Utilization of Glycine by Microorganisms along the Altitude Changbai Mountain, China: An Uptake Test Using 13C,15N Labeling and 13C-PLFA Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Collection
2.2. Soil Chemical Analysis
2.3. Gross N Mineralization
2.4. Different Form of 15N Glycine Uptake
2.5. 13C-PLFA Analysis
2.6. Statistical Analysis
3. Results
3.1. Glycine-Addition Effects: Gross N Mineralization
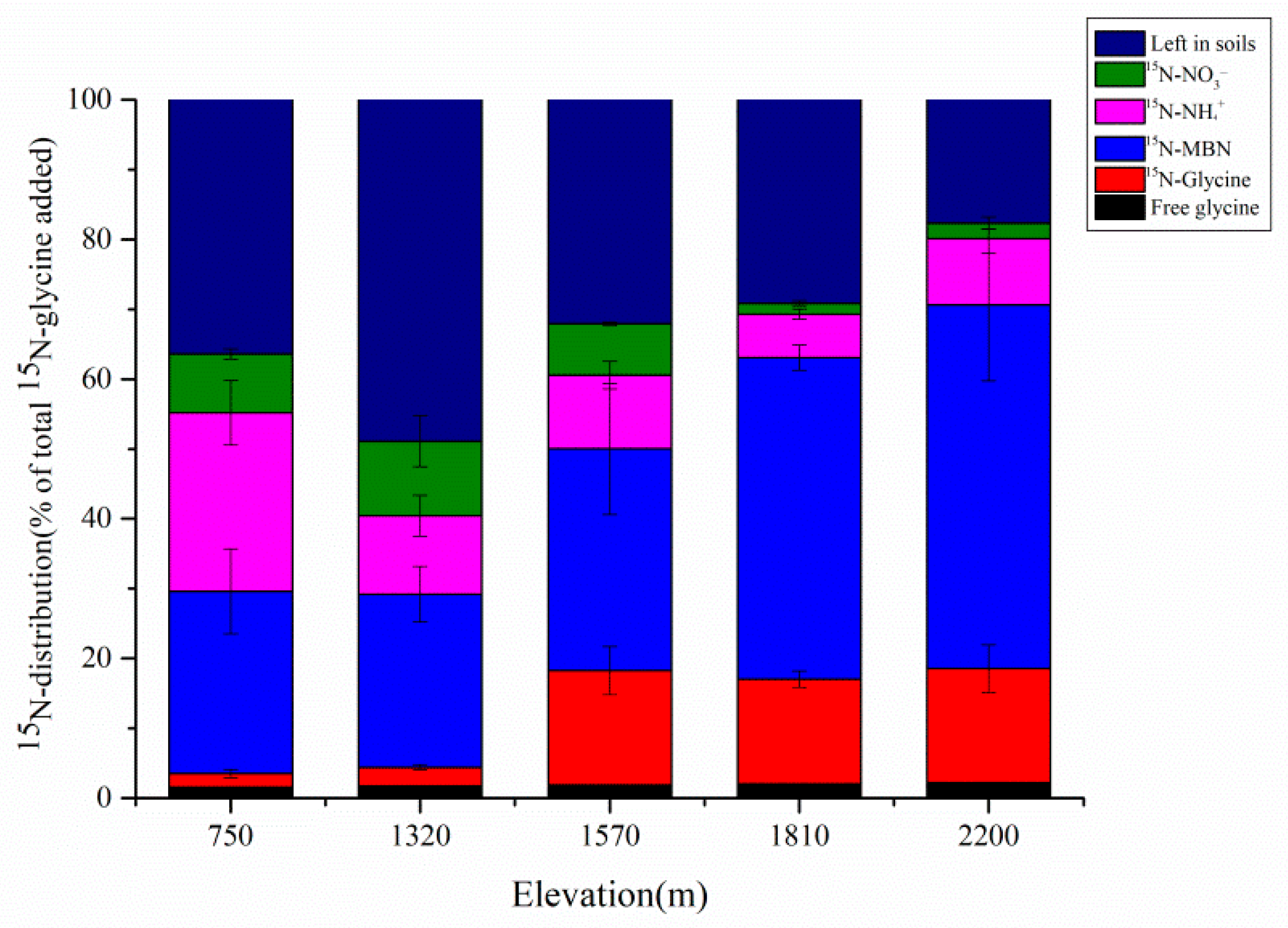
3.2. 15N-Distribution in Soils and Microorganisms
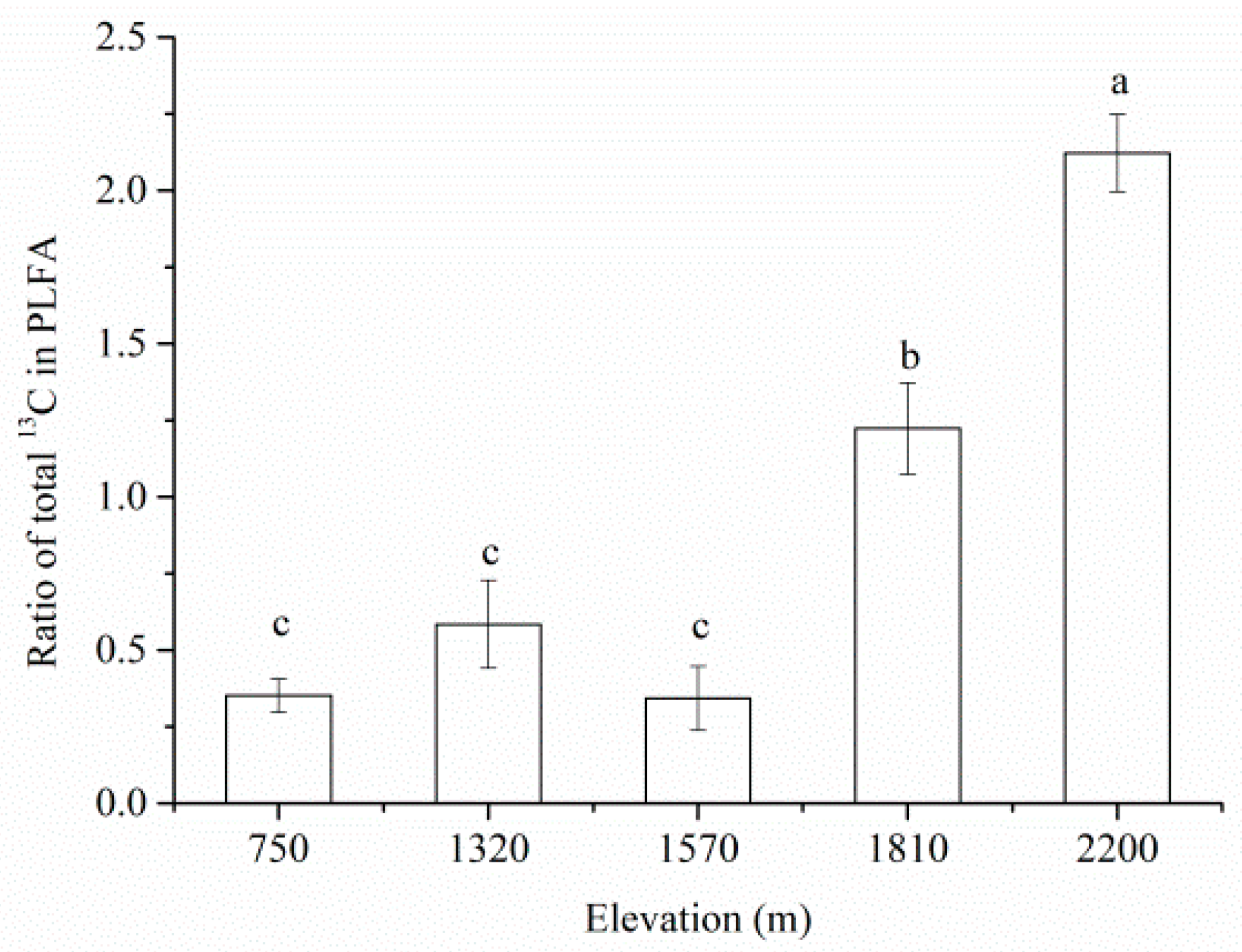
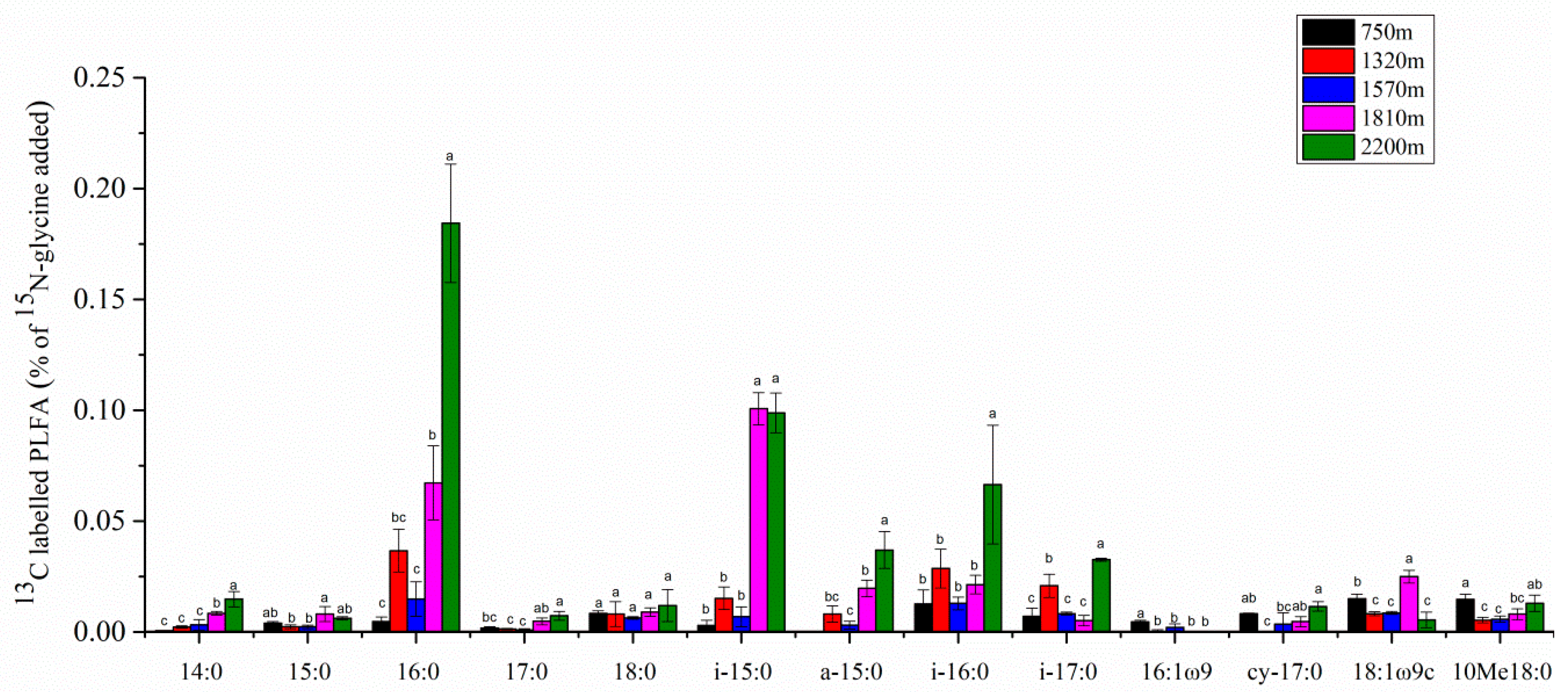
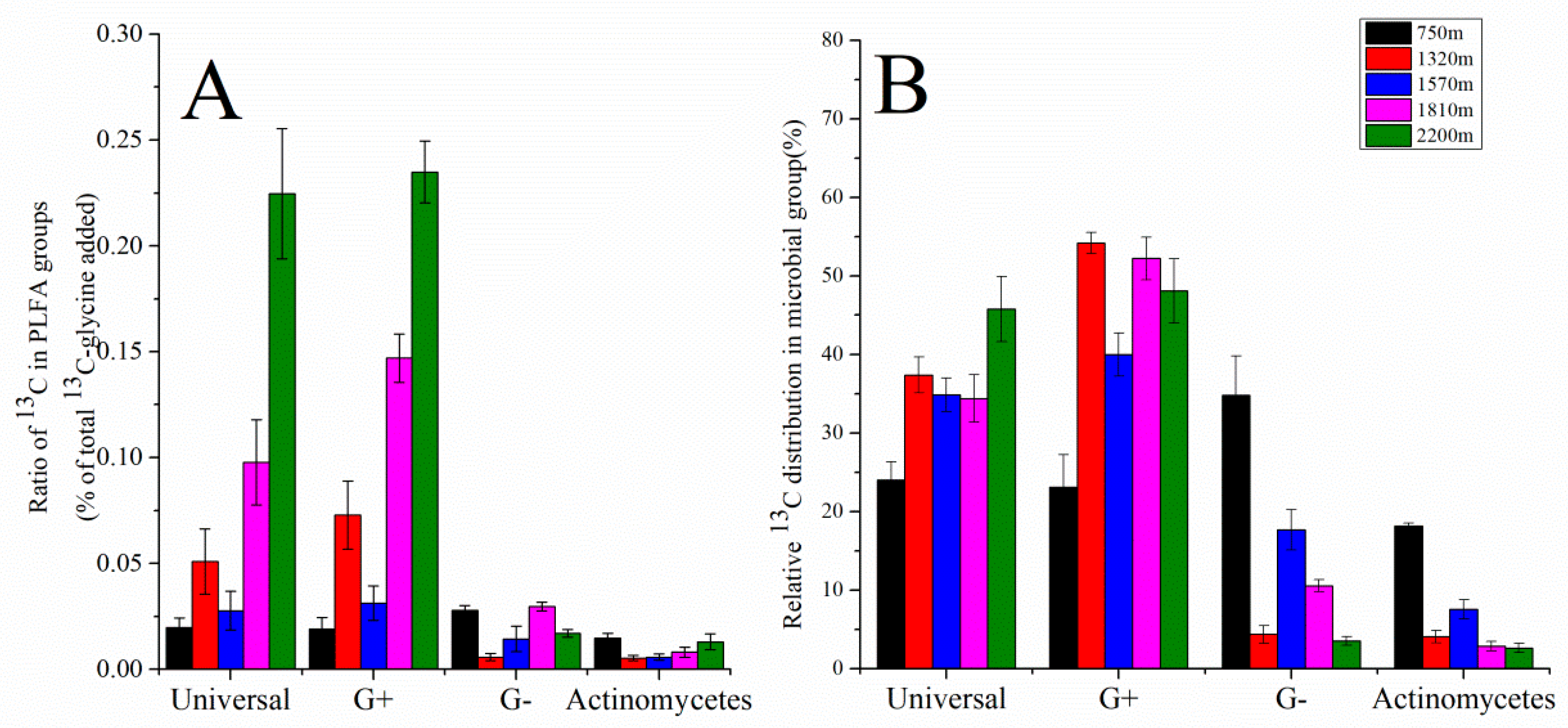
3.3. Analysis of 13C-PLFA Incorporation at Each Elevation Treatment
4. Discussion
4.1. Glycine-Addition Effects: The Increased Gross N Mineralization and Microbial Biomass N
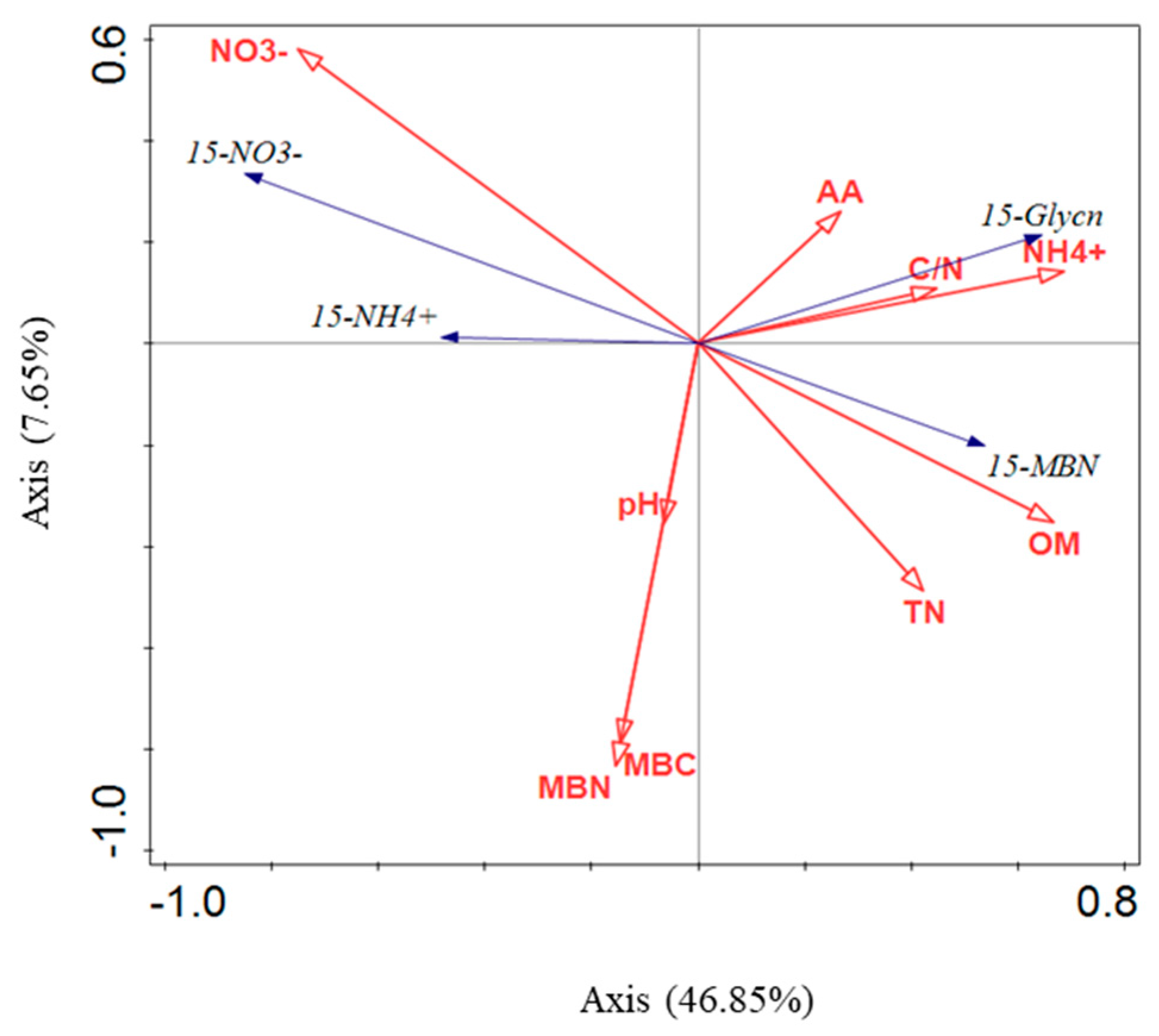
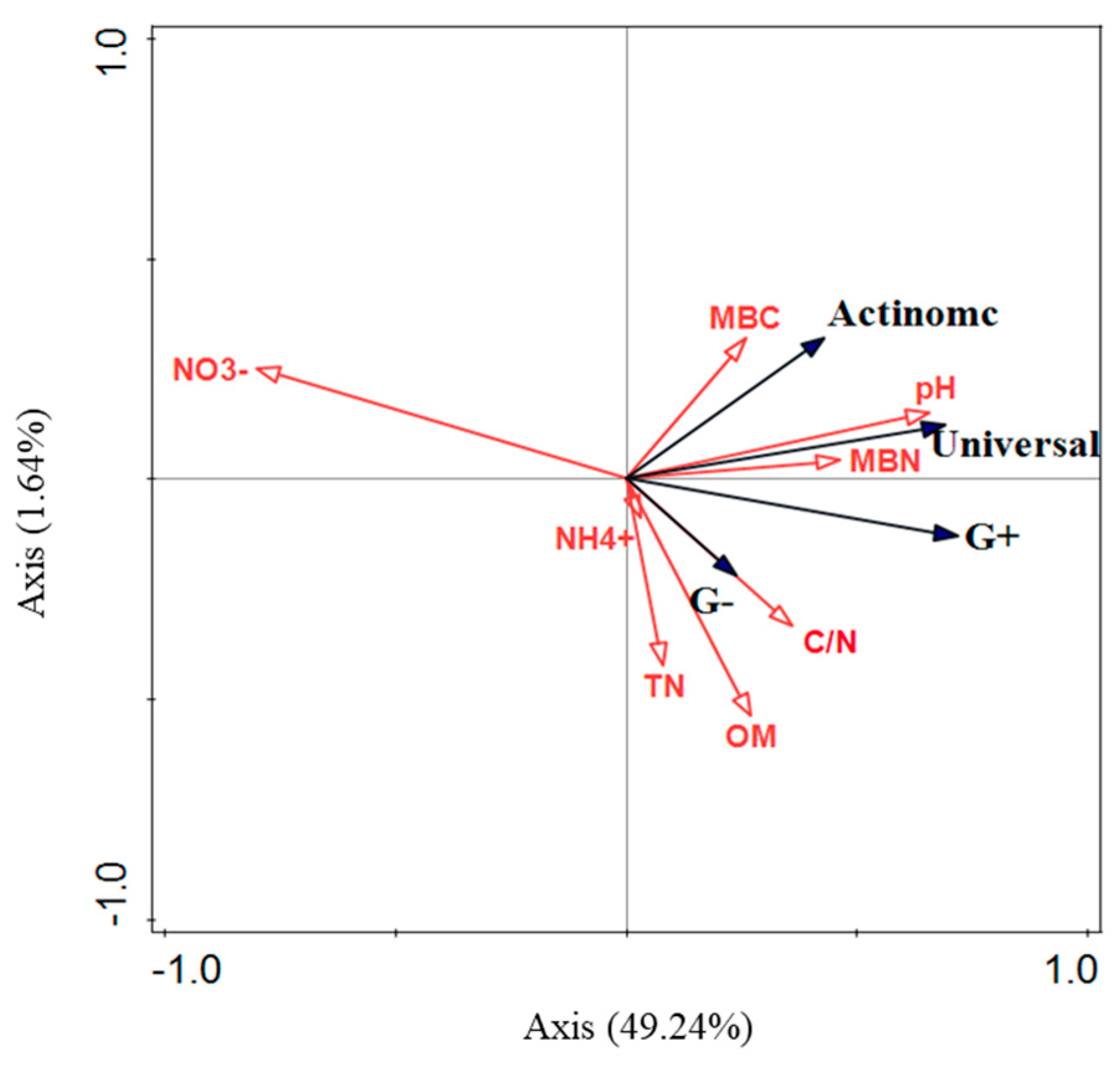
4.2. Glycine-Addition Uptake by Soil Microorganisms and Controlling Factors
4.3. Microorganisms Actively Utilize Supplied Glycine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Yang, Y.H.; Luo, Y.Q.; Fang, C.M.; Zhou, X.H.; Chen, J.K.; Yang, X.; Li, B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.E.; Bowman, W.D. Variation in nitrogen-15 natural abundance and nitrogen uptake traits among co-occurring alpine species: Do species partition by nitrogen form? Oecologia 2002, 130, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.W.; Farrell, M.; Roberts, P.; Farrar, J.; Grant, H.; Newsham, K.K.; Hopkins, D.W.; Bardgett, R.D.; Jones, D.L. Soil- and enantiomer-specific metabolism of amino acids and their peptides by Antarctic soil microorganisms. Soil Biol. Biochem. 2011, 43, 2410–2416. [Google Scholar] [CrossRef]
- Fischer, H.; Ingwersen, J.; Kuzyakov, Y. Microbial uptake of low-molecular-weight organic substances out-competes sorption in soil. Eur. J. Soil Sci. 2010, 61, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.L.; Hodge, A. Biodegradation kinetics and sorption reactions of three diferently charged amino acids in soil and their efects on plant organic nitrogen availability. Soil Biol. Biochem. 1999, 31, 1331–1342. [Google Scholar] [CrossRef]
- Roberts, P.; Newsham, K.K.; Bardgett, R.D.; Farrar, J.F.; Jones, D.L. Vegetation cover regulates the quantity, quality and temporal dynamics of dissolved organic carbon and nitrogen in Antarctic soils. Polar Biol. 2009, 32, 999–1008. [Google Scholar] [CrossRef]
