Biochar Rescues Native Trees in the Biodiversity Hotspot of Mauritius
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
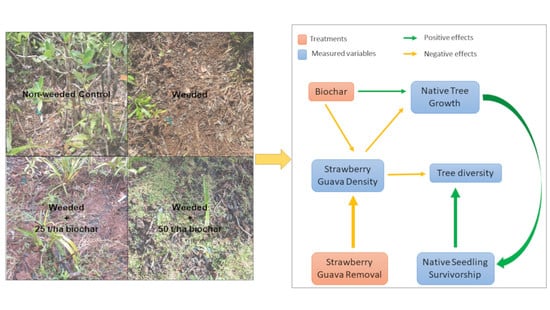
2.2. Experimental Design
2.3. Biochar Synthesis and Characterization
2.4. Soil Collection and Analysis
2.5. Plant Measurements
2.6. Statistical Analysis
3. Results
3.1. Soil and Biochar Properties
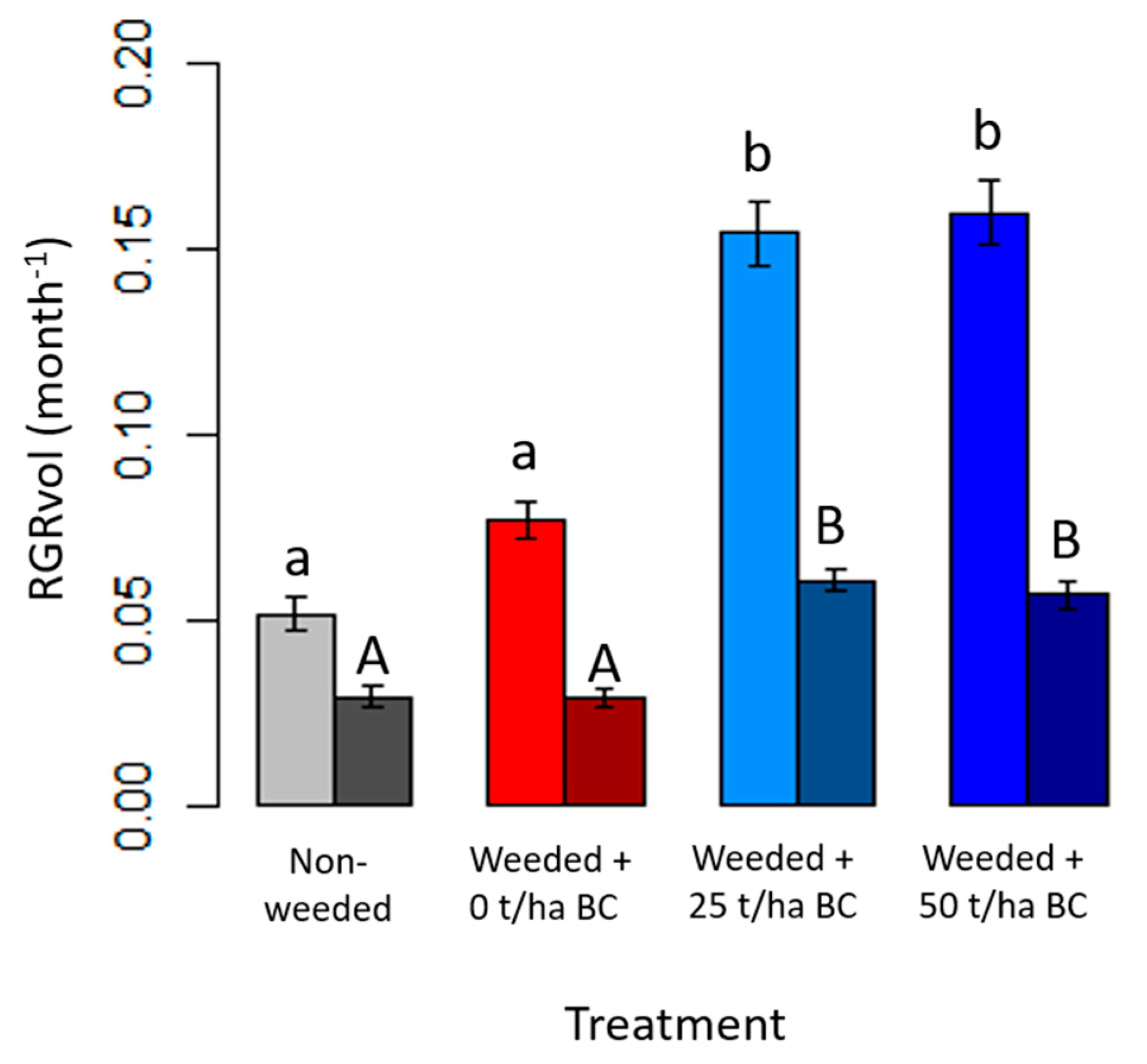
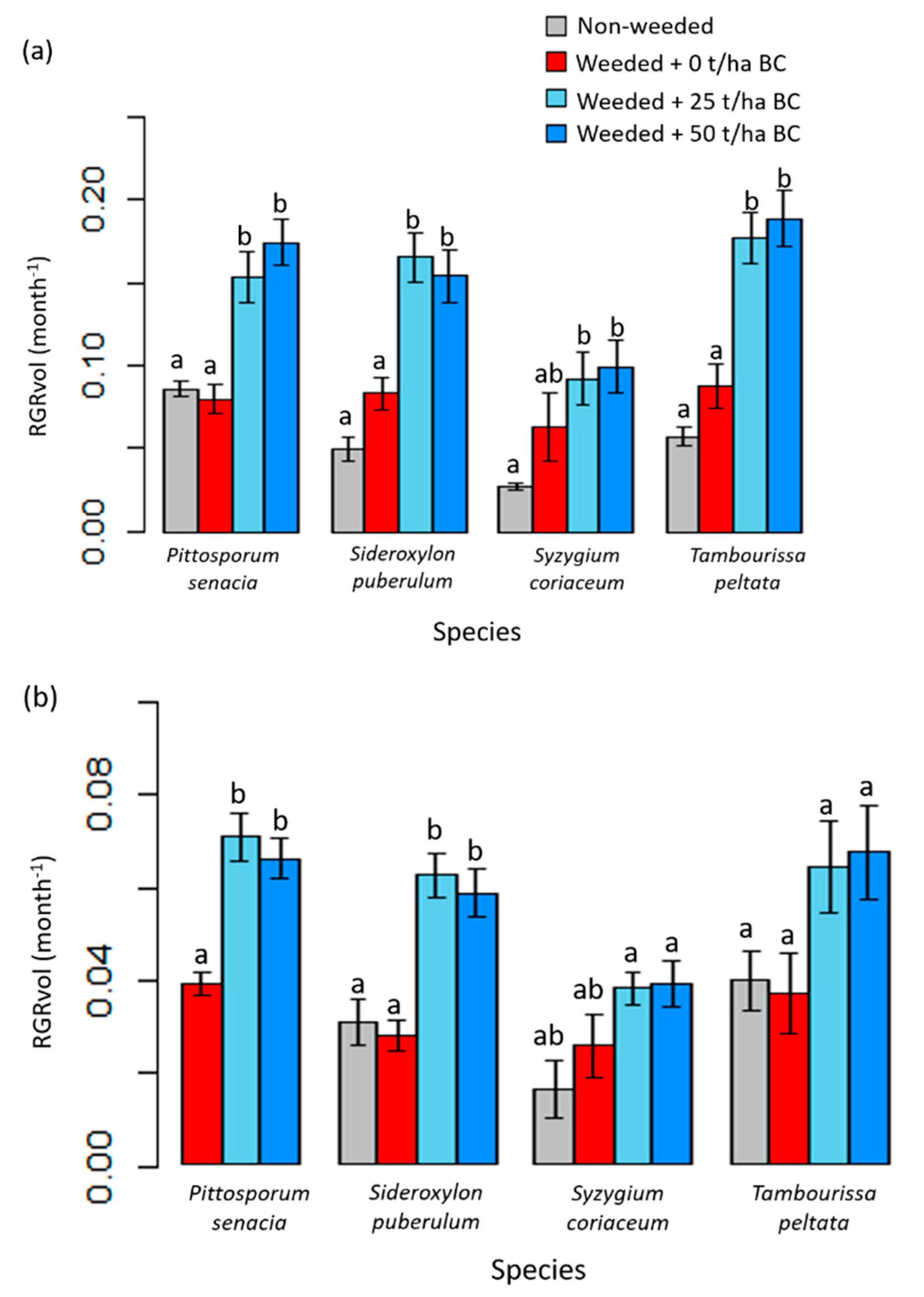
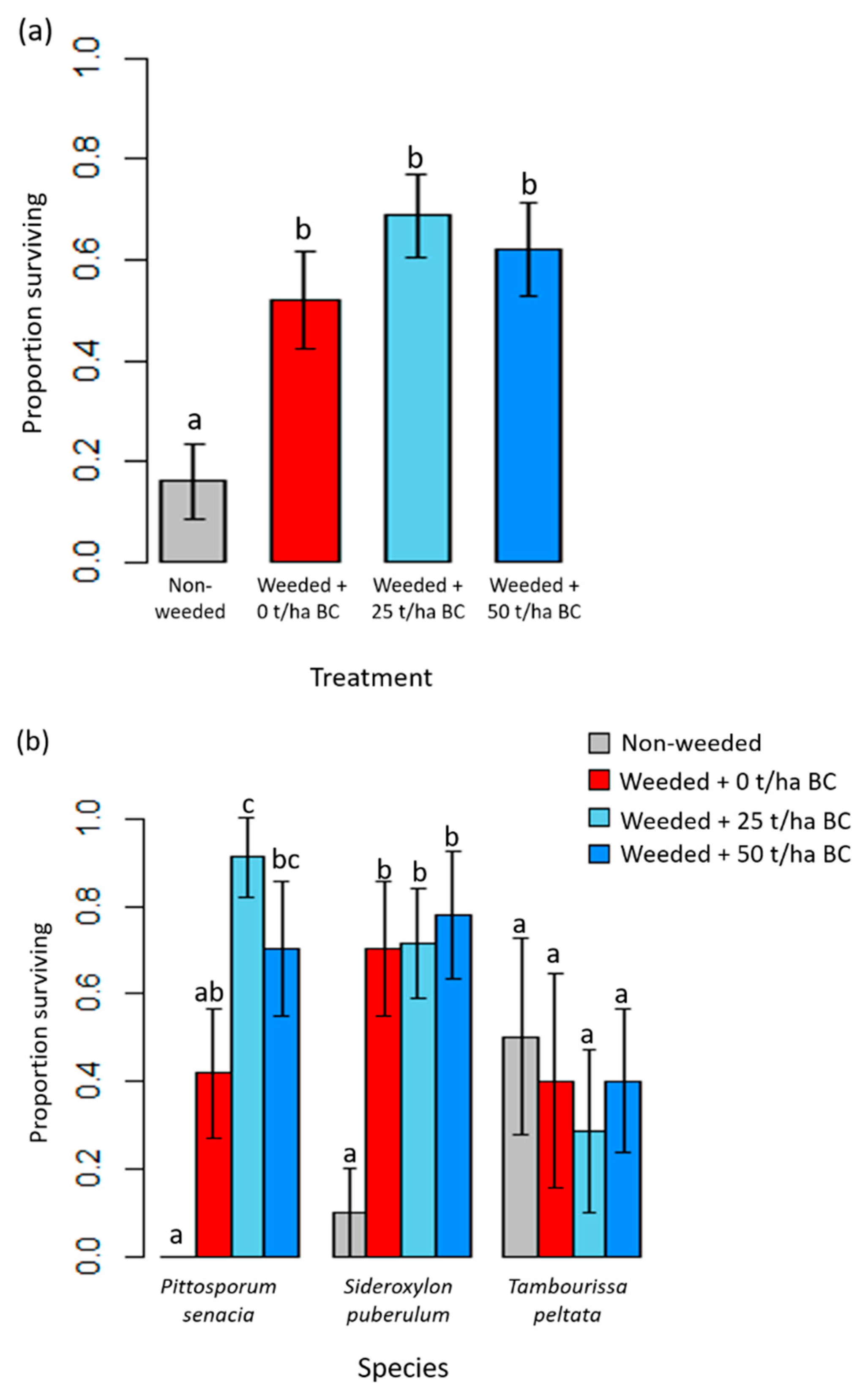
3.2. Native Tree Growth and Survivorship
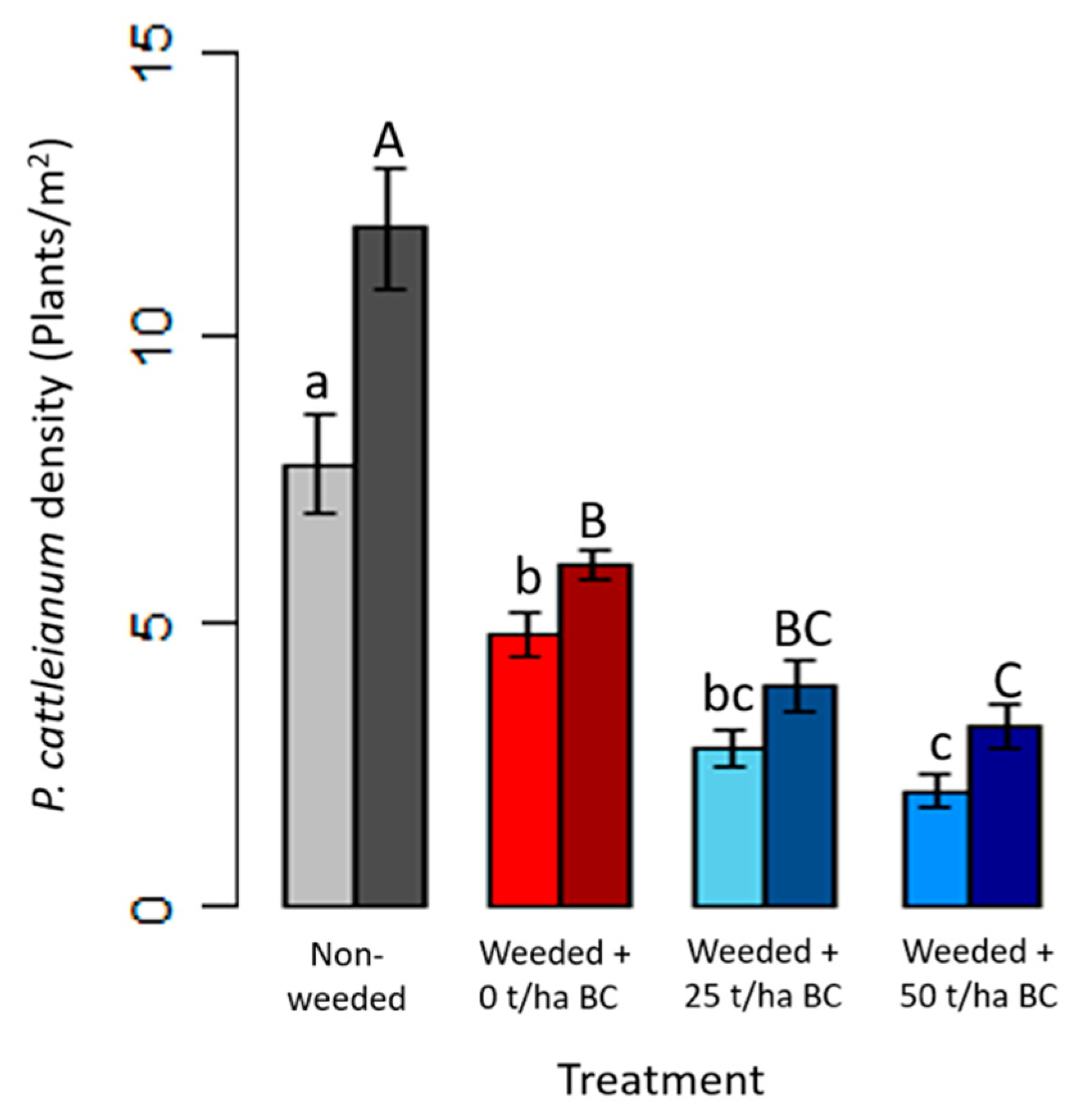
3.3. Strawberry Guava Density
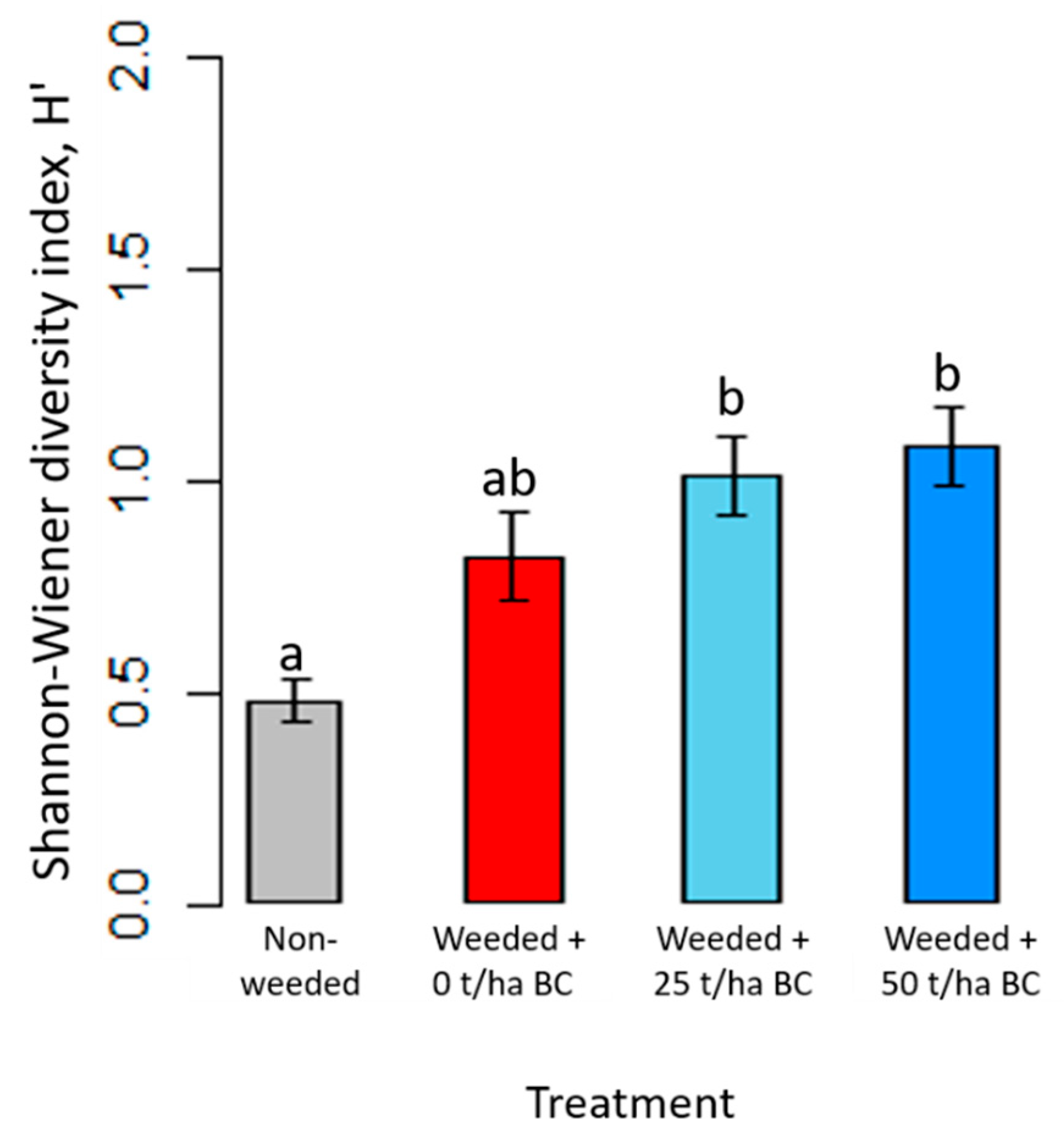
3.4. Diversity
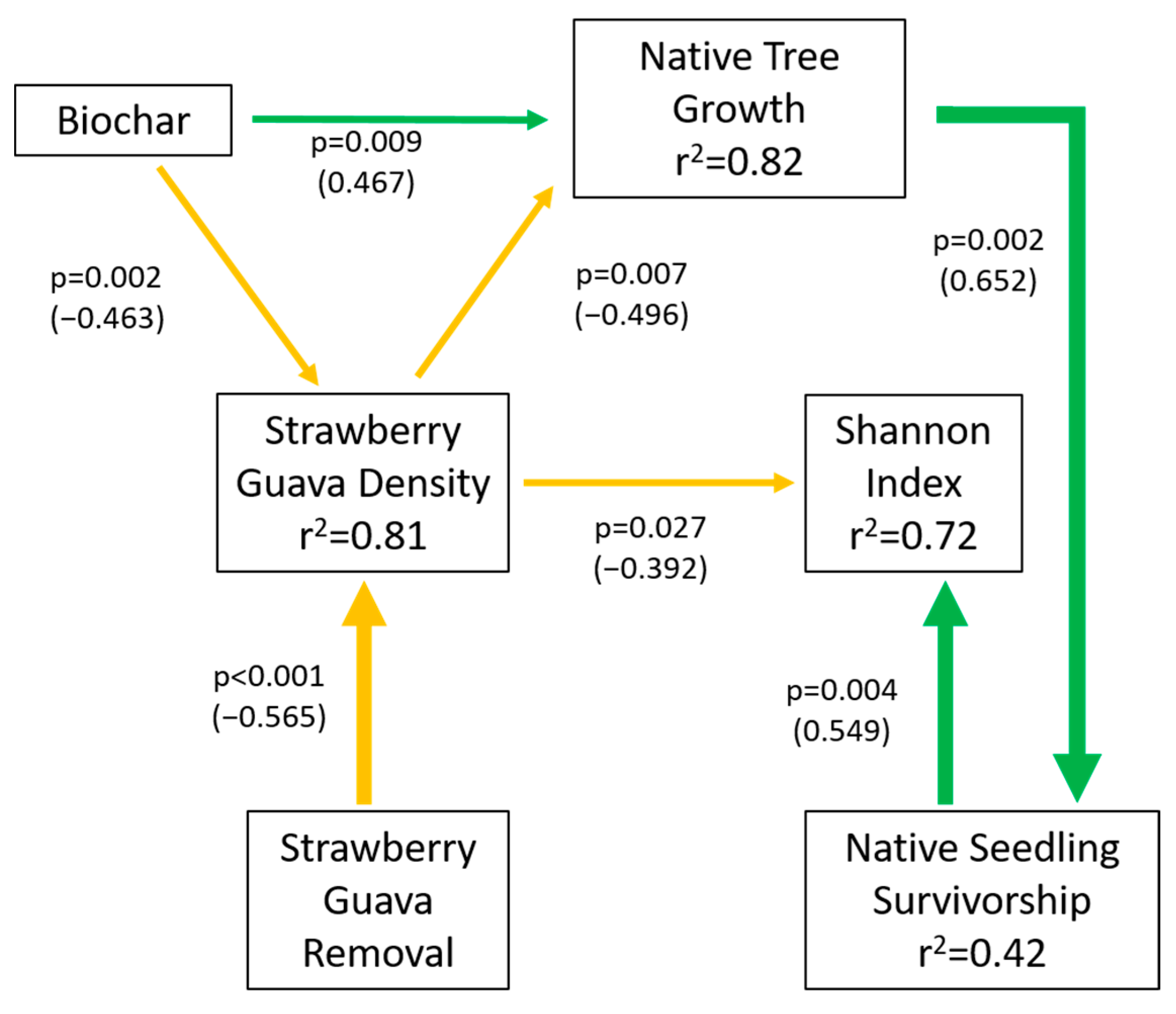
3.5. Structural Equation Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Van Kleunen, M.; Dawson, W.; Maurel, N. Characteristics of successful alien plants. Mol. Ecol. 2015, 24, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy is pervasive in invasive plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Hagan, D.L.; Jose, S.; Lin, C.H. Allelopathic exudates of cogongrass (Imperata cylindrica): Implications for the performance of native pine savanna plant species in the Southeastern US. J. Chem. Ecol. 2013, 39, 312–322. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, Z. Allelopathic effects of Chromolaena odorata on native and non-native invasive herbs. J. Food. Agric. Environ. 