Pilot Study of Sap Properties of Norway Spruce (Picea abies (L.) Karst.) Trees Used and Not Used for Sap-Feeding by Three-Toed Woodpeckers (Picoides tridactylus)
Abstract
1. Introduction
2. Materials and Methods
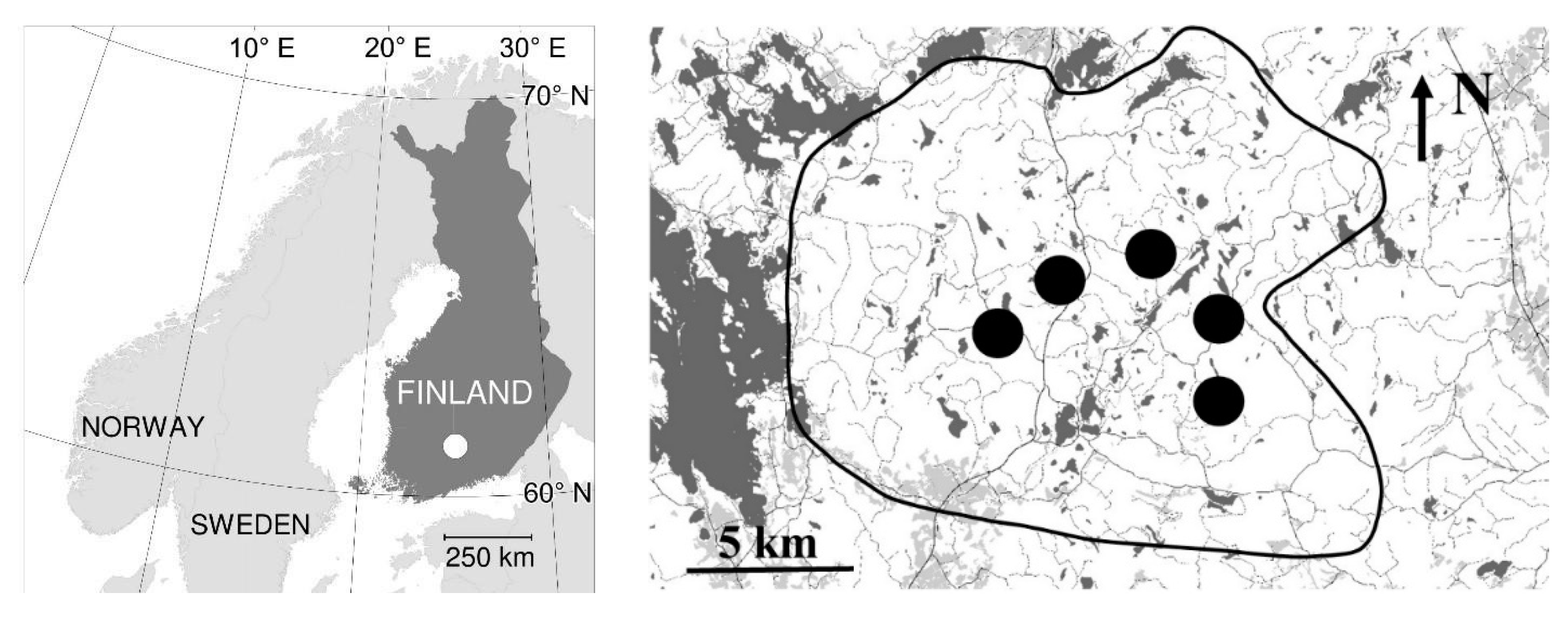
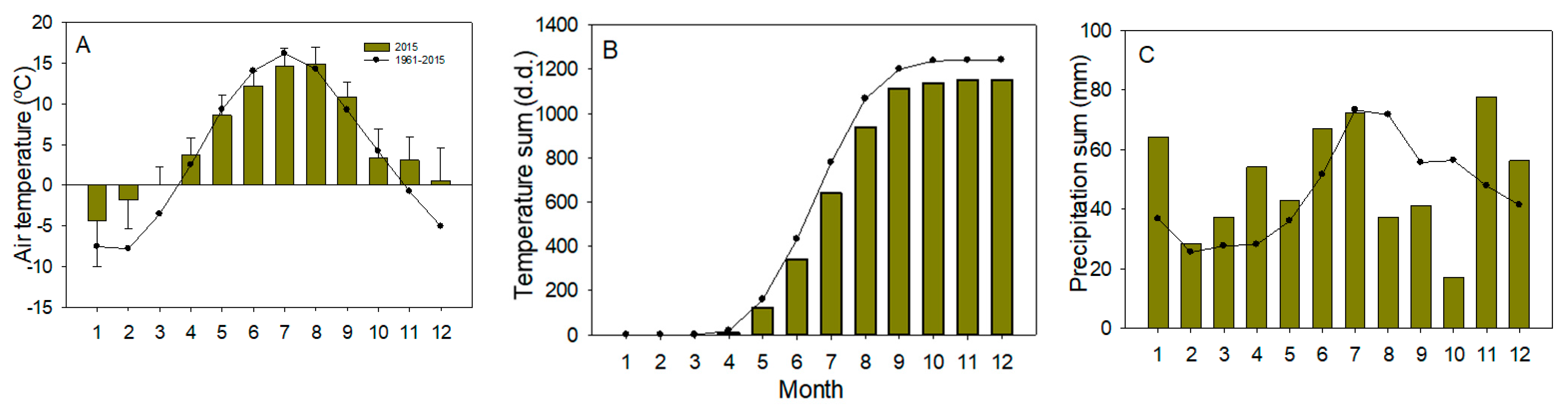
2.1. Research Area and Tree Material