- Ma, Q.X.; Wu, L.H.; Wang, J.; Ma, J.Z.; Zheng, N.G.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Fertilizer regime changes the competitive uptake of organic nitrogen by wheat and soil microorganisms: An in-situ uptake test using 13C, 15N labelling, and 13C-PLFA analysis. Soil Biol. Biochem. 2018, 125, 319–327. [Google Scholar] [CrossRef]
- Philben, M.; Ziegler, S.E.; Edwards, K.A.; Kahler, R.; Benner, R. Soil organic nitrogen cycling increases with temperature and precipitation along a boreal forest latitudinal transect. Biogeochemistry 2016, 127, 397–410. [Google Scholar] [CrossRef]
- Kaiser, K.; Canedo-Oropeza, M.; Mcmahon, R.; Amon, R.M.W. Origins and transformations of dissolved organic matter in large Arctic rivers. Sci. Rep. 2017, 7, 13064. [Google Scholar] [CrossRef] [Green Version]
- Nordin, A.; Schmidt, I.K.; Shaver, G.R. Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 2004, 85, 955–962. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Doane, T.A. Significance of organic nitrogen uptake from plant residues by soil microorganisms as affected by carbon and nitrogen availability. Soil Biol. Biochem. 2009, 41, 1281–1288. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.C.; Xu, X.L.; Wanek, W.; Jiang, N.; Wang, H.M.; Yang, X.D. Organic and inorganic nitrogen uptake by 21 dominant tree species in temperate and tropical forests. Tree Physiol. 2017, 37, 1515–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisseler, D.; Joergensen, R.G.; Ludwig, B. Temporal effect of straw addition on amino acid utilization by soil microorganisms. Eur. J. Soil Biol. 2012, 53, 107–113. [Google Scholar] [CrossRef]
- Nordin, A.; Hogberg, P.; Nasholm, T. Soil nitrogen form and plant nitrogen uptake along a boreal forest productivity gradient. Oecologia 2001, 129, 125–132. [Google Scholar] [CrossRef]
- Apostel, C.; Dippold, M.A.; Glaser, B.; Kuzyakov, Y. Biochemical pathways of amino acids in soil: Assessment by position-specific labeling and 13C-PLFA analysis. Soil Biol. Biochem. 2013, 67, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Glanville, H.C.; Hill, P.W.; Schnepf, A.; Oburger, E.; Jones, D.L. Combined use of empirical data and mathematical modelling to better estimate the microbial turnover of isotopically labelled carbon substrates in soil. Soil Biol. Biochem. 2016, 94, 154–168. [Google Scholar] [CrossRef] [Green Version]
- Jansson, S.L. Tracer studies on nitrogen transformations in soil with special attention to mineralization-immobilization relationships. Ann. Roy. Coll. Surg. 1958, 24, 101–306. [Google Scholar]
