Spatiotemporal Dynamics and Factors Driving the Distributions of Pine Wilt Disease-Damaged Forests in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Data
2.2. Environmental Factors
2.3. Piecewise Polynomial Fitting
2.4. Spatial Autocorrelation Analysis
2.5. MaxEnt Model
3. Results
3.1. Spatiotemporal Distribution of PWD-Damaged Forests
3.1.1. Temporal Distribution Characteristics
3.1.2. Spatial Distribution Characteristics
3.2. Factors Influencing the Distribution of PWD-Damaged Forests
3.2.1. MaxEnt Model with Short-Term Environmental Factors
3.2.2. MaxEnt Model with Long-Term Environmental Factors
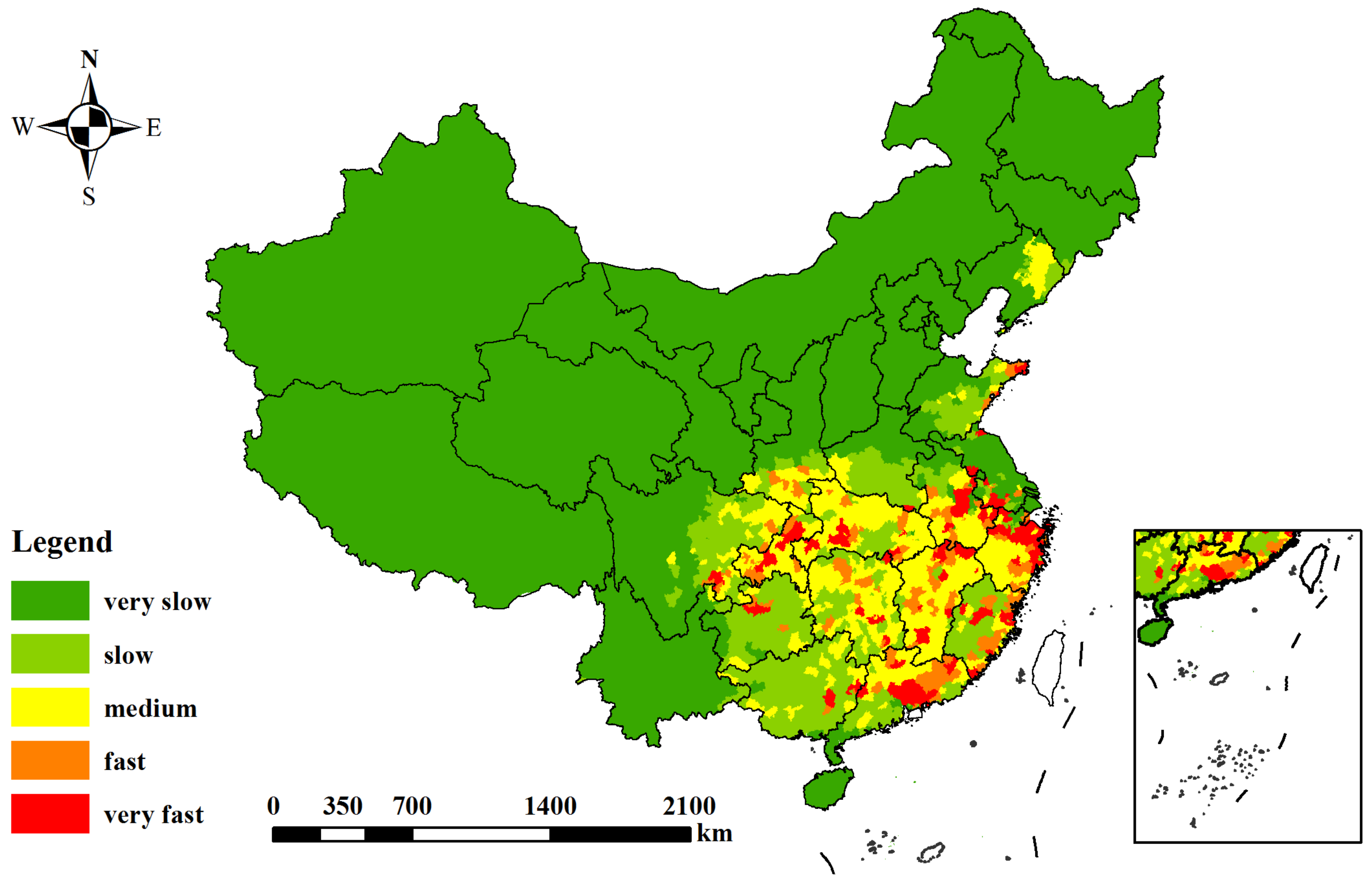
3.3. Risk Assessment of PWD-Damaged Forests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Y.; Ziegler, A.D.; Elsen, P.R.; Liu, Y.; He, X.; Spracklen, D.V.; Holden, J.; Jiang, X.; Zheng, C.; Zeng, Z. Upward expansion and acceleration of forest clearance in the mountains of Southeast Asia. Nat. Sustain. 2021, 4, 892–899. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A.R. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model—Pine Wilt disease as a model case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futai, K. Pine Wilt in Japan: From First Incidence to the Present. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Japan, Tokyo, 2008; pp. 5–12. [Google Scholar]
- Zhao, B.G. Pine Wilt Disease in China. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Japan, Tokyo, 2008; pp. 18–25. [Google Scholar]
- Tzean, S.; Jan, S. Pine wilt disease caused by pinewood nematode (Bursaphelenchus xylophilus) and its occurrence in Taiwan. Phytopathol. Entomol. NTU 1985, 12, 1–19. [Google Scholar]
- Han, H.; Chung, Y.J.; Shin, S.C. First Report of Pine Wilt Disease on Pinus koraiensis in Korea. Plant Dis. 2008, 92, 1251. [Google Scholar] [CrossRef]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- State Forestry and Grassland Administration. Pine Wilt Disease. 2021. Available online: https://www.forestry.gov.cn/main/5462/20210521/114505021470794.html (accessed on 13 June 2021).
- Ye, J. Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. Sci. Silvae Sin. 2019, 55, 1–10. [Google Scholar]
- Hao, Z.; Huang, J.; Zhou, Y.; Fang, G. Spatiotemporal pattern of pine wilt disease in the Yangtze river basin. Forests 2021, 12, 731. [Google Scholar] [CrossRef]
