Role of Egg Parasitoids in Controlling the Pine Processionary Moth in the Cedar Forests of Chréa National Park (Algeria)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Collection of Biological Material
2.3. Data Analysis
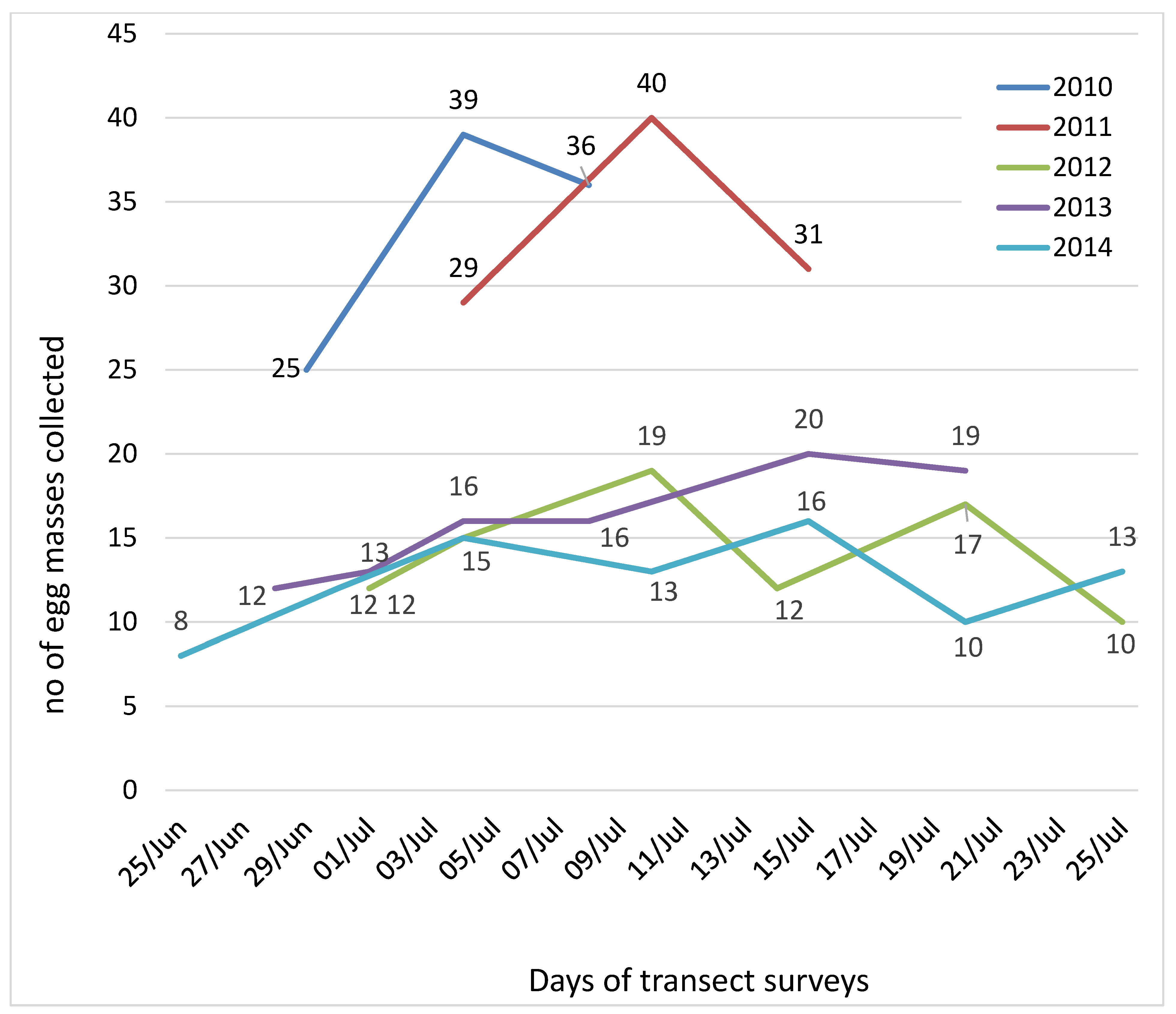
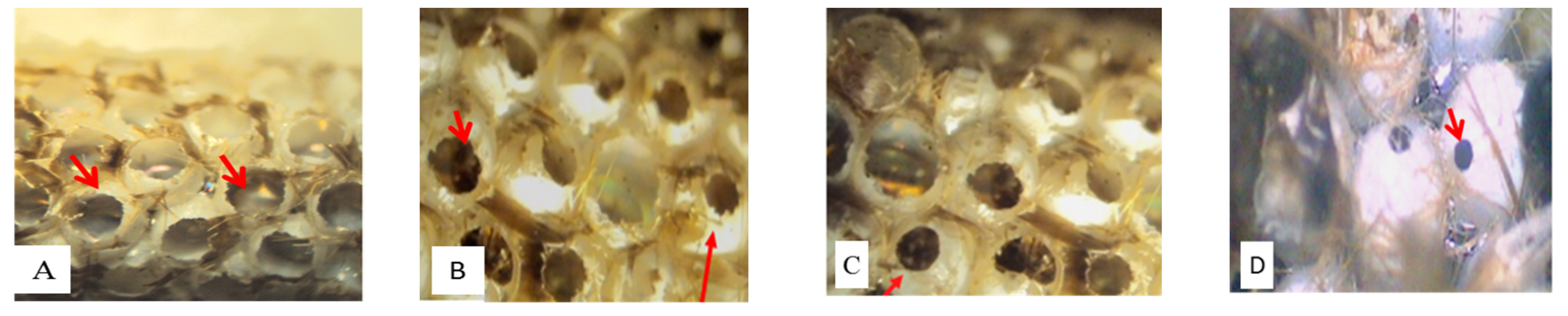
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bentouati, A. La situation du cèdre de l’Atlas dans les Aurès (Algérie). Forêt Méditerr. 2008, 29, 203–208. [Google Scholar]
- Beghami, R.; Bertella, N.; Laamari, M.; Bensaci, O.A. Bark beetle and woodborer insects’ outbreak as a potent driver of Atlas cedar (Cedrus atlantica (Endl.) Carriere) forests dieback in Aures, East Algeria. For. Sci. Technol. 2020, 16, 75–85. [Google Scholar] [CrossRef]
- Schmidt, G.H.; Mirchev, R.; Tsankov, G. The Egg Parasitoids of Thaumetopoea pityocampa in the Atlas Mountains near Marrakech (Morocco). Phytoparasitica 1997, 25, 275–281. [Google Scholar] [CrossRef]
- Sebti, S.; Chakali, G. Distribution and importance of the pine processionary moth winter nests Thaumetopoea pityocampa (Denis and Schiffermüller) (Lepidoptera: Notodontidae) in the forests cedar of the national park of Chréa (Algeria). Int. J. Agric. Sci. Res. 2014, 5, 77–84. [Google Scholar]
- Hezil, S.; Chakali, G.; Battisti, A. Plant phenotype affects oviposition behaviour of pine processionary moth and egg survival at the southern edge of its range. For.-Biogeosci. For. 2018, 11, 568–572. [Google Scholar] [CrossRef]
- Jactel, H.; Barbaro, L.; Battisti, A.; Bosc, A.; Branco, M.; Brockerhoff, E.; Castagneyrol, B.; Dulaurent, A.M.; Hodar, J.A.; Jacquet, J.S.; et al. Insect—Tree Interactions in Thaumetopoea pityocampa. In Processionary Moths and Climate Change: An Update; Springer: Versailles, France, 2015; pp. 265–310. [Google Scholar]