- Barraclough, D. The direct or MIT route for nitrogen immobilization: A 15N mirror image study with leucine and glycine. Soil Biol. Biochem. 1997, 29, 101–108. [Google Scholar] [CrossRef]
- Myrold, D.D.; Bottomley, P.J. Nitrogen mineralization and immobilization. In Nitrogen in Agricultural Systems; Schepers, J.S., Raun, W.R., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2008; Volume 49, pp. 157–172. [Google Scholar]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms: A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Baldos, A.P.; Corre, M.D.; Veldkamp, E. Response of N cycling to nutrient inputs in forest soils across a 1000–3000 m elevation gradient in the Ecuadorian Andes. Ecology 2015, 96, 749–761. [Google Scholar] [CrossRef]
- Booth, M.S.; Stark, J.M.; Rastetter, E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Urakawa, R.; Ohte, N.; Shibata, H.; Isobe, K.; Tateno, R.; Oda, T.; Hishi, T.; Fukushima, K.; Inagaki, Y.; Hirai, K.; et al. Factors contributing to soil nitrogen mineralization and nitrification rates of forest soils in the Japanese archipelago. For. Ecol. Manag. 2016, 361, 382–396. [Google Scholar] [CrossRef]
- Colman, B.P.; Schimel, J.P. Drivers of microbial respiration and net N mineralization at the continental scale. Soil Biol. Biochem. 2013, 60, 65–76. [Google Scholar] [CrossRef]
- Zhu, T.B.; Meng, T.Z.; Zhang, J.B.; Yin, Y.F.; Cai, Z.C.; Yang, W.Y.; Zhong, W.H. Nitrogen mineralization, immobilization turnover, heterotrophic nitrification, and microbial groups in acid forest soils of subtropical China. Biol. Fertil. Soils 2013, 49, 323–331. [Google Scholar] [CrossRef]
- Tahovská, K.; Kaňa, J.; Bárta, J.; Oulehle, F.; Richter, A.; Šantrůčková, H. Microbial N immobilization is of great importance in acidified mountain spruce forest soils. Soil Biol. Biochem. 2013, 59, 58–71. [Google Scholar] [CrossRef]
- Fujii, K.; Yamada, T.; Hayakawa, C.; Nakanishi, A.; Funakawa, S. Decoupling of protein depolymerization and ammonification in nitrogen mineralization of acidic forest soils. Appl. Soil Ecol. 2020, 153, 103572. [Google Scholar] [CrossRef]
- Cao, Y.S.; He, Z.L.; Zhu, T.B.; Zhao, F.L. Organic-C quality as a key driver of microbial nitrogen immobilization in soil: A meta-analysis. Geoderma 2021, 383, 114784. [Google Scholar] [CrossRef]
- Bengtsson, G.; Bengtson, P.; Månsson, K.F. Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol. Biochem. 2003, 35, 143–154. [Google Scholar] [CrossRef]
