Abstract
Middle-aged trees refer to trees aged between 50 and 99 years, which are the reserve resources of old trees (trees ≥ 100 years of age). They are vital parts of the urban ecosystem, with important ecological, landscape, cultural, and historical value. Conservation of middle-aged trees in urban areas is important for the development of large old trees in the future. In this study, we investigated the middle-aged trees in Changchun city and analyzed the species composition and diversity characteristics of different urban green space types and tree age classes. The results showed that there were 72 species and 22,376 plants of middle-aged trees in Changchun city, and the coniferous species prevailed. The top five species with a high importance value (IV) were Pinus tabuliformis var. mukdensis, Lavix olgensis, Salix matsudana, Ulmus pumila, and Abies holophylla. Green space type and tree age were important factors influencing the richness and diversity of middle-aged trees. Tree growth spaces were relatively sufficient, and land use was stable for park green spaces (PGS) and attached green spaces (AGS), which resulted in the abundant, richer, and diverse species richness (SR) of middle-aged trees. Road green spaces (RGS) and square green spaces (SGS) had fewer trees and lower species richness, Margalef richness index (dMa), Shannon–Wiener index (He) and evenness index (Je) which could be attributed to the high intensity of human interference and poor environmental quality. The SR of middle-aged trees decreased with an increase in age class, and the values of SR in Age Class 80–89 years and Age Class 90–99 years were lower than in Age Class 50–59 years. Age Class 70–79 years had the lowest values of dMa, He, and Je, which need to be protected urgently. The results of this study can provide a basis for the conservation and management of middle-aged trees in urban areas and the choice of species for urban greening.
1. Introduction
Middle-aged trees refer to trees aged between 50 and 99 years, which are the reserve resources of old trees (tree ≥ 100 years of age) [1,2]. These trees have great potential to become large old trees in the future [3]. Old trees are keystone structures in forests, woodlands, and agricultural ecosystems, playing unique ecological roles that are not provided by younger trees [4]. Some studies have shown that large and big-sized trees are key elements for carbon storage [5,6,7], and the rate of tree carbon accumulation increases continuously with tree size [7]. In addition, old trees can create microclimate and microhabitat heterogeneity, such that epiphyte species richness and abundance increase with tree size [4,8]; provide irreplaceable habitats; and act as stepping stones for many animals [4]. Moreover, old trees are an important part of cultural heritage and can provide people with aesthetic, symbolic, religious, and historical cues [9]. Middle-aged trees, as important reserve resources for future large old trees, have great potential to provide richer and multifunctional ecosystem services, such as high ecological, historical, cultural, and landscape value [3].
In the past 20 years, a series of studies have been carried out on old trees, mainly focusing on the investigation of old trees, species diversity, and spatial differentiation characteristics [10,11,12,13,14,15,16,17]. Some studies have shown that the destruction of old trees is serious, and the number of old trees has been reduced sharply [4,18]. With the recognition of the importance of old trees [11,18,19,20,21,22,23,24,25], a number of conservation management measures for old trees have been carried out [26,27,28]. In the urban ecosystem, old trees are also considered keystone structures that have an important function in urban landscapes [29], such as their role in carbon sequestration, stormwater runoff reduction, heat island effect alleviation, atmospheric pollution mitigation [30], and heritage effects [31,32,33]. In addition, they can provide important habitat resources for wildlife [29]. Compared with old trees, middle-aged trees have received less attention from the government and society [34]. Only a handful of studies have focused on the surveying, diversity analysis, and conservation of middle-aged trees in the urban ecosystem [18,22,25]. Although middle-aged trees are smaller in DBH and tree height than old trees, they have good growth status, stable community structures, and powerful ecological services [35]. However, the location, function, habitats, and management objectives vary greatly for different types of urban green spaces. Performing a thorough investigation of middle-aged trees in different types of green spaces and ascertaining their age structure, species composition, and diversity are necessary to maintain the stability and sustainable development of the urban ecosystem.
Changchun is one of the fastest growing cities in Northeast China and has a special historical process. Changchun was the capital of “Manchukuo” from 1931 to 1945, and the construction of an East Asian metropolis began in 1932 [23]. Some trees planted in the period of “Manchukuo” are still alive today. Learning from the experience of public green space construction in the former Soviet Union [36], a large number of trees were planted in the city’s green spaces in the early years of the founding of the People’s Republic of China, and these trees have become important middle-aged trees. In the past 40 years, Changchun has experienced a period of rapid urban development [24]. With the continuous expansion of the city, the inner space of the city has become increasingly crowded, and the environmental pressure on middle-aged trees is also increasing. How to protect and make good use of these precious resources has become an urgent issue. The purposes of this study were (1) to determine the species, richness, and importance value (IV) of the middle-aged trees in Changchun city; (2) to clarify the species composition and diversity characteristics of middle-aged trees in different types of green spaces; and (3) to clarify the species composition and diversity characteristics of middle-aged trees in different age classes. This study can provide a basic reference for the conservation of middle-aged trees and the plant community configuration for ecological garden construction in the rapid urban development period.
2. Materials and Methods
2.1. Study Area
Changchun city, the capital city of Jilin Province, is located in the middle latitude zone of the Northern Hemisphere (43°05′~45°15′ N, 124°18′~127°05′ E) and situated at east of Eurasia in the hinterland of the Northeast China plain. The built-up area is about 543 km2, and the urban population is around 4.451 million. Changchun has a mid-temperate continental monsoon climate, with an average annual temperature of 4.8 °C, annual precipitation of 522~615 mm, and annual sunshine hours of 2688 h. Soil types are mainly black soil, meadow soil, and chernozem [37].
2.2. Habitat Classification of Middle-Aged Trees
According to the location, function, habitats, management objectives and growth environment of middle-aged trees [38], and classification standard of urban green spaces [39], the growth environment of middle-aged trees was divided into four types of green spaces in Changchun city (Table 1). They were all public green spaces, including park green spaces (PGS), road green spaces (RGS), square green spaces (SGS), and attached green spaces (AGS).

Table 1.
Four tree habitats that accommodate middle-aged trees in Changchun city.
2.3. Field Survey
A field survey was conducted from April to September 2018. A total of 632 sample plots in 59 sites were surveyed, which included 411 plots in 12 sites for PGS, 171 plots in 36 sites for AGS, 21 plots in 6 sites for RGS, and 29 plots in 5 sites for SGS. The area of each plot was 400 m2. The area of each site was different according to the boundary, and woody plants in each plot were all investigated. The species names and abundances were recorded, and the diameter at breast height (DBH) was measured at 1.3 m above the ground. The trees in the sample plots were planted by the Garden Bureau, enterprises, and institutions and were not privately owned. The species were grouped into six classes according to the literature [11], namely, signature (>200 trees/species), dominant (100 to 199 trees/species), common (50 to 99 trees/species), occasional (10 to 49 trees/species), rare (2 to 9 trees/species), and solitary (1 tree/species). Tree age was reckoned from planting records, and trees with no planting records were determined using growth cones. For some species where tree age could not be determined, 30 trees for each species of different types of green spaces were selected to determine the tree age using growth cones, and the DBH was measured with diameter tape; then, the average annual growth of DBH of these species was calculated, and the tree age was estimated based on the average annual growth of DBH.
2.4. Data Analysis
After the field survey and data collation, species diversity indexes for the middle-aged trees in each site were calculated. The indexes included species richness (SR), Margalef richness index (dMa) [40], Shannon–Wiener index (He) [41,42], and evenness index (Je) [43]. These were used to analyze the difference in tree species composition and diversity by different types of urban forests and different age classes. The importance value (IV) for each species was also calculated to indicate their status and role in the ecosystem [30].
where S = total number of species in each site; Pi = proportion of individuals of ith species; RA = relative abundance; RAi = number of trees in ith species/total numbers of trees in eah site; RD = relative dominance, RDi = basal area at breast height of ith species/total basal area in each site; RFi = frequency of ith species/total species frequency in each site.
Shannon–Wiener index: He = −∑Pi lnPi
Evenness index: Je = He/lnS
Importance value: IV = (RAi + RDi + RFi)/3
One-way analysis of variance was performed to test the differences in the species diversity indexes of middle-aged trees among the different types of green spaces and among the different age classes. It is assumed that there is no significant difference in each diversity index between different types of urban forests and different age classes. Multiple comparison tests were used to test the significance of more than two categories. When the variances were homogeneous, Tukey tests were used; otherwise, Games–Howell tests were used. Principal component analysis (PCA) was applied to assess the effects of forest type and age class on middle-aged tree species composition, and species richness was the variable. Similarity percentage (SIMPER) analysis was adopted to assess the species differentiation among the different types of green spaces and age classes. Prior to SIMPER analysis, the species richness was square-root transformed and standardized by normalization. SIMPER analysis was used to examine the contribution of each species to the average Bray–Curtis dissimilarity between the different types of green spaces and the contribution of each species to the average Bray–Curtis dissimilarity among the different age classes. PCA ordination was performed with Canoco 5.0 (Centre for Biometry, Wageningen, The Netherlands). SIMPER analysis was performed with PRIMER version 5.0 (Primer-E Ltd., Roborough, UK). Heatmaps of species dissimilarity for both urban green space types and age classes were generated in R using the pheatmap package.
3. Results
3.1. Species Composition and Importance Value of Middle-Aged Trees in Changchun
There were 22,376 middle-aged trees in Changchun city, which belonged to 72 species, 42 genera, and 25 families. Among them, there were 15 coniferous species with 14,927 trees (66.71% in total), 53 deciduous broad-leaved species with 7307 trees (32.66%), and 4 shrubs with 142 trees (0.63%). The ratio of the number of coniferous trees to broad-leaved trees was 1.00:0.49, which indicates that the coniferous species prevailed. The results of species composition showed that the contributions of individual species were highly heterogeneous (Table 2). The signature group had 14 species but accounted for 91.74% of the number of the total trees and had an IV of 78.51%. Pinus tabuliformis var. mukdensis, Lavix olgensis, Salix matsudana, Ulmus pumila, Abies holophylla, Picea koraiensis Armeniaca sibirica, and Fraxinus mandshurica were the most important 8 species, and the IV of these species reached 64.45%. The dominant group and common group contained 7 and 9 species, which accounted for 3.96% and 2.83% of the total trees and had an IV of 7.33% and 7.51%, respectively. The occasional group had 15 species, which accounted for 1.19% of the total trees and had an IV of 3.74%. The rare category encompassed the highest species number, which comprised 26.39% of the species and 0.27% of the trees, with an IV of 2.41%. The 8 species in the solitary group contributed to only 0.04% of species and 0.51% of IV.

