Abstract
There is a complex cadmium (Cd) and iron (Fe) interaction in soil. To explore the influences of Fe application on the growth, Cd accumulation, and antioxidant capacity of poplar under Cd exposure, Populus tremula × P. alba ‘717’ was treated with different concentrations of Cd (0 and 100 μM) and Fe (50 and 150 μM). In addition, the root architecture, leaf chlorophyll content, Cd accumulation, and antioxidant enzyme activity were analyzed. The results showed that the high-dose Fe (150 μM) did not change poplar biomass in zero-Cd treatment but increased the chlorophyll content, total root surface area, net photosynthetic rate, and biomass accumulation of Cd-stressed poplar. In addition, under Cd stress, high-dose Fe increased the translocation factor (TF) of Cd, decreased root and leaf malondialdehyde (MDA) content, and enhanced root and leaf SOD activity. That is, high-dose Fe could alleviate the suppression of Cd on the growth of poplar and enhance the transport of Cd to aboveground tissues and the SOD activity in roots and leaves, thus alleviating the Cd-induced oxidative stress. This study will provide reference for the remediation of Cd-contaminated soils using poplar.
1. Introduction
Heavy metal accumulation caused by natural processes or anthropogenic activities severely affects the life and biodiversity on earth [1]. Especially, cadmium (Cd) pollution has attracted much attention from people of many countries [2,3,4,5]. Cd in soil can be absorbed by plant roots and enters the human body through the food chain, endangering human immune, nervous, and reproductive systems [6]. To reduce the Cd accumulation in soil and the threat of Cd pollution to human health, many physical, chemical, and biological remediation technologies have been developed [7,8]. Among them, phytoremediation is an effective, low-cost, and environment-friendly way [9] to remove heavy metals from soils by plants’ uptake. For example, Hu et al. [10] and Balestri et al. [11] reported that Pteris vittata and Sedum alfredii could adsorb a large amount of Cd in the soil. However, most known Cd-enriched plants are herbs. Due to the low biomass, the amount of Cd accumulation is very small compared with trees [2]. Therefore, some scholars began to study the remediation of Cd-contaminated soils using trees [12,13,14]. Marmiroli et al. reported that the total Cd contents extracted from Populus and Thlaspi were about 250 and 125 g ha−1 per year in similarly polluted soil, respectively [15]. Due to the fast growth and strong adaptability, the performance of poplar in Cd-contaminated soil remediation has been explored [16]. He et al. [17] found that due to Populus canescens had a large biomass: the total amount of Cd absorbed and transported to the aboveground part was considerable. The root and leaf Cd content of P. canescens exceeded 1000 and 100 μg g−1 DW, respectively. Therefore, poplar has great potential in phytoremediation.
Several studies have shown that Cd can enter plant cells through transport vectors of Fe2+, Zn2+, and Ca2+, disrupting the uptake of minerals by plants [18,19,20]. The strong binding ability of Cd to proteins could replace Fe in proteins and change protein function and chloroplast structure, thus inhibiting photosynthesis and reducing biomass accumulation [21,22]. Cd can also disturb the metabolism and increase the production of reactive oxygen species (ROS), which ultimately causes oxidative stress [23]. Many studies have shown that plants can adapt to environmental stresses through changing root architecture to obtain adequate nutrients [24]. In addition, the activities of antioxidant enzymes in plant cells, such as ascorbate peroxidase (APX), ascorbate oxidase (AAO), superoxide dismutase (SOD), and catalase (CAT), could also be induced to maintain intracellular redox balance [12].
Iron (Fe) is necessary for plant growth. The redox of Fe2+ and Fe3+ participates in plant electron transport chain, photosynthesis, respiration, and chloroplast synthesis [25,26]. A previous study found that Cd can disrupt the absorption and transportation of Fe by plants, causing the iron deficiency chlorosis of plants [27]. Fe deficiency further aggravates Cd-induced oxidative stress and inhibits plant growth [28,29]. Although Fe could restore the photosynthesis of plants, it also strengthens the absorption of Cd and the allocation to the aboveground part [30,31]. However, a previous study also proved that sufficient Fe could reduce the absorption of Cd by plants [32]. Fe is a key component of the iron superoxide dismutase (Fe-SOD) subunit, which is vital for ROS scavenging [33], and most Fe in the soil is in insoluble form that cannot be directly utilized by plants. Thus, exploring the effect of high-dose Fe on poplar growth and Cd accumulation is essential for poplar phytoremediation.
The root system directly contacts with soil Cd and is the channel for Cd to enter the plant’s aboveground part, so it is more sensitive to soil Cd [34]. Therefore, in this study, the effects of Fe treatments (50 and 150 μM) on the growth parameters, root architecture, Cd accumulation, and antioxidant enzyme activity of poplar under Cd exposure (100 μM) were explored, to clarify the mechanism of high-dose Fe alleviating Cd stress on poplar growth. This study hypothesized that: (1) high-dose Fe might affect the absorption and accumulation of Cd by poplar by changing poplar root architecture and (2) high-dose Fe might affect antioxidant enzyme activity to improve poplar tolerance to Cd stress. This research will provide reference for the phytoremediation of Cd-contaminated soil using poplar.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Tissue culture plants of Populus tremula × P. alba ‘717’ were used in this experiment. In March 2021, tissue culture plants were cultured under growth room condition (25/18 °C in the day and night; light duration: 14 h; light intensity: 150 μmol m−2 s−1; relative humidity: 50%–60%) for one month and then transplanted in 6 L pots filled with sands. Hoagland nutrient solution (1/4 concentration, 50 mL) was irrigated into each pot every day.
In May, when the height of seedlings reached 30–35 cm, 24 seedlings with similar size were transplanted in aerated Hoagland nutrient solution without Fe and equally divided into four groups, followed by Cd (0 and 100 μM CdCl2) and Fe (50 and 150 μM FeCl3) treatments. The Cd and Fe levels were determined according to previous studies [14,35,36], and 50 and 150 μM Fe doses were considered normal- and high-dose Fe, respectively. There were four treatments in total, including (1) Cd0Fe50: 0 μM CdCl2 + 50 μM FeCl3; (2) Cd0Fe150: 0 μM CdCl2 + 150 μM FeCl3; (3) Cd100Fe50: 100 μM CdCl2 + 50 μM FeCl3; and (4) Cd100Fe150: 100 μM CdCl2 + 150 μM FeCl3. The nutrient solution was renewed every three days, and Cd and Fe were added again after the nutrient solution was replaced. After 5 weeks, all poplar plants were harvested (Figure 1).