- Månsson, K.; Bengtson, P.; Falkengren-Grerup, U.; Bengtsson, G. Plant-microbial competition for nitrogen uncoupled from soil C:N ratios. Oikos 2009, 118, 1908–1916. [Google Scholar] [CrossRef]
- Hooker, T.D.; Stark, J.M. Carbon flow from plant detritus and soil organic matter to microbes: Linking carbon and nitrogen cycling in semiarid soils. Soil Sci. Soc. Am. J. 2012, 76, 903–914. [Google Scholar] [CrossRef]
- Fritz, C.; Wichern, F. In the land of plenty: Catch crops trigger nitrogen uptake by soil microorganisms. Plant Soil 2018, 423, 549–562. [Google Scholar] [CrossRef]
- Warren, C.R. Uptake of inorganic and amino acid nitrogen from soil by Eucalyptus regnans and Eucalyptus pauciflora seedlings. Tree Physiol. 2009, 29, 401–409. [Google Scholar] [CrossRef]
- Cookson, W.R.; Osman, M.; Marschner, P.; Clark, I.M.; Murphy, D.V.; Stockdale, E.A.; Watson, C.A. Controls on soil nitrogen cycling and microbial community composition across land use and incubation temperature. Soil Biol. Biochem. 2007, 39, 744–756. [Google Scholar] [CrossRef]
- Frostegard, A.; Tunlid, A.; Baath, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Kemmitt, S.J.; Michallon, L.; Guo, S.L.; Wen, Q.; Brookes, P.C.; Evershed, R.P. The variable response of soil microorganisms to trace concentrations of low molecular weight organic substrates of increasing complexity. Soil Biol. Biochem. 2013, 64, 57–64. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Steeter, T.C.; Bol, R. Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 2003, 84, 1277–1287. [Google Scholar] [CrossRef]
- Rinnan, R.; Baath, E. Differential utilization of carbon substrates by bacteria and fungi in tundra soil. Appl. Environ. Microbiol. 2009, 75, 3611–3620. [Google Scholar] [CrossRef] [Green Version]
- Gunina, A.; Dippold, M.A.; Glaser, B.; Kuzyakov, Y. Fate of low molecular weight organic substances in an arable soil: From microbial uptake to utilisation and stabilisation. Soil Biol. Biochem. 2014, 77, 304–313. [Google Scholar] [CrossRef]
- Broughton, R.C.I.; Newsham, K.K.; Hill, P.W.; Stott, A.; Jones, D.L. Differential acquisition of amino acid and peptide enantiomers within the soil microbial community and its implications for carbon and nitrogen cycling in soil. Soil Biol. Biochem. 2015, 88, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Abbruzzese, V.; Semple, K.T.; Haygarth, P.M.; Aller, M.F.; Russell, E.; Surridge, B.W.J. Effects of substrate quality on carbon partitioning and microbial community composition in soil from an agricultural grassland. Appl. Soil Ecol. 2021, 161, 103881. [Google Scholar] [CrossRef]