- Sbabdji, M.; El Hadi, O.; Haddad, A.; Kadik, B.; Lambs, L. Cedar tree growth (Cedrus atlantica Manetti) in Chréa National Park, Algeria, and the influence of defoliation by the pine processionary caterpillar (Thaumetopoea pityocampa Schiff.). Rev. D’ecol. 2009, 64, 323–332. [Google Scholar]
- Jacquet, J.S.; Orazio, C.; Jactel, H. Defoliation by processionary moth significantly reduces tree growth: A quantitative review. Ann. For. Sci. 2012, 69, 857–866. [Google Scholar] [CrossRef]
- El Yousfi, M. La santé du Cèdre de l’Atlas au Maroc. Ann. De La Rech. For. Maroc 1994, 27, 593–611. [Google Scholar]
- Ayache, S.; El Mokhefi, M.; Bonifácio, L.; Chakali, G. Egg batches parasitism of processionary moth Thaumetopoea pityocampa (Lepidoptera, Thaumetopoeidae) from two Atlas cedar ecotypes in Algeria. Zoodiversity 2021, 55, 239–250. [Google Scholar] [CrossRef]
- Huchon, H.; Démolin, G. La bio écologie de la processionnaire du pin. Dispersion potentielle-Dispersion actuelle. Rev. For. Fr. 1970, 22, 220–234. [Google Scholar] [CrossRef]
- Newton, I. Population Limitation in Birds; Academic Press: London, UK, 1998; pp. 1–597. [Google Scholar]
- Grison, P. Lutte intégrée en forêt. Rev. For. Fr. 1970, 22, 256–271. [Google Scholar] [CrossRef]
- Geri, C. Répartition et évolution des populations de la processionnaire du pin, Thaumetopoea pityocampa Schiff., (Lépidoptère, Thaumetopoeidae) dans les montagnes corses. Acta Oecol.—Oecol. Appl. 1983, 4, 247–268. [Google Scholar]
- Hódar, J.A.; Zamora, R.; Cayuela, L. Climate change and the incidence of a forest pest in Mediterranean ecosystems: Can the North Atlantic Oscillation be used as a predictor? Clim. Chang. 2012, 113, 699–711. [Google Scholar] [CrossRef]
