light competition in plant communities. Ecol. Lett. 2007, 10, 54–62. [Google Scholar] [CrossRef]
- Morin, X. Species richness promotes canopy packing: A promising step towards a better understanding of the mechanisms driving the diversity effects on forest functioning. Funct. Ecol. 2015, 29, 993–994. [Google Scholar] [CrossRef] [Green Version]
- Fotis, A.T.; Murphy, S.J.; Ricart, R.D.; Krishnadas, M.; Whitacre, J.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Above-ground biomass is driven by mass-ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol. 2018, 106, 561–570. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 2017, 7, 39102. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bao, W.; Bongers, F.; Chen, B.; Chen, G.; Guo, K.; Jiang, M.; Lai, J.; Lin, D.; Liu, C. Drivers of tree carbon storage in subtropical forests. Sci. Total Environ. 2019, 654, 684–693. [Google Scholar] [CrossRef]
- Scholten, T.; Goebes, P.; Kühn, P.; Seitz, S.; Assmann, T.; Bauhus, J.; Bruelheide, H.; Buscot, F.; Erfmeier, A.; Fischer, M. On the combined effect of soil fertility and topography on tree growth in subtropical forest ecosystems—A study from SE China. J. Plant Ecol. 2017, 10, 111–127. [Google Scholar] [CrossRef]
- Zekeng, J.C.; van der Sande, M.T.; Fobane, J.L.; Sebego, R.; Mphinyane, W.N.; Ebanga, P.A.; Mbolo, M. Environmental, structural and taxonomic diversity factors drive aboveground carbon stocks in a semi-deciduous tropical rainforest strata in Cameroon. bioRxiv 2021. [Google Scholar] [CrossRef]
- Boerner, R.J. Unraveling the Gordian Knot: Interactions among vegetation, topography, and soil properties in the central and southern Appalachians. J. Torrey Bot. Soc. 2006, 133, 321–361. [Google Scholar] [CrossRef]
- Liu, N.; Nan, H. Carbon stocks of three secondary coniferous forests along an altitudinal gradient on Loess Plateau in inland China. PLoS ONE 2018, 13, e0196927. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Hayat, Z.; Ahmad, W.; Ramzan, M.; Shah, Z.; Sharif, M.; Mian, I.A.; Hanif, M. Effect of slope position on physico-chemical properties of eroded soil. Soil Environ. 2013, 32, 22–28. [Google Scholar]
- Liu, L.; Zeng, F.; Song, T.; Wang, K.; Du, H. Stand structure and abiotic factors modulate karst forest biomass in Southwest China. Forests 2020, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Pan, Y.; Bao, H.; Liang, S.; Jiang, Y.; Tu, H.; Nong, J.; Huang, W. Variations in Soil Physico-Chemical Properties along Slope Position Gradient in Secondary Vegetation of the Hilly Region, Guilin, Southwest China. Sustainability 2020, 12, 1303. [Google Scholar] [CrossRef] [Green Version]
- Wubie, M.A.; Assen, M. Effects of land cover changes and slope gradient on soil quality in the Gumara watershed, Lake Tana basin of North–West Ethiopia. Model. Earth Syst. Environ. 2020, 6, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Cuni-Sanchez, A.; Pfeifer, M.; Marchant, R.; Calders, K.; Sørensen, C.L.; Pompeu, P.V.; Lewis, S.L.; Burgess, N.D. New insights on above ground biomass and forest attributes in tropical montane forests. For. Ecol. Manag. 2017, 399, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, S.; Baishya, R. Stand structure and species diversity regulate biomass carbon stock under major Central Himalayan forest types of India. Ecol. Processes 2021, 10, 14. [Google Scholar] [CrossRef]
- Dimobe, K.; Kuyah, S.; Dabré, Z.; Ouédraogo, A.; Thiombiano, A. Diversity-carbon stock relationship across vegetation types in W National park in Burkina Faso. For. Ecol. Manag. 2019, 438, 243–254. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.-R.; Chen, H.Y.; Chang, S.X.; Zhao, Y.-T.; Yang, X.-D.; Xu, M.-S. Stand structural diversity rather than species diversity enhances aboveground carbon storage in secondary subtropical forests in Eastern China. Biogeosciences 2016, 13, 4627–4635. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.-C.; Zheng, X.-X. Forest Health Assessment in the Jingouling Forest Farm of Changbai Mountain Based on GIS and RS. Adv. J. Food Sci. Technol. 2014, 6, 408–412. [Google Scholar]
- Chen, C.; Zhu, J. Biomass Manual of Main Trees in Northeastern China; China Forestry Press: Beijing, China, 1989. [Google Scholar]
- Wang, C. Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For. Ecol. Manag. 2006, 222, 9–16. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, L.; Fan, C.; Zheng, J.; Guo, Z. On carbon sequestration rates and differences of main tree species in Changbai Mountain forest area. J. Beihua Univ. 2018, 19, 164–169. [Google Scholar]
- Chu, C.J.; Weiner, J.; Maestre, F.T.; Xiao, S.; Wang, Y.S.; Li, Q.; Yuan, J.L.; Zhao, L.Q.; Ren, Z.W.; Wang, G. Positive interactions can increase size inequality in plant populations. J. Ecol. 2009, 97, 1401–1407. [Google Scholar] [CrossRef]
