Effects of Nitrogen Addition on Microbial Carbon Use Efficiency of Soil Aggregates in Abandoned Grassland on the Loess Plateau of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Vegetation Community Characteristics
2.4. Soil Sampling and Aggregate Fractionation Sieving
2.5. Soil Property Analyses
2.6. Soil Enzyme Activity Assays
2.7. Calculation of Microbial Carbon Use Efficiency
2.8. Statistical Analyses
3. Results
3.1. Changes in Vegetation Community and Soil Characteristics
3.2. Variations in Soil Aggregate Carbon Composition and Microbial CUE under Different N Addition Concentration
3.3. Effects of Vegetation Community, Soil Characteristics, and Carbon Components on Microbial Carbon Use Efficiency
4. Discussion
4.1. Linkages of Soil Aggregate Carbon Components and Microbial Carbon Use Efficiency
4.2. The Direct and Indirect Effect of Environmental Variability on the Linkages of C Aggregate and Microbial CUE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Sun, S.; Han, J.; Yan, J.; Liu, W.; Wei, Y.; Lu, N.; Sun, Y. Impacts of Chinese Grain for Green program and climate change on vegetation in the Loess Plateau during 1982–2015. Sci. Total Environ. 2019, 660, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Fu, B.; Piao, S.; Wang, S.; Ciais, P.; Zeng, Z.; Lü, Y.; Zeng, Y.; Li, Y.; Jiang, X.; et al. Revegetation in China’s Loess Plateau is approaching sustainable water resource limits. Nat. Clim. Chang. 2016, 6, 1019–1022. [Google Scholar] [CrossRef]
- Gang, C.; Zhao, W.; Zhao, T.; Zhang, Y.; Gao, X.; Wen, Z. The impacts of land conversion and management measures on the grassland net primary productivity over the Loess Plateau, Northern China. Sci. Total Environ. 2018, 645, 827–836. [Google Scholar] [CrossRef]
- Hu, X.; Li, Z.; Chen, J.; Nie, X.; Liu, J.; Wang, L.; Ning, K. Carbon sequestration benefits of the grain for Green Program in the hilly red soil region of southern China. Int. Soil Water Conserv. Res. 2021, 9, 271–278. [Google Scholar] [CrossRef]
- Ayoubi, S.; Khormali, F.; Sahrawat, K.L.; DeLima, A.C.R. Assessing Impacts of Land Use Change on Soil Quality Indicators in a Loessial Soil in Golestan Province, Iran. J. Agric. Sci. Technol. 2011, 13, 727–742. [Google Scholar]
- Sinsabaugh, R.L.; Turner, B.L.; Talbot, J.M.; Waring, B.G.; Powers, J.S.; Kuske, C.R.; Moorhead, D.L.; Follstad Shah, J.J. Stoichiometry of microbial carbon use efficiency in soils. Ecol. Monogr. 2016, 86, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sang, C.; Yang, J.; Qu, L.; Xia, Z.; Sun, H.; Jiang, P.; Wang, X.; He, H.; Wang, C. Stoichiometric imbalance and microbial community regulate microbial elements use efficiencies under nitrogen addition. Soil Biol. Biochem. 2021, 156, 108207. [Google Scholar] [CrossRef]