- Battisti, A.; Stastny, M.; Netherer, S.; Robinet, C.; Schopf, A.; Roques, A.; Larsson, S. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol. Appl. 2005, 15, 2084–2096. [Google Scholar] [CrossRef]
- Battisti, A.; Stastny, M.; Buffo, E.; Larsson, S. A rapid altitudinal range expansion in the pine recessionary moth produced by the 2003 climatic anomaly. Glob. Chang. Biol. 2006, 12, 662–671. [Google Scholar] [CrossRef]
- Hódar, J.A.; Castro, J.; Zamora, R. Pine processionary caterpillar Thaumetopoea pityocampa as a new threat for relict Mediterranean Scots pine forests under climatic warming. Biol. Conserv. 2003, 110, 123–129. [Google Scholar] [CrossRef]
- Battisti, A. Forests and climate change—lessons from insects. Ifor. Biogeosci. For. 2008, 1, 1–5. [Google Scholar] [CrossRef]
- Harrington, R.; Fleming, R.A.; Woiwod, I.P. Climate change impacts on insect management and conservation in temperate regions: Can they be predicted? Agric. For. Entomol. 2001, 3, 233–240. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, A.; Menzel, C.; Parmesan, T.J.C.; Beebee, J.M.; Fromentin, O.; Hoegh-Guldberg, F.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Stireman, J.O.; Dyer, L.A.; Janzen, D.H.; Singer, M.S.; Li, J.T.; Marquis, R.J.; Ricklefs, R.E.; Gentry, G.L.; Hallwachs, W.; Coley, P.D.; et al. Climatic unpredictability and parasitism of caterpillars: Implications of global warming. Proc. Natl. Acad. Sci. USA 2005, 102, 17384–17387. [Google Scholar] [CrossRef] [PubMed]
- Van Asch, M.; van Tienderen, P.H.; Holleman, L.J.M.; Visser, M. Predicting adaptation of phenology in response to climate change, an insect herbivore example. Glob. Chang. Biol. 2007, 13, 1596–1604. [Google Scholar] [CrossRef]
- STATISTICA Software; Version 6.1; StatSoft, Inc.: Tulsa, OK, USA, 2003.
