Soil Environment and Fauna Communities in Europe after Afforestation of Post-Agricultural Lands—A Review
Abstract
1. Introduction
2. Data Collection, Selection Criteria and Meta-Analysis
2.1. Search Methods
- The article must be published, peer-reviewed and written in English.
- The article must have provided documentation of fauna occurrence, abundance, diversity, or other estimates of occupancy in regrowth forest habitat.
- The article must have made a clear distinction between fauna response to regrowth forests and mature forests or cleared and arable land.
- The article must have explicitly identified prior cropping or grazing land use for at least one category of regrowth forest.
2.2. Data Grouping and Data Analysis
3. Habitat Characteristics
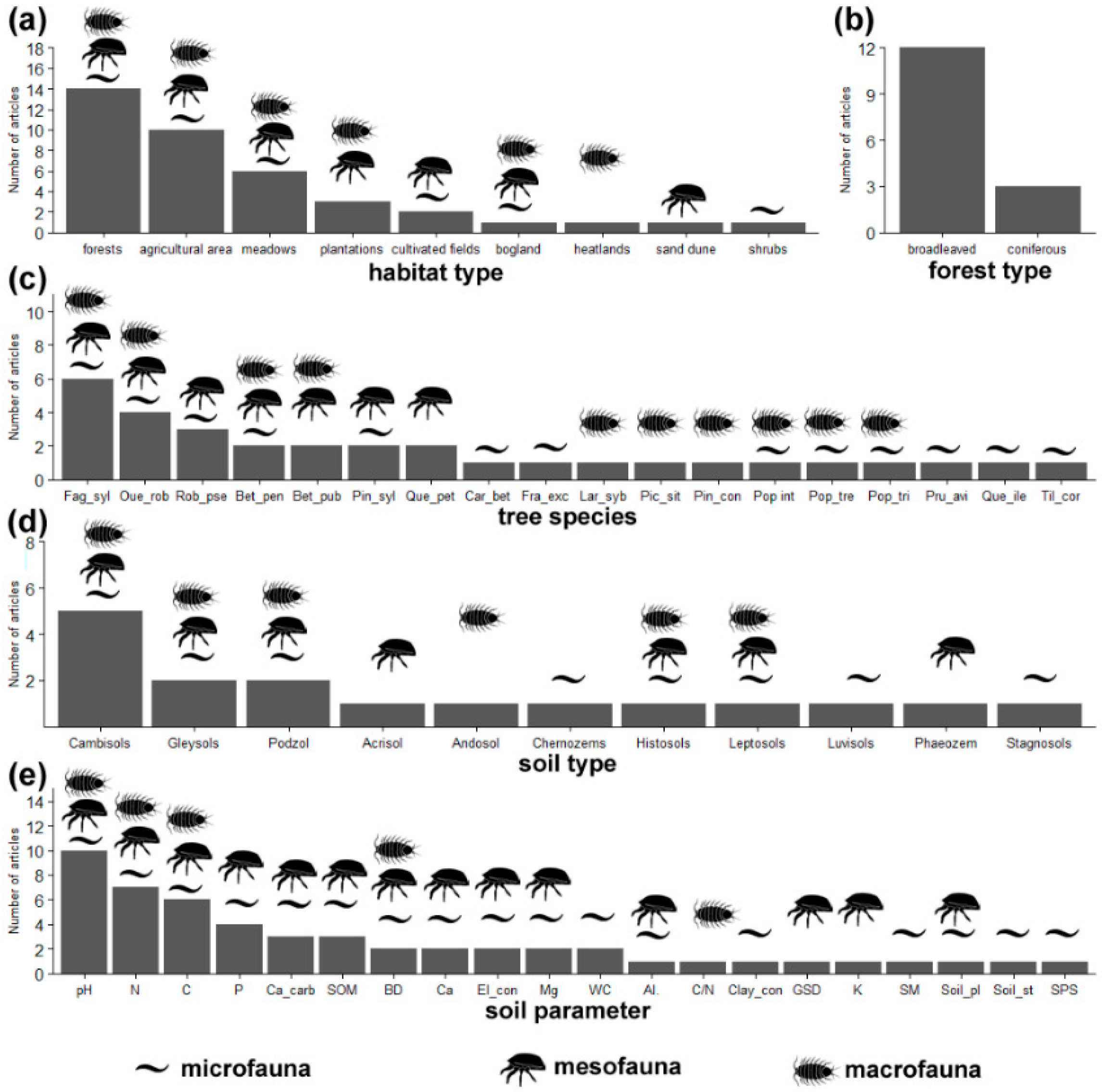
3.1. Location and Types of Examined Habitats
3.2. Forest Types on Post-Agricultural Lands
4. Effect of Afforestation on Soil Chemistry
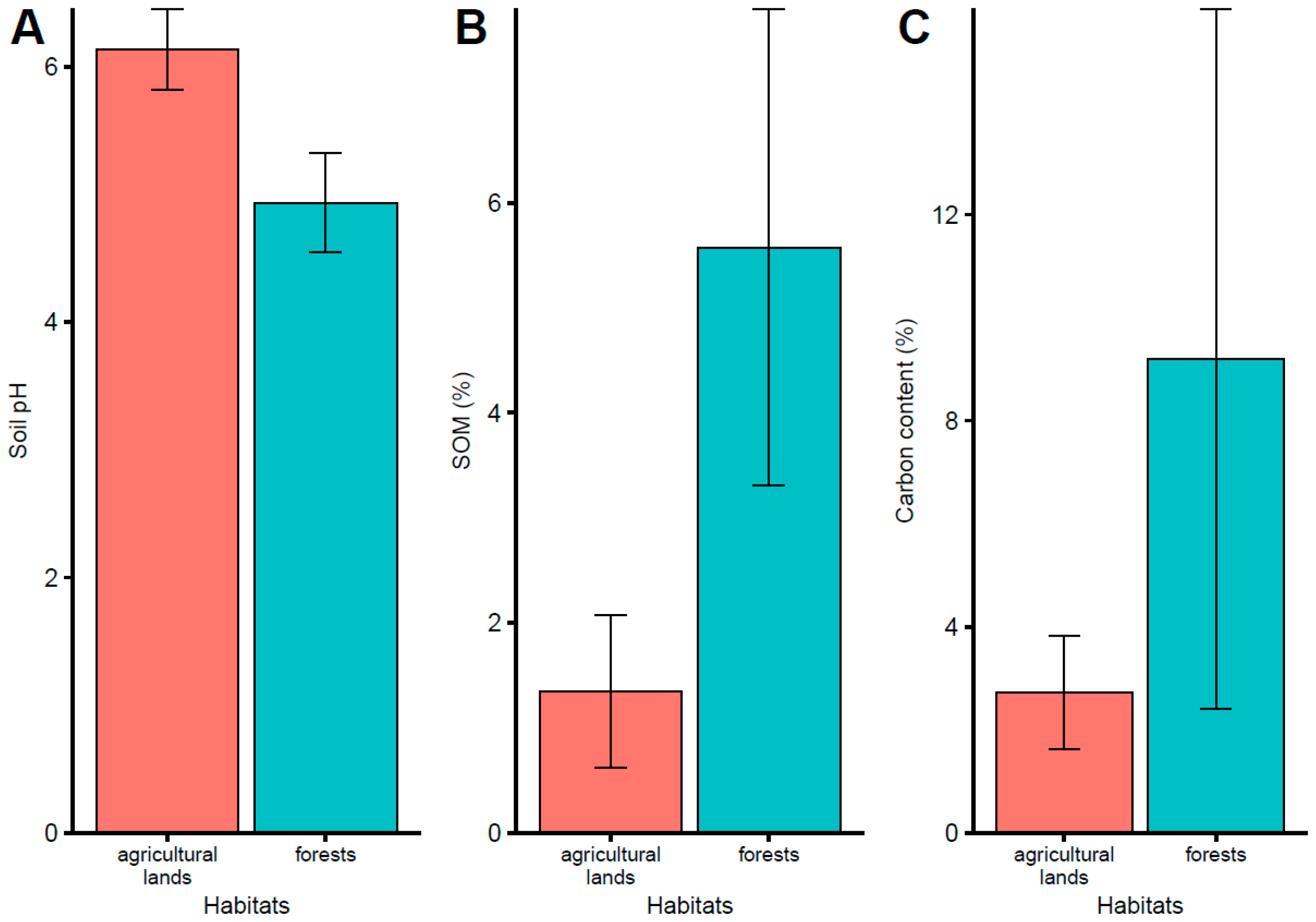
4.1. Soil Acidification
4.2. Nitrogen Content
4.3. Phosphorous Content
4.4. Carbon Content
4.5. Aluminum, Calcium, Potassium, and Magnesium Concentration
4.6. Carbon to Nitrogen Ratio and Nitrogen to Phosphorous Ratio
4.7. Soil Moisture
5. Effect of Afforestation on Soil Fauna
5.1. Soil Fauna Classes
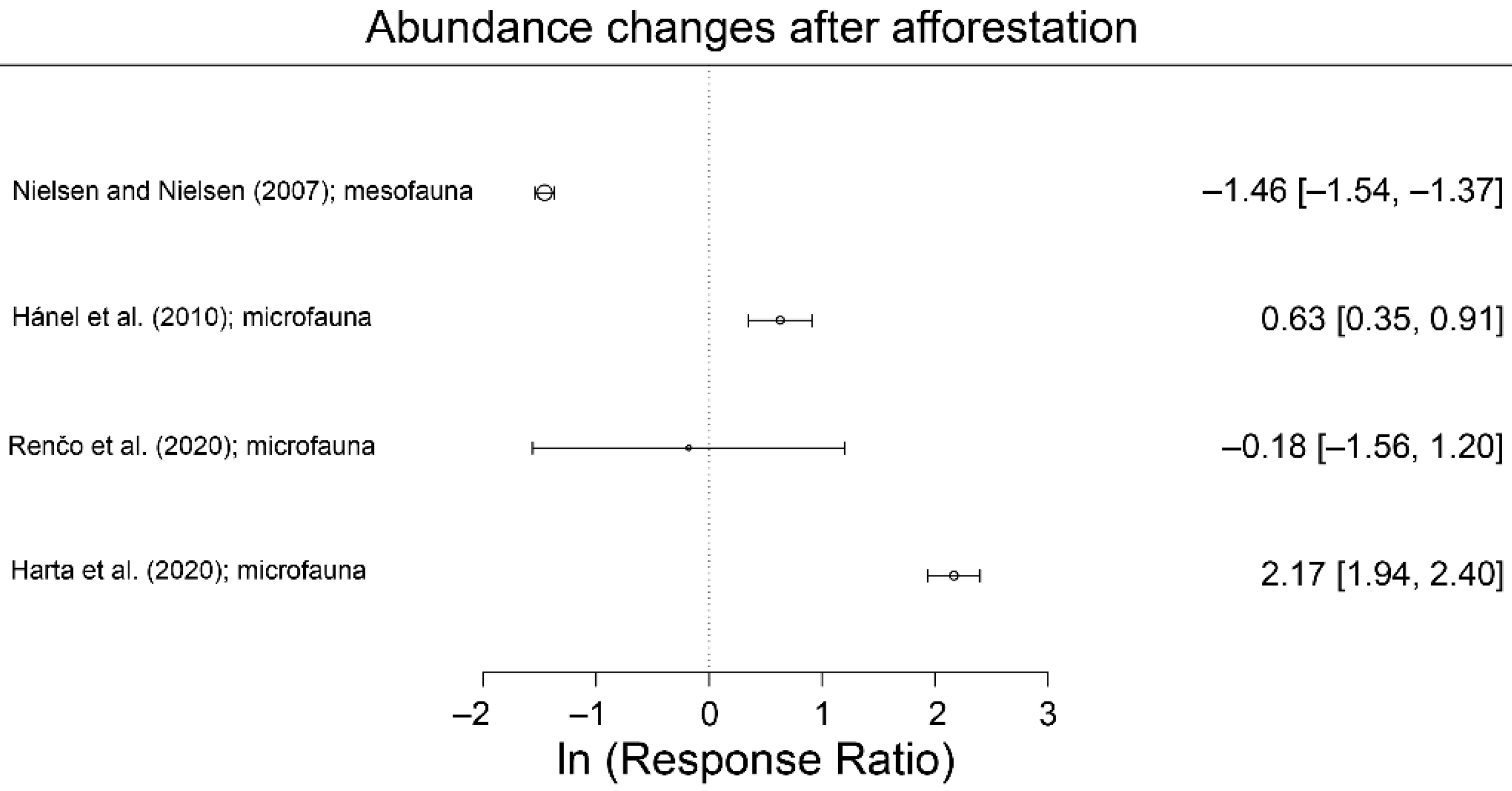
5.2. Soil Microfauna
5.3. Soil Mesofauna
5.4. Soil Macrofauna
6. Planning Future Studies in Forest on Post-Agricultural Lands
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rittenhouse, C.D.; Rissman, A.R. Forest Cover, Carbon Sequestration, and Wildlife Habitat: Policy Review and Modeling of Tradeoffs among Land-Use Change Scenarios. Environ. Sci. Policy 2012, 21, 94–105. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2000; FAO: Rome, Italy, 2001. [Google Scholar]
- Jagodziński, A.M.; Zasada, M.; Bronisz, K.; Bronisz, A.; Bijak, S. Biomass Conversion and Expansion Factors for a Chronosequence of Young Naturally Regenerated Silver Birch (Betula Pendula Roth) Stands Growing on Post-Agricultural Sites. For. Ecol. Manag. 2017, 384, 208–220. [Google Scholar] [CrossRef]