- Spohn, M.; Pötsch, E.M.; Eichorst, S.A.; Woebken, D.; Wanek, W.; Richter, A. Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol. Biochem. 2016, 97, 168–175. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Poeplau, C.; Helfrich, M.; Dechow, R.; Szoboszlay, M.; Tebbe, C.C.; Don, A.; Greiner, B.; Zopf, D.; Thumm, U.; Korevaar, H.; et al. Increased microbial anabolism contributes to soil carbon sequestration by mineral fertilization in temperate grasslands. Soil Biol. Biochem. 2019, 130, 167–176. [Google Scholar] [CrossRef]
- Widdig, M.; Schleuss, P.; Biederman, L.A.; Borer, E.T.; Crawley, M.J.; Kirkman, K.P.; Seabloom, E.W.; Wragg, P.D.; Spohn, M. Microbial carbon use efficiency in grassland soils subjected to nitrogen and phosphorus additions. Soil Biol. Biochem. 2020, 146, 107815. [Google Scholar] [CrossRef]
- Luo, R.; Kuzyakov, Y.; Liu, D.; Fan, J.; Luo, J.; Lindsey, S.; He, J.; Ding, W. Nutrient addition reduces carbon sequestration in a Tibetan grassland soil: Disentangling microbial and physical controls. Soil Biol. Biochem. 2020, 144, 107764. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E. Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol. Biochem. 2016, 99, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Devêvre, O.C.; RHorwáth, W. Decomposition of rice straw and microbial carbon use e ciency under di erent soil temperatures and moistures. Soil Biol. Biochem. 2000, 32, 1773–1785. [Google Scholar] [CrossRef]
- Li, T.; Wang, R.; Cai, J.; Meng, Y.; Wang, Z.; Feng, X.; Liu, H.; Turco, R.F.; Jiang, Y. Enhanced carbon acquisition and use efficiency alleviate microbial carbon relative to nitrogen limitation under soil acidification. Ecol. Processes 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z. Distribution of microbial biomass and activity within soil aggregates as affected by tea plantation age. CATENA 2017, 153, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Han, X.; You, M.; Yan, J.; Lu, X.; Horwath, W.R.; Zou, W. Soil macroaggregates and organic-matter content regulate microbial communities and enzymatic activity in a Chinese Mollisol. J. Integr. Agric. 2019, 18, 2605–2618. [Google Scholar] [CrossRef]
- Lu, X.; Hou, E.; Guo, J.; Gilliam, F.S.; Li, J.; Tang, S.; Kuang, Y. Nitrogen addition stimulates soil aggregation and enhances carbon storage in terrestrial ecosystems of China: A meta-analysis. Glob. Chang. Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; van Groenigen, K.J.; Hungate, B.A.; Cao, J.; Zhou, X.; Wang, R. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Qin, W.; Xu, H.; Zhang, Z.; Zhou, H.; Zhu, B. Sensitivity of soil carbon dynamics to nitrogen and phosphorus enrichment in an alpine meadow. Soil Biol. Biochem. 2020, 150, 107984. [Google Scholar] [CrossRef]