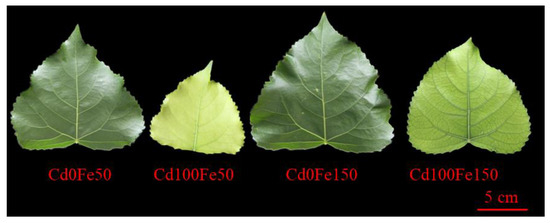
Figure 1.
The leaves of poplar under different Cd and Fe combined treatments. Cd0Fe50: 0 μM CdCl2 + 50 μM FeCl3; Cd0Fe150: 0 μM CdCl2 + 150 μM FeCl3; Cd100Fe50: 100 μM CdCl2 + 50 μM FeCl3; Cd100Fe150: 100 μM CdCl2 + 150 μM FeCl3. The same below.
2.2. Determination of Photosynthetic Parameters and Sampling
Three leaves of each seedling (leaf plastochron index: 7–9) were sampled, and the method of Luo et al. [37] was employed to determine the net photosynthetic rate (Pn), transpiration rate (E), and stomatal conductance (gs) using a LI-6400 portable photosynthetic apparatus (LI-COR Inc., Lincoln, NE, USA) in the morning (9:00–11:00).
Then, the roots of each seedling were soaked in 50 mM CaCl2 for 5 min, washed twice, and dried with absorbent paper, to remove root surface metal ions. A portion of the seeding roots (ca. 2 g) was used for scanning (see Section 2.3). After that, the seedlings were separated into roots, woods, barks, and leaves; weighed; and recorded. After wrapping in tin foil and freezing in liquid nitrogen, the tissues were ground at low temperature using a ball mill (Tissuelyser-64L, Jingxin, Shanghai, China) and stored at −80 °C. About 200 mg was then dried to constant weight and weighed, followed by the calculation of the dry-wet ratio.
2.3. Determination of Root Morphology
The roots sampled for scanning above mentioned were spread out in water to avoid root overlap. Then the root was scanned using the WinRHIZO root analysis system (Regent Instruments, Québec, QC, Canada) to determine the total root length (TRL), total root surface area (TRSA), total root volume (TRV), and total root tips (TRT) [24].
2.4. Assays of Chlorophyll Content
Leaf chlorophyll content (chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid (Car)) were determined [14]. Forty milligrams of leaves was soaked in pure acetone and (400 μL) for 5 min. Then, 9.6 mL of 80% acetone solution was added. The mixture was shaken in dark conditions overnight. Fifteen minutes after the sample turned white, the absorbance at 663, 646, and 470 nm were measured using a spectrophotometer (Unico, Beijing, China).
2.5. Determination of the Concentrations of Cd and Fe
One hundred milligrams of root and leaf samples was digested using, respectively, solutions composed of perchloric acid (2 mL) and concentrated nitric acid (8 mL). Then, the ICP-MS inductively coupled plasma mass spectrometry (Agilent7800, Santa Clara, CA, USA) was used to determine the Cd and Fe concentrations [13].
2.6. Determination of MDA Content
One hundred milligrams of root and leaf samples was extracted in 10% TCA for 30 min. The supernatant was mixed with a solution composed of 0.6% TBA and 10% TCA after centrifugation. After that, the mixture was subjected to a 15 min boiling water bath, followed by cooling in ice and centrifugation. Finally, the absorbance of the supernatant was determined at 450, 532, and 600 nm [38].
2.7. Assays of Antioxidant Enzyme Activity
One hundred milligrams of root and leaf samples was extracted using an extract solution composed of polyvinylpolypyrrolidone (200 mg), potassium phosphate (0.1 M, pH 7.8), and Triton X-100 (v/v = 0.5%). After centrifugation, the soluble protein content was determined using the supernatant, with bovine serum as the standard. The antioxidant enzyme activity was determined using the extracted soluble protein [39].
Following Tamás et al. [40], the reaction system for the determination of AAO (EC 1.10.3.3) activity included enzyme solution, potassium phosphate buffer (0.1 M, pH 5.6), EDTA-Na2 (5 mM), and sodium ascorbate (50 mM). The absorbance was determined at 265 nm using a spectrophotometer. The oxidization of 1 μmol ascorbic acid (AsA) per milligram of protein per minute was defined as one unit of AAO activity.
Following He et al. [41], the reaction system for the determination of APX (EC 1.11.1.11) activity consisted of enzyme solution, phosphate buffer (0.05 M, pH 7.0), EDTA (0.1 mM), sodium ascorbate (0.1 mM), and H2O2 (2.5 mM). The absorbance was determined at 290 nm using a spectrophotometer. The decomposition of 1 μmoL of AsA per milligram of protein per minute was defined as one unit of APX activity.
Following Gong at al. [42], the SOD (EC1.15.1.1) activity was determined. The reaction system included enzyme solution, potassium phosphate (0.05 M, pH 7.8), methionine (0.22 M), nitro blue tetrazolium chloride monohydrate (NBT) (1.25 mM), and riboflavin (33 μM). The absorbance was determined at 560 nm using a spectrophotometer. The 50% inhibition of NBT photoreduction was defined as one unit of SOD activity.
2.8. Analysis of BCF, BAF, TF and TI
The bioconcentration factor (BCF), bioaccumulation factor (BAF), translocation factor (TF), and tolerance index (TI) were calculated [1]:
where CCdR, CCdW, CCdB, CCdR, CCdL, and CSubstrate are the Cd concentrations in poplar root, wood, bark, leaves, and growing substrate, respectively, and DBMCd-treatment and DBMControl are the dry biomass (DBM) of each Cd treatment and the control, respectively.
BCFR = CCdR/CSubstrate
BAFW = CCdW/CSubstrate
BAFB = CCdB/CSubstrate
BAFL = CCdL/CSubstrate
TFW = CCdW/CCdR
TFB = CCdB/CCdR
TFL = CCdL/CCdR
TI = DBMCd-treatment/DBMControl
2.9. Statistical Analysis
Data normality was first analyzed. All the data were analyzed using Statgraphics software (STN, USA). The significance of the differences was determined with two-factor analyses of variance at p < 0.05, and for significant differences, the means were analyzed by a posteriori comparison.
3. Results
3.1. Effect of Fe Application on Cd-Stressed Poplar Growth
The biomass of roots (0.63 g), woods (0.59 g), barks (0.50 g), and leaves (0.50 g) for Cd100Fe50 decreased by 42%, 56%, 44%, and 51%, respectively, compared with those for Cd0Fe50 (Figure 2a–d). The biomass of roots (0.82 g), woods (0.71 g), barks (0.65 g), and leaves (3.79 g) for Cd100Fe150 decreased by 19%, 44%, 30%, and 30%, respectively, compared with those for Cd0Fe150 (Figure 2a–d). There was no difference between Cd0Fe50 and Cd0Fe150 (Figure 2a–d). However, the biomass of roots, woods, barks, and leaves for Cd100Fe150 increased by 29%, 20%, 29%, and 38%, respectively, compared with those for Cd100Fe50 (Figure 2a–d). The TI for Cd100Fe50 decreased by 50% compared with that for Cd0Fe50. Similarly, The TI for Cd100Fe150 decreased by 31% compared with that for Cd0Fe150 (Figure 2e). Under Cd-free condition, there was no difference in the TI between Cd0Fe150 and Cd0Fe50. The TI for Cd100Fe150 increased by 34% compared with that for Cd100Fe50 (Figure 2e).