2013, 11, 878–882. [Google Scholar]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Huenneke, L.F.; Vitousek, P.M. Seedling and clonal recruitment of the invasive tree Psidium cattleianum: Implications for management of native Hawaiian forests. Biol. Conserv. 1990, 53, 199–211. [Google Scholar] [CrossRef]
- Mascaro, J.; Becklund, K.K.; Hughes, R.F.; Schnitzer, S.A. Limited native plant regeneration in novel, exotic-dominated forests on Hawai’i. For. Ecol. Manag. 2008, 256, 593–606. [Google Scholar] [CrossRef]
- Patel, S. Exotic tropical plant Psidium cattleianum: A review on prospects and threats. Rev. Environ. Sci. Biotechnol. 2012, 11, 243–248. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Vázquez, C. Characterization of volatiles in strawberry guava (Psidium cattleianum Sabine) Fruit. J. Agric. Food Chem. 2001, 49, 5883–5887. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Z.; Wang, C.J. Expansion risk of invasive plants in regions of high plant diversity: A global assessment using 36 species. Ecol. Inform. 2018, 46, 8–18. [Google Scholar] [CrossRef]
- van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef]
- Denslow, J.S. Weeds in paradise: Thoughts on the invasibility of tropical islands. Ann. Mo. Bot. Gard. 2003, 90, 119–127. [Google Scholar] [CrossRef]
- Lorence, D.H.; Sussman, R.W. Exotic species invasion into Mauritius wet forest remnants. J. Trop. Ecol. 1986, 2, 147–162. [Google Scholar] [CrossRef]