2.2. Sampling of Phloem
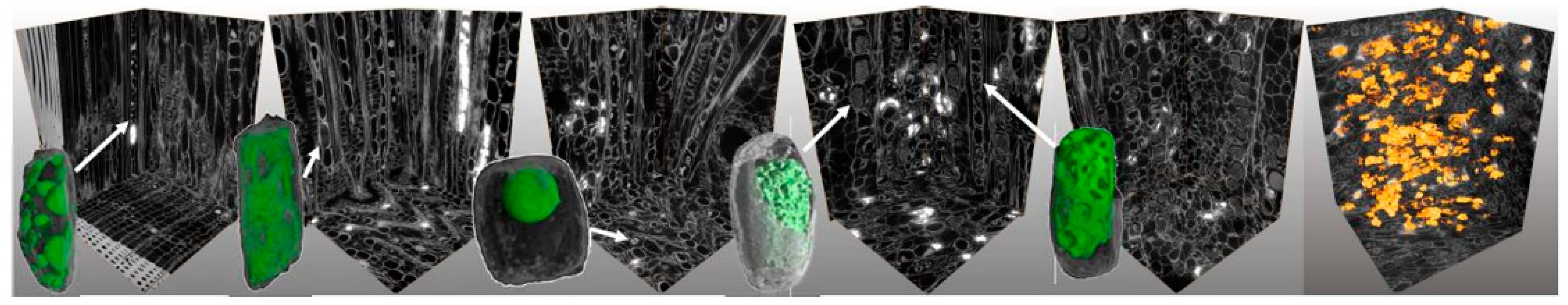
2.3. Samples for Phloem 3D Structure
2.4. Analysis of Phloem Water Content
2.5. Osmolality Analysis
2.6. Carbohydrate Analysis of Phloem Sap
2.7. Carbohydrate Analysis of Phloem Tissue
2.8. 3D-Microtomographic Analysis of Phloem Cellular Features