- Nie, M.; Pendall, E.; Bell, C.; Wallenstein, M.D. Soil aggregate size distribution mediates microbial climate change feedbacks. Soil Biol. Biochem. 2014, 68, 357–365. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, Q.; Hu, N.; Li, Z.; Lou, Y.; Li, Y.; Xue, D.; Chen, Y.; Wu, C.; et al. Long-term effects of nitrogen fertilization on aggregation and localization of carbon, nitrogen and microbial activities in soil. Sci. Total Environ. 2018, 624, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Yanlong, J.; Qiufeng, W.; Jianxing, Z.; Zhi, C.; Nianpeng, H.; Guirui, Y. A spatial and temporal dataset of atmospheric inorganic nitrogen wet deposition in China (1996–2015). Sci. Data Bank 2019, 1, 4–13. [Google Scholar]
- Bach, E.M.; Hofmockel, K.S. Soil aggregate isolation method affects measures of intra-aggregate extracellular enzyme activity. Soil Biol. Biochem. 2014, 69, 54–62. [Google Scholar] [CrossRef]
- Ren, C.; Kang, D.; Wu, J.P.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil Carbon Fractions Based on their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 7, 1459–1466. [Google Scholar] [CrossRef]
- Dalai, R.C.; Sahrawat, K.L.; Myers, R.J.K. Inclusion of nitrate and nitrite in the Kjeldahl nitrogen determination of soils and plant materials using sodium thiosulphate. Commun. Soil Sci. Plant Anal. 1984, 15, 1453–1461. [Google Scholar] [CrossRef] [Green Version]
- Koide, R.T.; Petprakob, K.; Peoples, M. Quantitative analysis of biochar in field soil. Soil Biol. Biochem. 2011, 43, 1563–1568. [Google Scholar] [CrossRef]
- Shidan, B. Methods for Soil Agricultural and Chemical Analysis; China Agricultural Press: Beijing, China, 2000; pp. 30–34. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 6, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 6, 837–842. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction-An automated procedure. Soil Biol. Biochem. 1990, 8, 1167–1169. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Gao, D.; Wang, X.; Liu, W.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Ecoenzymatic stoichiometry and nutrient dynamics along a revegetation chronosequence in the soils of abandoned land and Robinia pseudoacacia plantation on the Loess Plateau, China. Soil Biol. Biochem. 2019, 134, 1–14. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.R.; Carrillo, Y.; Dijkstra, F.A. Drought-induced and seasonal variation in carbon use efficiency is associated with fungi: Bacteria ratio and enzyme production in a grassland ecosystem. Soil Biol. Biochem. 2021, 155, 108159. [Google Scholar] [CrossRef]
- Geyer, K.M.; Dijkstra, P.; Sinsabaugh, R.; Frey, S.D. Clarifying the interpretation of carbon use efficiency in soil through methods comparison. Soil Biol. Biochem. 2019, 128, 79–88. [Google Scholar] [CrossRef]