- Schumacher, E.; Kueffer, C.; Tobler, M.; Gmür, V.; Edwards, P.J.; Dietz, H. Influence of drought and shade on seedling growth of native and invasive trees in the Seychelles. Biotropica 2008, 40, 543–549. [Google Scholar] [CrossRef]
- Florens, F.B.V.; Baider, C.; Seegoolam, N.B.; Zmanay, Z.; Strasberg, D. Long-term declines of native trees in an oceanic island’s tropical forests invaded by alien plants. Appl. Veg. Sci. 2017, 20, 94–105. [Google Scholar] [CrossRef]
- Monty, M.L.F.; Florens, F.B.V.; Baider, C. Invasive alien plants elicit reduced production of flowers and fruits in various native forest species on the tropical island of Mauritius (Mascarenes, Indian Ocean). Trop. Conserv. Sci. 2013, 6, 35–49. [Google Scholar] [CrossRef]
- Zimmerman, N.; Hughes, R.F.; Cordell, S.; Hart, P.; Chang, H.K.; Perez, D.; Like, R.K.; Ostertag, R. Patterns of primary succession of native and introduced plants in lowland wet forests in eastern Hawai‘i. Biotropica 2008, 40, 277–284. [Google Scholar] [CrossRef]
- Florens, F.V.; Baider, C.; Martin, G.M.; Strasberg, D. Surviving 370 years of human impact: What remains of tree diversity and structure of the lowland wet forests of oceanic island Mauritius? Biodivers. Conserv. 2012, 21, 2139–2167. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Baider, C.; Florens, F.V.; Baret, S.; Beaver, K.; Matatiken, D.; Strasberg, D.; Kueffer, C. Status of plant conservation in oceanic islands of the Western Indian Ocean. In Proceedings of the 4th Global Botanic Gardens Congress, Dublin, Ireland, 13–18 June 2010; Volume 2, pp. 1–7. [Google Scholar]