Table 2.
Species frequency, relative frequency (RF), relative abundance (RA), relative dominance (RD), and species importance value (IV) in Changchun.
The species composition of middle-aged trees was heavily biased toward native species. A total of 57 species were native and owned 18,593 trees, contributing to 79.17% of species and 83.09% of trees. There were 11 species of 3765 trees from other regions of China, accounting for 15.27% of total species and 16.82% of total trees. Only 4 species were alien with 18 trees, accounting for 5.56% and 0.08% of the total species and trees, respectively. The alien species were Pinus strobus, Acer negundo, Robinia pseudoacacia, and Populus canadensis, respectively.
The analysis of IV for different families showed that Pinaceae had the highest IV, with a value of 49.37%, followed by Salicaceae, with a value of 15.17%. For different genera, Pinus had the highest IV of 22.84%, followed by Larix and Salix, with a value of 13.31% and 10.32%, respectively. In addition, the species with a higher IV in the different types of green spaces varied greatly (Table 3). The IV of Pinus tabuliformis var. mukdensis in SGS, AGS, and PGS was the highest and ranked second in RGS. Lavix olgensis had the highest IV in RGS and ranked second in PGS.

Table 3.
Two preponderant species of middle-aged trees by type of green spaces in Changchun city. RGS, road green spaces; SGS, square green spaces; AGS, attached green spaces; PGS, park green spaces.
3.2. Structural Characteristics and Distribution of Middle-Aged Trees among Different Types of Green Spaces
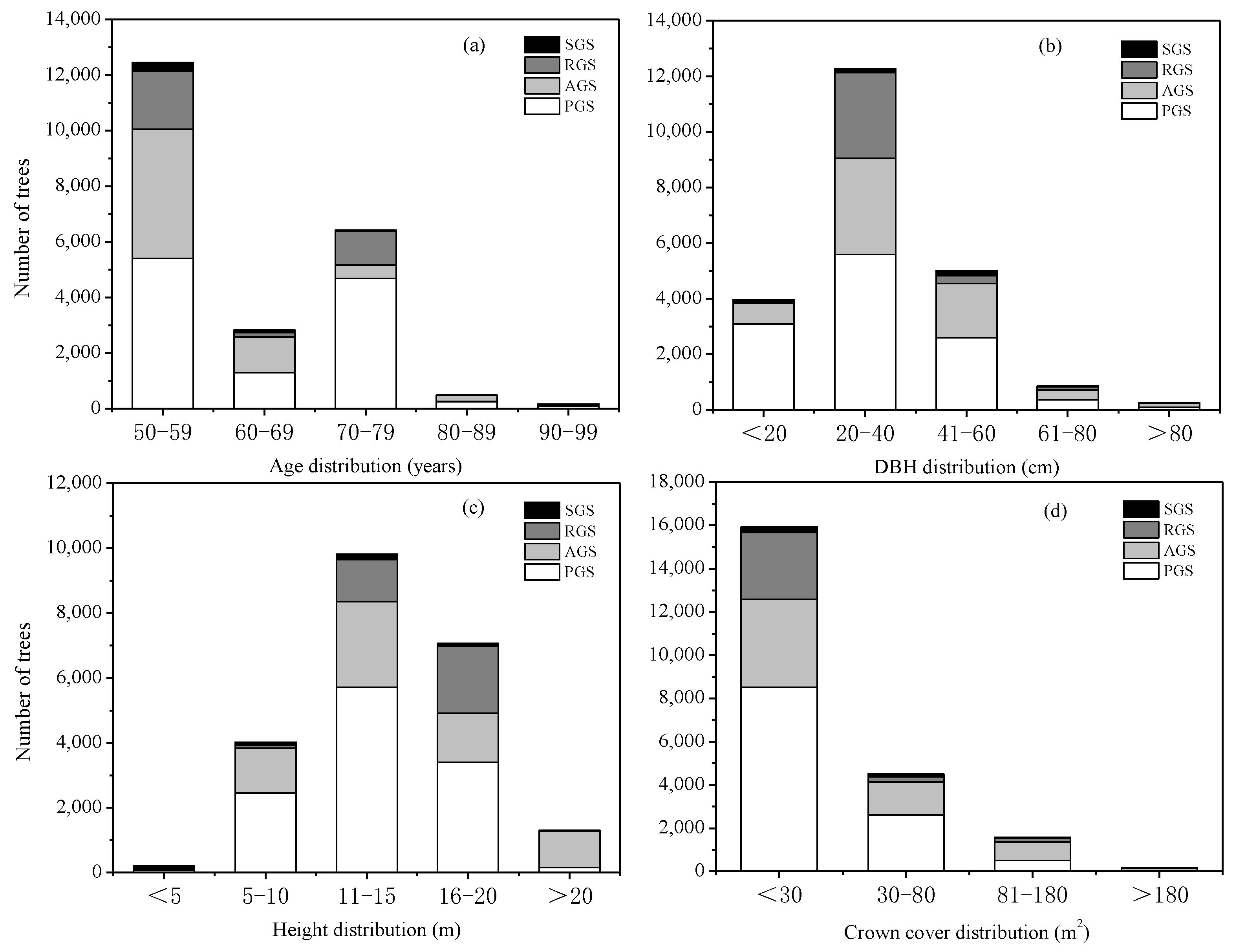
The distribution of middle-aged trees in the different types of green spaces showed that PGS had the largest number of trees, followed by AGS and RGS, while SGS had the lowest number of trees, with 11,748, 6676, 3481, and 471 trees, accounting for 52.50%, 29.84%, 15.56%, and 2.10% of the total middle-aged trees, respectively (Figure 1a). Furthermore, the number of middle-aged trees between 50–59 years was the highest, accounting for 55.66% of the total trees, followed by Age Class 70–79 years and Age Class 60–69 years, accounting for 28.72% and 12.67% of the total trees, respectively (Figure 1a). However, the number of trees in Age Class 80–89 years and Age Class 90–99 years was lower than in other age classes, which accounted for 2.19% and 0.77%, respectively. As for different types of green spaces, PGS had the highest number of trees in different age classes, but SGS was the lowest.
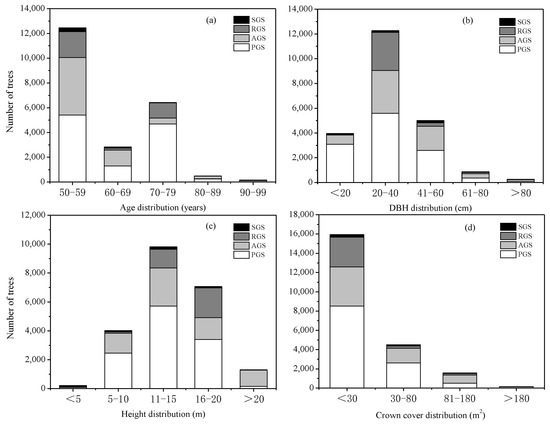
Figure 1.
Age, DBH, height, and crown cover distribution of middle-aged trees. (a) Number of middle-aged trees in different age classes; (b) number of middle-aged trees in different DBH grades; (c) number of middle-aged trees in different height grades; (d) number of middle-aged trees in different crown cover grades. SGS, square green spaces; RGS, road green spaces; AGS, attached green spaces; PGS, park green spaces.
The distribution of middle-aged trees in different DBH grades showed that the number of trees at the DBH grade of 20–40 cm was the highest, followed by the DBH grade of 41–60 cm and the grade of <20 cm, and the number of trees at the DBH grade of >80 cm was the lowest, which accounted for 54.85%, 22.35%, 17.74%, and 1.18% of the total trees, respectively (Figure 1b). At the range of DBH <20 cm, 21–40 cm, and 41–60 cm, PGS had the largest number of trees, and SGS had the lowest number of trees. However, at the range of 61–80 cm and >80 cm, AGS had the highest number of trees, followed by PGS and RGS, and SGS had the lowest number of trees.
The distribution of middle-aged trees in different height grades showed that the number of trees at the height of 11–15 m was the largest, followed by the grade of 16–20 m, 5–10 m, and >20 m; however, the number of trees in the grade of <5 m was the lowest, which accounted for 43.95%, 31.62%, 17.65%, 5.81%, and 0.98%, respectively (Figure 1c). For the different types of green spaces, at the grades of 5–10 cm, 11–15 cm, and 16–20 cm, the number of trees all ranked as PGS > AGS > RGS > SGS, at the range of <5 m, which ranked as SGS > PGS > AGS > RGS. However, for the trees at the range of >20 m, the AGS had the highest number of trees, accounting for 86.13% of this grade, followed by the PGS, accounting for 12.63%, and the SGS only accounted for 0.08% of this grade’s trees.
The distribution of middle-aged trees in different crown cover grades showed that trees distributed at the range of <30 m2 had the highest number, followed by the grades of 30–80 m2 and 81–180 m2. The number of trees at the range of >180 m2 was the lowest, which accounted for 72.37%, 20.44%, 7.14%, and 0.05%, respectively (Figure 1d). At the grades of <30 m2 and 30–80 m2, the number of trees was ranked as PGS > AGS > RGS > SGS, and at the range of 81–180 m2 and >180 m2, the number of trees was ranked as AGS > PGS > RGS > SGS.