- Allison, S.D. Modeling adaptation of carbon use efficiency in microbial communities. Front. Microbiol. 2014, 5, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öquist, M.G.; Erhagen, B.; Haei, M.; Sparrman, T.; Ilstedt, U.; Schleucher, J.; Nilsson, M.B. The effect of temperature and substrate quality on the carbon use efficiency of saprotrophic decomposition. Plant Soil 2017, 414, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Borase, D.N.; Nath, C.P.; Hazra, K.K.; Senthilkumar, M.; Singh, S.S.; Praharaj, C.S.; Singh, U.; Kumar, N. Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzymes activity. Ecol. Indic. 2020, 114, 106322. [Google Scholar] [CrossRef]
- Tajik, S.; Ayoubi, S.; Lorenz, N. Soil microbial communities affected by vegetation, topography and soil properties in a forest ecosystem. Appl. Soil Ecol. 2020, 149, 103514. [Google Scholar] [CrossRef]
- Silva-Sánchez, A.; Soares, M.; Rousk, J. Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality. Soil Biol. Biochem. 2019, 134, 25–35. [Google Scholar] [CrossRef]
- Jones, D.L.; Cooledge, E.C.; Hoyle, F.C.; Griffiths, R.I.; Murphy, D.V. pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biol. Biochem. 2019, 138, 107584. [Google Scholar] [CrossRef]
- Soares, M.; Rousk, J. Microbial growth and carbon use efficiency in soil: Links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol. Biochem. 2019, 131, 195–205. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; He, H. Effects of rehabilitation through afforestation on soil aggregate stability and aggregate-associated carbon after forest fires in subtropical China. Geoderma 2020, 376, 114548. [Google Scholar] [CrossRef]
- Wang, X.; Qi, J.; Zhang, X.; Li, S.; Latif Virk, A.; Zhao, X.; Xiao, X.; Zhang, H. Effects of tillage and residue management on soil aggregates and associated carbon storage in a double paddy cropping system. Soil Tillage Res. 2019, 194, 104339. [Google Scholar] [CrossRef]
- Tian, J.; Pausch, J.; Yu, G.; Blagodatskaya, E.; Gao, Y.; Kuzyakov, Y. Aggregate size and their disruption affect 14C-labeled glucose mineralization and priming effect. Appl. Soil Ecol. 2015, 90, 1–10. [Google Scholar] [CrossRef]
- Mutuo, P.K.; Shepherd, K.D.; Albrecht, A.; Cadisch, G. Prediction of carbon mineralization rates from different soil physical fractions using diffuse reflectance spectroscopy. Soil Biol. Biochem. 2006, 38, 1658–1664. [Google Scholar] [CrossRef]
- Zeraatpisheh, M.; Ayoubi, S.; Mirbagheri, Z.; Mosaddeghi, M.R.; Xu, M. Spatial prediction of soil aggregate stability and soil organic carbon in aggregate fractions using machine learning algorithms and environmental variables. Geoderma Reg. 2021, 27, e00440. [Google Scholar] [CrossRef]
- Ayoubi, S.; Mirbagheri, Z.; Mosaddeghi, M.R. Soil organic carbon physical fractions and aggregate stability influenced by land use in humid region of northern Iran. Int. Agrophysics 2020, 34, 343–353. [Google Scholar] [CrossRef]