- Butler, J.L.; Williams, M.A.; Bottomley, P.J.; Myrold, D.D. Microbial community dynamics associated with rhizosphere carbon flow. Appl. Environ. Microbiol. 2003, 69, 6793–6800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andresen, L.C.; Dungait, J.A.J.; Bol, R.; Selsted, M.B.; Ambus, P.; Michelsen, A. Bacteria and fungi respond differently to multifactorial climate change in a temperate heathland, traced with 13C-glycine and FACE CO2. PLoS ONE 2014, 9, e85070. [Google Scholar] [CrossRef]
- Bray, S.R.; Kitajima, K.; Mack, M.C. Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biol. Biochem. 2012, 49, 30–37. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.K.; Liang, W.J.; Jiang, Y.; Dai, G.H.; Han, S.J. Distribution of soil organic carbon fractions along the altitudinal gradient in the northern slope of Changbai Mountain. Pedoshphere 2011, 21, 615–620. [Google Scholar] [CrossRef]
- Lu, M.Z.; Cheng, S.L.; Fang, H.J.; Xu, M.; Yang, Y.; Li, Y.N.; Zhang, J.B.; Müller, C. Organic nitrogen addition causes decoupling of microbial nitrogen cycles by stimulating gross nitrogen transformation in a temperate forest soil. Geoderma 2021, 385, 114886. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, G.R.; Yu, Z.L.; Zhao, S.D. Analysis on factors affecting net primary productivity distribution in Changbai Mountain based on process model for landscape scale. J. Appl. Ecol. 2003, 14, 659–664. [Google Scholar]
- Tong, F.C.; Xiao, Y.H.; Wang, Q.L. Soil nematode community structure on the northern slope of Changbai Moutain, Northeast China. J. Forest Res. 2010, 21, 93–98. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, C.; He, H.; Zhang, X. Variations in soil microbial communities and residues along an altitude gradient on the northern slope of Changbai Mountain, China. PLoS ONE 2013, 8, e66184. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Sebilo, M.; Mayer, B.; Grably, M.; Billiou, D.; Mariotti, A. The use of the ‘ammonium diffusion’ method for δ15N-NH4+ and δ15N-NO3− measurements: Comparison with other techniques. Environ. Chem. 2004, 1, 99–103. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Investigating amino acid utilization by soil microorganisms using compound specific stable isotope analysis. Soil Biol. Biochem. 2014, 74, 100–105. [Google Scholar] [CrossRef]