- Lawson, S.S.; Michler, C.H. Afforestation, Restoration and Regeneration—Not All Trees Are Created Equal. J. For. Res. 2014, 25, 3–20. [Google Scholar] [CrossRef]
- Harta, I.; Simon, B.; Vinogradov, S.; Winkler, D. Collembola Communities and Soil Conditions in Forest Plantations Established in an Intensively Managed Agricultural Area. J. For. Res. 2020, 32, 1819–1832. [Google Scholar] [CrossRef]
- Pawson, S.M.; Brin, A.; Brockerhoff, E.G.; Lamb, D.; Payn, T.W.; Paquette, A.; Parrotta, J.A. Plantation Forests, Climate Change and Biodiversity. Biodivers. Conserv. 2013, 22, 1203–1227. [Google Scholar] [CrossRef]
- Krawczyk, R. Afforestation and Secondary Succession. For. Res. Pap. 2014, 75, 423–427. [Google Scholar] [CrossRef]
- Holubík, O.; Podrázský, V.; Vopravil, J.; Khel, T.; Remeš, J. Effect of Agricultural Lands Afforestation and Tree Species Composition on the Soil Reaction, Total Organic Carbon and Nitrogen Content in the Uppermost Mineral Soil Profile. Soil Water Res. 2014, 9, 192–200. [Google Scholar] [CrossRef]
- Rykowski, K. Problemy Ochrony Lasu Na Gruntach Porolnych. Sylwan 1990, 134, 75–88. [Google Scholar]
- Virto, I.; Imaz, M.J.; Fernández-Ugalde, O.; Gartzia-Bengoetxea, N.; Enrique, A.; Bescansa, P. Soil Degradation and Soil Quality in Western Europe: Current Situation and Future Perspectives. Sustainability 2015, 7, 313–365. [Google Scholar] [CrossRef]
- Gorzelak, A. Ekologiczne Uwarunkowania Kształtowania Lasów Na Gruntach Porolnych. Sylwan 1996, 140, 29–34. [Google Scholar]
- Bernacki, E. Koncepcje Hodowli Lasu Na Gruntach Porolnych. Sylwan 1990, 134, 51–59. [Google Scholar]
- Sobczak, R. O Przywracaniu Lasów Na Grunty Porolne. Sylwan 1996, 140, 35–41. [Google Scholar]
- Chazdon, R.L.; Lindenmayer, D.; Guariguata, M.R.; Crouzeilles, R.; Benayas, J.M.R.; Chavero, E.L. Fostering Natural Forest Regeneration on Former Agricultural Land through Economic and Policy Interventions. Environ. Res. Lett. 2020, 15, 043002. [Google Scholar] [CrossRef]