2.9. Statistical Analysis
3. Results
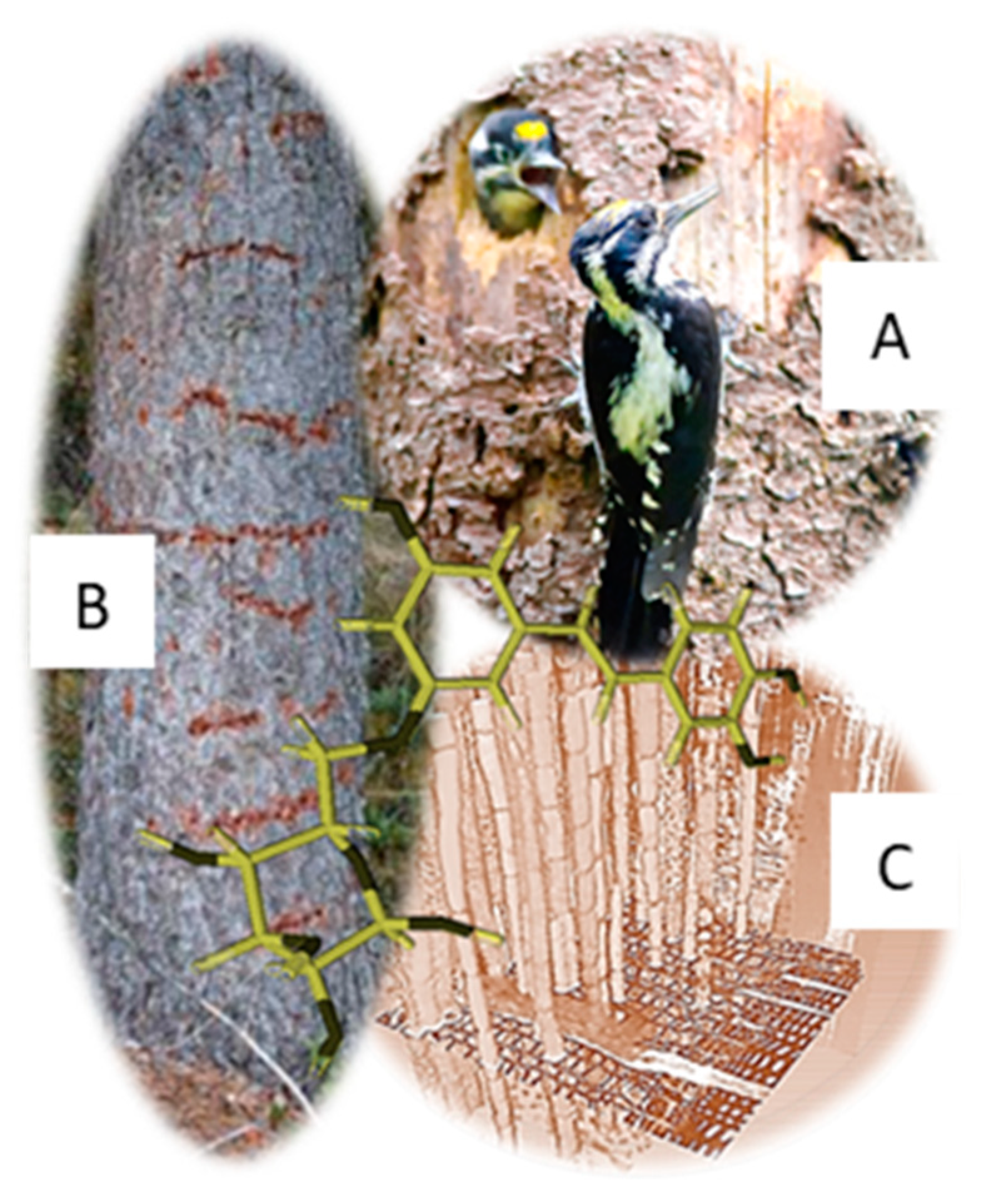
3.1. Visual Appearance of Bark Used by the TTWs for Phloem Sap Feeding
3.2. Cellular Structures of Norway Spruce Phloem
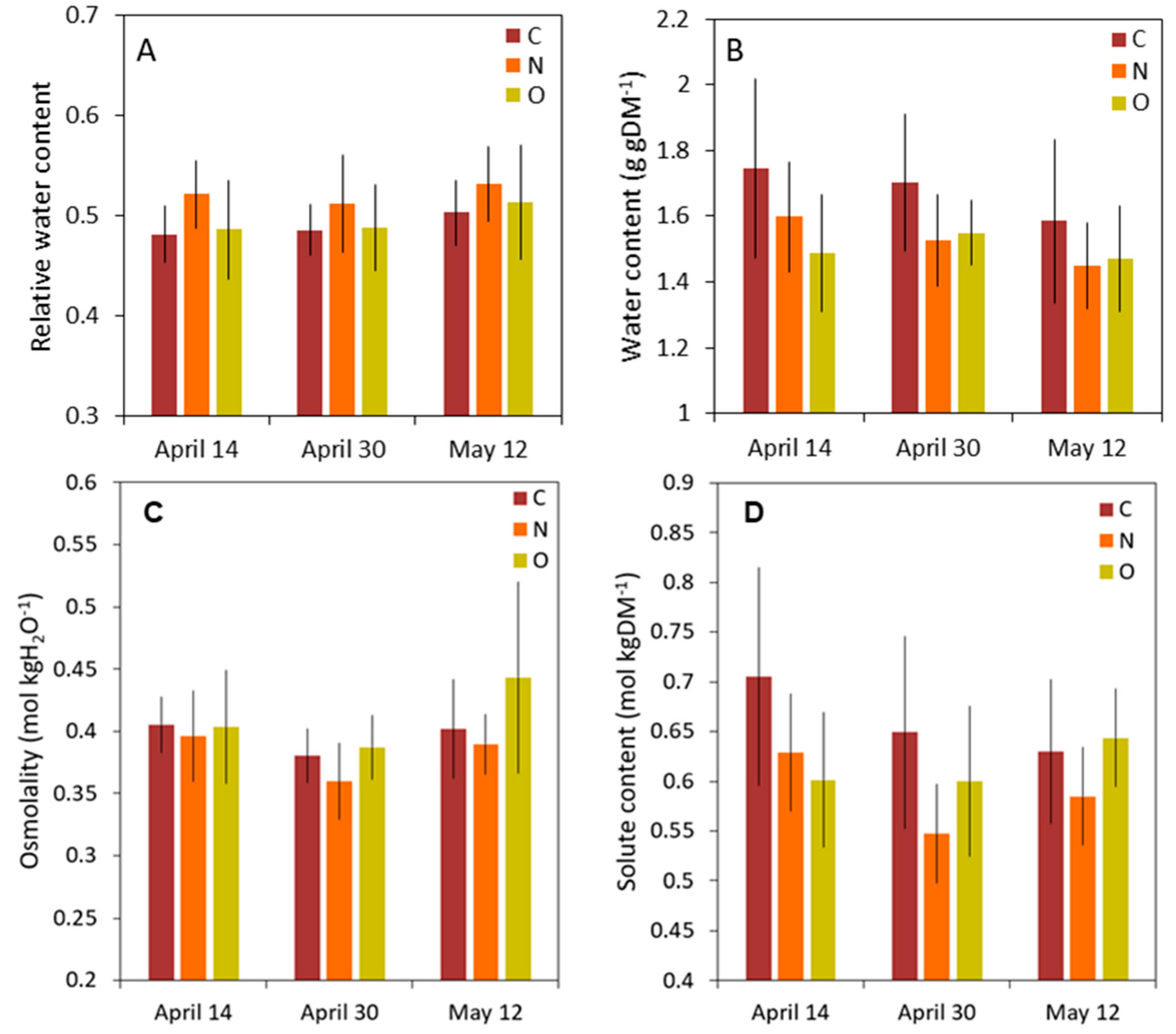
3.3. Phloem Water Content and Osmolality of Phloem Sap
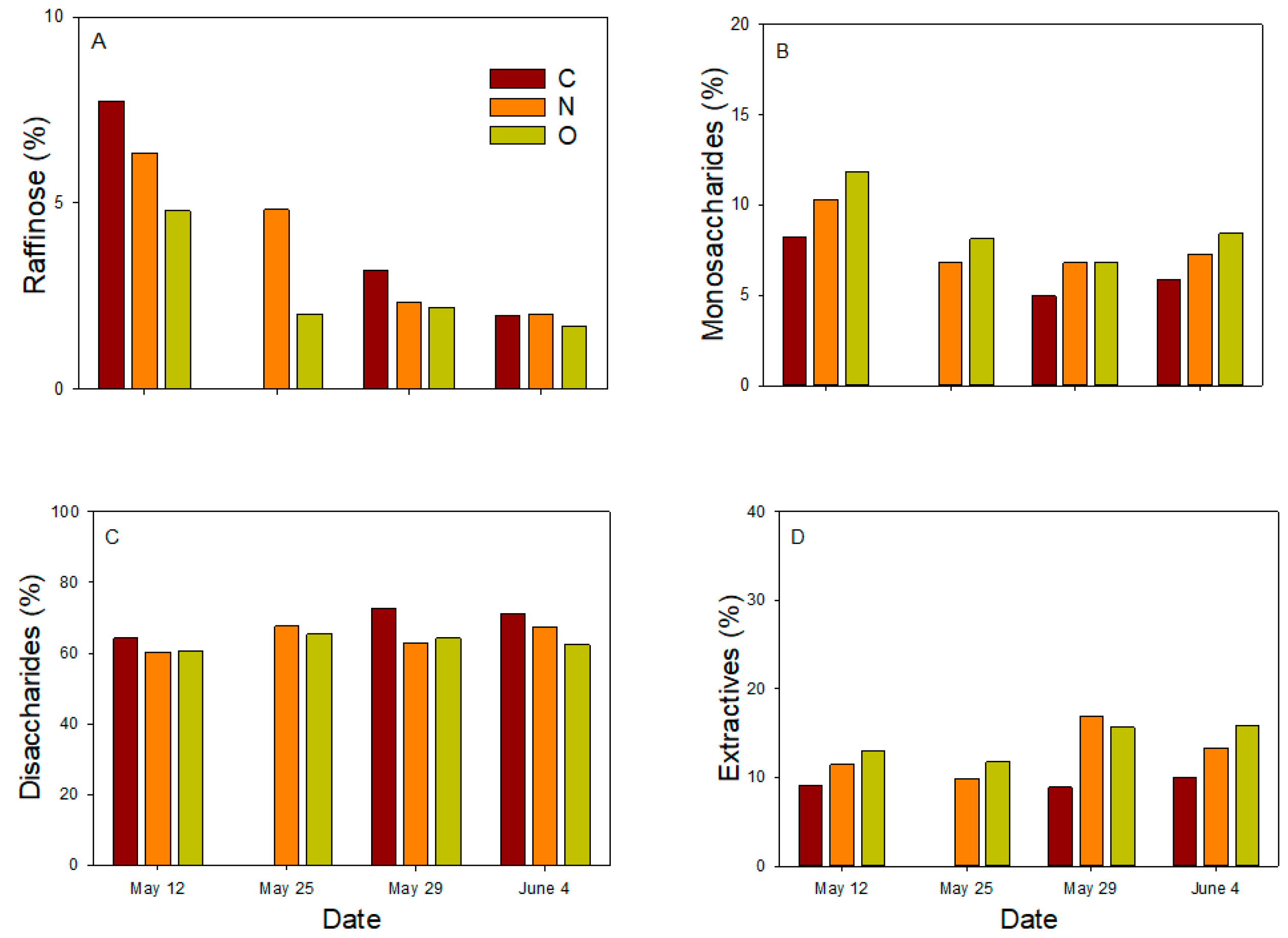