- Su, F.; Xu, S.; Sayer, E.J.; Chen, W.; Du, Y.; Lu, X. Distinct storage mechanisms of soil organic carbon in coniferous forest and evergreen broadleaf forest in tropical China. J. Environ. Manag. 2021, 295, 113142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.; Wang, X.; Li, W.; Shi, P. Aggregate-associated soil organic carbon dynamics as affected by erosion and deposition along contrasting hillslopes in the Chinese Corn Belt. CATENA 2021, 199, 105106. [Google Scholar] [CrossRef]
- Yang, C.; Liu, N.; Zhang, Y. Soil aggregates regulate the impact of soil bacterial and fungal communities on soil respiration. Geoderma 2019, 337, 444–452. [Google Scholar] [CrossRef]
- Li, N.; Yao, S.; Qiao, Y.; Zou, W.; You, M.; Han, X.; Zhang, B. Separation of soil microbial community structure by aggregate size to a large extent under agricultural practices during early pedogenesis of a Mollisol. Appl. Soil Ecol. 2015, 88, 9–20. [Google Scholar] [CrossRef]
- Helgason, B.L.; Walley, F.L.; Germida, J.J. No-till soil management increases microbial biomass and alters community profiles in soil aggregates. Appl. Soil Ecol. 2010, 46, 390–397. [Google Scholar] [CrossRef]
- Tian, J.; Pausch, J.; Yu, G.; Blagodatskaya, E.; Kuzyakov, Y. Aggregate size and glucose level affect priming sources: A three-source-partitioning study. Soil Biol. Biochem. 2016, 97, 199–210. [Google Scholar] [CrossRef]
- Bimüller, C.; Kreyling, O.; Kölbl, A.; von Lützow, M.; Kögel-Knabner, I. Carbon and nitrogen mineralization in hierarchically structured aggregates of different size. Soil Tillage Res. 2016, 160, 23–33. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Hall, E.K.; Wanek, W.; Szukics, U.; Hämmerle, I.; Ellersdorfer, G.; Böck, S.; Strauss, J.; Sterflinger, K.; Richter, A.; et al. The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 2010, 73, 430–440. [Google Scholar] [CrossRef]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, H.; Denef, K.; Six, J.; Frey, S.D.; Merckx, R.; Paustian, K. Influence of microbial populations and residue quality on aggregate stability. Appl. Soil Ecol. A Sect. Agric. Ecosyst. Environ. 2001, 16, 195–208. [Google Scholar] [CrossRef]
- Jansson, M.; Bergström, A.; Lymer, D.; Vrede, K.; Karlsson, J. Bacterioplankton Growth and Nutrient Use Efficiencies under Variable Organic Carbon and Inorganic Phosphorus Ratios. Microb. Ecol. 2006, 52, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ågren, G.I.; Bosatta, E.; Magill, A.H. Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 2001, 128, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Porporato, A. Soil carbon and nitrogen mineralization: Theory and models across scales. Soil Biol. Biochem. 2009, 41, 1355–1379. [Google Scholar] [CrossRef]
- Russell, J.B.; Cook, G.M. Energetics of bacterial growth: Balance of anabolic and catabolic reactions. Microbiol. Rev. 1995, 59, 48–62. [Google Scholar] [CrossRef] [PubMed]