3.3. Species Diversity Characteristics of Middle-Aged Trees among Different Types of Green Spaces and Different Age Classes
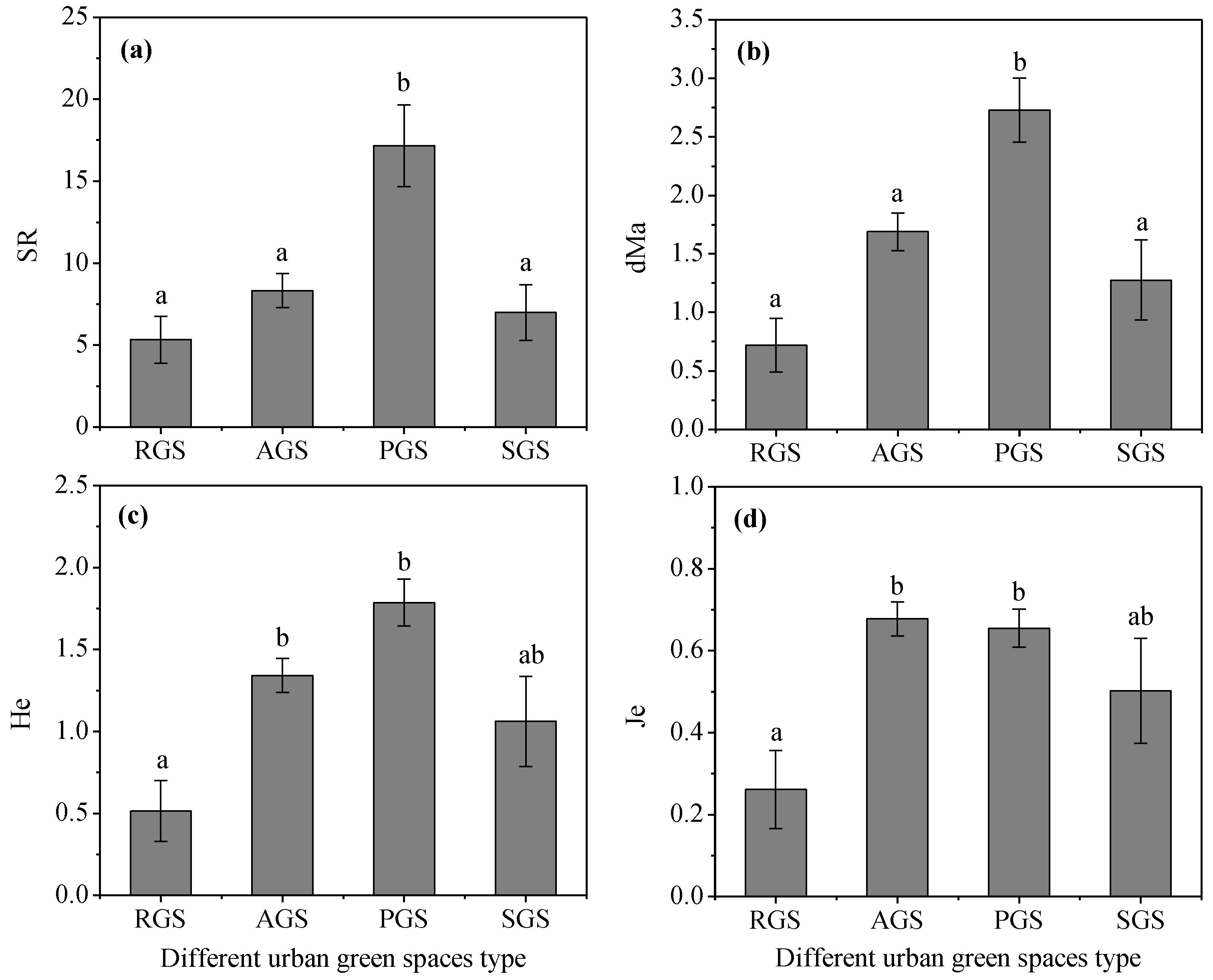
The species richness and diversity characteristics of middle-aged trees in different types of green spaces varied greatly (Figure 2). For the mean value of SR and dMa, the PGS was the highest and significantly higher than other types of green spaces (p < 0.05), but there was no significant difference among AGS, RGS, and SGS (p > 0.05). For the Shannon–Wiener index He, PGS also had the highest mean value of 1.79, followed by AGS, and RGS was the lowest, with a mean value of only 0.51. However, AGS had the highest value of 0.68 for Je, followed by PGS, and RGS had the lowest value of only 0.26. Furthermore, the mean value of He and Je in AGS and PGS was significantly higher than RGS (p < 0.05). These results indicate that the SR in PGS were richer and diverse and evenly distributed, but in RGS, they were poor and uneven.
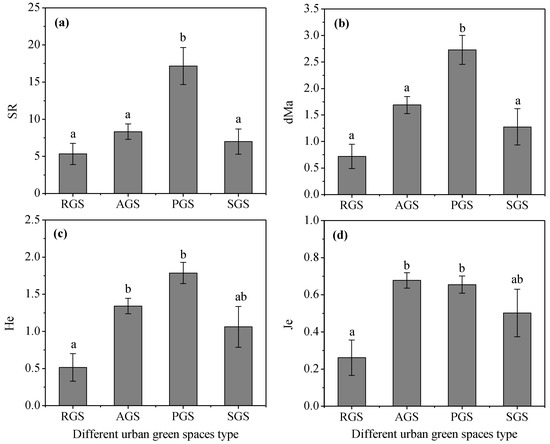
Figure 2.
SR and diversity indexes of middle-aged trees among different types of green spaces in Changchun city. Note: Data in the figure are means ± standard errors; different lowercase letters indicate significant differences (p < 0.05). (a) SR, species richness; (b) dMa, Margalef richness index; (c) He, Shannon–Wiener index; (d) Je, evenness index. RGS, road green spaces; AGS, attached green spaces; PGS, park green spaces; SGS, square green spaces.
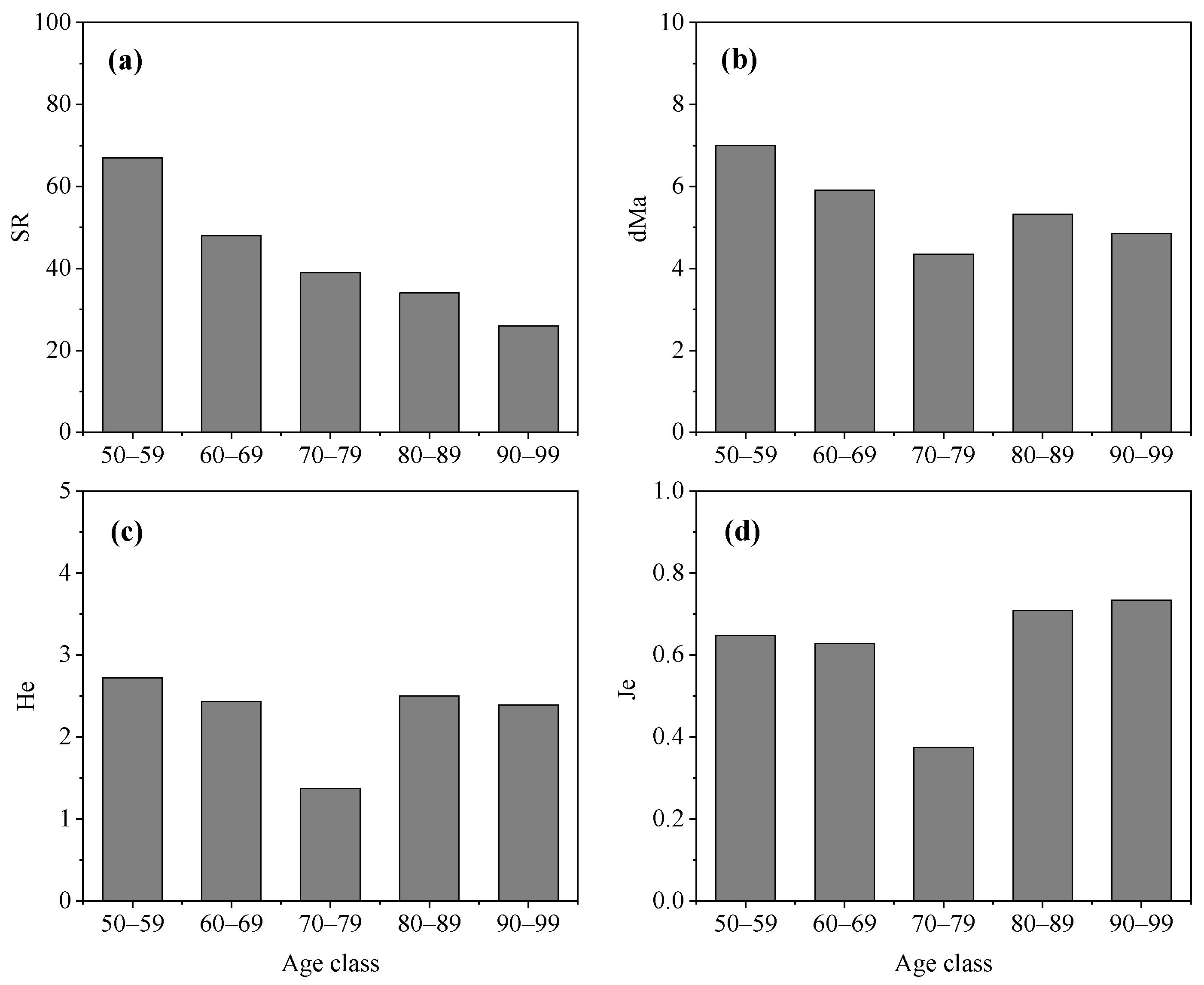
The diversity characteristics of middle-aged trees among different age classes varied greatly (Figure 3). The SR of middle-aged trees decreased with an increase in age class. The value of SR in Age Class 50–59 years was 67 species and higher than in Age Class 90–99. However, Age Class 70–79 years had the lowest value of dMa, He, and Je. The indexes of dMa, and He had a similar trend in different age classes. They were highest in Age Class 50–59 years, followed by Age Class 60–69 years, Age Class 80–89 years, and Age Class 90–99 years. Age Class 80–89 years and Age Class 90–99 years had a lower value of SR, dMa, and He but had a higher value of Je compared with other three age classes.