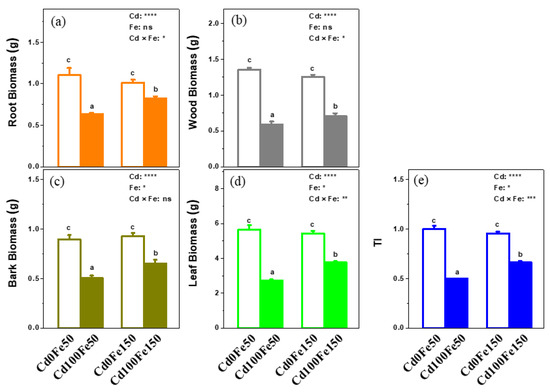
Figure 2.
The biomass of root (a), wood (b), bark (c), leaves (d), and TI © of poplar under different Cd and Fe combined treatments. Data were means ± SE (n = 6). Different lowercase letters on the bars indicate significant differences in ANOVA (p < 0.05). p values of two-way ANOVA on Cd, Fe, and their interaction (Cd × Fe) were indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant). The same below.
3.2. Effect of Fe Application on Cd-Stressed Poplar Root Architecture
The TRL of poplar for Cd100Fe50 decreased by 58%, compared with that for Cd0Fe50. Similarly, the TRL of poplar for Cd100Fe150 decreased by 32% compared with that for Cd0Fe150. The TRL of poplar for Cd0Fe150 decreased by 25% compared with that for Cd0Fe50. However, there was no difference in the TRL of poplar between Cd100Fe150 and Cd100Fe50 (Figure 3a).
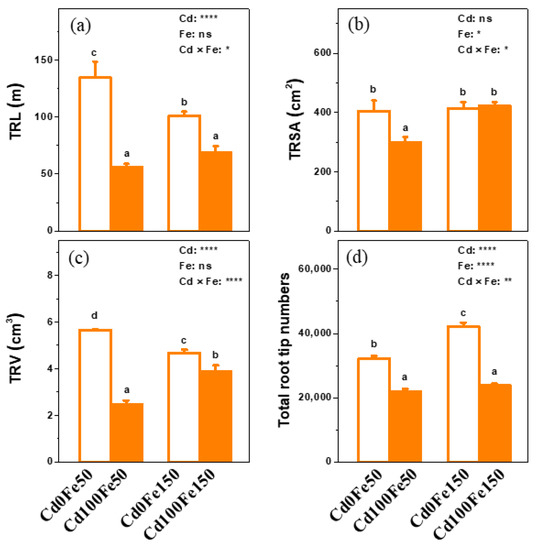
Figure 3.
The TRL (a), TRSA (b), TRV (c) and total root tip number (d) of poplar under different Cd and Fe combined treatments. Data were means ± SE (n = 6). Different lowercase letters on the bars indicate significant differences in ANOVA (p < 0.05). p values of two-way ANOVA on Cd, Fe, and their interaction (Cd × Fe) were indicated (*, p < 0.05; **, p < 0.01; ****, p < 0.0001; ns, not significant).
The TRSA of poplar for Cd100Fe50 decreased by 26% compared with that for Cd0Fe50. There was no difference between Cd100Fe150 and Cd0Fe150 or between Cd0Fe50 and Cd0Fe150. However, the TRSA of poplar for Cd100Fe150 increased by 42% compared with that for Cd100Fe50 (Figure 3b).
The TRV of poplar for Cd100Fe50 decreased by 56% compared with that for Cd0Fe50. Similarly, the TRV of poplar for Cd100Fe150 decreased by 17% compared with that for Cd0Fe150. The TRV of poplar for Cd0Fe150 decreased by 17% compared with that for Cd0Fe50. However, the TRV of poplar for Cd100Fe150 increased by 56% compared with that for Cd100Fe50 (Figure 3c).
The total number of poplar root tips for Cd100Fe50 decreased by 32% compared with that for Cd0Fe50. Similarly, the total number of poplar root tips for Cd100Fe150 decreased by 43% compared with that for Cd0Fe150. However, the total number of root tips of poplar for Cd0Fe150 increased by 32% compared with that for Cd0Fe50. There was no difference between Cd100Fe50 and Cd100Fe150 (Figure 3d).
3.3. Effects of Fe on Chlorophyll Content and Photosynthetic Parameters of Cd-Stressed Poplar
The leaf Pn, E, and gs for Cd100Fe50 decreased by 48%, 41%, and 27%, respectively, compared with those for Cd0Fe50. Similarly, the Pn, E, and gs for Cd100Fe150 decreased by 41%, 47% and 38%, respectively, compared with those for Cd0Fe150. However, the Pn, E, and gs of leaves for Cd0Fe150 increased by 21%, 47%, and 33%, respectively, compared with those for Cd0Fe50. In addition, the Pn, E, and gs of leaves for Cd100Fe150 increased by 37%, 33%, and 13%, respectively, compared with those in the Cd100Fe50 (Figure 4).
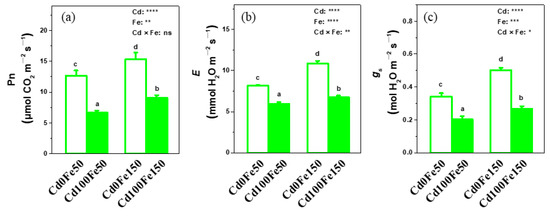
Figure 4.
The Pn (a), E (b) and gs (c) of poplar under different Cd and Fe combined treatments. Data were means ± SE (n = 6). Different lowercase letters on the bars indicate significant differences in ANOVA (p < 0.05). p values of two-way ANOVA on Cd, Fe, and their interaction (Cd × Fe) were indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant).
The contents of Chl a (4.61 mg g−1 DW), Chl b (1.01 mg g−1 DW), and Car (0.85 mg g−1 DW) in poplar leaves for Cd100Fe50 decreased by 54%, 41%, and 51%, respectively, compared with those for Cd0Fe50. Similarly, the contents of Chl a (2.82 mg g−1 DW), Chl b (0.77 mg g−1 DW), and Car (0.61 mg g−1 DW) in poplar leaves for Cd100Fe150 decreased by 42%, 30%, and 29%, respectively, compared with those for Cd0Fe150. However, the content of Chl b for Cd0Fe150 increased by 10%, while the contents of Chl a and Car did not change significantly compared with those for Cd0Fe50. Additionally, the contents of Chl a, Chl b, and Car in poplar leaves for Cd100Fe150 increased by 34%, 29%, and 45%, respectively, compared with those for Cd100Fe50 (Figure 5).