- De Vries, F.T.; Bloem, J.; van Eekeren, N.; Brusaard, L.; Hoffland, E. Fungal biomass in pastures increases with age and reduced N input. Soil Biol. Biochem. 2007, 39, 1620–1630. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Jiang, Y.; Wang, G.; Su, Y.; Smoak, J.M.; Liu, M.; Duan, B. Effects of N addition and clipping on above and belowground plant biomass, soil microbial community structure, and function in an alpine meadow on the Qinghai-Tibetan Plateau. Eur. J. Soil Biol. 2021, 106, 103344. [Google Scholar] [CrossRef]
- Zhi, C.; Guirui, Y. Spatial variations and controls of carbon use efciency in China’s terrestrial ecosystems. Sci. Rep. 2019, 9, 19516. [Google Scholar]
- Yu, G.; Zhu, X.; Fu, Y.; He, H.; Wang, Q.; Wen, X.; Li, X.; Zhang, L.; Zhang, L.; Su, W.; et al. Spatial patterns and climate drivers of carbon fluxes in terrestrial ecosystems of China. Glob. Chang. Biol. 2013, 19, 798–810. [Google Scholar] [CrossRef] [PubMed]
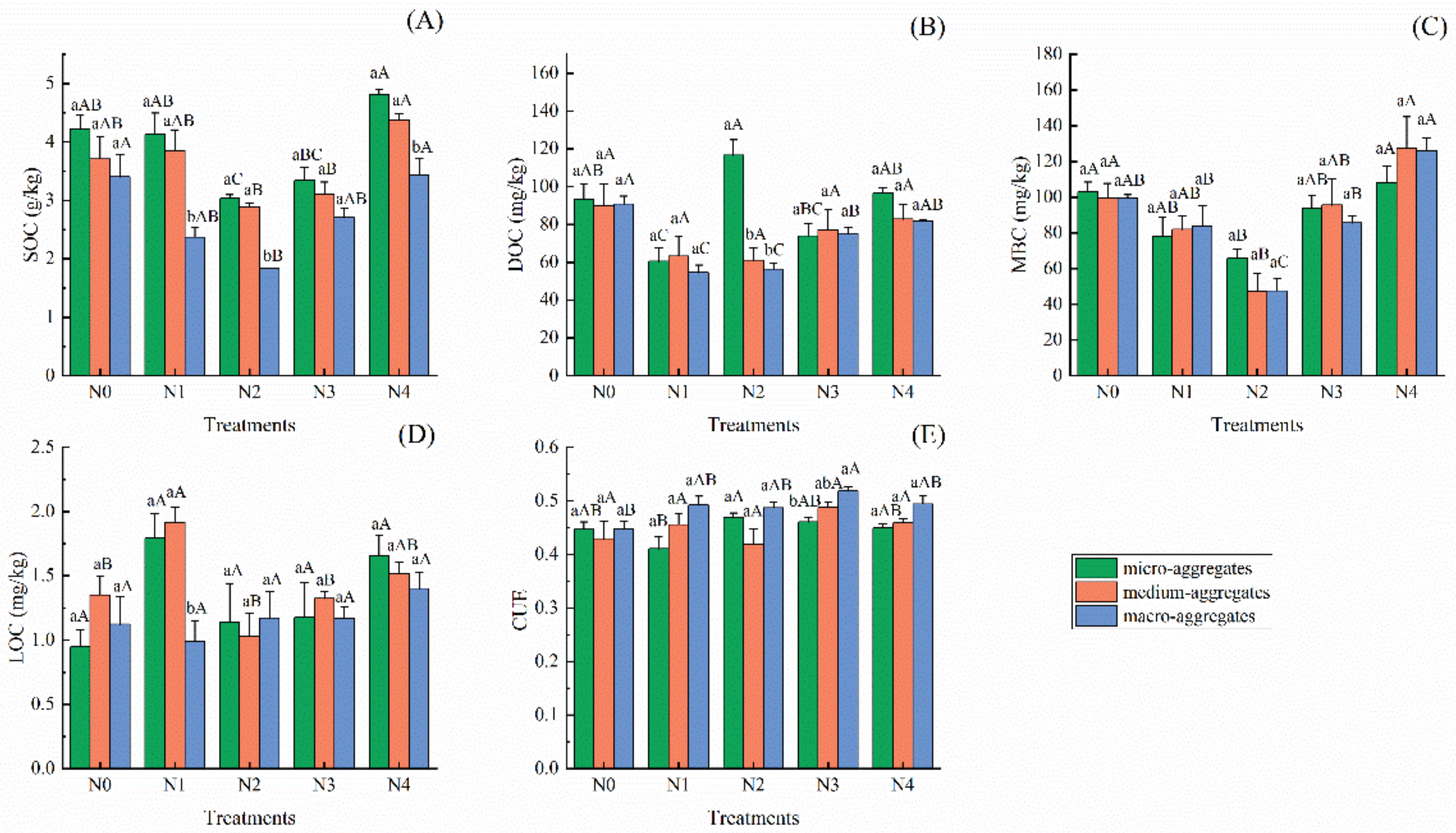
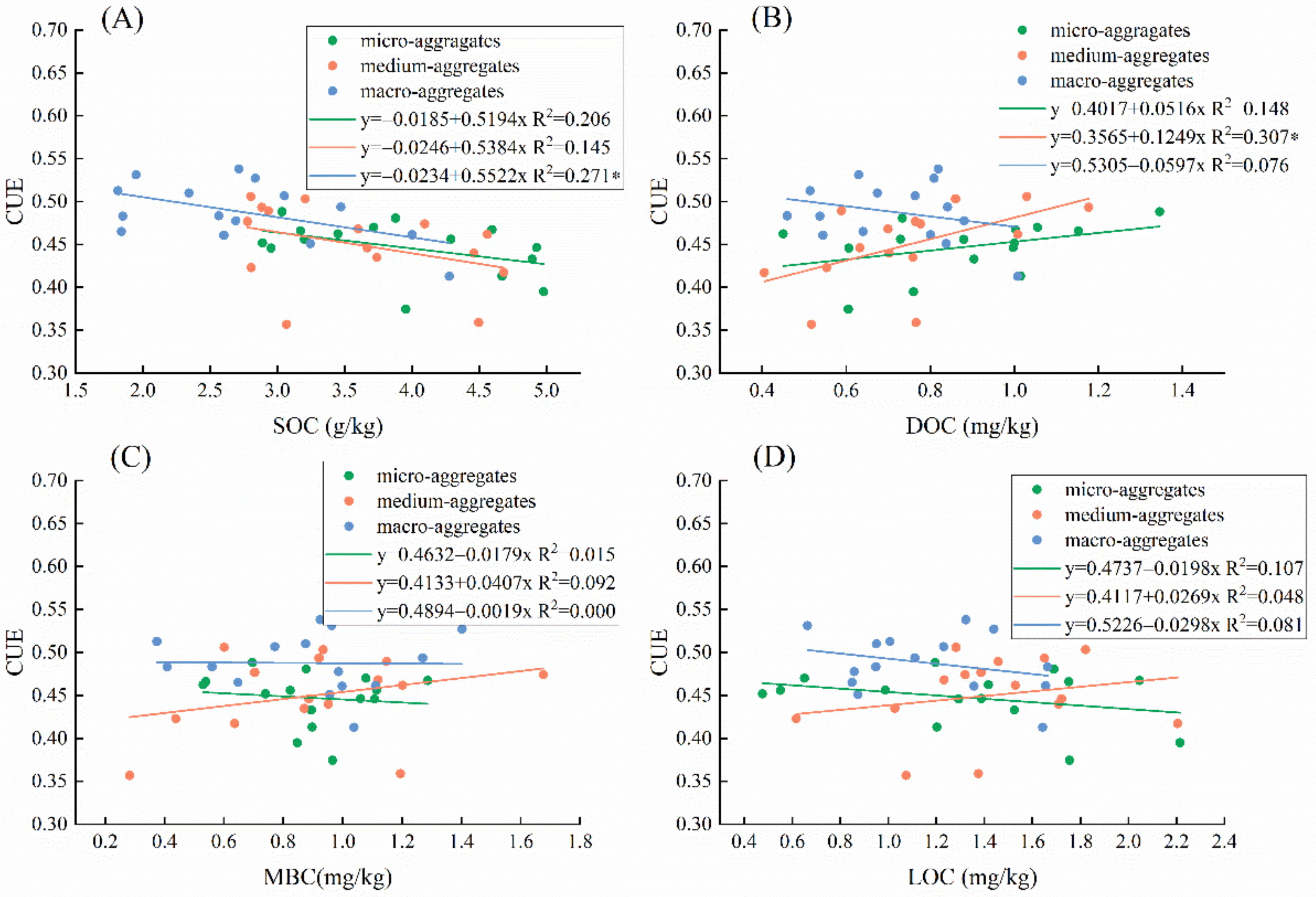
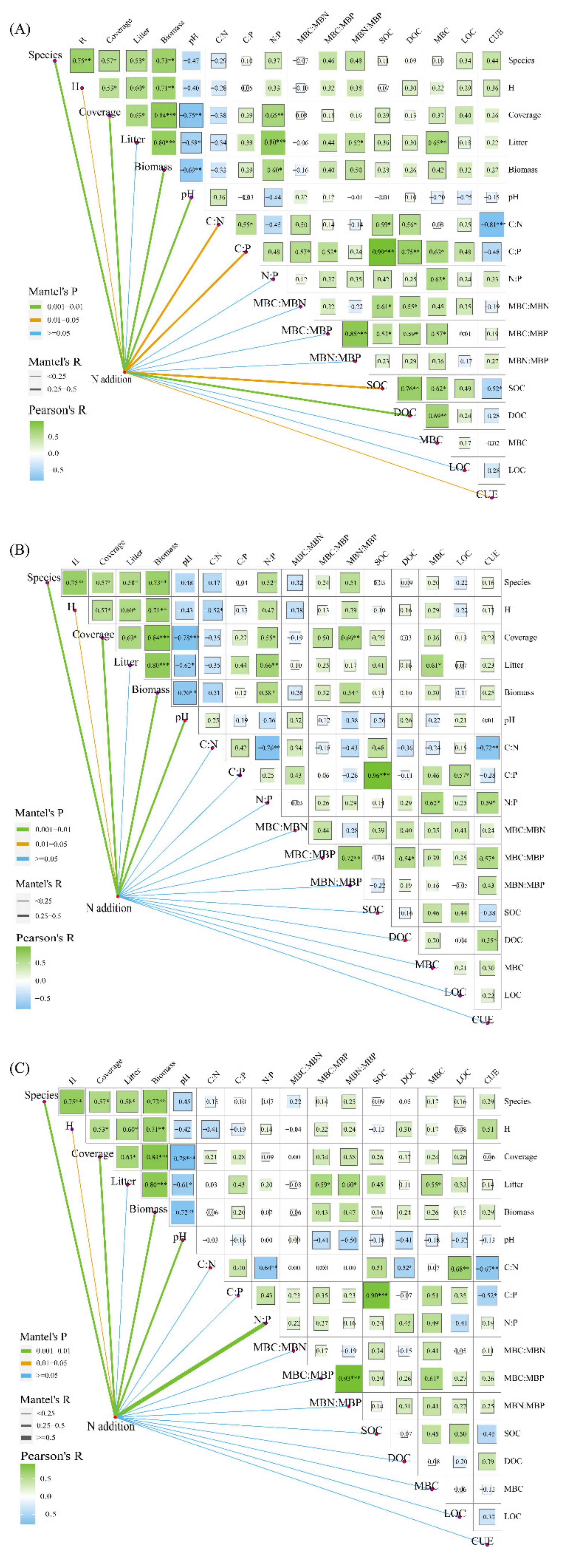
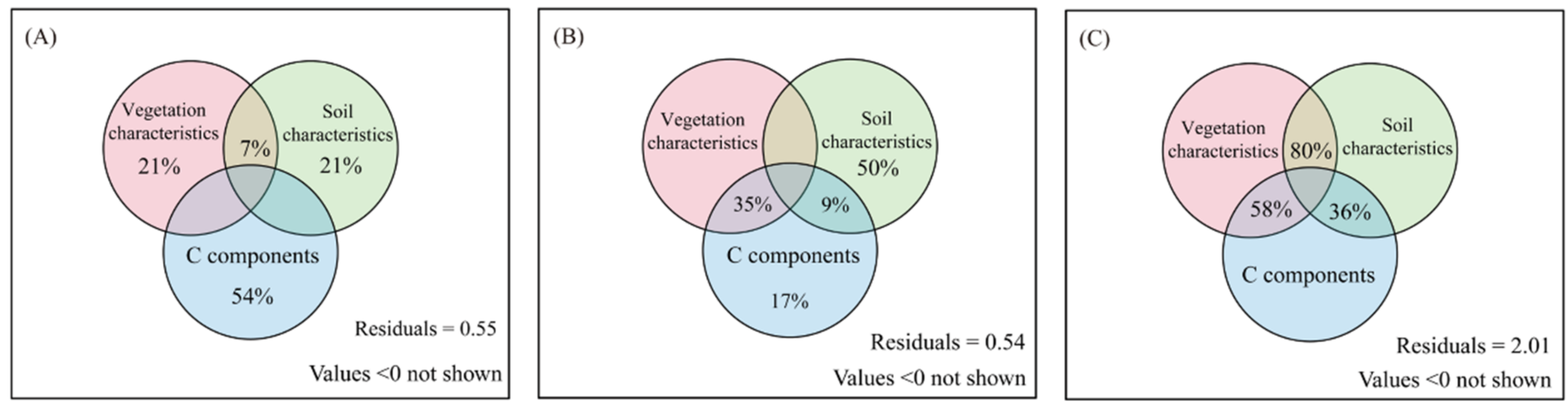
| Index | N Addition | ||||
|---|---|---|---|---|---|
| N0 | N1 | N2 | N3 | N4 | |
| Coverage (%) | 62.92 ± 5.50 C | 71.67 ± 3.23 BC | 71.50 ± 3.18 BC | 82.00 ± 1.48 AB | 89.50 ± 1.41 A |
| Species Number | 14.67 ± 0.72 C | 15.00 ± 0.47 C | 18.00 ± 1.25 BC | 22.00 ± 0.82 A | 21.00 ± 0.94 AB |
| Shannon–Wiener index | 3.00 ± 0.15 AB | 2.78 ± 0.10 B | 3.19 ± 0.15 AB | 3.46 ± 0.10 A | 3.47 ± 0.06 A |
| Biomass (g·m−2) | 128.33 ± 15.83 C | 134.63 ± 1.71 C | 189.73 ± 25.77 BC | 254.23 ± 10.33 B | 335.73 ± 28.24 A |
| Litter (g·m−2) | 45.35 ± 2.55 B | 45.21 ± 2.09 B | 45.43 ± 1.88 B | 54.75 ± 6.12 B | 95.86 ± 4.62 A |
| pH | 8.57 ± 0.02 A | 8.51 ± 0.03 AB | 8.43 ± 0.05 BC | 8.44 ± 0.02 BC | 8.35 ± 0.02 C |
| SBD (g·cm−3) | 119.48 ± 1.84 A | 122.43 ± 0.66 A | 119.17 ± 4.65 A | 117.88 ± 4.18 A | 119.26 ± 2.31 A |
| SOC (g·kg−1) | 3.88 ± 0.31 A | 3.60 ± 0.33 A | 2.55 ± 0.06 B | 3.84 ± 0.22 A | 3.57 ± 0.13 A |
| DOC (mg·kg−1) | 220.41 ± 5.82 A | 227.23 ± 9.03 A | 227.76 ± 5.89 A | 242.25 ± 10.78 A | 240.86 ± 2.88 A |
| TP (g·kg−1) | 0.67 ± 0.01 A | 0.64 ± 0.03 A | 0.68 ± 0.01 A | 0.70 ± 0.01 A | 0.69 ± 0.00 A |
| TN (g·kg−1) | 0.52 ± 0.01 B | 0.46 ± 0.01 BC | 0.40 ± 0.01 C | 0.51 ± 0.01 AB | 0.66 ± 0.03 A |
| AP (mg·kg−1) | 4.89 ± 0.47 B | 5.75 ± 0.11 AB | 6.53 ± 1.01 AB | 7.52 ± 0.78 A | 7.75 ± 0.40 A |
| NH4+-N (mg·kg−1) | 4.27 ± 0.45 A | 3.94 ± 0.29 A | 5.97 ± 1.23 A | 5.12 ± 1.06 A | 5.03 ± 1.45 A |
| NO3−-N (mg·kg−1) | 4.57 ± 0.14 A | 3.48 ± 0.26 BC | 2.82 ± 0.19 C | 3.71 ± 0.24 AB | 4.30 ± 0.22 AB |
| MBC (mg·kg−1) | 102.43 ± 8.44 AB | 76.90 ± 3.14 B | 49.82 ± 4.11 C | 99.87 ± 5.96 AB | 120.98 ± 8.90 A |