- Smart, K.F.; Aggio, R.B.; Van Houtte, J.R.; Villas-Bôas, S.G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 1709–1729. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.L.; Beare, M.H. Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci. Soc. Am. J. 1993, 57, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Thornton, B.; Zhang, Z.; Mayes, R.W.; Högberg, M.N.; Midwood, A.J. Can gas chromatography combustion isotope ratio mass spectrometry be used to quantify organic compound abundance? Rapid Commun. Mass Spectrom. 2011, 25, 2433–2438. [Google Scholar] [CrossRef]
- Bååth, E. The use of neutral lipid fatty acids to indicate the physiological conditions of soil fungi. Microb. Ecol. 2003, 45, 373–383. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Mazzarino, M.J.; Bertiller, M.B.; Sain, C.; Satti, P.; Coronato, F. Soil nitrogen dynamics in northeastern Patagonia steppe under different precipitation regimes. Plant Soil 1998, 202, 125–131. [Google Scholar] [CrossRef]
- Hart, S.C.; Nason, G.E.; Myrold, D.D.; Perry, D.A. Dynamics of gross nitrogen transformations in an old-growth forest: The carbon connection. Ecology 1994, 75, 880–891. [Google Scholar] [CrossRef]
- Owen, A.G.; Jones, D.L. Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol. Biochem. 2001, 33, 651–657. [Google Scholar] [CrossRef]
- Jones, D.L.; Shannon, D.; Murphy, D.V.; Farrar, J. Role of dissolved organic nitrogen (DON) in soil N cycling in grassland soils. Soil Biol. Biochem. 2004, 36, 749–756. [Google Scholar] [CrossRef]
- Rothstein, D.E. Effects of amino-acid chemistry and soil properties on the behavior of free amino acids in acidic forest soils. Soil Biol. Biochem. 2010, 42, 1743–1750. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Dippold, M.A.; Kuzyakov, Y. Biogeochemical transformations of amino acids in soil assessed by position-specific labeling. Plant Soil 2013, 373, 385–401. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Wang, J.Y.; Wang, S.Q.; Chang, S.X.; Cai, Z.C.; Zhang, J.B.; Niu, S.L.; Hu, S.J. Nitrogen deposition differentially affects soil gross nitrogen transformations in organic and mineral horizons. Earth Sci. Rev. 2020, 201, 103033. [Google Scholar] [CrossRef]
- Jones, D.L.; Kemmitt, S.J.; Wright, D.; Cuttle, S.P.; Bol, R.; Edwards, A.C. Rapid intrinsic rates of amino acid biodegradation in soils are unaffected by agricultural management strategy. Soil Biol. Biochem. 2005, 37, 1267–1275. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen limitation of decomposition and decay: How can it occur? Glob. Change Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.E.; Burke, I.C. Potential nitrogen immobilization in grassland soils across a soil organic matter gradient. Soil Biol. Biochem. 2000, 32, 1707–1716. [Google Scholar] [CrossRef]