- Virah-Sawmy, M.; Mauremootoo, J.; Marie, D.; Motala, S.; Sevathian, J.C. Rapid degradation of a Mauritian rainforest following 60 years of plant invasion. Oryx 2009, 43, 599. [Google Scholar] [CrossRef]
- Sujeeun, L.; Thomas, S.C. Potential of biochar to mitigate allelopathic effects in tropical island invasive plants: Evidence from seed germination trials. Trop. Conserv. Sci. 2017, 10, 194008291769726. [Google Scholar] [CrossRef]
- Adam, F.; Vahirua-Lechat, I.; Deslandes, E.; Menut, C. Aromatic plants of French Polynesia. V. chemical composition of essential oils of leaves of Psidium guajava L. and Psidium cattleyanum Sabine. J. Essent. Oil Res. 2011, 23, 98–101. [Google Scholar] [CrossRef]
- Kong, C.; Hu, F.; Xu, T.; Lu, Y. Allelopathic potential and chemical constituents of volatile oil from Ageratum conyzoides. J. Chem. Ecol. 1999, 25, 2347–2356. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Hister, C.A.L.; Trapp, K.C.; Tedesco, S.B. Potencial alelopático e antiproliferativo de extratos aquosos das folhas de Psidium cattleianum Sabine sobre Lactuca sativa L. Braz. J. Biol. Sci. 2016, 14, 131. [Google Scholar]