- Chazdon, R.; Brancalion, P. Restoring Forests as a Means to Many Ends. Science 2019, 364, 24–25. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- André, H.M.; Ducarme, X.; Lebrun, P. Soil Biodiversity: Myth, Reality or Conning? Oikos 2002, 96, 3–24. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Nagy, L.G.; Tóth, R.; Kiss, E.; Slot, J.; Gácser, A.; Kovács, G. Six Key Traits of Fungi: Their Evolutionary Origins and Genetic Bases. Microbiol. Spectr. 2017, 5, 35–56. [Google Scholar] [CrossRef]
- Johann, E. Forest History in Europe. In Biological Resources and Migration; Werner, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 73–82. [Google Scholar]
- de la Peña, E.; Baeten, L.; Steel, H.; Viaene, N.; Sutter, N.D.; Schrijver, A.D.; Verheyen, K. Beyond Plant–Soil Feedbacks: Mechanisms Driving Plant Community Shifts Due to Land-Use Legacies in Post-Agricultural Forests. Funct. Ecol. 2016, 30, 1073–1085. [Google Scholar] [CrossRef]
- Háněl, L. An Outline of Soil Nematode Succession on Abandoned Fields in South Bohemia. Appl. Soil Ecol. 2010, 46, 355–371. [Google Scholar] [CrossRef]
- Nielsen, B.O.; Nielsen, L.B. Soil Diptera of a Beech Stand and an Arable Field: A Comparison of Dipteran Emergence in Neighbouring Sites. Pedobiologia 2007, 51, 33–43. [Google Scholar] [CrossRef]
- Heiniger, C.; Barot, S.; Ponge, J.-F.; Salmon, S.; Meriguet, J.; Carmignac, D.; Suillerot, M.; Dubs, F. Collembolan Preferences for Soil and Microclimate in Forest and Pasture Communities. Soil Biol. Biochem. 2015, 86, 181–192. [Google Scholar] [CrossRef]
- Jackson, L.E.; Bowles, T.M.; Ferris, H.; Margenot, A.J.; Hollander, A.; Garcia-Palacios, P.; Daufresne, T.; Sánchez-Moreno, S. Plant and Soil Microfaunal Biodiversity across the Borders between Arable and Forest Ecosystems in a Mediterranean Landscape. Appl. Soil Ecol. 2019, 136, 122–138. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Brown, V.K.; Reader, P.M. The Relationships of Plant and Insect Diversities in Succession. Biol. J. Linn. Soc. 1979, 12, 327–348. [Google Scholar] [CrossRef]