- Robinet, C.; Imbert, C.-E.; Rousselet, J.; Sauvard, D.; Garcia, J.; Goussard, F.; Roques, A. Human-mediated long-distance jumps of the pine processionary moth in Europe. Biol. Invasions 2012, 14, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Wang, Z.; Wang, H.; Hao, Y.; Shi, J. Relationship between pine wilt disease outbreaks and climatic variables in the three Gorges reservoir region. Forests 2019, 10, 816. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T. Responses of water-stressed Pinus thunbergii to inoculation with avirulent pine wood nematode (Bursaphelenchus xylophilus): Water relations and xylem histology J. For. Res. 1996, 1, 223–226. [Google Scholar] [CrossRef]
- Rutherford, T.A.; Webster, J.M. Distribution of pine wilt disease with respect to temperature in North America, Japan, and Europe. Can. J. For. Res. 1987, 17, 1050–1059. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Pan, L.; Meng, F.; Zhang, X. Cold adaptive potential of pine wood nematodes overwintering in plant hosts. Biol. Open 2019, 8, bio041616. [Google Scholar] [CrossRef] [Green Version]
- Jikumaru, S.; Togashi, K. Temperature effects on the transmission of Bursaphelenchus xylophilus (Nemata: Aphelenchoididae) by Monochamus alternatus (Coleoptera: Cerambycidae). J. Nematol. 2000, 32, 110–116. [Google Scholar]
- Wang, B.; Ma, L.; Wang, F.; Wang, B.; Hao, X.; Xu, J.; Ma, Y. Low Temperature Extends the Lifespan of Bursaphelenchus xylophilus through the cGMP Pathway. Int. J. Mol. Sci. 2017, 18, 2320. [Google Scholar] [CrossRef] [Green Version]
- Robinet, C.; Roques, A.; Pan, H.; Fang, G.; Ye, J.; Zhang, Y.; Sun, J. Role of human-mediated dispersal in the spread of the Pinewood Nematode in China. PLoS ONE 2009, 4, e4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Kiyohara, T. Influence of water stress on development of pine wilting disease caused by Bursaphelenchus lignicolus. Eur. J. Plant Pathol. 1978, 8, 97–107. [Google Scholar] [CrossRef]
- Rebetez, M.; Dobbertin, M. Climate change may already threaten Scots pine stands in the Swiss Alps. Theor. Appl. Climatol. 2004, 79, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jaime, L.; Batllori, E.; Margalef-Marrase, J.; Pérez Navarro, M.Á.; Lloret, F. Scots pine (Pinus sylvestris L.) mortality is explained by the climatic suitability of both host tree and bark beetle populations. For. Ecol. Manag. 2019, 448, 119–129. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Battisti, A.; Stastny, M.; Buffo, E.; Larsson, S. A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Glob. Change Biol. 2006, 12, 662–671. [Google Scholar] [CrossRef]