- Wikler, C.; Smith, C.W.; Pedrosa-Macedo, J.H. The stem-gall wasp Eurytoma sp. (Hymenoptera: Eurytomidae): A potential biological control agent against Psidium cattleianum. In Proceedings of the IX International Symposium on Biological Control of Weeds, Stellenbosch, South Africa, 19–26 January 1996; pp. 219–221. [Google Scholar]
- Safford, R.J. A survey of the occurrence of native vegetation remnants on Mauritius in 1993. Biol. Conserv. 1997, 80, 181–188. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015; ISBN 9780367779184. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, T.S.; Suansa, N.I. Application of biochar to alleviate effects of allelopathic chemicals on seed germination and seedling growth. BioResources 2020, 15, 382–400. [Google Scholar]
- Bieser, J.M.; Al-Zayat, M.; Murtada, J.; Thomas, S.C. Biochar mitigation of allelopathic effects in three invasive plants: Evidence from seed germination trials. Can. J. Soil Sci. 2022, in press. [CrossRef]
- Shen, Y.; Tang, H.; Wu, W.; Shang, H.; Zhang, D.; Zhan, X.; Xing, B. Role of nano-biochar in attenuating the allelopathic effect from Imperata cylindrica on rice seedlings. Environ. Sci. Nano 2020, 7, 116–126. [Google Scholar] [CrossRef]
- Chu, C.; Mortimer, P.E.; Wang, H.; Wang, Y.; Liu, X.; Yu, S. Allelopathic effects of Eucalyptus on native and introduced tree species. For. Ecol. Manag. 2014, 323, 79–84. [Google Scholar] [CrossRef]