- Makeschin, F. Effects of Energy Forestry on Soils. Biomass Bioenergy 1994, 6, 63–79. [Google Scholar] [CrossRef]
- Scheu, S.; Schulz, E. Secondary Succession, Soil Formation and Development of a Diverse Community of Oribatids and Saprophagous Soil Macro-Invertebrates. Biodivers. Conserv. 1996, 5, 235–250. [Google Scholar] [CrossRef]
- Sigurðsson, B.D.; Guðleifsson, B.E. Impact of Afforestation on Earthworm Populations in Iceland. Icel. Agric. Sci. 2013, 26, 21–36. [Google Scholar]
- Keith, A.M.; Boots, B.; Hazard, C.; Niechoj, R.; Arroyo, J.; Bending, G.D.; Bolger, T.; Breen, J.; Clipson, N.; Doohan, F.M.; et al. Cross-Taxa Congruence, Indicators and Environmental Gradients in Soils under Agricultural and Extensive Land Management. Eur. J. Soil Biol. 2012, 49, 55–62. [Google Scholar] [CrossRef]
- Szczepko, K.; Kruk, A.; Bartos, M.; Wiśniowski, B. Factors Influencing the Diversity of Cuckoo Wasps (Hymenoptera: Chrysididae) in the Post-Agriculture Area of the Kampinos National Park, Poland. Insect Conserv. Divers. 2013, 6, 339–353. [Google Scholar] [CrossRef]
- Renčo, M.; Gömöryová, E.; Cerevková, A. The Effect of Soil Type and Ecosystems on the Soil Nematode and Microbial Communities. Helminthologia 2020, 57, 129–144. [Google Scholar] [CrossRef]
- Gormsen, D.; Hedlund, K.; Huifu, W. Diversity of Soil Mite Communities When Managing Plant Communities on Set-aside Arable Land. Appl. Soil Ecol. 2006, 31, 147–158. [Google Scholar] [CrossRef]
- Jaffuel, G.; Blanco-Pérez, R.; Hug, A.-S.; Chiriboga, X.; Meuli, R.G.; Mascher, F.; Turlings, T.C.J.; Campos-Herrera, R. The Evaluation of Entomopathogenic Nematode Soil Food Web Assemblages across Switzerland Reveals Major Differences among Agricultural, Grassland and Forest Ecosystems. Agric. Ecosyst. Environ. 2018, 262, 48–57. [Google Scholar] [CrossRef]
- Podrázský, V.; Remeš, J.; Hart, V.; Moser, W.K. Production and Humus Form Development in Forest Stands Established on Agricultural Lands-Kostelec Nad Černými Lesy Region. J. For. Sci. 2009, 55, 299–305. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Elias, D.M.O.; Grant, H.K.; McNamara, N.P. Plant Identity Control on Soil Food Web Structure and C Transfers under Perennial Bioenergy Plantations. Soil Biol. Biochem. 2019, 138, 107603. [Google Scholar] [CrossRef]