| MBN (mg·kg−1) | 20.07 ± 1.67 BC | 15.21 ± 0.59 CD | 14.08 ± 0.68 D | 24.99 ± 1.69 AB | 27.75 ± 1.60 A |
| MBP (mg·kg−1) | 3.93 ± 0.38 B | 3.21 ± 0.12 B | 3.30 ± 0.16 B | 5.17 ± 0.10 A | 5.57 ± 0.12 A |
| BG (nmol·h−1·g−1) | 23.83 ± 2.33 AB | 19.73 ± 1.06 B | 13.42 ± 0.87 C | 20.12 ± 0.84 AB | 28.51 ± 0.47 A |
| LAP (nmol·h−1·g−1) | 18.24 ± 0.93 B | 17.58 ± 0.13 B | 12.89 ± 0.85 C | 16.97 ± 1.18 B | 27.48 ± 0.45 A |
| NAG (nmol·h−1·g−1) | 4.16 ± 0.67 B | 4.17 ± 0.58 B | 3.90 ± 0.70 B | 7.22 ± 0.93 AB | 10.30 ± 1.07 A |
| ALP (nmol·h−1·g−1) | 41.92 ± 4.13 AB | 39.30 ± 2.29 AB | 32.98 ± 2.65 B | 39.73 ± 4.19 AB | 46.72 ± 1.38 A |
| LOC (mg kg−1) | 0.94 ± 0.06 B | 1.02 ± 0.03 AB | 1.04 ± 0.04 AB | 1.15 ± 0.03 AB | 1.19 ± 0.08 A |
| CUE | 0.45 ± 0.01 A | 0.47 ± 0.01 A | 0.46 ± 0.00 A | 0.47 ± 0.01 A | 0.47 ± 0.01 A |
| N addition | pH | TN (g·kg−1) | TP (g·kg−1) | NH4+-N (g·kg−1) | NO3−-N (g·kg−1) | AP (g·kg−1) | MBN (mg·kg−1) | MBP (mg·kg−1) | BG (nmol·h−1·g−1) | LAP (nmol·h−1·g−1) | NAG (nmol·h−1·g−1) | ALP (nmol·h−1·g−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N0 | micro- | 8.55 ± 0.02 aA | 0.67 ± 0.02 aA | 0.59 ± 0.01 aA | 5.07 ± 0.22 aA | 4.73 ± 0.05 aA | 5.33 ± 0.95 aA | 15.80 ± 0.76 aA | 6.10 ± 0.66 aA | 27.92 ± 2.29 aAB | 16.25 ± 0.95 aB | 4.64 ± 0.92 aB | 47.26 ± 1.24 aA |
| medium | 8.55 ± 0.01 aA | 0.38 ± 0.02 bCD | 0.59 ± 0.00 aA | 2.55 ± 0.19 aA | 4.78 ± 0.30 aA | 5.03 ± 0.64 aB | 14.48 ± 2.36 aA | 6.40 ± 0.71 aA | 21.06 ± 4.02 aAB | 14.39 ± 1.19 aB | 3.48 ± 0.47 aB | 29.66 ± 0.08 bAB | |
| macro- | 8.56 ± 0.02 aA | 0.41 ± 0.02 bBC | 0.55 ± 0.01 aA | 4.17 ± 0.95 aA | 4.24 ± 0.22 aA | 4.06 ± 0.37 aA | 15.89 ± 1.56 aAB | 5.13 ± 0.71 aA | 15.42 ± 3.37 aAB | 10.03 ± 0.63 bC | 2.37 ± 0.20 aB | 26.79 ± 0.95 bBC | |
| N1 | micro- | 8.49 ± 0.03 aAB | 0.38 ± 0.01 aD | 0.61 ± 0.03 aA | 3.50 ± 0.29 aA | 3.75 ± 0.37 aAB | 6.44 ± 1.21 aA | 14.83 ± 1.56 aA | 5.13 ± 0.43 aA | 19.88 ± 3.36 aBC | 16.51 ± 1.38 aB | 4.07 ± 0.56 aB | 26.66 ± 0.84 bB |
| medium | 8.47 ± 0.03 aAB | 0.49 ± 0.05 aBC | 0.55 ± 0.01 aA | 3.74 ± 0.38 aA | 3.70 ± 0.09 aB | 5.20 ± 0.50 aB | 14.94 ± 2.75 aA | 6.43 ± 1.18 aA | 15.84 ± 2.08 aB | 13.71 ± 0.59 aB | 3.26 ± 0.43 aB | 43.11 ± 6.04 aA | |
| macro- | 8.48 ± 0.03 aAB | 0.48 ± 0.01 aBC | 0.56 ± 0.01 aA | 4.16 ± 0.90 aA | 3.21 ± 0.44 aAB | 5.75 ± 0.64 aA | 15.75 ± 2.92 aAB | 6.17 ± 1.83 aA | 14.82 ± 2.31 aB | 13.49 ± 1.16 aBC | 2.99 ± 0.31 aB | 29.80 ± 2.21 abB | |
| N2 | micro- | 8.40 ± 0.05 aBC | 0.49 ± 0.01 aC | 0.57 ± 0.03 aA | 5.66 ± 1.24 aA | 3.24 ± 0.30 aB | 5.82 ± 1.05 aA | 14.71 ± 0.48 aA | 6.57 ± 1.84 aA | 14.77 ± 0.69 aC | 15.16 ± 1.42 aB | 5.50 ± 0.74 aB | 36.55 ± 3.23 aAB |
| medium | 8.41 ± 0.04 aBC | 0.34 ± 0.02 bD | 0.60 ± 0.01 aA | 6.03 ± 1.30 aA | 3.08 ± 0.35 aB | 6.68 ± 1.02 aAB | 12.02 ± 0.35 aA | 4.27 ± 0.53 aA | 13.17 ± 0.92 abB | 13.22 ± 1.82 aB | 4.98 ± 0.21 aAB | 23.31 ± 1.80 bB | |