- Del Fabbro, C.; Güsewell, S.; Prati, D. Allelopathic effects of three plant invaders on germination of native species: A field study. Biol. Invasions 2014, 16, 1035–1042. [Google Scholar] [CrossRef]
- Panagos, P.; Jones, A.; Bosco, C.; Senthil Kumar, P.S. European digital archive on soil maps (EuDASM): Preserving important soil data for public free access. Int. J. Digit. Earth 2011, 4, 434–443. [Google Scholar] [CrossRef]
- Hansen, D.M.; Olesen, J.M.; Jones, C.G. Trees, birds and bees in Mauritius: Exploitative competition between introduced honeybees and endemic nectarivorous birds? J. Biogeogr. 2002, 29, 721–734. [Google Scholar] [CrossRef]
- Kohyama, T.; Hotta, M. Significance of allometry in tropical saplings. Funct. Ecol. 1990, 4, 515–521. [Google Scholar] [CrossRef]
- Mokria, M.; Mekuria, W.; Gebrekirstos, A.; Aynekulu, E.; Belay, B.; Gashaw, T.; Bräuning, A. Mixed-species allometric equations and estimation of aboveground biomass and carbon stocks in restoring degraded landscape in northern Ethiopia. Environ. Res. Lett. 2018, 13, 024022. [Google Scholar] [CrossRef]
- Zeng, H.Q.; Liu, Q.J.; Feng, Z.W.; Ma, Z.Q. Biomass equations for four shrub species in subtropical China. J. For. Res. 2010, 15, 83–90. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. lme4: Linear Mixed-Effects Models Using Eigen and S4, R Package Version, 1(7). Available online: https://cran.r-project.org/web/packages/lme4/lme4.pdf (accessed on 20 October 2021).