- Jackson, B. Regulation of Litter Decomposition in Forest Ecosystems of Sweden and New Zealand; Department of Forest Ecology and Management, Swedish University of Agricultural Sciences (SLU): Umeå, Sweden, 2012. [Google Scholar]
- Rengel, Z. Handbook of Soil Acidity; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-203-91231-7. [Google Scholar]
- Xu, D.; Carswell, A.; Zhu, Q.; Zhang, F.; de Vries, W. Modelling Long-Term Impacts of Fertilization and Liming on Soil Acidification at Rothamsted Experimental Station. Sci. Total Environ. 2020, 713, 136249. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-System Perspective of the Global Nitrogen Cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Stevens, C.J. Nitrogen in the Environment. Science 2019, 363, 578–580. [Google Scholar] [CrossRef]
- Huang, L.-M.; Jia, X.-X.; Zhang, G.-L.; Shao, M.-A. Soil Organic Phosphorus Transformation during Ecosystem Development: A Review. Plant Soil 2017, 417, 17–42. [Google Scholar] [CrossRef]
- Sylvain, Z.A.; Wall, D.H.; Cherwin, K.L.; Peters, D.P.C.; Reichmann, L.G.; Sala, O.E. Soil Animal Responses to Moisture Availability Are Largely Scale, Not Ecosystem Dependent: Insight from a Cross-Site Study. Glob. Chang. Biol. 2014, 20, 2631–2643. [Google Scholar] [CrossRef]
- Korboulewsky, N.; Perez, G.; Chauvat, M. How Tree Diversity Affects Soil Fauna Diversity: A Review. Soil Biol. Biochem. 2016, 94, 94–106. [Google Scholar] [CrossRef]
- Bradford, M.A.; Jones, T.H.; Bardgett, R.D.; Black, H.I.J.; Boag, B.; Bonkowski, M.; Cook, R.; Eggers, T.; Gange, A.C.; Grayston, S.J.; et al. Impacts of Soil Faunal Community Composition on Model Grassland Ecosystems. Science 2002, 298, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Maaroufi, N.I.; De Long, J.R. Global Change Impacts on Forest Soils: Linkage between Soil Biota and Carbon-Nitrogen-Phosphorus Stoichiometry. Front. For. Glob. Chang. 2020, 3, 16. [Google Scholar] [CrossRef]