- Aukema, B.H.; Carroll, A.L.; Zheng, Y.; Zhu, J.; Raffa, K.F.; Dan Moore, R.; Stahl, K.; Taylor, S.W. Movement of outbreak populations of mountain pine beetle: Influences of spatiotemporal patterns and climate. Ecography 2008, 31, 348–358. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Zhao, M.; Zhao, X. A harmonized global nighttime light dataset 1992–2018. Sci. Data 2020, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Marbuah, G.; Gren, I.-M.; McKie, B.G.; Buisson, L. Economic activity and distribution of an invasive species: Evidence from night-time lights satellite imagery data. Ecol. Econ. 2021, 185, 107037. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Matsuhashi, S.; Hirata, A.; Akiba, M.; Nakamura, K.; Oguro, M.; Takano, K.T.; Nakao, K.; Hijioka, Y.; Matsui, T. Developing a point process model for ecological risk assessment of pine wilt disease at multiple scales. For. Ecol. Manag. 2020, 463, 118010. [Google Scholar] [CrossRef]
- Kumar, S.; Graham, J.; West, A.M.; Evangelista, P.H. Using district-level occurrences in MaxEnt for predicting the invasion potential of an exotic insect pest in India. Comput. Electron. Agric. 2014, 103, 55–62. [Google Scholar] [CrossRef]
- Medley, K.A. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob. Ecol. Biogeogr. 2010, 19, 122–133. [Google Scholar] [CrossRef]
- Hutchinson, M.F.; Xu, T. Anusplin version 4.2 User Guide. Centre for Resource and Environmental Studies, The Australian National University: Canberra, Australia, 2004; p. 54. [Google Scholar]
- Pramanik, M.; Paudel, U.; Mondal, B.; Chakraborti, S.; Deb, P. Predicting climate change impacts on the distribution of the threatened Garcinia indica in the Western Ghats, India. Clim. Risk Manag. 2018, 19, 94–105. [Google Scholar] [CrossRef]
- Mateo, R.G.; Vanderpoorten, A.; Muñoz, J.; Laenen, B.; Désamoré, A. Modeling species distributions from heterogeneous data for the biogeographic regionalization of the European bryophyte flora. PLoS ONE 2013, 8, e55648. [Google Scholar] [CrossRef] [Green Version]
- Cunis, T.; Burlion, L.; Condomines, J.-P. Piecewise polynomial modeling for control and analysis of aircraft dynamics beyond stall. J. Guid. Control Dyn. 2019, 42, 949–957. [Google Scholar] [CrossRef]