- Lefchek, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Grace, J.B.; Bollen, K.A. Interpreting the results from multiple regression and structural equation models. Bull. Ecol. Soc. Am. 2005, 86, 283–295. [Google Scholar] [CrossRef]
- Greene, B.T.; Blossey, B. Lost in the weeds: Ligustrum sinense reduces native plant growth and survival. Biol. Invasions 2012, 14, 139–150. [Google Scholar] [CrossRef]
- Gould, A.M.A.; Gorchov, D.L. Effects of the exotic invasive shrub Lonicera maackii on the survival and fecundity of three species of native annuals. Am. Midl. Nat. 2000, 144, 36–50. [Google Scholar] [CrossRef]
- Baider, C.; Florens, F.B.V. Control of invasive alien weeds averts imminent plant extinction. Biol. Invasions 2011, 13, 2641–2646. [Google Scholar] [CrossRef]
- Belote, R.T.; Weltzin, J.F. Interactions between two co-dominant, invasive plants in the understory of a temperate deciduous forest. Biol. Invasions 2006, 8, 1629–1641. [Google Scholar] [CrossRef]
- Gorchov, D.L.; Trisel, D.E. Competitive effects of the invasive shrub, Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of native tree seedlings. Plant Ecol. 2003, 166, 13–24. [Google Scholar] [CrossRef]
- Dacoreggio, M.V.; Moroni, L.S.; Kempka, A.P. Antioxidant, antimicrobial and allelopathic activities and surface disinfection of the extract of Psidium cattleianum sabine leaves. Biocatal. Agric. Biotechnol. 2019, 21, 101295. [Google Scholar] [CrossRef]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol. Manag. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Medina, A.L.; Haas, L.I.R.; Chaves, F.C.; Salvador, M.; Zambiazi, R.C.; da Silva, W.P.; Nora, L.; Rombaldi, C.V. Araçá (Psidium cattleianum Sabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chem. 2011, 128, 916–922. [Google Scholar] [CrossRef]
- Siemens, T.J.; Blossey, B. An evaluation of mechanisms preventing growth and survival of two native species in invasive Bohemian knotweed (Fallopia ×bohemica, Polygonaceae). Am. J. Bot. 2007, 94, 776–783. [Google Scholar] [CrossRef]
- Hale, S.E.; Endo, S.; Arp, H.P.H.; Zimmerman, A.R.; Cornelissen, G. Sorption of the monoterpenes α-pinene and limonene to carbonaceous geosorbents including biochar. Chemosphere 2015, 119, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Zackrisson, O.; Nilsson, M.C. The charcoal effect in boreal forests: Mechanisms and ecological consequences. Oecologia 1998, 115, 419–426. [Google Scholar] [CrossRef]
- Pluchon, N.; Gundale, M.J.; Nilsson, M.-C.; Kardol, P.; Wardle, D.A. Stimulation of boreal tree seedling growth by wood-derived charcoal: Effects of charcoal properties, seedling species and soil fertility. Funct. Ecol. 2014, 28, 766–775. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.-K.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Gautam, S. Pyrolysis of coconut husk biomass: Analysis of its biochar properties. Energy Sources Part Recovery Util. Environ. Eff. 2017, 39, 761–767. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—a meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef]
- Drake, J.A.; Carrucan, A.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Biochar application during reforestation alters species present and soil chemistry. Sci. Total Environ. 2015, 514, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.V.; Halim, M.A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; Lentze, R.D.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Siddique, I.; Vieira, I.C.G.; Schmidt, S.; Lamb, D.; Carvalho, C.J.R.; de Figueiredo, R.O.; Blomberg, S.; Davidson, E.A. Nitrogen and phosphorus additions negatively affect tree species diversity in tropical forest regrowth trajectories. Ecology 2010, 91, 2121–2131. [Google Scholar] [CrossRef]
- Bieser, J.M.; Thomas, S.C. Biochar and high-carbon wood ash effects on soil and vegetation in a boreal clearcut. Can. J. For. Res. 2019, 49, 1124–1134. [Google Scholar] [CrossRef]