- Cong, G.; Zhang, Z.D.; Zhang, J.J.; Xu, L.; He, N.P. Research on Characteristics of Soil Organic Carbon in Different Forest Types in Changbai Mountain. J. Soil Water Conserv. 2019, 33, 179–184. [Google Scholar]
- Yang, L.J.; Zhang, L.L.; Geisseler, D.; Wu, Z.J.; Gong, P.; Xue, Y.; Yu, C.X.; Juan, Y.H.; Horwath, W.R. Available C and N affect the utilization of glycine by soil microorganisms. Geoderma 2016, 283, 32–38. [Google Scholar] [CrossRef]
- Farrell, M.; Macdonald, L.M.; Hill, P.W.; Wanniarachchi, S.D.; Farrar, J.; Bardgett, R.D.; Jones, D.L. Amino acid dynamics across a grassland altitudinal gradient. Soil Biol. Biochem. 2014, 76, 179–182. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Gavrichkova, O. Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob. Chang. Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Denef, K.; Roobroeck, D.; Wadu, M.C.M.; Lootens, P.; Boeckx, P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol. Biochem. 2009, 41, 144–153. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hester, A.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Hewison, R.L.; Potts, J.M. Explaining the variation in the soil microbial community: Do vegetation composition and soil chemistry explain the same or different parts of the microbial variation? Plant Soil 2012, 351, 355–362. [Google Scholar] [CrossRef]
- Margesin, R.; Jud, M.; Tscherko, D.; Schinner, F. Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol. Ecol. 2009, 67, 208–221. [Google Scholar] [CrossRef] [Green Version]


| 750 m | 1320 m | 1570 m | 1810 m | 2200 m | |
|---|---|---|---|---|---|
| Soil moisture content | 0.32 ± 0.02 b | 0.33 ± 0.04 b | 0.34 ± 0.01 a,b | 0.41 ± 0.2 a,b | 0.47 ± 0.04 a |
| pH | 4.68 ± 0.18 b | 4.83 ± 0.17 b | 4.27 ± 0.13 c | 4.21 ± 0.12 c | 5.37 ± 0.15 a |
| Total C (%) | 5.38 ± 1 b | 3.04 ± 0.13 b | 4.73 ± 0.34 b | 10.68 ± 1.94 a | 6.47 ± 1.45 b |
| Total N (%) | 0.46 ± 0.07 a | 0.16 ± 0.01 b | 0.28 ± 0.01 b | 0.51 ± 0.11 a | 0.39 ± 0.06 a |
| Soil C/N | 11.63 ± 0.47 c | 19.59 ± 0.82 a,b | 17.21 ± 0.89 b | 20.77 ± 2.24 a | 16.37 ± 1.12 b |
| NH4+-N (mg kg−1) | 18.8 ± 6.48 a | 9.24 ± 1.53 b | 27.44 ± 4.22 a | 32.41 ± 8.07 a | 23.67 ± 5.48 a |
| NO3—N (mg kg−1) | 49.49 ± 3.02 a,b | 42.79 ± 6.31 b | 58.7 ± 7.55 a | 5.26 ± 2.05 c | 5.64 ± 2 c |
| MBC (mg kg−1) | 1208.95 ± 131.91 a | 803.56 ± 146.96 b | 597.03 ± 58.55 b | 881.25 ± 187.64 b | 1083.45 ± 166.75 a,b |