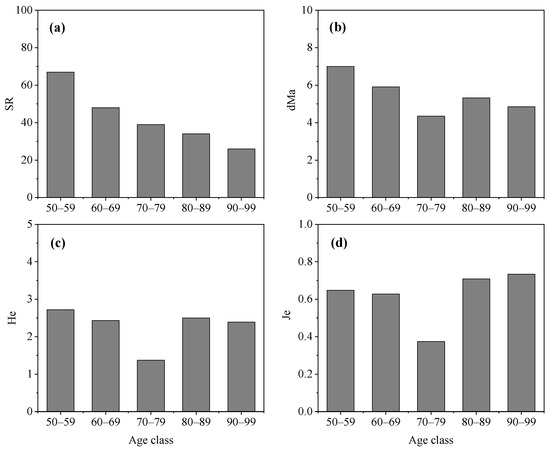
Figure 3.
Diversity characteristics of middle-aged trees among different mean age classes in Changchun city. (a) SR, species richness; (b) dMa, Margalef richness index; (c) He, Shannon–Wiener index; (d) Je, evenness index.
3.4. PCA Analysis of Species Composition of Middle-Aged Trees among Different Types of Green Spaces and Different Age Classes
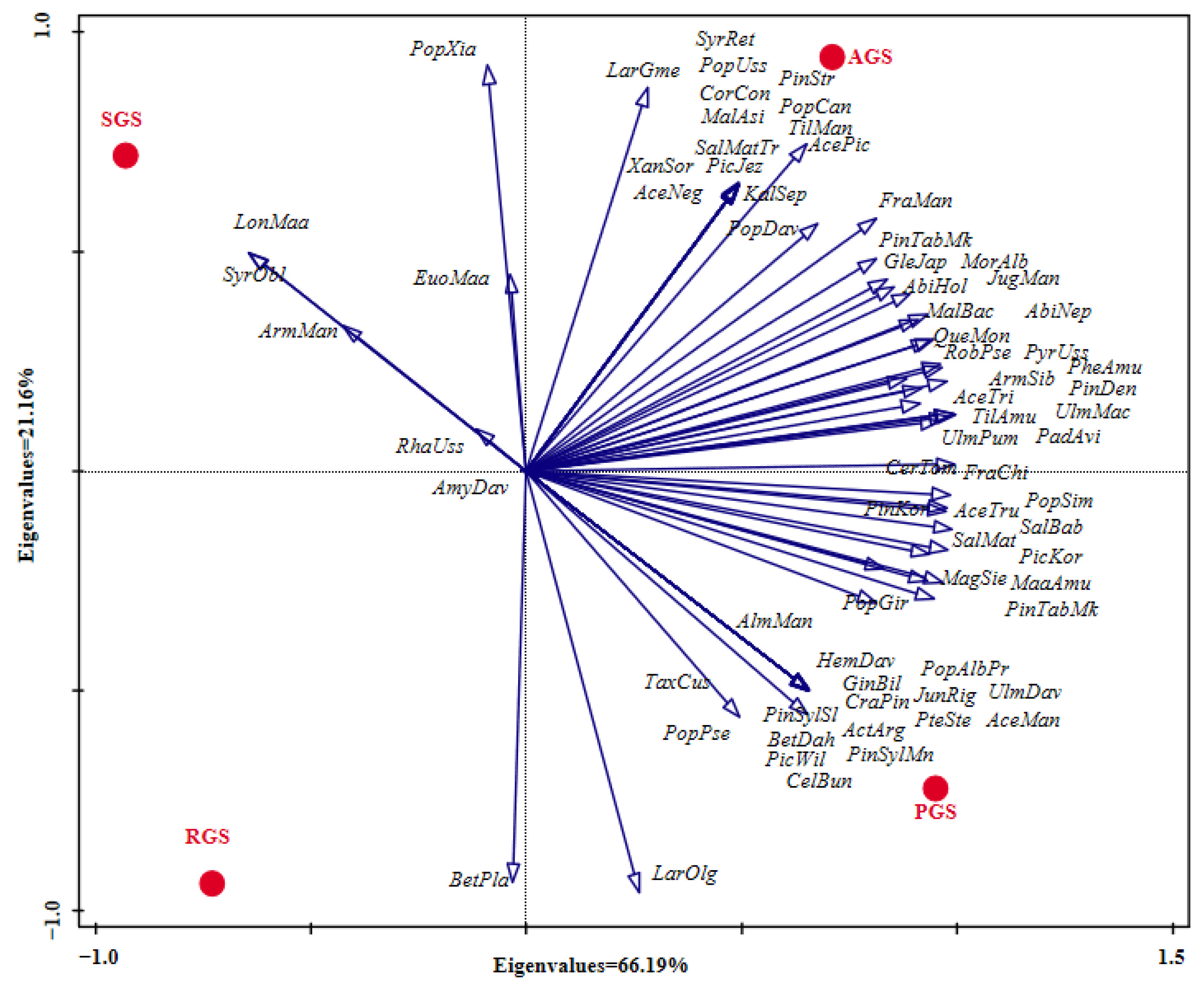
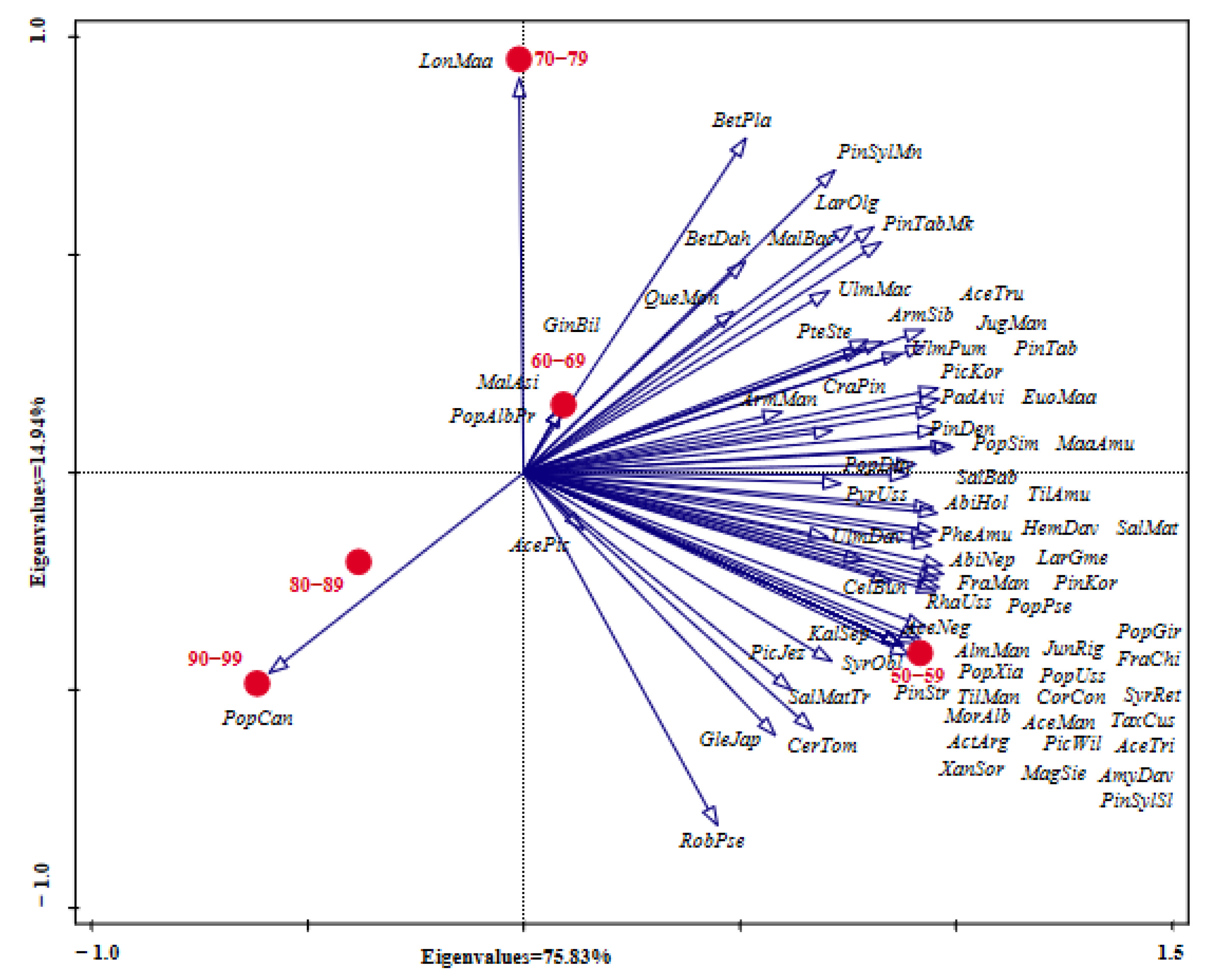
PCA analysis of middle-aged tree species composition among the different types of green spaces showed that the species richness of PGS was the highest, followed by AGS, while SGS and RGS were relatively lower, with species richness of 57, 53, 22 and 19, respectively (Figure 4). For the type of AGS, the abundance of Fra man, Abi hol, Sal mat, Phe amu, Arm sib, Mal bac, Lar gme, Qur mon, Pyr uss, Jug man, and Ace pic was higher than in the other types of green spaces. Furthermore, Pic jez, Ace neg, Pop uss, Sal mat tor, Cor con, Pin str, Syr ret, Til man, Kal sep, Mal asi, Pop can, and Xan sor were the unique species. For the type of PGS, the abundance of Lar olg was the highest, followed by Pin tab muk, Pin kor, Pic kor, Ulm pum, Abi hol, Maa amu, Sal mat, Til amu, and Pin syl mon., among the above species, except for the abundance of Abi hol and Sal mat, which were lower than in the type of AGS, the abundance of the other species was the highest in the type of PGS. In addition, the species of Pin syl syl, Bet dah, Pte ste, Cra pin, Act arg, Hem dav, Jun rig, Ulm dav, Tax cus, Alm man, Cel bun, Pic wil, Ace man, Pop alb pyr, and Gin bil was the unique species in the type of PGS. For the type of RGS, the abundance of the top eight species was Lar olg, Pin tab muk, Pin syl mon, Bet pla, Sal mat, Pop pse Arm sib, and Euo maa, respectively. Among them, Pop pse and Bet pla had a higher abundance in RGS than in the other types of green spaces, but the abundance of Pin tab muk, Sal mat, Arm sib, and Pic kor was lower than in the types of PGS and AGS. However, for the type of SGS, Syr obl and Lon Maa were the unique species, but the abundance of Arm man, Pop xia, and Rha uss was higher than in the other types of green spaces.