- Mangel, M. Evolution of host selection in parasitoids: Does the state of the parasitoid matter? Am. Nat. 1989, 133, 688–705. [Google Scholar] [CrossRef]
- Courtney, S.P.; Kibota, T.T. Mother doesn’t know best: Selection of hosts by ovipositing insects. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 161–187. [Google Scholar]
- Bovey, P. Impact de l’Insecte déprédateur sur la Forêt. Rev. For. Fr. 1970, 33, 199–203. [Google Scholar] [CrossRef]
- Wellington, W.G. Qualitative changes in populationin unstable environments. Can. Entomol. 1964, 96, 436–451. [Google Scholar] [CrossRef]
- Wellington, W.G. Some maternal influence on progeny quality in the western tent caterpillar, Malacosoma pluvial Dyar. Can. Entomol. 1965, 97, 1–14. [Google Scholar] [CrossRef]
- Klomp, H. Intraspecific competition and the regulation of insect numbers. Annu. Rev. Entomol. 1964, 9, 17–40. [Google Scholar] [CrossRef]
- Hawley, A.W. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: Epidemiological consequences. J. Anim. Ecol. 1985, 54, 955–964. [Google Scholar] [CrossRef]
- Mangel, M.; Rosenheim, J.A.; Alder, F.R. Clutch size offspring performance and intergenerational fitness. Behav. Ecol. 1994, 5, 412–417. [Google Scholar] [CrossRef][Green Version]
- Parker, G.A.; Begon, M. Optimal egg size and clutch size: Effects of environment and maternal phenotype. Am. Nat. 1986, 128, 573–592. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations and Communities; Blackwell: Hoboken, NJ, USA, 1986; pp. 1–945. [Google Scholar]
- Sirot, E.; Bernstein, C. Food searching and superparasitism in solitary parasitoids. Acta Oecol. 1997, 18, 63–72. [Google Scholar] [CrossRef]
- Brockelmann, W.Y. Competition, the fitness of offspring, and the optimal clutch size. Am. Nat. 1975, 109, 677–699. [Google Scholar] [CrossRef]
- Sather, B.E. Environmental stochasticity and population dynamics of large herbivores: A search for mechanisms. Trends Ecol. Evol. 1997, 12, 143–149. [Google Scholar] [CrossRef]
- Gaillard, J.M.; Festa-Bianchet, M.; Yoccoz, N.G.; Loison, A.; Toïgo, C. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Syst. 2000, 31, 367–393. [Google Scholar] [CrossRef]
- Barford, E. Crop pests advancing with global warming. Nature 2013. [Google Scholar] [CrossRef]
- Rocha, S.; Kerdelhué, C.; Ben Jamaa, M.L.; Dhahri, S.; Burban, C.; Branco, M. Effect of heat waves on embryo mortality in the pine processionary moth. Bull. Entomol. Res. 2017, 107, 583–591. [Google Scholar] [CrossRef]
- Freese, G.; Zwölfer, H. The problem of optimal clutch size in a tritrophicsystem: The oviposition strategy of the thistle gallfly Urophora cardui (Diptera:Tephritidae). Oecologia 1996, 108, 293–302. [Google Scholar] [CrossRef]
- Roff, D.A. The Evolution of Life Histories: Theory and Analysis; Chapman & Hall: New York, NY, USA, 1992; pp. 1–548. [Google Scholar]
- Lawson, S.A.; Furuta, K.; Katagiri, K. Effect of tree host and beetle density on reproduction and survival of Ips typographus japonicas Niijima (Coleoptera, Scolytidae) in Hokkaido, Japan. J. Appl. Entomol. 1995, 119, 383–390. [Google Scholar] [CrossRef]
- Sallé, A.; Baylac, M.; Lieutier, F. Size and shape changes of Ips typographus L. (Coleoptera: Scolytinae) in relation to population level. Agric. For. Entomol. 2005, 7, 297–306. [Google Scholar] [CrossRef]
- Sallé, A.; Raffa, K.F. Interactions among intraspecific competition, emergence patterns, and host selection behaviour in Ips pini (Coleoptera: Scolytinae). Ecol. Entomol. 2007, 32, 162–171. [Google Scholar] [CrossRef]