| macro- | 8.41 ± 0.05 aBC | 0.37 ± 0.05 abC | 0.55 ± 0.01 aA | 6.17 ± 1.47 aA | 2.38 ± 0.15 aB | 6.83 ± 1.62 aA | 12.61 ± 2.09 aB | 4.67 ± 0.83 aA | 10.10 ± 1.09 bB | 12.80 ± 1.10 aBC | 2.97 ± 0.25 bB | 20.02 ± 1.91 bC | |
| N3 | micro- | 8.43 ± 0.02 aABC | 0.47 ± 0.01 bC | 0.58 ± 0.03 aA | 4.89 ± 0.82 aA | 3.71 ± 0.42 aAB | 8.94 ± 2.09 aA | 16.87 ± 2.95 aA | 6.30 ± 0.54 aA | 21.58 ± 1.82 aBC | 18.95 ± 1.95 aB | 7.20 ± 0.84 aAB | 29.96 ± 3.54 aB |
| medium | 8.42 ± 0.02 aBC | 0.59 ± 0.02 aAB | 0.58 ± 0.03 aA | 5.01 ± 0.86 aA | 4.01 ± 0.03 aAB | 6.06 ± 0.40 aAB | 20.36 ± 4.13 aA | 5.20 ± 1.11 aA | 18.22 ± 2.00 abB | 15.98 ± 1.06 aB | 5.44 ± 0.85 aAB | 39.34 ± 5.49 aA | |
| macro- | 8.42 ± 0.02 aBC | 0.50 ± 0.01 bB | 0.58 ± 0.02 aA | 5.44 ± 1.38 aA | 3.42 ± 0.29 aAB | 6.43 ± 0.53 aA | 15.07 ± 1.42 aB | 3.60 ± 0.08 aA | 11.96 ± 1.18 bB | 15.02 ± 1.08 aB | 5.08 ± 1.32 aAB | 23.65 ± 2.27 aBC | |
| N4 | micro- | 8.32 ± 0.02 aC | 0.60 ± 0.03 bB | 0.62 ± 0.03 aA | 5.32 ± 1.45 aA | 4.28 ± 0.41 aAB | 8.00 ± 0.34 aA | 19.69 ± 1.91 aA | 4.93 ± 0.70 aA | 31.19 ± 0.60 aA | 26.89 ± 1.38 aA | 9.62 ± 0.83 aA | 42.85 ± 3.66 aA |
| medium | 8.32 ± 0.02 aC | 0.64 ± 0.03 abA | 0.58 ± 0.00 aA | 5.25 ± 1.35 aA | 4.67 ± 0.24 aA | 8.58 ± 0.52 aA | 21.55 ± 1.82 aA | 6.77 ± 0.92 aA | 28.22 ± 0.71 aA | 22.89 ± 0.32 abA | 7.04 ± 0.62 aA | 44.19 ± 0.20 aA | |
| macro- | 8.34 ± 0.02 aC | 0.72 ± 0.01 aA | 0.54 ± 0.00 aA | 4.47 ± 1.33 aA | 3.88 ± 0.25 aA | 6.94 ± 0.39 aA | 23.29 ± 1.07 aA | 5.57 ± 0.96 aA | 23.12 ± 1.22 bA | 20.12 ± 0.96 bA | 6.61 ± 0.63 aA | 41.72 ± 0.81 aA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Lu, X.; Yang, J.; Zhang, D.; Ren, C.; Wang, X.; Zhang, X.; Deng, J. Effects of Nitrogen Addition on Microbial Carbon Use Efficiency of Soil Aggregates in Abandoned Grassland on the Loess Plateau of China. Forests 2022, 13, 276. https://doi.org/10.3390/f13020276
Zhao X, Lu X, Yang J, Zhang D, Ren C, Wang X, Zhang X, Deng J. Effects of Nitrogen Addition on Microbial Carbon Use Efficiency of Soil Aggregates in Abandoned Grassland on the Loess Plateau of China. Forests. 2022; 13(2):276. https://doi.org/10.3390/f13020276
Chicago/Turabian StyleZhao, Xue, Xiaoyue Lu, Jiayi Yang, Dan Zhang, Chengjie Ren, Xiukang Wang, Xiaoxi Zhang, and Jian Deng. 2022. "Effects of Nitrogen Addition on Microbial Carbon Use Efficiency of Soil Aggregates in Abandoned Grassland on the Loess Plateau of China" Forests 13, no. 2: 276. https://doi.org/10.3390/f13020276
APA StyleZhao, X., Lu, X., Yang, J., Zhang, D., Ren, C., Wang, X., Zhang, X., & Deng, J. (2022). Effects of Nitrogen Addition on Microbial Carbon Use Efficiency of Soil Aggregates in Abandoned Grassland on the Loess Plateau of China. Forests, 13(2), 276. https://doi.org/10.3390/f13020276