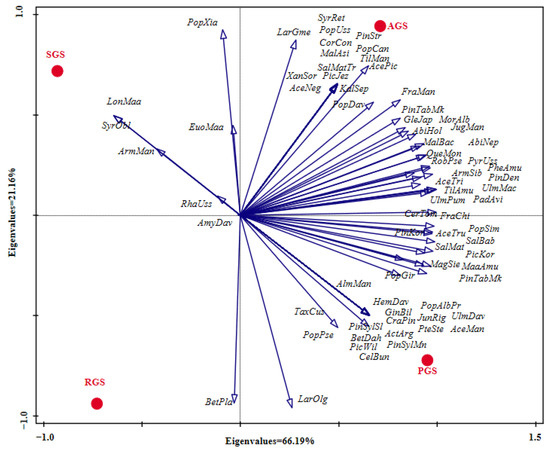
Figure 4.
PCA analysis of the species composition among different types of green spaces. RGS, road green spaces; AGS, attached green spaces; PGS, park green spaces; SGS, square green spaces. For abbreviations of species names, refer to Table A1.
PCA analysis of middle-aged tree species composition among the different age classes showed that the species richness in Age Class 50–59 years was the highest, and the main species abundance was higher than that in other age classes, such as Abi hol, Pin kor, Fra man, Maa amu, Til amu, Lar gme, Phe amu, Pop pse, Sal Mat, and so on. In addition, Syr obl, Ace man, Ace neg, Ace tri, Alm man, Amy dav, Cor con, Jun rig, Mag sie, and Pin syl syl were the unique species in Age Class 50–59 years (Figure 5). In Age Class 60–69 years, the species abundance of Arm sib, Que mon, Bet dah, Arm man, and Ulm dav was higher than in the other age classes, and Gin bil, Mal asi, and Pop alb pyr were the unique species. In Age Class 70–79 years, the abundance of Lar olg, Pin syl mon, Mal bac, and Bet pla was higher than in other classes, and Lon maa was the unique species. In Age Class 80–89 and Age Class 90–99, the abundance of the main species was lower than in the other three age classes, and Pop can was the only unique species.
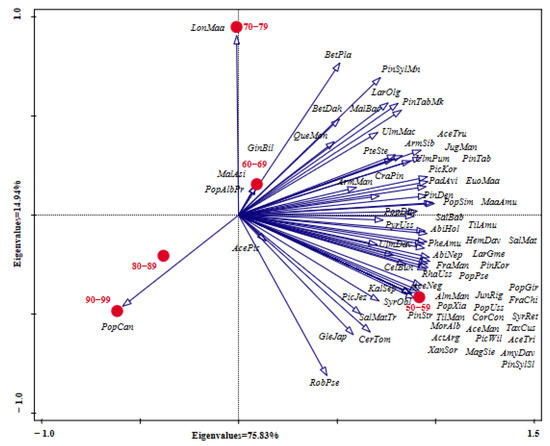
Figure 5.
PCA analysis of the species composition among different age classes (years). For abbreviations of species names, refer to Table A1.
3.5. Species Dissimilarity of Middle-Aged Trees among Different Types of Green Spaces and Different Age Classes
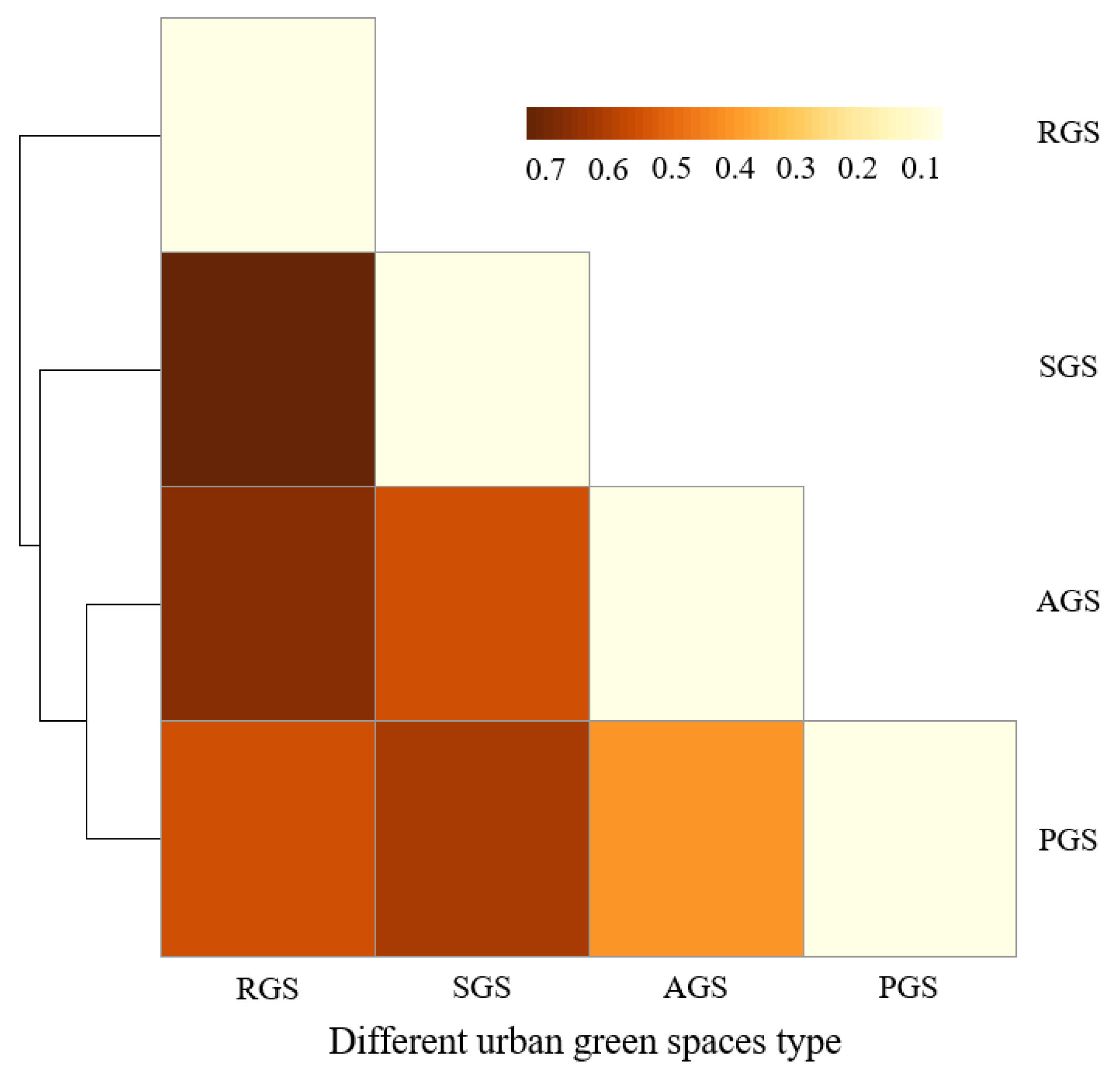
The spatial differentiation of middle-aged trees among the different types of urban green spaces was analyzed with SIMPER analysis (Figure 6). While the dissimilarity was 37.20% between AGS and PGS, between other types of green spaces, it was higher than 50%. This indicates that the species composition of AGS and PGS had a higher similarity. Lar olg, Fra man, Pin kor, Phe amu, Sal mat, and Abi hol were the main contributing species, which contributed to 43.01% of the total dissimilarity (Table 4). The highest dissimilarity was detected between RGS and SGS, with a value of up to 70.99%, followed by the dissimilarity between RGS and AGS, with a value of 64.17%. The dissimilarity between RGS and PGS was 52.05%. The highest dissimilarity between RGS and SGS can be attributed to the species of Lar olg, Syr obl, Pop xia, Abi hol, Pin syl mon, and Pin tab muk, contributing to 52.09% of the total dissimilarity. Between RGS and AGS, the species of Lar olg, Fra man, Abi hol, Phe amu, Ulm pum, and Sal mat contributed to 50.47% of the total dissimilarity. Furthermore, the dissimilarity between SGS and PGS and between SGS and AGS was at a moderate level, with a value of 52.29% and 58.86%, respectively.
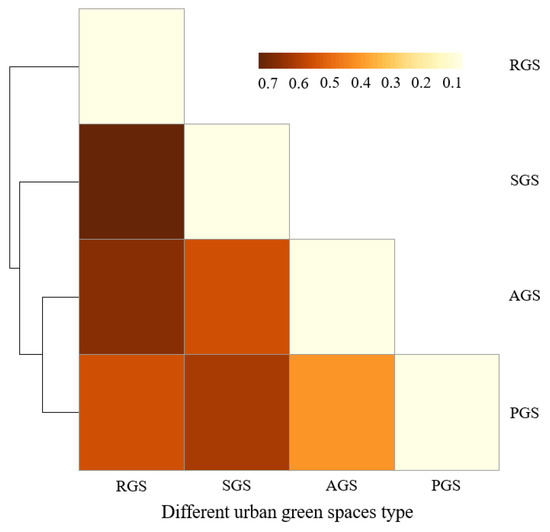
Figure 6.
Dissimilarity among four types of green spaces based on species richness of middle-aged trees found by SIMPER analysis. RGS, road green spaces; SGS, square green spaces; AGS, attached green spaces; PGS, park green spaces.

Table 4.
Main contributing species of middle-aged trees to the dissimilarity of different types of green spaces. RGS, road green spaces; SGS, square green spaces; AGS, attached green spaces; PGS, park green spaces. Values in parentheses represent the contribution of each species to the dissimilarity. For abbreviations of species names, refer to Table A1.