- Gao, J.; Ji, W.; Zhang, L.; Shao, S.; Wang, Y.; Shi, F. Fast piecewise polynomial fitting of time-series data for streaming computing. IEEE Access 2020, 8, 43764–43775. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, F.; Li, G.; Qin, W.; Li, S.; Gao, H.; Cai, Z.; Lin, G.; Zhang, T. Maxent modeling for predicting the spatial distribution of three raptors in the Sanjiangyuan National Park, China. Ecol. Evol. 2019, 9, 6643–6654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007, 7, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briscoe Runquist, R.D.; Lake, T.; Tiffin, P.; Moeller, D.A. Species distribution models throughout the invasion history of Palmer amaranth predict regions at risk of future invasion and reveal challenges with modeling rapidly shifting geographic ranges. Sci. Rep. 2019, 9, 2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-S.; Choi, W.I.; Nam, Y.; Park, Y.-S. Predicting potential occurrence of pine wilt disease based on environmental factors in South Korea using machine learning algorithms. Ecol. Inform. 2021, 64, 101378. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum entropy modeling to predict the impact of climate change on pine wilt disease in China. Front. Plant Sci. 2021, 12, 764. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Obenauer, J.F.; Andrew Joyner, T.; Harris, J.B. The importance of human population characteristics in modeling Aedes aegypti distributions and assessing risk of mosquito-borne infectious diseases. Trop. Med. Health 2017, 45, 38. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Wu, J.; Li, W.; Peng, J.; Liu, H. Evaluating Saturation Correction Methods for DMSP/OLS Nighttime Light Data: A Case Study from China’s Cities. Remote Sens. 2014, 6, 9853–9872. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, P.H.; Kumar, S.; Stohlgren, T.J.; Young, N.E. Assessing forest vulnerability and the potential distribution of pine beetles under current and future climate scenarios in the Interior West of the US. For. Ecol. Manag. 2011, 262, 307–316. [Google Scholar] [CrossRef]
- Zhao, L.; Mota, M.; Vieira, P.; Butcher, R.A.; Sun, J. Interspecific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol. 2014, 30, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.E.; Franklin, S.B. Understanding the relative roles of disturbance and species interactions in shaping Mississippi River island plant communities. Community Ecol. 2011, 12, 108–116. [Google Scholar] [CrossRef]