| Groups of Organisms | Experimental Type | Agriculture Activities | Period of Sampling | Forest Type and Age Class of Stands | Analyzed Physical Parameters | Location | Authors |
|---|---|---|---|---|---|---|---|
| Macrofauna | Secondary succession studies | treated with weed-killer, shallow ploughed, harrowed, lightly rolled | ~1.5 year | Forest type: broadleaved Age class: middle aged forest | - | United Kingdom | Southwood et al. [26] |
| Macrofauna | Energetic plants species studies | Arable field | 4 years | Forest type: broadleaved Age class: young forest | pH, Carbon, NO3 | Germany | Makeschin [27] |
| Mesofauna and macrofauna | Secondary succession studies | Arable field, sown with grain | 1 year | Forest type: broadleaved Age class: middle aged and mature forest | Carbon, Nitrogen, Soil Bulk Density | Germany | Scheu and Schulz [28] |
| Mesofauna | Secondary succession studies | Fields fertilized, harrowed, ploughed and cultivated | 1 year | Forest type: broadleaved Age class: no data | pH | Sweden, Netherlands, Spain | Gormsen et al. [33] |
| Macrofauna | Secondary succession studies | Arable filed, treated with pesticides, ploughed, cropped with winter barley | 7 years | Forest type: broadleaved Age class: mature forest | pH | Denmark | Nielsen and Nielsen [23] |
| Microfauna | Secondary succession studies | Abandoned arable field | 12 years | Forest type: broadleaved Age class: middle aged and mature forest | pH SOC | Czech Republic | Háněl [22] |
| Microfauna, Mesofauna, Macrofauna | Impact of land use type | - | - | - | - | Ireland | Keith et al. [30] |
| Macrofauna | Impact of afforestation | Sheep grazing | 2 years | Forest type: coniferous and broadleaved Age class: young and middle aged forest | pH, Carbon, Nitrogen, C/N | Iceland | Sigurdsson and Gudleifsson [29] |
| Macrofauna | - | - | 6 years | - | - | Poland | Szczepko et al. [31] |
| Mesofauna | Impact of soil | Pastures | 1 year | Forest type: broadleaved Age class: mature forest | pH | France | Heiniger et al. [24] |