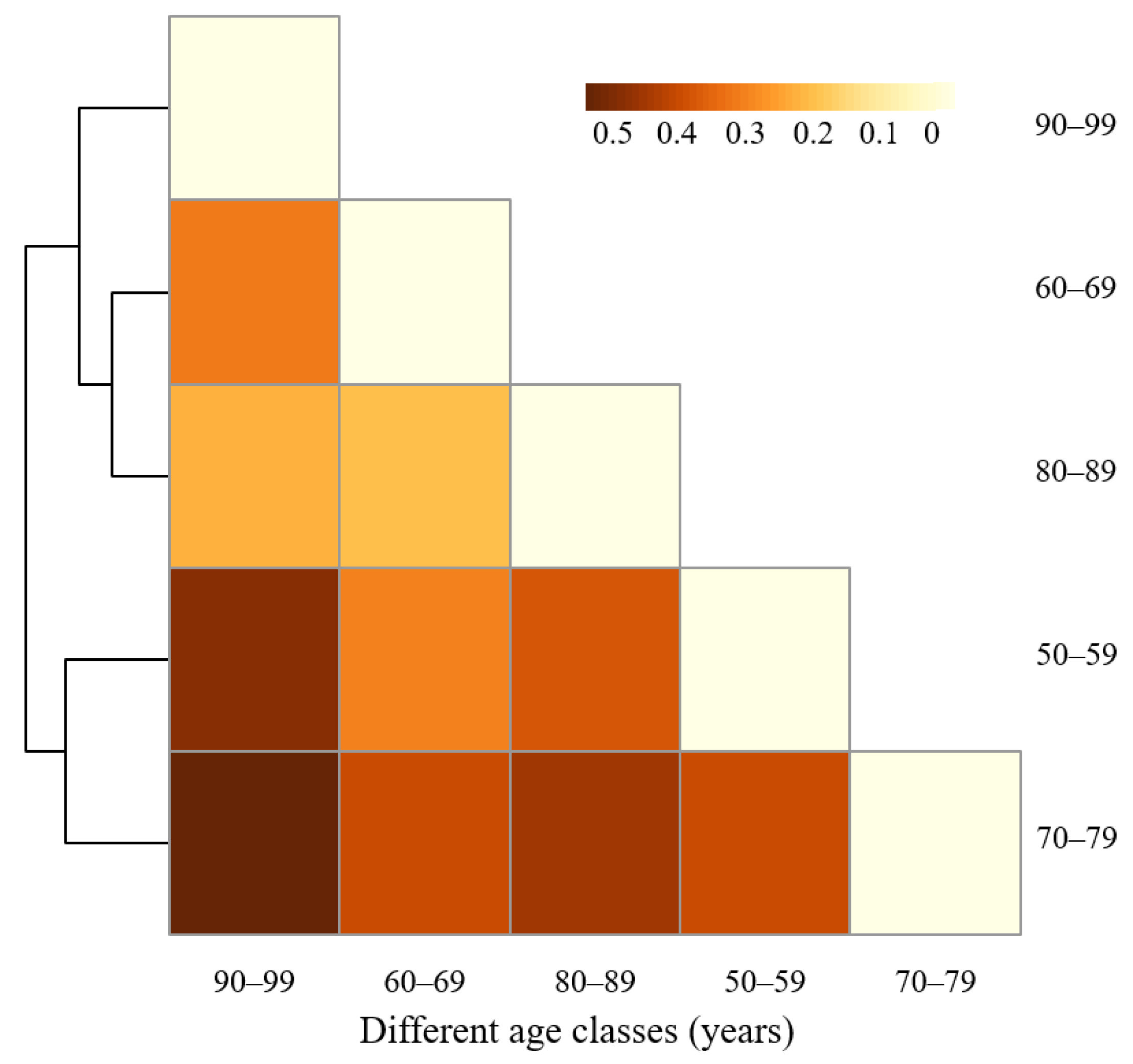
The species dissimilarity of middle-aged trees between age classes was also analyzed by SIMPER analysis (Figure 7). The lowest dissimilarity was found between Age Class 60–69 years and Age Class 80–89 years (23.38%). The main contributing species were Pin tab muk, Ace pic, Ulm pum, Abi hol, and Phe amu, which contributed to 42.80% of the total dissimilarity (Table 5). In addition, the dissimilarity was 25.60% between Age Class 80–89 years and Age Class 90–99 years. However, the middle-aged tree species dissimilarity between Age Class 70–79 years and Age Class 90–99 years was the largest, which reached 59.23%, followed by the dissimilarity between Age Class 50–59 years and Age Class 90–99 years, and the dissimilarity between Age Class 70–79 years and Age Class 80–89 years, with a value of 54.41% and 50.39%, respectively. This indicates that the middle-aged tree species composition differed greatly between these age classes. The highest species dissimilarity (59.23%) of middle-aged trees between Age Class 70–79 years and Age Class 90–99 years can be attributed to the species of Lar olg, Ulm pum, Sal mat, Pin syl mon, and Pin tab muk, which contributed to 45.88% of the total dissimilarity.
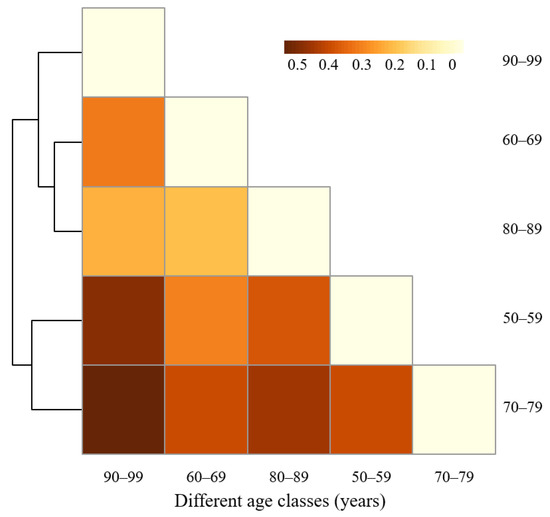
Figure 7.
Dissimilarity among age classes based on species richness of middle-aged trees found by SIMPER analysis.

Table 5.
Main contributing middle-aged tree species to the dissimilarity of different age classes. Values in parentheses represent the contribution of each species to the dissimilarity. For abbreviations of species names, refer to Table A1.
4. Discussion
4.1. General Species Composition of Middle-Aged Trees in Changchun
Middle-aged trees are an important part of the urban ecosystem. A total of 72 species and 22,376 middle-aged trees were found in Changchun, which was much higher than the existing old trees (25 species and 773 trees) [44]. At the same time, the number of middle-aged trees in Changchun was higher than in Quzhou, Shanghai, and Yangzhou [18,22,25]. The richer species and larger number of middle-aged trees can be attributed to the fact that Changchun began to build an East Asian metropolis in the 1930s. Its urban planning work started earlier than most Chinese cities and took a leading position in the whole of Asia. Systematic green space construction not only left a large number of tree resources, but also retained a large number of urban green spaces for the city. Compared with old trees mostly scattered in the city, middle-aged trees exist as a community in urban green spaces and grow vigorously and strongly. In this study, the five middle-aged tree species with the highest importance value were Pinus tabuliformis var. mukdensis, Lavix olgensis, Salix matsudana, Ulmus pumila, and Abies holophylla. Among these five species, Pinus tabuliformis var. mukdensi, Lavix olgensis, and Abies holophylla were planted widely due to their excellent landscape properties and stronger resistance to pollution, drought, and poor soil, as well as remaining evergreen in all seasons. Salix matsudana and Ulm pum have strong resistance to cold and stressful urban environments, which enables them to grow and develop normally with human disturbance [44]. The above species are all the priority choice and key protection tree species for urban greening in the future.
Urban areas are hot spots for the intentional or unintentional introduction and naturalization of alien species [45]. The choice of plant species was a rather earnest preference for native species in the urban forests of Changchun [46]. In this study, the results of lower alien species richness and tree count were consistent with previous studies. Regional climate and human preferences for species influenced the introduction of alien species [46,47].
4.2. Species Composition, Diversity Attributes, and Differentiation of Middle-Aged Trees in Different Types of Green Spaces
Higher spatial heterogeneity in cities leads to rich plant species diversity [48]. Previous studies have shown that species composition and diversity characteristics vary significantly among different urban forest types [46]. The combination of urban forest management systems and habitat conditions and their changes through time has engendered distinctive forest types in terms of ecological, amenity, and environmental functions [49]. In this study, middle-aged tree species composition and diversity among different types of green spaces also varied significantly. PGS had the highest number of middle-aged trees at different age classes, DBH, height, and crown cover grades, as well as having the highest SR, He, and Je. This can be attributed to several reasons. On the one hand, the predominant species of Pinus tabuliformis var. mukdensi and Lavix olgensis of PGS have a long history of planting, as well as excellent adaptability to harsh urban environments. For example, Pinus tabuliformis var. mukdensi was widely planted in Changchun in the period of “Manchukuo” [50]. Lavix olgensis was planted in large quantities in the early years of the People’s Republic of China. On the other hand, the conservation, protection, and management measures of PGS were professional and regular. PGS can provide adequate spaces and high-quality habitats for the long-term growth of a large number of middle-aged trees. Correspondingly, the effects between organisms and the environment are interactive. Middle-aged trees are tall and leafy. They not only provide stronger ecological services for human beings, but also play an important role in urban biodiversity protection and landmark plant landscape construction [51]. For RGS and SGS, the significantly lower number of middle-aged trees, SR, dMa, He, and Je is mainly due to the poor growth environment, monotonous species selection and simple community configuration, and lower level of protection and maintenance, as well as limited living spaces. Compared with other types of urban green spaces, tree planting and management in AGS are mainly decided by enterprises and institutions. Individual preference, land use spaces, management, and greening funding investment influenced the number of trees and the level of SR, He, and Je of middle-aged trees.