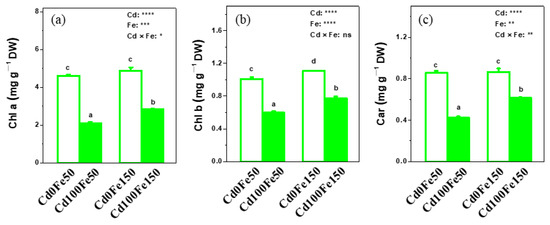
Figure 5.
Chl a (a), Chl b (b), and Car (c) in the leaves of poplar under different Cd and Fe combined treatments. Data were means ± SE (n = 6). Different lowercase letters on the bars indicate significant differences in ANOVA (p < 0.05). p values of two-way ANOVA on Cd, Fe, and their interaction (Cd × Fe) were indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant).
3.4. Effect of Fe on the Cd Accumulation of Cd-Stressed Poplar
No Cd was found in the poplar for Cd0Fe150 and Cd0Fe50 (Figure 6a–d). The root Cd content (231.98 μg g−1 DW) in the Cd100Fe150 reduced by 66%, while the Cd contents in the woods (179.57 μg g−1 DW), barks (464.86 μg g−1 DW), and leaves (167.53 μg g−1 DW) increased by 44%, 76%, and 56%, respectively, compared with those for Cd100Fe50 (Figure 6a–d).
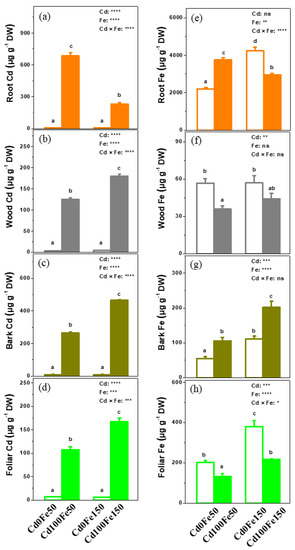
Figure 6.
The Cd (a–d) and Fe (e–h) contents in the roots, woods, barks, and leaves of poplar under different Cd and Fe combined treatments. Data were means ± SE (n = 6). Different lowercase letters on the bars indicate significant differences in ANOVA (p < 0.05). p values of two-way ANOVA on Cd, Fe, and their interaction (Cd × Fe) were indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant).
The root and bark Fe content for Cd100Fe50 increased by 71% and 92%, respectively, while the wood and leaf Fe content decreased by 37% and 34%, respectively, compared with those for Cd0Fe50. The Cd content in poplar roots, woods, and leaves for Cd100Fe150 reduced by 30%, 23%, and 43%, respectively, while the content of Fe in the barks increased by 81% compared with those for Cd0Fe150. The Fe content in the roots, barks, and leaves of poplar for Cd0Fe150 increased by 92%, 103%, and 89%, respectively, compared with those for Cd0Fe50, but there was no difference in the Fe content in woods. The content of Fe in the barks and leaves for Cd100Fe150 increased by 91% and 62%, respectively, but that in the roots reduced by 22%, compared with those for Cd100Fe50 (Figure 6e–h).
The BCFR for Cd100Fe150 (20.71) reduced by 66%, while the BAFW, BAFB, and BAFL increased by 44%, 76%, and 56%, respectively, compared with those for Cd100Fe50. The TFW (0.78), TFB (2.02), and TFL (0.73) for Cd100Fe150 increased by 325%, 420%, and 361%, respectively, compared with those for Cd100Fe50 (Table 1).

Table 1.
BAF, BCF, and TF for Cd in the roots (R), woods (W), barks (B), and leaves (L) of poplar under different Cd and Fe combined treatments.
3.5. Effect of Fe application on MDA Content and Antioxidant Enzyme Activities of Cd-Stressed Poplar
The MDA content in poplar roots for Cd100Fe50 increased by 101% compared with that for Cd0Fe50 (Figure 7a). The MDA content in poplar roots for Cd100Fe150 increased by 60% compared with that for Cd0Fe150 (Figure 7a). There was no difference in the root MDA content between Cd0Fe50 and Cd0Fe150 (Figure 7a). However, the MDA content in poplar roots for Cd100Fe150 reduced by 24% compared with that for Cd100Fe50 (Figure 7a).
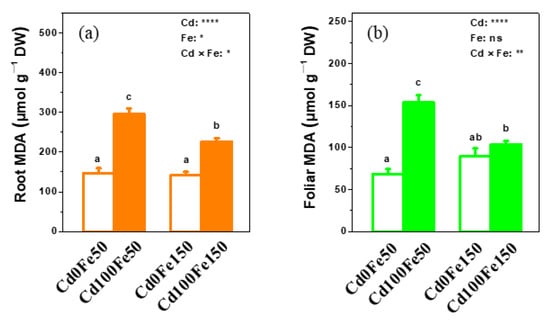
Figure 7.
The contents of MDA in the roots (a) and leaves (b) of poplar under different Cd and Fe combined treatments. Data were means ± SE (n = 6). Different lowercase letters on the bars indicate significant differences in ANOVA (p < 0.05). p values of two-way ANOVA on Cd, Fe, and their interaction (Cd × Fe) were indicated (*, p < 0.05; **, p < 0.01; ****, p < 0.0001; ns, not significant).
The MDA content in poplar leaves for Cd100Fe50 increased by 124% compared with that for Cd0Fe50 (Figure 7b). There was no difference between Cd0Fe150 and Cd100Fe150 and between Cd0Fe50 and Cd0Fe150 (Figure 7b). The MDA content in poplar leaves for Cd100Fe150 reduced by 30% compared with that for Cd100Fe50 (Figure 7b).
The AAO and SOD activities in poplar roots for Cd100Fe50 reduced by 24% and 33%, respectively, compared with those for Cd0Fe50, while there was no difference in root APX activity (Figure 8a–c). The AAO, APX, and SOD activities in poplar roots for Cd100Fe150 reduced by 36%, 21%, and 23%, respectively, compared with those for Cd0Fe150 (Figure 8a–c). The APX and SOD activities in poplar roots for Cd0Fe150 increased by 9% and 11%, respectively, compared with those for Cd0Fe50, while there was no difference in root AAO activity (Figure 8a–c). The AAO and APX activities in poplar roots for Cd100Fe150 reduced by 20% and 19%, respectively, while the SOD activity in poplar roots increased by 27% compared with those for Cd100Fe50 (Figure 8a–c).