| Mesofauna | Secondary succession studies | Wheat field, grassland | 2 years | - | - | Switzerland | Jaffuel et al. [34] |
| Macrofauna | Impact of afforestation | Arable field cropped with winter wheat | 1 year | Forest type: broadleaved Age class: young forest | pH, Carbon, Nitrogen, Bulk density | UK | Briones et al. [36] |
| Microfauna | Secondary succession studies | Arable field, pastures | 1 year | Forest type: broadleaved Age class: no data | pH, Carbon, Nitrogen, C/N | France | Jackson et al. [25] |
| Mesofauna | Impact of afforestation | Arable field, abandoned field | 1 year | Forest type: broadleaved Age class: young and mature forest | pH, SOM, Ca, Mg, N, CaCO3 | Hungary | Harta et al. [5] |
| Microfauna | Secondary succession studies | Arable field, Meadow | 1 year | Forest type: broadleaved Age class: no data | pH, Carbon. Nitrogen, Sulphur, C/N, SM | Slovakia | Renčo et al. [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malica, J.; Urbanowski, C.K.; Rączka, G.; Skorupski, M.; Pers-Kamczyc, E.; Kamczyc, J. Soil Environment and Fauna Communities in Europe after Afforestation of Post-Agricultural Lands—A Review. Forests 2022, 13, 1713. https://doi.org/10.3390/f13101713
Malica J, Urbanowski CK, Rączka G, Skorupski M, Pers-Kamczyc E, Kamczyc J. Soil Environment and Fauna Communities in Europe after Afforestation of Post-Agricultural Lands—A Review. Forests. 2022; 13(10):1713. https://doi.org/10.3390/f13101713
Chicago/Turabian StyleMalica, Jacek, Cezary K. Urbanowski, Grzegorz Rączka, Maciej Skorupski, Emilia Pers-Kamczyc, and Jacek Kamczyc. 2022. "Soil Environment and Fauna Communities in Europe after Afforestation of Post-Agricultural Lands—A Review" Forests 13, no. 10: 1713. https://doi.org/10.3390/f13101713
APA StyleMalica, J., Urbanowski, C. K., Rączka, G., Skorupski, M., Pers-Kamczyc, E., & Kamczyc, J. (2022). Soil Environment and Fauna Communities in Europe after Afforestation of Post-Agricultural Lands—A Review. Forests, 13(10), 1713. https://doi.org/10.3390/f13101713