Species dissimilarities in different habitats were mainly contributed by dominant and common species [11]. In this study, species dissimilarity of middle-aged trees between AGS and PGS was the lowest. There were more species in common between these two types of urban green spaces, contributing to the lowest dissimilarity between AGS and PGS, but the number of the main contributing species of Lar olg, Fra man, Pin kor, Phe amu, Sal mat, and Abi hol varied greatly between AGS and PGS. Thus, strengthening the selection and protection of Lar olg and Pin kor for AGS and enhancing the management and protection of Fra man, Phe amu, Sal mat, and Abi hol for PGS is needed. The highest dissimilarity was detected between RGS and SGS, indicated by the higher similarity of species composition between RGS and SGS. This is mainly due to the wide application of coniferous species of Lar olg, Pin syl mon, and Pin tab muk in these two types of green spaces in history. Deciduous species of Fra man, Abi hol, Phe amu, Ulm pum, and Sal mat are suggested to be used in RGS and SGS in the future. Habitats of different types of green spaces vary greatly. Species differentiation by types of green spaces implies the need to preserve not only middle-aged trees, but more importantly their habitat and setting [11].
4.3. Species Composition, Diversity Attributes, and Differentiation of Middle-Aged Trees in Different Age Classes
Understanding the characteristics of species composition and diversity attributes in different age classes was vital to maintaining the dynamic balance of biological populations in the urban ecosystem. Previous studies have shown that species differentiation is found among different administrative districts, which are closely related to the district construction history [11,44]. However, there are few studies on the characteristics of species composition and diversity of middle-aged trees in different tree age classes. In this study, we found the SR and number of middle-aged trees decreased with an increase in age classes. The total number of middle-aged trees in Age Class 80–89 years and Age Class 90–99 years accounted for 2.96%. This result indicates that the protection of middle-aged trees in Age Class 80–89 years and Age Class 90–99 years is urgently needed. Furthermore, Age Class 70–79 years had the second highest number of middle-aged trees but had the lowest dMa, He, and Je, reflecting that species distribution is extremely uneven in this age class. The species diversity of He and Je can be enhanced by increasing the protection of rare and unique species in different age classes. Planting rare and native tree species would be an effective way to mitigate the biotic homogenization of urban green spaces [52].
In this study, species dissimilarity of middle-aged trees between Age Class 60–69 years and Age Class 80–89 years was the lowest. The uneven distribution of Pin tab muk, Ace pic, Ulm pum, Abi hol, and Phe amu contributed more to the dissimilarity. For example, the abundance of Pin tab muk, Ulm pum, Abi hol, and Phe amu was much higher in Age Class 60–69 years than in Age Class 80–89 years, but the abundance of Ace pic was higher in Age Class 80–89 years than in Age Class 60–69 years. The second lowest dissimilarity between Age Class 80–89 years and Age Class 90–99 years indicates that the similarity of middle-aged tree species composition was higher between these age classes. It is urgent to protect and maintain the main contributing species of Ace pic, Phe amu, Mal bac, Gle jap, and Sal bab in these two age classes. These results also demonstrated that more attention should be paid to species selection and cultivation at multiple age classes during urban green space planning and community structure configuration.
5. Conclusions and Implications
The middle-aged tree species is richer in Changchun. A total of 72 species and 22,376 middle-aged trees were found in Changchun, which was much higher than the existing old trees. Great spatial differentiation of middle-aged trees existed among the different types of green spaces. Growth spaces, environment, and management measures were the main influencing factors. Decreasing the differentiation between types of green spaces, strengthening the selection and protection of Lar olg and Pin kor for AGS, and enhancing the selection and protection of Fra man, Phe amu, Sal mat, and Abi hol for PGS are urgently needed. Deciduous species of Fra man, Abi hol, Phe amu, Ulm pum, and Sal mat are suggested to be used in RGS and SGS. Middle-aged tree species diversity also varies greatly among different age classes. Protection of middle-aged trees in Age Class 80–89 years and Age Class 90–99 years is urgently needed. Furthermore, species distribution is extremely uneven in Age Class 70–79 years. Increasing the protection of rare and unique species is important to enhance He and Je in Age Class 70–79 years. The selection and configuration of tree species at multiple age classes should be considered in plant community construction.
With rapid urbanization, the survival pressure of middle-aged trees is increasing. In order to better protect middle-aged trees, we suggest (1) to formulate protection and management measures for middle-aged trees, such as the improvement of soil, the application of fertilizer, the expansion of the tree pool, and the pruning of branches and leaves [44]; (2) to set up protection signs for trees in Age Class 80–89 years and Age Class 90–99 years; and (3) to establish a long-term dynamic monitoring platform to observe the growth, health, habitats, and changes of middle-aged trees. Monitoring data should include geographical location information (location, green space type, geographic coordinates), growth and structural attributes (DBH, tree height, crown width, tree age, growth status), environmental information (soil properties, human disturbance, land use change), and maintenance management information (watering, fertilization, pest control, rejuvenation measures).
Author Contributions
Y.Y., X.S., C.Z. and D.Z. lead the data collection. Z.W. and J.W. analyzed the data and drew the figures. The manuscript was written by Y.Y. The article was revised by D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research article was supported by the Key Project of Changchun Garden Bureau (2019JBH27L14), the “Climbing” Program of Changchun University (ZKP202015), and the Natural Science Foundation of Jilin Province (20220101315JC).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Species botanical names and abbreviations of middle-aged trees in Changchun city.
Table A1.
Species botanical names and abbreviations of middle-aged trees in Changchun city.
| Species Name | Abbreviation | Species Name | Abbreviation |
|---|---|---|---|
| Abies holophylla Maxim. | Abi hol | Phellodendron amurense Rupr. | Phe amu |
| Abies nephrolepis (Trautv.) Maxim | Abi nep | Picea jezoensis | Pic jez |
| Acer mandshuricum Maxim. | Ace man | Picea koraiensis Nakai | Pic kor |
| Acer negundo L. | Ace neg | Picea wilsonii Mast. | Pic wil |
| Acer pictum subsp. mono (Maxim.) H. Ohashi | Ace pic | Pinus densiflora Sieb.et Zucc. | Pin den |
| Acer triflorum Kom. | Ace tri | Pinus koraiensis Siebold et Zuccarini | Pin kor |
| Acer truncatum Bunge | Ace tru | Pinus strobus L. | Pin str |
| Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq. | Act arg | Pinus sylvestris var. mongolica Litv. | Pin syl mon |
| Alnus mandshurica (Callier) Hand.-Mazz. | Alm man | Pinus sylvestris var. sylvestriformis (Takenouchi) Cheng et C. D. Chu | Pin syl syl |
| Amygdalus davidiana (Carr.) C. de Vos | Amy dav | Pinus tabulaeformis Carriere | Pin tab |
| Armeniaca mandshurica (Maxim.) Skv. | Arm man | Pinus tabuliformis var. mukdensis (Uyeki ex Nakai) Uyeki | Pin tab muk |
| Armeniaca sibirica (L.) Lam. | Arm sib | Populus × canadensis Moench | Pop can |
| Betula dahurica Pall. | Bet dah | Populus × xiaohei T. S. Hwang et Liang | Pop xia |
| Betula platyphylla Suk. | Bet pla | Populus alba var. pyramidalis Bunge | Pop alb pyr |
| Celtis bungeana Bl. | Cel bun | Populus davidiana Dode | Pop dav |
| Cerasus tomentosa (Thunb.) Wall | Cer tom | Populus girinensis Skv. | Pop gir |
| Cornus controversa Hemsl. | Cor con | Populus pseudo simonii Kitagawa | Pop pse |
| Crataegus pinnatifida Bge. | Cra pin | Populus simonii Carr. | Pop sim |
| Euonymus maackii Rupr. | Euo maa | Populus ussuriensis Kom. | Pop uss |
| Fraxinus chinensis subsp. rhynchophylla (Hance) E. Murray | Fra chi | Pterocarya stenoptera C. DC. | Pte ste |
| Fraxinus mandshurica Rupr. | Fra man | Pyrus ussuriensis Maxim. | Pyr uss |
| Ginkgo biloba L. | Gin bil | Quercus mongolica Fischer ex Ledebour | Que mon |
| Gleditsia japonica Miq. | Gle jap | Rhamnus ussuriensis J. Vass. | Rha uss |
| Hemiptelea davidii (Hance) Planch. | Hem dav | Robinia pseudoacacia L. | Rob pse |
| Juglans mandshurica Maxim. | Jug man | Salix babylonica L. | Sal bab |
| Juniperus rigida Sieb. et Zucc. | Jun rig | Salix matsudana f. tortuosa (Vilm.) Rehd. | Sal mat tor |
| Kalopanax septemlobus (Thunb.) Koidz | Kal sep | Salix matsudana Koidz | Sal mat |
| Larix gmelinii (Rupr.) Kuzen. | Lar gme | Syringa oblata Lindl. | Syr obl |
| Lavix olgensis Henry | Lar olg | Syringa reticulata subsp. amurensis (Ruprecht) P. S. Green & M. C. Chang | Syr ret |
| Lonicera maackii (Rupr.) Maxim. | Lon maa | Taxus cuspidata Sieb. et Zucc | Tax cus |
| Maackia amurensis Rupr. et Maxim | Maa amu | Tilia amurensis Rupr. | Til amu |
| Magnolia sieboldii | Mag sie | Tilia mandshurica Rupr. et Maxim. | Til man |
| Malus asiatica Nakai | Mal asi | Ulmus davidiana var. japonica (Rehd.) Nakai | Ulm dav |
| Malus baccata (L.) Borkh | Mal bac | Ulmus macrocarpa Hance | Ulm mac |
| Morus alba L. | Mor alb | Ulmus pumila L. | Ulm pum |
| Padus avium Miller | Pad avi | Xanthoceras sorbifolium Bunge | Xan sor |
References
- LY/T2738-2016; Forestry-Industrial Standard of the People’s Republic of China Technical Regulation for Surveyign of Old and Notable Trees. National Forestry and Grassland Administration: Beijing, China, 2016.