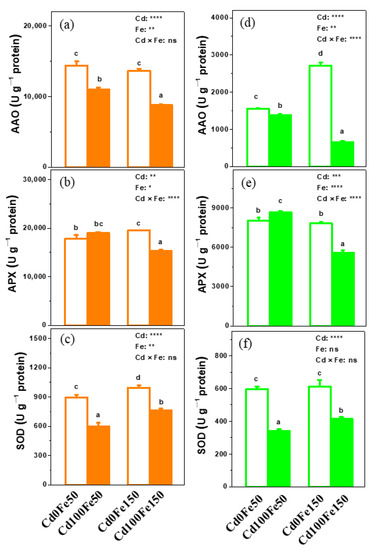
Figure 8.
The activities of AAO (a,d), APX (b,e), and SOD (c,f) in the roots and leaves of poplar under different Cd and Fe combined treatments. Data were means ± SE (n = 6). Different lowercase letters on the bars indicate significant differences in ANOVA (p < 0.05). p values of two-way ANOVA on Cd, Fe, and their interaction (Cd × Fe) were indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant).
The AAO and SOD activities in poplar leaves for Cd100Fe50 reduced by 11% and 43%, respectively, while the APX activity increased by 8% compared with those for Cd0Fe50 (Figure 8d–f). The AAO, APX, and SOD activities in poplar leaves for Cd100Fe150 reduced by 76%, 29%, and 32%, respectively, compared with those for Cd0Fe150 (Figure 8d–f). The AAO activity in poplar leaves for Cd0Fe150 increased by 75% compared with that for Cd0Fe50, but there was no difference in the APX and SOD activities in leaves (Figure 8d–f). The AAO and APX activities in poplar leaves for Cd100Fe150 reduced by 53% and 35%, respectively, while the SOD activity increased by 22% compared with those for Cd100Fe50 (Figure 8d–f).
4. Discussion
4.1. Cd and Fe Interaction Affects the Growth, Photosynthesis, and Pigment Accumulation of Poplar
Cd stress can inhibit plant growth and photosynthesis, and even cause plant death [2,13,36]. This study found that Cd significantly inhibited poplar growth and reduced the biomass of roots, woods, barks, and leaves. Fe is necessary for plant growth. Sperotto et al. [43] found that proper high-dose Fe was beneficial to plant growth, but excessive Fe was toxic to plants. This study found that 150 μM Fe did not reduce the biomass of poplar roots, woods, barks, and leaves. This indicates that 150 μM is the suitable Fe level for poplar. Separately, TI can indicate a plant’s resistance to Cd toxicity [1]. In this study, under Cd stress, 150 μM Fe increased the biomass accumulation, and TI of poplar compared with 50 μM Fe. These results indicate that proper high-dose Fe is helpful to enhance poplar’s tolerance to Cd stress, and 150 μM is not excessive for poplar growth.
The analysis of root architecture showed that Cd significantly reduced the TRL, TRSA, TRV, and root tip number of poplar. This may be related to the toxicity of Cd [44]. In the absence of Cd, high-dose Fe decreased the TRV and TRL but increased the TRT. This indicates that high-dose Fe (150 μM) could make poplar roots shorter, thinner, and more lateral. In this study, under Cd stress, high-dose Fe increased the TRV of the poplar root system. This indicates that high-dose Fe could effectively alleviate the inhibition of Cd on poplar root growth. Meanwhile, the root system is the key part for plants to adsorb nutrients from soil, and large TRSA helps plants to adsorb nutrients [24,45]. In this study, under Cd stress, the high-dose Fe increased the TRSA. This may be due to the higher root biomass under Cd stress [45]. The increased TRSA can not only enhance the absorption of nutrients, but also enhance the absorption and accumulation of Cd by poplar.
Previous research has shown that plants with higher Pn have higher biomass [46,47]. Cd stress can lead to the reduction of biomass by inhibiting plant photosynthesis [27]. In this study, Cd significantly inhibited the Pn of poplar regardless of normal or high concentration of Fe, which was in line with the biomass results. Studies have shown that Cd could greatly inhibit the synthesis of chlorophyll, reduce the chlorophyll content, and cause the iron deficiency chlorosis of plants [38,44]. This study obtained similar results. Qureshi et al. [48] reported that Fe exists in heme- and iron-sulfur proteins as a cofactor that plays an important role in photosynthesis in chloroplasts. In addition, Fe participates in the transport of photosynthetic electrons in the photosynthetic electron transport chain [49]. In this study, under Cd stress, high-dose Fe increased the contents of Chl a, Chl b, and Car and enhanced the Pn and biomass. This indicates that high-dose Fe (150 μM) could effectively alleviate the iron deficiency chlorosis induced by Cd, enhance poplar tolerance to Cd, and restore the normal growth of poplar.
4.2. Fe application Enhances Cd Enrichment in Aboveground Parts of Poplar
Cd can be absorbed by plant roots, transported and accumulated in the aboveground tissues [8,40,50,51]. Studies have shown that the interaction between Cd and Fe in soil is complex. Because Cd2+ and Fe2+ have similar ionic radii, Cd2+ can enter plant cells through IRTs, ZIPs, NRAMPs, and other Fe2+ transport carriers [2,32,52], and Fe deficiency can increase the Cd accumulation in Arabidopsis [46]. In this study, under Cd stress, high-dose Fe increased the transpiration rate of poplar. Thus, more Cd could be transferred to the aboveground part with sap flow. The plant’s phytoextraction capacity is defined as TF [1]. In this study, the TFW, TFB, and TFL of Cd under high-dose Fe were significantly higher than those under normal-dose Fe. This indicates that high-dose Fe (150 μM) could effectively enhance the transport of Cd to the aboveground part of poplar. Separately, the Cd absorption capacity of plants from substrate/soil is defined as BCF [1]. In this study, most BAFs were lower than BCFs. It indicates that there is a limitation in the Cd transport from roots to aboveground parts, which results in more Cd accumulating in the root instead of the aboveground part [53]. However, it was found that after high-dose Fe, the BAF increased. This indicates that high-dose Fe may break some restrictions and enhance the transport and accumulation of Cd to the aboveground part of poplar. Although Cd-enriched herb plants have higher Cd contents, poplar trees can enrich a larger amount of Cd due to their larger biomass, which has important value in phytoremediation [54,55,56]. This study result indicates that a proper-high amount of Fe can be applied in the poplar phytoremediation of Cd-contaminated soils, so as to enhance the remediation efficiency.