- Moreau, G.; Auty, D.; Pothier, D.; Shi, J.; Lu, J.; Achim, A.; Xiang, W. Long-term tree and stand growth dynamics after thinning of various intensities in a temperate mixed forest. For. Ecol. Manag. 2020, 473, 118311. [Google Scholar] [CrossRef]
- Fox, J.; Monette, G. Generalized collinearity diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Fox, J. Applied Regression Analysis and Generalized Linear Models; Sage Publications: Thousand Oaks, CA, USA, 2015. [Google Scholar]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Galicia, L.; López-Blanco, J.; Zarco-Arista, A.; Filips, V.; Garcıa-Oliva, F. The relationship between solar radiation interception and soil water content in a tropical deciduous forest in Mexico. Catena 1999, 36, 153–164. [Google Scholar] [CrossRef]
- Vayreda, J.; Gracia, M.; Canadell, J.G.; Retana, J. Spatial patterns and predictors of forest carbon stocks in Western Mediterranean. Ecosystems 2012, 15, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Ullah, F.; Gilani, H.; Sanaei, A.; Hussain, K.; Ali, A. Stand structure determines aboveground biomass across temperate forest types and species mixture along a local-scale elevational gradient. For. Ecol. Manag. 2021, 486, 118984. [Google Scholar] [CrossRef]
- Ruiz-Benito, P.; Gómez-Aparicio, L.; Paquette, A.; Messier, C.; Kattge, J.; Zavala, M.A. Diversity increases carbon storage and tree productivity in S panish forests. Glob. Ecol. Biogeogr. 2014, 23, 311–322. [Google Scholar] [CrossRef]
- Mensah, S.; Noulekoun, F.; Ago, E.E. Aboveground tree carbon stocks in West African semi-arid ecosystems: Dominance patterns, size class allocation and structural drivers. Glob. Ecol. Conserv. 2020, 24, e01331. [Google Scholar] [CrossRef]
- Mensah, S.; Salako, V.K.; Seifert, T. Structural complexity and large-sized trees explain shifting species richness and carbon relationship across vegetation types. Funct. Ecol. 2020, 34, 1731–1745. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [Green Version]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity—productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef] [Green Version]
- Weiner, J. Neighbourhood interference amongst Pinus rigida individuals. J. Ecol. 1984, 72, 183–195. [Google Scholar] [CrossRef]
- Bourdier, T.; Cordonnier, T.; Kunstler, G.; Piedallu, C.; Lagarrigues, G.; Courbaud, B. Tree size inequality reduces forest productivity: An analysis combining inventory data for ten European species and a light competition model. PLoS ONE 2016, 11, e0151852. [Google Scholar] [CrossRef]
- Jucker, T.; Bouriaud, O.; Coomes, D.A. Crown plasticity enables trees to optimize canopy packing in mixed-species forests. Funct. Ecol. 2015, 29, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.; Lutz, J.A.; Král, K.; Vrška, T.; Yin, X.; Myers, J.A.; Abiem, I.; Alonso, A.; Bourg, N.; Burslem, D.F. Direct and indirect effects of climate on richness drive the latitudinal diversity gradient in forest trees. Ecol. Lett. 2019, 22, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Schall, P.; Schulze, E.-D.; Fischer, M.; Ayasse, M.; Ammer, C. Relations between forest management, stand structure and productivity across different types of Central European forests. Basic Appl. Ecol. 2018, 32, 39–52. [Google Scholar] [CrossRef]
- Reich, P.B.; Bakken, P.; Carlson, D.; Frelich, L.E.; Friedman, S.K.; Grigal, D.F. Influence of logging, fire, and forest type on biodiversity and productivity in southern boreal forests. Ecology 2001, 82, 2731–2748. [Google Scholar] [CrossRef]
- Phillips, O.L.; Sullivan, M.J.; Baker, T.R.; Monteagudo Mendoza, A.; Vargas, P.N.; Vásquez, R. Species matter: Wood density influences tropical forest biomass at multiple scales. Surv. Geophys. 2019, 40, 913–935. [Google Scholar] [CrossRef] [Green Version]
- Bradford, J.B.; Fraver, S.; Milo, A.M.; D’Amato, A.W.; Palik, B.; Shinneman, D.J. Effects of multiple interacting disturbances and salvage logging on forest carbon stocks. For. Ecol. Manag. 2012, 267, 209–214. [Google Scholar] [CrossRef]
- Russell, M.B.; D’Amato, A.W.; Albers, M.A.; Woodall, C.W.; Puettmann, K.J.; Saunders, M.R.; VanderSchaaf, C.L. Performance of the Forest Vegetation Simulator in managed white spruce plantations influenced by eastern spruce budworm in northern Minnesota. For. Sci. 2015, 61, 723–730. [Google Scholar] [CrossRef]
- Khan, M.N.I.; Islam, M.R.; Rahman, A.; Azad, M.S.; Mollick, A.S.; Kamruzzaman, M.; Sadath, M.N.; Feroz, S.; Rakkibu, M.G.; Knohl, A. Allometric relationships of stand level carbon stocks to basal area, tree height and wood density of nine tree species in Bangladesh. Glob. Ecol. Conserv. 2020, 22, e01025. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).