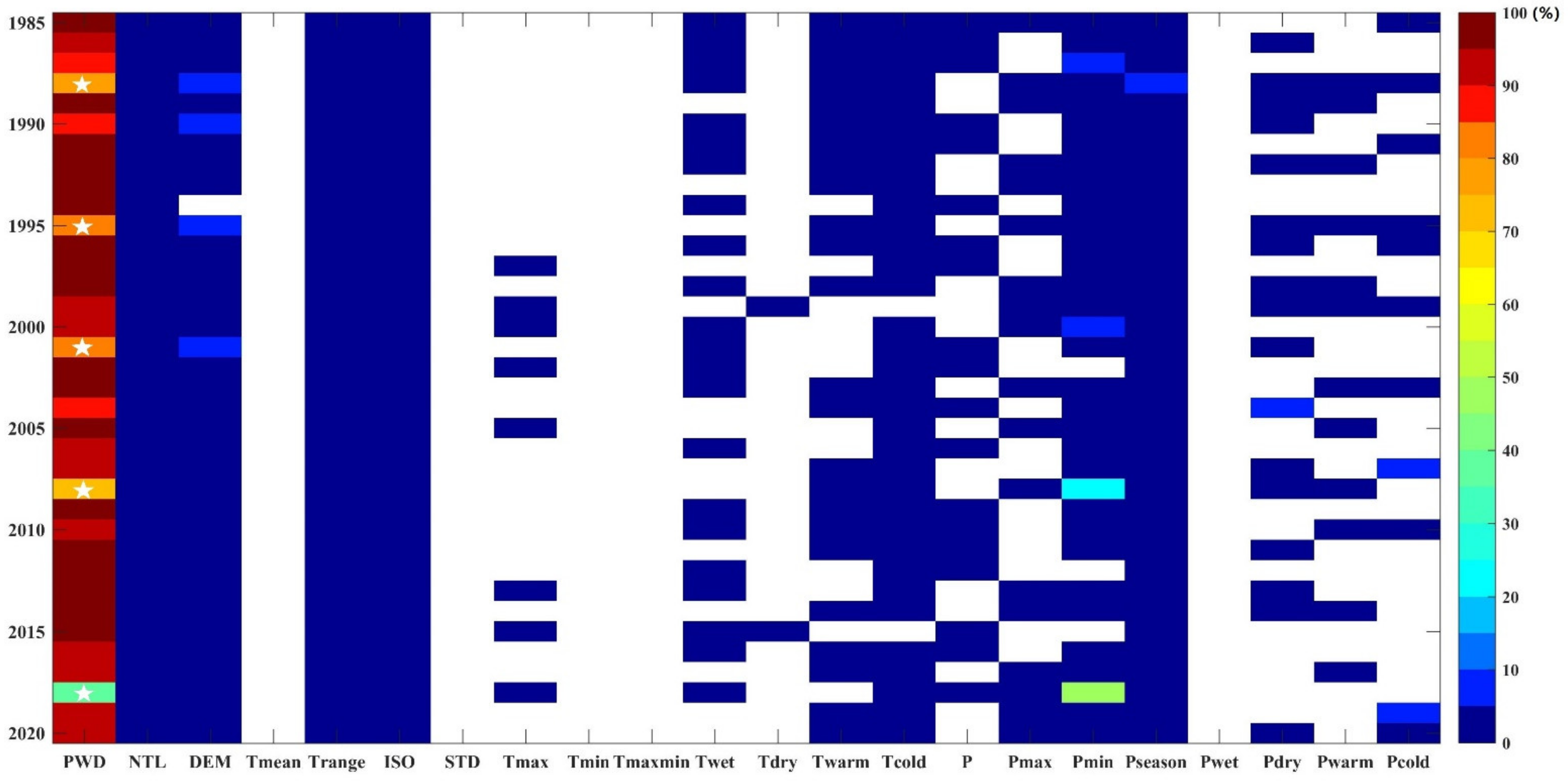


| Type | Definition |
|---|---|
| 19 Bioclimatic variables | Bio1 (Tmean) = annual mean temperature; Bio2 (Trange) = mean diurnal range (mean of monthly) (maximum temperature − minimum temperature)); Bio3 (ISO) = isothermality (Bio2/Bio7) × 100; Bio4 (STD) = temperature seasonality (standard deviation × 100); Bio5 (Tmax) = maximum temperature in warmest month; Bio6 (Tmin) = minimum temperature in coldest month; Bio7 (Tmaxmin) = annual temperature range (Bio5−Bio6); Bio8 (Twet) = mean temperature in wettest quarter; Bio9 (Tdry) = mean temperature in driest quarter; Bio10 (Twarm) = mean temperature in warmest quarter; Bio11 (Tcold) = mean temperature in coldest quarter; Bio12 (P) = annual precipitation; Bio13 (Pmax) = precipitation in wettest month; Bio14 (Pmin) = precipitation in driest month; Bio15 (Pseason) = precipitation seasonality (coefficient of variation); Bio16 (Pwet) = precipitation in wettest quarter; Bio17 (Pdry) = precipitation in driest quarter; Bio18 (Pwarm) = precipitation in warmest quarter; and Bio19 (Pcold) = precipitation in coldest quarter |
| DEM | Digital elevation model |
| Human activity | Nighttime light |
| Temporal dependence | Presence/absence of PWD-damaged forest in the cell for the previous year |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Peng, W.; Liu, X.; He, G.; Cai, Y. Spatiotemporal Dynamics and Factors Driving the Distributions of Pine Wilt Disease-Damaged Forests in China. Forests 2022, 13, 261. https://doi.org/10.3390/f13020261
Wang W, Peng W, Liu X, He G, Cai Y. Spatiotemporal Dynamics and Factors Driving the Distributions of Pine Wilt Disease-Damaged Forests in China. Forests. 2022; 13(2):261. https://doi.org/10.3390/f13020261
Chicago/Turabian StyleWang, Wei, Wanting Peng, Xiuyu Liu, Geng He, and Yongli Cai. 2022. "Spatiotemporal Dynamics and Factors Driving the Distributions of Pine Wilt Disease-Damaged Forests in China" Forests 13, no. 2: 261. https://doi.org/10.3390/f13020261
APA StyleWang, W., Peng, W., Liu, X., He, G., & Cai, Y. (2022). Spatiotemporal Dynamics and Factors Driving the Distributions of Pine Wilt Disease-Damaged Forests in China. Forests, 13(2), 261. https://doi.org/10.3390/f13020261