As Cd2+ can enter plant cells through the transport protein of Fe2+, it will disturb the absorption of Fe2+ by plants [36,57,58]. This study obtained similar results: Although high-dose Fe increased the Fe content in roots, barks, and leaves under zero Cd condition, it only increased the Fe content in barks and leaves under Cd exposure. Studies have shown that under Cd stress, increasing the Fe content of aboveground parts, especially increasing leaf Fe content, is very important for maintaining chlorophyll synthesis and improving photosynthetic rate [44,59,60]. Sarvari et al. [34] held that an appropriate Cd: Fe ratio was conducive to reducing the damage of Cd to plants. This study results showed that the poplar growing under the condition of Cd/Fe ratio = 2:3 was better than the poplar growing under the condition of Cd/Fe ratio = 2:1.
4.3. Antioxidant Defense of Poplar under Cd and Fe Interaction
Cd can induce the accumulation of a large amount of ROS after entering cells, resulting in membrane lipid peroxidation and MDA accumulation [15,61]. In this study, Cd induced the accumulation of large amounts of MDA under both normal- and high-dose Fe. In addition, in the absence of Cd, high-dose Fe did not change poplar root and leaf MDA content. This indicates that 150 μM Fe is not excessive for poplar growth and could not cause oxidative damage. Interestingly, this study found that under Cd stress, the root and leaf MDA content in the high-dose Fe group was significantly lower than that of poplar in the normal-dose Fe group. This indicates that under Cd stress, high-dose Fe could alleviate the Cd-induced oxidative damage to a certain extent and promote the growth of poplar.
To cope with the Cd-induced oxidative stress, plant antioxidant enzymes are activated to scavenge ROS, but the enzymes’ responses are different under different degrees of Cd stress [12]. In this study, Cd significantly suppressed root and leaf AAO and SOD activities under normal- and high-dose Fe. However, under normal-dose Fe, the Cd-stressed poplar root and leaf APX activity increased. This indicates that APX may play a more important role in mitigating Cd-induced oxidative stress under normal-dose Fe compared with other antioxidant enzymes. In the absence of Cd, high-dose Fe increased root APX and SOD activities and leaf AAO and APX activities, thus enhancing poplar’s antioxidant capacity. However, under Cd stress, high-dose Fe decreased root and leaf AAO and APX activities but increased the activity of SOD in roots and leaves, which helped to scavenge ROS. A previous study reported that under abiotic stress, plants could maintain the ROS balance by a ROS-scavenging system that mainly includes three types of SODs (Cu/Zn-SOD, Mn-SOD, and Fe-SOD) [62]. Among them, Fe-SOD is an important component in chloroplasts, peroxisomes, and mitochondria [32]. Therefore, in this study, under Cd stress, high-dose Fe may have increased the activity of Fe-SOD to enhance the ROS scavenging ability.
5. Conclusions
In this study, Cd (100 μM) could suppress the photosynthesis and reduce the total root surface area of P. tremula × P. alba ‘717’, thus suppressing the absorption of nutrients, CO2 assimilation, and the growth of poplar. Under Cd stress, high-dose Fe (150 μM) increased the net photosynthetic rate, total root surface area, and nutrient absorption, thus alleviating the toxicity of Cd. That is, the Fe dose of 150 μM is not excessive for poplar growth but promotes biomass accumulation. In addition, the high-dose Fe increased the BAF and TF, thus breaking some restrictions and enhancing the transport of Cd to the aboveground part of poplar. In addition, under Cd stress, high-dose Fe enhanced the antioxidant capacity of poplar by increasing the activity of Fe-SOD. Therefore, it is necessary to further explore the effects of high-dose Fe (150 μM) on the activity of Fe-SOD under Cd stress. This study has a guiding significance for the use of poplar in the high-efficiency remediation of Cd-contaminated soil.
Author Contributions
S.D. and M.L. designed the experiments, analyzed the data, drafted the manuscript and other authors revised the draft. C.L., D.Z. and B.W. carried out the experiments. S.D. finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was jointly supported by the National Natural Science Foundation of China (Grant No. 32001347) and the Henan Provincial Science and Technology Research Project (Grant No. 212102110190).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akram, M.A.; Wahid, A.; Abrar, M.; Manan, A.; Naeem, S.; Zahid, M.A.; Gilani, M.M.; Paudyal, R.; Gong, H.Y.; Ran, J.Z.; et al. Comparative study of six maize (Zea mays L.) cultivars concerning cadmium uptake, partitioning and tolerance. Appl. Ecol. Environ. Res. 2021, 19, 2305–2331. [Google Scholar] [CrossRef]
- Luo, Z.B.; He, J.; Polle, A.; Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Barrutia, O.; Artetxe, U.; Hernandez, A.; Olano, J.M.; Garcia-Plazaola, J.I.; Garbisu, C.; Becerril, J.M. Native plant communities in an abandoned Pb-Zn mining area of northern Spain: Implications for phytoremediation and germplasm preservation. Int. J. Phytoremediat. 2011, 13, 256–270. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Herawati, N.; Suzuki, S.; Hayashi, K.; Rivai, I.F.; Koyama, H. Cadmium, copper, and zinc levels in rice and soil of Japan, Indonesia, and China by soil type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Douchiche, O.; Chaibi, W.; Morvan, C. Cadmium tolerance and accumulation characteristics of mature flax, cv. Hermes: Contribution of the basal stem compared to the root. J. Hazard. Mater. 2012, 235–236, 101–107. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Ma, C.; Zhang, Y.; Polle, A.; Rennenberg, H.; Cheng, X.; Luo, Z.B. Overexpression of bacterial gamma-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 2015, 205, 240–254. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2020, 249, 71–131. [Google Scholar]
- Hu, Y.; Tian, S.; Foyer, C.H.; Hou, D.; Wang, H.; Zhou, W.; Liu, T.; Ge, J.; Lu, L.; Lin, X. Efficient phloem transport significantly remobilizes cadmium from old to young organs in a hyperaccumulator Sedum alfredii. J. Hazard. Mater. 2019, 365, 421–429. [Google Scholar] [CrossRef]