- van de Voorde, T.F.J.; Bezemer, T.M.; Groenigen, J.W.V.; Jeffery, S.; Mommer, L. Soil biochar amendment in a nature restoration area: Effects on plant productivity and community composition. Ecol. Appl. 2014, 24, 1167–1177. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Taylor, J.P.; Smith, L.M.; Haukos, D.A. Seedling competition between native cottonwood and exotic saltcedar: Implications for restoration. Biol. Invasions 2009, 11, 1777–1787. [Google Scholar] [CrossRef]
- Li, W.; Luo, J.; Tian, X.; Soon Chow, W.; Sun, Z.; Zhang, T.; Peng, S.; Peng, C. A new strategy for controlling invasive weeds: Selecting valuable native plants to defeat them. Sci. Rep. 2015, 5, 11004. [Google Scholar] [CrossRef]
- Mangla, S.; Sheley, R.L.; James, J.J.; Radosevich, S.R. Intra and interspecific competition among invasive and native species during early stages of plant growth. Plant Ecol. 2011, 212, 531–542. [Google Scholar] [CrossRef]
- Witkowski, E.T.F. Growth and competition between seedlings of Protea repens (L.) L. and the alien invasive, Acacia saligna (Labill.) Wendl. in relation to nutrient availability. Funct. Ecol. 1991, 5, 101–110. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Lowe, P.N.; Lauenroth, W.K.; Burke, I.C. Effects of nitrogen availability on competition between Bromus tectorum and Bouteloua gracilis. Plant Ecol. 2003, 167, 247–254. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, R.; Guo, X.; Liang, X.; Wang, R.; Liu, J. Shifts in growth and competitive dominance of the invasive plant Alternanthera philoxeroides under different nitrogen and phosphorus supply. Environ. Exp. Bot. 2017, 135, 118–125. [Google Scholar] [CrossRef]
- Normand, F.; Habib, R. Nitrogen fertilisation induces floriferous flush in strawberry guava (Psidium cattleianum). Agronomie 2001, 21, 735–742. [Google Scholar] [CrossRef][Green Version]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Adams, M.M.; Benjamin, T.J.; Emery, N.C.; Brouder, S.J.; Gibson, K.D. The effect of biochar on native and invasive prairie plant species. Invasive Plant Sci. Manag. 2013, 6, 197–207. [Google Scholar] [CrossRef]
- Chiriboga, M.; Guo, H.; Campos-Herrera, R.; Röder, G.; Imperiali, N.; Keel, C.; Maurhofer, M.; Turlings, T.C.J. Root-colonizing bacteria enhance the levels of (E)-β-caryophyllene produced by maize roots in response to rootworm feeding. Oecologia 2018, 187, 459–468. [Google Scholar] [CrossRef]
- Köllner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.V.; Thomas, S.C. Dose-dependence of growth and ecophysiological responses of plants to biochar. Sci. Total Environ. 2019, 658, 1344–1354. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Gale, N.V.; Thomas, S.C. Spatial heterogeneity in soil pyrogenic carbon mediates tree growth and physiology following wildfire. J. Ecol. 2021, 109, 1479–1490. [Google Scholar] [CrossRef]
| Treatment | |||||
|---|---|---|---|---|---|
| Attribute | Biochar (BC) | Non-Weeded | Weeded +BC0 | Weeded +BC25 | Weeded +BC50 |
| pH | 8.33 ± 0.08 | 6.04 ± 0.17 b | 4.75 ± 0.08 c | 6.8 ± 0.22 a | 7.04 ± 0.08 a |
| EC (µS/cm) | 1194 ± 110 | 118 ± 22.1 a | 80.6 ± 6.86 a | 214 ± 27.4 b | 249 ± 12.1 b |
| OM (%) | 87 ± 1.52 | 20.6 ± 4.1 a | 25.6 ± 4.23 a | 40.6 ± 8.43 a | 35.7 ± 1.37 a |
| Carbon (%) | 60.5 ± 0.79 | 9.53 ± 0.34 a | 7.93 ± 0.89 a | 8.35 ± 0.53 a | 10.3 ± 1.78 a |
| Nitrogen (%) | 0.71 ± 0.01 | 0.46 ± 0.01 a | 0.40 ± 0.01 ab | 0.32 ± 0.01 b | 0.38 ± 0.05 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujeeun, L.; Thomas, S.C. Biochar Rescues Native Trees in the Biodiversity Hotspot of Mauritius. Forests 2022, 13, 277. https://doi.org/10.3390/f13020277
Sujeeun L, Thomas SC. Biochar Rescues Native Trees in the Biodiversity Hotspot of Mauritius. Forests. 2022; 13(2):277. https://doi.org/10.3390/f13020277
Chicago/Turabian StyleSujeeun, Leeladarshini, and Sean C. Thomas. 2022. "Biochar Rescues Native Trees in the Biodiversity Hotspot of Mauritius" Forests 13, no. 2: 277. https://doi.org/10.3390/f13020277
APA StyleSujeeun, L., & Thomas, S. C. (2022). Biochar Rescues Native Trees in the Biodiversity Hotspot of Mauritius. Forests, 13(2), 277. https://doi.org/10.3390/f13020277