| MBN (mg kg−1) | 203.26 ± 41 a | 199.18 ± 25.41 a | 76.57 ± 21.36 b | 181.05 ± 65.24 a | 208.57 ± 23.8 a |
| Soil Variable by Elevation | Control | Glycine |
|---|---|---|
| 750 m | 0.0018 ± 0.0013 | 0.0034 ± 00008 |
| 1320 m | 0.0007 ± 0.0002 | 0.0113 ± 0.0050 * |
| 1570 m | 0.0086 ± 0.0033 | 0.212 ± 0.0111 * |
| 1810 m | 0.0023 ± 0.0017 | 0.0028 ± 0.0006 |
| 2200 m | 0.0009 ± 0.0003 | 0.0031 ± 0.001 * |
| Elevation | Microbial C | Microbial N | Microbial C:N Ratio |
|---|---|---|---|
| 750 m | |||
| Gross N mineralization | 0.6 | −0.79 * | 0.598 |
| Microbial C | 0.194 | 0.827 * | |
| Microbial N | −0.389 | ||
| 1320 m | |||
| Gross N mineralization | 0.768 * | 0.125 | 0.710 * |
| Microbial C | 0.341 | 0.776 * | |
| Microbial N | −0.327 | ||
| 1570 m | |||
| Gross N mineralization | −0.73 * | −0.088 | −0.623 |
| Microbial C | 0.561 | 0.228 | |
| MicrobialN | −0.668 | ||
| 1810 m | |||
| Gross N mineralization | −0.699 | 0.023 | −0.703 |
| Microbial C | 0.221 | 0.881 ** | |
| Microbial N | −0.265 | ||
| 2200 m | |||
| Gross N mineralization | −0.697 | −0.173 | −0.832 * |
| Microbial C | 0.752* | 0.933 ** | |
| Microbial N | 0.466 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Wu, Z.; Zhang, L.; Bai, W.; Li, D.; Yang, L.; Gong, P.; Wei, Z.; Song, Y.; Cui, L.; et al. Utilization of Glycine by Microorganisms along the Altitude Changbai Mountain, China: An Uptake Test Using 13C,15N Labeling and 13C-PLFA Analysis. Forests 2022, 13, 307. https://doi.org/10.3390/f13020307
Xue Y, Wu Z, Zhang L, Bai W, Li D, Yang L, Gong P, Wei Z, Song Y, Cui L, et al. Utilization of Glycine by Microorganisms along the Altitude Changbai Mountain, China: An Uptake Test Using 13C,15N Labeling and 13C-PLFA Analysis. Forests. 2022; 13(2):307. https://doi.org/10.3390/f13020307
Chicago/Turabian StyleXue, Yan, Zhijie Wu, Lili Zhang, Wei Bai, Dongpo Li, Lijie Yang, Ping Gong, Zhanbo Wei, Yuchao Song, Lei Cui, and et al. 2022. "Utilization of Glycine by Microorganisms along the Altitude Changbai Mountain, China: An Uptake Test Using 13C,15N Labeling and 13C-PLFA Analysis" Forests 13, no. 2: 307. https://doi.org/10.3390/f13020307
APA StyleXue, Y., Wu, Z., Zhang, L., Bai, W., Li, D., Yang, L., Gong, P., Wei, Z., Song, Y., Cui, L., Wu, K., & Xiao, F. (2022). Utilization of Glycine by Microorganisms along the Altitude Changbai Mountain, China: An Uptake Test Using 13C,15N Labeling and 13C-PLFA Analysis. Forests, 13(2), 307. https://doi.org/10.3390/f13020307