- Ma, J.; Wang, Q.L.; Qing, S.Q.; Yu, S.C.; Zhao, Z. Establishment and application of health evaluation system for reserve resources of Platycladus orientalis. J. Northwest For. Univ. 2022, 37, 90–97. [Google Scholar]
- Chen, S.; Guo, H.X.; Guan, K.L.; Deng, Y.W.; Xie, T.F.; Tan, G.W. Characteristics and Distribution Patterns of Ancient Tree Reserve Resources in Luhe County. Chin. J. Trop. Agric. 2022, 42, 49–55. [Google Scholar]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F. Global decline in large old trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef]
- Sist, P.; Mazzei, L.; Blanc, L.; Rutishauser, E. Large trees as key elements of carbon storage and dynamics after selective logging in the Eastern Amazon. For. Ecol. Manag. 2014, 318, 103–109. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.-L.; He, J.-K.; Kong, F.-M.; Yu, J.-H.; Jiang, H.-S. Big-sized trees overrule remaining trees’ attributes and species richness as determinants of aboveground biomass in tropical forests. Glob. Change Biol. 2019, 25, 2810–2824. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.L.; Das, A.; Condit, R.; Russo, S.; Baker, P.; Beckman, N.G.; Coomes, D.; Lines, E.; Morris, W.; Rüger, N. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 90–93. [Google Scholar] [CrossRef]
- Woods, C.L.; Cardelús, C.L.; DeWalt, S.J. Microhabitat associations of vascular epiphytes in a wet tropical forest canopy. J. Ecol. 2015, 103, 421–430. [Google Scholar] [CrossRef]
- Gilhen-Baker, M.; Roviello, V.; Beresford-Kroeger, D.; Roviello, G.N. Old growth forests and large old trees as critical organisms connecting ecosystems and human health. A review. Environ. Chem. Lett. 2022, 20, 1529–1538. [Google Scholar] [CrossRef]
- Chen, Q.J.; Guo, S.; Chen, C.P. Present situation and distribution of ancient and famous tree resources in Guangdong province. For. Investig. Plan. 2019, 44, 172–175+180. [Google Scholar]
- Jim, C.Y.; Zhang, H. Species diversity and spatial differentiation of old-valuable trees in urban Hong Kong. Urban For. Urban Green. 2013, 12, 171–182. [Google Scholar] [CrossRef]
- Fang, Q.C. Investigation and protection of ancient and famous trees in Hefei. Anhui Agric. Sci. Bull. 2020, 26, 72–73+100. [Google Scholar]
- Liu, J.; Zeng, D.H.; Tang, X.; Chen, W.Y. Research and analyze the present situation of ancient and famous tress in San Ya city. Trop. For. 2013, 41, 41–44. [Google Scholar]
- Pan, S.H. A preliminary report on the investigation of ancient and famous trees resources in Dalian area. J. Liaoning For. Sci. Technol. 2017, 2, 44–47. [Google Scholar]
- Wu, M.L.; Lin, L.N. Investigation and analysis of ancient and precious trees in Zhangzhou city. Fujian Sci. Technol. Trop. Crops 2018, 43, 31–38. [Google Scholar]
- Lai, P.Y.; Jim, C.Y.; Tang, G.D.; Hong, W.J.; Zhang, H. Spatial differentiation of heritage trees in the rapidly-urbanizing city of Shenzhen, China. Landsc. Urban Plan. 2019, 181, 148–156. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, P.Y.; Jim, C.Y. Species diversity and spatial pattern of old and precious trees in Macau. Landsc. Urban Plan. 2017, 162, 56–67. [Google Scholar] [CrossRef]
- Lv, M.L.; Jiang, W.H. A case study of of ancient and famous trees and its subsequent resource in Kecheng district of Quzhou City, Zhejiang Province. Acta Agric. Jiangxi 2007, 19, 112–117. [Google Scholar]
- Chen, J. Current situation and countermeasures of protection and management of ancient and famous trees in Gaochun district of Nanjing. J. South China Agric. 2018, 12, 56–58. [Google Scholar]
- Deng, J.H. The measures for conservation and rejuvenation of ancient and valuable trees—A case study of conservation and rejuvenation of the ancient Ficus concinna in Songkou Yongtai. J. Fujian For. 2020, 3, 44–48. [Google Scholar]
- Lai, W.J.; He, X.P.; Mao, Z.C.; Zhang, Y. Status Quo of Ancient and Famous Tree Resources in Ninghai County and Protection and Management Countermeasures. Anhui Agric. Sci. 2018, 46, 73–78. [Google Scholar]
- Lu, A.Z. The Actually and Conservation Technique Study of Ancient and Famous Trees and Its Subsequent Resource in Shanghai District. Master’s Thesis, Zhejiang University, Hangzhou, China, 2008. [Google Scholar]
- Wang, J. Selection and analysis of road greening tree species in Changchun city. J. Flowers 2016, 12, 70–71. [Google Scholar]
- Wang, Y.; Liu, Y.J. Research on the development of urbanization in Changchun. J. Chang. Univ. 2016, 26, 34–38. [Google Scholar]
- Zhao, J.; Zhao, D.; Sheng, L.; Wei, T.; Hui, J.; Yu, J. The actuality of ancient trees and its subsequent resources in Yangzhou City, Jiangsu Province. South China For. Sci. 2017, 45, 65–69. [Google Scholar]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F.; Likens, G.E.; Banks, S.C.; Blanchard, W.; Gibbons, P.; Ikin, K.; Blair, D.; McBurney, L. New policies for old trees: Averting a global crisis in a keystone ecological structure. Conserv. Lett. 2014, 7, 61–69. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Manning, A.D.; Gibbons, P. Single large or several small? Applying biogeographic principles to tree-level conservation and biodiversity offsets. Biol. Conserv. 2015, 191, 558–566. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F. The unique challenges of conserving large old trees. Trends Ecol. Evol. 2016, 31, 416–418. [Google Scholar] [CrossRef]
- Stagoll, K.; Lindenmayer, D.B.; Knight, E.; Fischer, J.; Manning, A.D. Large trees are keystone structures in urban parks. Conserv. Lett. 2012, 5, 115–122. [Google Scholar] [CrossRef]
- Chang, Y.F.; Wang, Z.H.; Zhang, D.; Zhai, C.; Wang, T.; Yang, Y.H.; Wu, J.J. Analysis of Urban Woody Plant Diversity among Different Administrative Districts and the Enhancement Strategy in Changchun City, China. Sustain. 2022, 14, 7624. [Google Scholar] [CrossRef]
- Jim, C.Y.; Chen, W.Y. Legacy effect of trees in the heritage landscape of a peri-urban golf course. Urban Ecosyst. 2016, 19, 1717–1734. [Google Scholar] [CrossRef]
- Seburanga, J.L.; Zhang, Q. Heritage trees and landscape design in urban areas of Rwanda. J. For. Res. 2013, 24, 561–570. [Google Scholar] [CrossRef]
- Chen, W.Y.; Hua, J. Heterogeneity in resident perceptions of a bio-cultural heritage in Hong Kong: A latent class factor analysis. Ecosyst. Serv. 2017, 24, 170–179. [Google Scholar] [CrossRef]
- Ma, J. Study on Health Diagnosis of Reserve Resources of Platycladus orientalis and Sabina Chinensis. Master’s Thesis, Northwest A&F University, Xianyang, China, 2022. [Google Scholar]
- Zou, F.S. Shanghai Ancient Trees (Subsequent) Resources Present Situation Investigation and Analysis. Master’s Thesis, Shanghai Jiao Tong University, Shanghai, China, 2017. [Google Scholar]
- Li, Y.Y. The evolvement course and enlightenment of urban garden policy in new China. Sichuan For. Sci. Technol. 2012, 33, 104–107. [Google Scholar]
- Changchun Bureau of Statistics. Changchun Statistical Yearbook; Changchun Bureau of Statistics: Changchun, China, 2020. [Google Scholar]
- Jim, C.Y. Spatial differentiation and landscape-ecological assessment of heritage trees in urban Guangzhou (China). Landsc. Urban Plan. 2004, 69, 51–68. [Google Scholar] [CrossRef]
- CJJ/T85-2017; Standard for Classifification of Urban Green Space. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2017.
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley and Sons: New York, NY, USA, 1974; p. 547. [Google Scholar] [CrossRef]
- Shannon, C.E. The mathematical theory of communication. 1963. MD Comput. 1997, 14, 306–317. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13. [Google Scholar] [CrossRef]
- Yang, Y.B.; Bao, G.D.; Zhang, D.; Zhai, C. Spatial Distribution and Driving Factors of Old and Notable Trees in a Fast-Developing City, Northeast China. Sustain. 2022, 14, 7937. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, H.; He, X.; Ren, Z.; Zhai, C.; Yu, X.; Mao, Z.; Wang, P. Effects of forest type and urbanization on species composition and diversity of urban forest in Changchun, Northeast China. Urban Ecosyst. 2016, 19, 455–473. [Google Scholar] [CrossRef]
- Qian, S.H.; Qi, M.; Huang, L.; Zhao, L.; Lin, D.; Yang, Y.C. Biotic homogenization of China’s urban greening: A meta-analysis on woody species. Urban For. Urban Green. 2016, 18, 25–33. [Google Scholar] [CrossRef]
- Wania, A.; Kühn, I.; Klotz, S. Plant richness patterns in agricultural and urban landscapes in Central Germany—Spatial gradients of species richness. Landsc. Urban Plan. 2006, 75, 97–110. [Google Scholar] [CrossRef]
- Jim, C.Y.; Liu, H. Species diversity of three major urban forest types in Guangzhou City, China. For. Ecol. Manag. 2001, 146, 99–114. [Google Scholar] [CrossRef]
- Yu, Y.C. Japanese construction of Manchukuo—Take Changchun urban planning as an example. Archit. Cult. 2018, 4, 159–160. [Google Scholar]
- Núñez, M.B.S.; Tarazona, T.; Silla, F.; Delgado, L.E.S. Árboles viejos como indicadores de biodiversidad de vertebrados forestales amenazados de la provincia de Salamanca (España). Pirin. 2016, 171, 20. [Google Scholar] [CrossRef]
- Wang, X.; Svenning, J.-C.; Liu, J.; Zhao, Z.; Zhang, Z.; Feng, G.; Si, X.; Zhang, J. Regional effects of plant diversity and biotic homogenization in urban greenspace–The case of university campuses across China. Urban For. Urban Green. 2021, 62, 127170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).