- Balestri, M.; Ceccarini, A.; Forino, L.M.; Zelko, I.; Martinka, M.; Lux, A.; Ruffini Castiglione, M. Cadmium uptake, localization and stress-induced morphogenic response in the fern Pteris vittata. Planta 2014, 239, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Xu, T.; Li, X.; Zhang, Y.; Ren, R.; Heng, Q.; Chen, W.; Zhang, S.; Wang, M.; Kou, L.; et al. The influence of phosphorus on leaf function, cadmium accumulation and stress tolerance of poplar leaves under cadmium exposure. Environ. Exp. Bot. 2022, 204, 105087. [Google Scholar] [CrossRef]
- Shi, W.; Liu, W.; Ma, C.; Zhang, Y.; Ding, S.; Yu, W.; Deng, S.; Zhou, J.; Li, H.; Luo, Z.B. Dissecting microRNAs-mRNAs regulatory networks underlying sulfur assimilation and cadmium accumulation in poplar leaves. Plant Cell Physiol. 2020, 61, 1614–1630. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Ma, C.; Shi, W.; Liu, W.; Lu, Y.; Liu, Q.; Luo, Z.B. Exogenous glutathione enhances cadmium accumulation and alleviates its toxicity in Populus canescens. Tree Physiol. 2017, 37, 1697–1712. [Google Scholar] [CrossRef]
- Marmiroli, M.; Pietrini, F.; Maestri, E.; Zacchini, M.; Marmiroli, N.; Massacci, A. Growth, physiological and molecular traits in Salicaceae trees investigated for phytoremediation of heavy metals and organics. Tree Physiol. 2011, 31, 1319–1334. [Google Scholar] [CrossRef]
- Luo, J.; Liang, Z.; Wu, M.; Mei, L. Genome-wide identification of BOR genes in poplar and their roles in response to various environmental stimuli. Environ. Exp. Bot. 2019, 164, 101–113. [Google Scholar] [CrossRef]
- He, J.; Ma, C.; Ma, Y.; Li, H.; Kang, J.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Cadmium tolerance in six poplar species. Environ. Sci. Pollut. R. 2013, 20, 163–174. [Google Scholar] [CrossRef]
- Migocka, M.; Kosieradzka, A.; Papierniak, A.; Maciaszczykdziubinska, E.; Posyniak, E.; Garbiec, A.; Filleur, S. Two metal-tolerance proteins, MTP1 and MTP4, are involved in Zn homeostasis and Cd sequestration in cucumber cells. J. Exp. Bot. 2015, 66, 581–596. [Google Scholar] [CrossRef]
- Tian, S.; Liang, S.; Qiao, K.; Wang, F.; Zhang, Y.; Chai, T. Co-expression of multiple heavy metal transporters changes the translocation, accumulation, and potential oxidative stress of Cd and Zn in rice (Oryza sativa). J. Hazard. Mater. 2019, 380, 120853. [Google Scholar] [CrossRef]
- She, W.; Cui, G.; Li, X.; Su, X.; Jie, Y.; Yang, R. Characterization of cadmium concentration and translocation among ramie cultivars as affected by zinc and iron deficiency. Acta Physiol. Plant. 2018, 40, 104. [Google Scholar] [CrossRef]
- Guo, Z.; Lv, J.; Zhang, H.; Hu, C.; Qin, Y.; Dong, H.; Zhang, T.; Dong, X.; Du, N.; Piao, F. Red and blue light function antagonistically to regulate cadmium tolerance by modulating the photosynthesis, antioxidant defense system and Cd uptake in cucumber (Cucumis sativus L.). J. Hazard. Mater. 2022, 429, 128412. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Song, Y.; Du, Q.; Yang, X.; Dong, C.; Chen, J.; Xie, J.; Li, B.; Zhang, D. Population genomic analysis of gibberellin-responsive long non-coding RNAs in Populus. J. Exp. Bot. 2016, 67, 2467–2482. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhi, Y.; Dai, Y.; Lv, J.; Li, Y.; Wu, Z. The detoxification mechanisms of low-accumulating and non-low-accumulating medicinal plants under Cd and Pb stress. RSC Adv. 2020, 10, 43882–43893. [Google Scholar] [CrossRef]
- Chen, Y.; Nguyen, T.H.N.; Qin, J.; Jiao, Y.; Li, Z.; Ding, S.; Lu, Y.; Liu, Q.; Luo, Z.B. Phosphorus assimilation of Chinese fir from two provenances during acclimation to changing phosphorus availability. Environ. Exp. Bot. 2018, 153, 21–34. [Google Scholar] [CrossRef]
- Basa, B.; Lattanzio, G.; Solti, Á.; Tóth, B.; Abadía, J.; Fodor, F.; Sárvári, É. Changes induced by cadmium stress and iron deficiency in the composition and organization of thylakoid complexes in sugar beet (Beta vulgaris L.). Environ. Exp. Bot. 2014, 101, 1–11. [Google Scholar] [CrossRef]
- Hu, X.; Page, M.T.; Sumida, A.; Tanaka, A.; Terry, M.J.; Tanaka, R. The iron-sulfur cluster biosynthesis protein SUFB is required for chlorophyll synthesis, but not phytochrome signaling. Plant J. 2017, 89, 1184–1194. [Google Scholar] [CrossRef]
- Solti, Á.; Sárvári, É.; Tóth, B.; Mészáros, I.; Fodor, F. Incorporation of iron into chloroplasts triggers the restoration of cadmium induced inhibition of photosynthesis. J. Plant Physiol. 2016, 202, 97–106. [Google Scholar] [CrossRef][Green Version]
- Muneer, S.; Hakeem, K.R.; Mohamed, R.; Lee, J.H. Cadmium toxicity induced alterations in the root proteome of green gram in contrasting response towards iron supplement. Int. J. Mol. Sci. 2014, 15, 6343–6355. [Google Scholar] [CrossRef]
- Solti, A.; Sarvari, E.; Szollosi, E.; Toth, B.; Meszaros, I.; Fodor, F.; Szigeti, Z. Stress hardening under long-term cadmium treatment is correlated with the activation of antioxidative defence and iron acquisition of chloroplasts in Populus. Z. Nat. C 2016, 71, 323–334. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N.K. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Su, Y.; Liu, J.; Lu, Z.; Wang, X.; Zhang, Z.; Shi, G. Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ. Exp. Bot. 2014, 97, 40–48. [Google Scholar] [CrossRef]
- He, X.L.; Fan, S.K.; Zhu, J.; Guan, M.Y.; Liu, X.X.; Zhang, Y.S.; Jin, C.W. Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil. 2017, 416, 453–462. [Google Scholar] [CrossRef]
- Molina-Rueda, J.J.; Tsai, C.J.; Kirby, E.G. The Populus superoxide dismutase gene family and its responses to drought stress in transgenic poplar overexpressing a pine cytosolic glutamine synthetase (GS1a). PLoS ONE 2013, 8, e56421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, H.; Shao, L.; Wang, H.; Zhang, Y.; Zhu, T.; Ma, L.; Ding, Q.; Ma, L. Root characteristics critical for cadmium tolerance and reduced accumulation in wheat (Triticum aestivum L.). J. Environ. Manag. 2022, 305, 114365. [Google Scholar] [CrossRef]
- Astolfi, S.; Ortolani, M.R.; Catarcione, G.; Paolacci, A.R.; Cesco, S.; Pinton, R.; Ciaffi, M. Cadmium exposure affects iron acquisition in barley (Hordeum vulgare) seedlings. Physiol. Plantarum. 2014, 152, 646–659. [Google Scholar] [CrossRef]