- Auger-Rozenberg, M.A.; Barbaro, L.; Battisti, A.; Blache, S.; Charbonnier, Y.; Denux, O.; Garcia, J.; Goussard, F.; Imbert, C.E.; Kerdelhué, C.; et al. Ecological responses of parasitoids, predators and associated insect communities to the climate-driven expansion of pine processionary moth. In Processionary Moths and Climate Change: An Update; Roques, A., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 311–358. [Google Scholar]
- Battisti, A. Field studies on the behaviour of two egg parasitoids of the pine processionary moth Thaumetopoea pityocampa. Entomophaga 1989, 34, 29–38. [Google Scholar] [CrossRef]
- Mirchev, P.; Georgiev, G.; Tsankov, G. Long-term studies on parasitoids of pine processionary pine (Thaumetopoea pityocampa) eggs in a new locality in Bulgaria. J. Entomol. Res. Soc. 2017, 19, 15–25. [Google Scholar]
- De Boer, J.G.; Harvey. J.A. Range-expansion in processionary moths and biological control. Insects 2020, 11, 267. [Google Scholar] [CrossRef]
- Sadat, F.; Nazari, A.; Jafari, S.; Karahroudi, Z.R. How long-term mass rearing affects the quality of the Trichogramma embryophagum (Hartig) (Hymenoptera: Trichogrammatidae) reared on Sitotroga cerealella (Olivier) eggs. Egypt J. Biol. Pest Control 2021, 31, 119–130. [Google Scholar] [CrossRef]
- Golbaghi, F.H.; Goldansaz, S.H.; Rahmani, S.; Attaran, M.R. Preference and performance of Trichogramma embryophagum when parasitizing Cydia pomonella and two stored-product moths. Bull. Insectol. 2020, 73, 79–86. [Google Scholar]
- Tiberi, R. Modificazioni della distribuzione dei parassiti oofagi in ovaturedi Thaumetopoea pityocampa, conseguenti al potenziamento artificial di Tetrastichus servadeii Dom. Redia 1980, 63, 307–321. [Google Scholar]
- Simonato, M.; Pilati, M.; Magnoux, E.; Courtin, C.; Sauné, L.; Rousselet, J.; Battisti, A.; Auger-Rozenberg, M.A.; Kerdelhué, C. A population genetic study of the egg parasitoid Baryscapus servadeii reveals large scale automictic parthenogenesis and almost fixed homozygosity. Biol. Control 2019, 139, 104097. [Google Scholar] [CrossRef]
- Bellin, S.; Schmidt, G.H.; Douma-Petridou, E. Structure, ooparasitoid spectrum and rate of parasitism of egg batches of Thaumetopoea pityocampa (Den. & Schiff.) (Lep. Thaumetopoeidae) in Greece. J. Appl. Entomol. 1990, 110, 113–120. [Google Scholar]
- Tiberi, R. Egg parasitoids of the pine processionary caterpillar, Thaumetopoea pityocampa (Den. & Schiff.) (Lepidoptera: Thaumetopoeidae) in Italy: Distribution and activity in different areas. J. Appl. Entomol. 1990, 110, 14–18. [Google Scholar]
- Zamoum, M.; Guendouz, H.; Deia, D. Structure des communautés d’ennemis naturels de Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae) sur pin d’Alep en Algérie subsaharienne. Entomol. Bari 2007, 40, 139–151. [Google Scholar]
- Mirchev, P.; Tsankov, G.; Avci, M.; Matova, M. Study of some aspects of ecology of pine processionary moth, Thaumetopoea pityocampa (den. & Schiff.) (Lepidoptera: Thaumetopoeidae) and its egg parasitoids in Turkey. Silva Balc. 2007, 8, 66–78. [Google Scholar]
- Petrucco-Toffolo, E.; Basso, A.; Kerdelhué, C.; Ipekdal, K.; Mendel, Z.; Simonato, M.; Battisti, A. Evidence of potential hybridization in the Thaumetopoea pityocampa-wilkinsoni complex. Agric. For. Entomol. 2018, 20, 9–17. [Google Scholar] [CrossRef]
- Daniel, Z.; Battisti, A.; Hellrigl, K.; Minerbi, S. Egg parasitoids of the pine processionary moth and their occurrence in Venosta/Vinschgau. For. Obs. 2006, 2, 81–88. [Google Scholar]