- Sarvari, E.; Solti, A.; Basa, B.; Meszaros, I.; Levai, L.; Fodor, F. Impact of moderate Fe excess under Cd stress on the photosynthetic performance of poplar (Populus jacquemontiana var. glauca cv. Kopeczkii). Plant Physiol. Biochem. 2011, 49, 499–505. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J. Growth performance, photosynthesis, and root characteristics are associated with nitrogen use efficiency in six poplar species. Environ. Exp. Bot. 2019, 164, 40–51. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, J.; Teng, Y.; He, J.; Christie, P.; Zhu, L.; Ren, W.; Zhang, M.; Deng, S. Effect of silicon on growth, physiology, and cadmium translocation of tobacco (Nicotiana tabacum L.) in cadmium-contaminated soil. Pedosphere 2018, 28, 680–689. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J.; Masclaux-Daubresse, C.; Wang, N.; Wang, H.; Zheng, B. Morphological and physiological responses to contrasting nitrogen regimes in Populus cathayana is linked to resources allocation and carbon/nitrogen partition. Environ. Exp. Bot. 2019, 162, 247–255. [Google Scholar] [CrossRef]
- Tamás, L.; Bočová, B.; Huttová, J.; Mistrík, I.; Ollé, M. Cadmium-Induced Inhibition of Apoplastic Ascorbate Oxidase in Barley Roots. Plant Growth Regul. 2006, 48, 41–49. [Google Scholar] [CrossRef]
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Jiang, X.; Liu, T.; Polle, A.; Liang, Z.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus canescens. Physiol. Plantarum. 2011, 143, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Miao, L.; Kong, W.; Bai, J.G.; Wang, X.; Wei, M.; Shi, Q. Nitric oxide, as a downstream signal, plays vital role in auxin induced cucumber tolerance to sodic alkaline stress. Plant Physiol. Biochem. 2014, 83, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.A.; Ricachenevsky, F.K.; Stein, R.J.; Waldow, V.; Fett, J.P. Iron stress in plants: Dealing with deprivation and overload. Plant Stress. 2010, 4, 57–69. [Google Scholar]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. R. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Kawa, D.; Julkowska, M.; Sommerfeld, H.M.; Horst, A.T.; Haring, M.A.; Testerink, C. Phosphate-dependent root system architecture responses to salt stress. Plant Physiol. 2016, 172, 690–706. [Google Scholar] [CrossRef]
- Pâques, L.E.; Lejeune, V.; Veisse, D. Do biomass partitioning and growth efficiency contribute to growth heterosis in inter-specific hybrid larch Larix eurolepis? Forestry 2022, 95, 466–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Li, T.; Marcelis, L.F.M. NaCl affects photosynthetic and stomatal dynamics by osmotic effects and reduces photosynthetic capacity by ionic effects in tomato. J. Exp. Bot. 2022, 73, 3637–3650. [Google Scholar] [CrossRef]
- Qureshi, M.I.; D’Amici, G.M.; Fagioni, M.; Rinalducci, S.; Zolla, L. Iron stabilizes thylakoid protein–pigment complexes in Indian mustard during Cd-phytoremediation as revealed by BN-SDS-PAGE and ESI-MS/MS. J. Plant Physiol. 2010, 167, 761–770. [Google Scholar] [CrossRef]
- Thomine, S.; Vert, G. Iron transport in plants: Better be safe than sorry. Curr. Opin. Plant Biol. 2013, 16, 322–327. [Google Scholar] [CrossRef]
- Luo, J.S.; Huang, J.; Zeng, D.L.; Peng, J.S.; Zhang, G.B.; Ma, H.L.; Guan, Y.; Yi, H.Y.; Fu, Y.L.; Han, B.; et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645–654. [Google Scholar] [CrossRef]
- Shi, W.G.; Liu, W.; Yu, W.; Zhang, Y.; Ding, S.; Li, H.; Mrak, T.; Kraigher, H.; Luo, Z.B. Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus canescens. J. Hazard. Mater. 2019, 362, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, X.; Fang, J.; Lu, Z.; Qiu, W.; Liu, M.; Sang, J.; Jiang, J.; Zhuo, R. Sedum alfredii SaNramp6 metal transporter contributes to cadmium accumulation in transgenic Arabidopsis thaliana. Sci. Rep. 2017, 7, 13318. [Google Scholar] [CrossRef]
- Ling, T.; Gao, Q.; Du, H.; Zhao, Q.; Ren, J. Growing, physiological responsesand cd uptake of corn (Zea mays L.) under different Cd supply. Chem. Speciat. Bioavailab. 2017, 29, 216–221. [Google Scholar] [CrossRef]
- Ali, N.; Hadi, F. Phytoremediation of cadmium improved with the high production of endogenous phenolics and free proline contents in Parthenium hysterophorus plant treated exogenously with plant growth regulator and chelating agent. Environ. Sci. Pollut. R. 2015, 22, 13305–13318. [Google Scholar] [CrossRef] [PubMed]
- Baldantoni, D.; Cicatelli, A.; Bellino, A.; Castiglione, S. Different behaviours in phytoremediation capacity of two heavy metal tolerant poplar clones in relation to iron and other trace elements. J. Environ. Manag. 2014, 146, 94–99. [Google Scholar] [CrossRef]
- Capuana, M. Heavy metals and woody plants—Biotechnologies for phytoremediation. iForest 2011, 4, 7–15. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Lima, J.E.; Nicolaus, V.W. Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell 2012, 24, 33–49. [Google Scholar] [CrossRef]
- Rui, H.; Chen, C.; Zhang, X.; Shen, Z.; Zhang, F. Cd-induced oxidative stress and lignification in the roots of two Vicia sativa L. varieties with different Cd tolerances. J. Hazard. Mater. 2016, 301, 304–313. [Google Scholar] [CrossRef]
- Brier, N.D.; Gomand, S.V.; Donner, E.; Paterson, D.; Smolders, E.; Delcour, J.A.; Lombi, E. Element distribution and iron speciation in mature wheat grains (Triticum aestivum L.) using synchrotron X-ray fluorescence microscopy mapping and XANES imaging. Plant Cell Environ. 2016, 39, 1835–1847. [Google Scholar] [CrossRef]
- Fodor, F.; Gáspár, L.; Morales, F.; Gogorcena, Y.; Lucena, J.J.; Cseh, E.; Kröpfl, K.; Abadía, J.; Sárvári, É. Effects of two iron sources on iron and cadmium allocation in poplar (Populus alba) plants exposed to cadmium. Tree Physiol. 2005, 25, 1173–1180. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, D.; Wang, W.; Song, Y.; Lu, M.; Ding, S. Foliar Application of Selenium Reduces Cadmium Accumulation in Walnut Seedlings. Forests 2022, 13, 1493. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Deng, F.; Yuan, R.; Shen, F. Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypium hirsutum. BMC Genom. 2017, 18, 376. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).