- Tunca, H.; Buradino, M.; Colombel, E.A.; Tabone, E. Tendency and consequences of superparasitism for the parasitoid Ooencyrtus pityocampae (Hymenoptera: Encyrtidae) in parasitizing a new laboratory host, Philosamia ricini (Lepidoptera: Saturniidae). Eur. J. Entomol. 2016, 113, 51–59. [Google Scholar] [CrossRef]

| Years | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|
| Mean air temp. (°C) | |||||
| June | 19.4 ± 1.88 | 20.1 ± 1.68 | 23.7 ± 1.61 | 18.2 ± 1.96 | 20.1 ± 1.71 |
| July | 25.9 ± 1.43 | 23.8 ± 1.38 | 25.2 ± 1.39 | 23.3 ± 1.28 | 23.6 ± 1.44 |
| August | 24.2 ± 1.42 | 25.5 ± 1.34 | 26.9 ± 1.33 | 23.5 ± 1.18 | 24.8 ± 1.39 |
| Max. air temp. (°C) | |||||
| June | 26.3 ± 1.70 | 27.1 ± 1.53 | 30.2 ± 1.33 | 27.2 ± 1.77 | 27.6 ± 1.56 |
| July | 33.6 ± 1.52 | 32.1 ± 1.44 | 35.3 ± 1.39 | 31.6 ± 1.60 | 32.3 ± 1.90 |
| August | 31.9 ± 1.54 | 32.5 ± 1.61 | 36.1 ± 1.65 | 32.5 ± 1.44 | 33.5 ± 1.68 |
| Egg mass sample size | 100 | 100 | 85 | 96 | 87 |
| Egg mass density at breast height (per ha) | 13 | 13 | 6 | 6 | 5 |
| Egg mass length (mm) | 26.55 ± 4.83 b | 28.14 ± 5.92 a | 25.91 ± 4.80 b | 22.68 ± 3.76 c | 23.30 ± 3.96 c |
| (18.0–38.0) | (16.2–46.3) | (17.0–41.0) | (17.0–39.0) | (17.9–41.1) | |
| Twig diameter (mm) | 3.77 ± 1.04 ab | 3.95 ± 1.07 a | 3.68 ± 1.04 bc | 3.76 ± 1.05 ab | 3.27 ± 1.01 c |
| (1.62–7.26) | (1.93–7.18) | (1.72–6.92) | (1.69–7.12) | (1.63–6.98) | |
| Number moth eggs | 22,558 | 23,759 | 18,755 | 18,429 | 17,133 |
| Fecundity (moth eggs per mass) | 225 ± 47 ab | 237 ± 45 a | 220 ± 41 b | 191 ± 43 c | 196 ± 54 c |
| (90–312) | (121–348) | (74–331) | (80–289) | (63–287) | |
| Hatched eggs | 203 ± 61 (90.22%) a | 212 ± 64 (89.45%) a | 97 ± 93 (44.09%) b | 65 ± 64 (34.03%) c | 66 ± 60 (33.67%) c |
| (27–312) | (15–334) | (0–276) | (0–229) | (4–242) | |
| Unhatched egg | 12 ± 23 (5.33%) a | 17 ± 23 (7.17%) ab | 101 ± 95 (45.91%) d | 29 ± 34 (15.18%) b | 69 ± 42 (35.20%) c |
| (0–160) | (0–130) | (0–273) | (0–244) | (2–177) | |
| Parasitized eggs | 10 ± 27 (4.53%) cd | 9 ± 24 (3.86%) d | 23 ± 41 (10.33%) c | 98 ± 63 (51.14%) a | 62 ± 48 (31.58%) b |
| (0–215) | (0–173) | (0–200) | (0–233) | (0–174) | |
| Eggs parasitized by B. servadeii | 4 ± 9 (1.62%) c | 2 ± 8 (1.15%) c | 14 ± 37 (6.30%) c | 78 ± 69 (40.46%) a | 36 ± 42 (18.12%) b |
| (0–49) | (0–54) | (0–200) | (0–233) | (0–126) | |
| Eggs parasitized by O. pityocampa | 2 ± 11 (0.96%) b | 2 ± 8 (0.75%) b | 4 ± 20 (1.97%) ab | 6 ± 17 (3.07%) ab | 9 ± 25 (4.65%) b |
| (0–89) | (0–173) | (0–179) | (0–96) | (0–119) | |
| Eggs parasitized by T. embryophagum | 4 ± 24 (1.95%) b | 5 ± 22 (1.96%) b | 4 ± 11 (2.06%) b | 15 ± 38 (7.61%) a | 17 ± 42 (8.81%) a |
| (0–215) | (0–54) | (0–80) | (0–196) | (0–174) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebti, S.; Bonifácio, L.; Chakali, G. Role of Egg Parasitoids in Controlling the Pine Processionary Moth in the Cedar Forests of Chréa National Park (Algeria). Forests 2022, 13, 211. https://doi.org/10.3390/f13020211
Sebti S, Bonifácio L, Chakali G. Role of Egg Parasitoids in Controlling the Pine Processionary Moth in the Cedar Forests of Chréa National Park (Algeria). Forests. 2022; 13(2):211. https://doi.org/10.3390/f13020211
Chicago/Turabian StyleSebti, Safia, Luís Bonifácio, and Gahdab Chakali. 2022. "Role of Egg Parasitoids in Controlling the Pine Processionary Moth in the Cedar Forests of Chréa National Park (Algeria)" Forests 13, no. 2: 211. https://doi.org/10.3390/f13020211
APA StyleSebti, S., Bonifácio, L., & Chakali, G. (2022). Role of Egg Parasitoids in Controlling the Pine Processionary Moth in the Cedar Forests of Chréa National Park (Algeria). Forests, 13(2), 211. https://doi.org/10.3390/f13020211