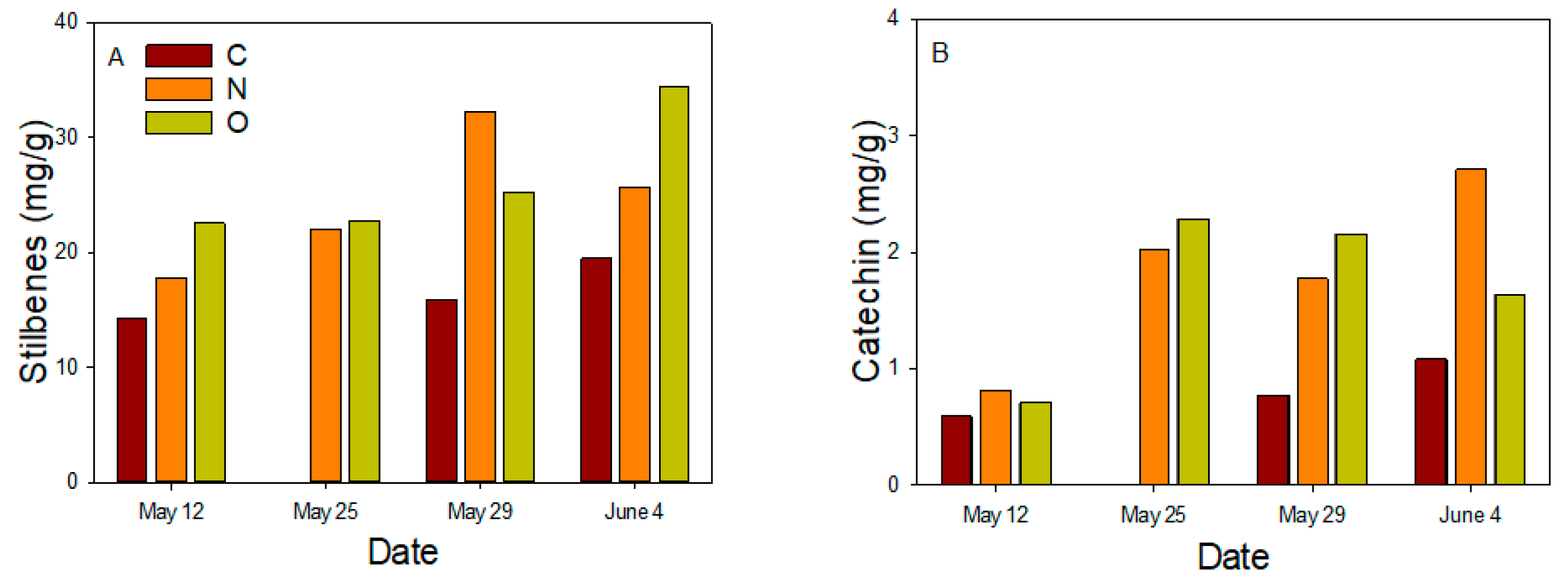
3.4. Chemical Properties of Phloem Sap
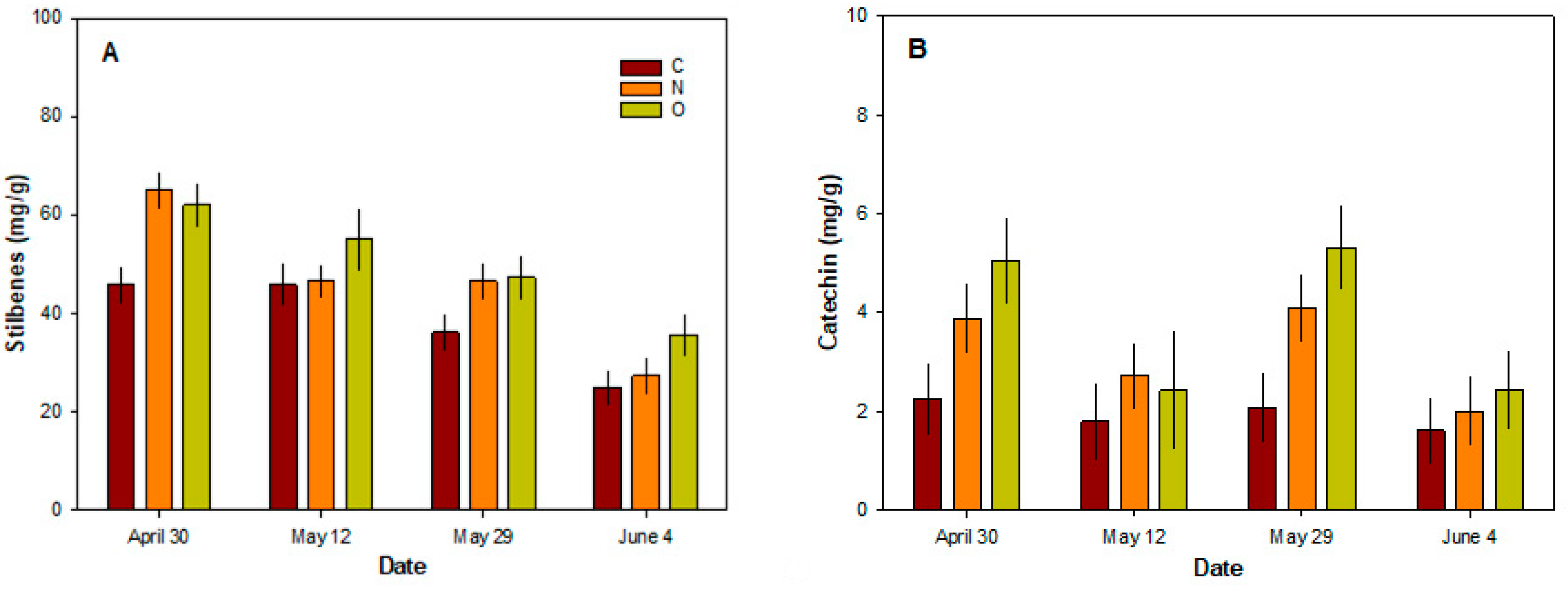
3.5. Chemical Properties of Phloem Tissue
4. Discussion
4.1. Patterns of Phloem Sap Feeding in the Three-Toed Woodpecker
4.2. Resilient Trees: Growth Vigor and Defense Metabolites
4.3. The Phenology of Trees and Seasonal Variation in Sap Chemistry and Availability
4.4. Possible Benefits of Sap for Three-Toed Woodpeckers
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glutz von Blotzheim, U.N.; Bauer, K.M. Handbuch der Vögel Mitteleuropas; Akademische Verlagsgesellschaft, Frankfurt a.M.: Leipzig, Germany, 1980; Volume 9. [Google Scholar]
- Cramp, S. (Ed.) Handbook of the Birds of Europe, the Middle East and North Africa; Oxford Univ. Press: Oxford, UK, 1985; Volume 4. [Google Scholar]
- Pakkala, T.; Hanski, I.; Tomppo, E. Spatial ecology of the three-toed woodpecker in managed forest landscapes. Silva Fenn. 2002, 36, 279–288. [Google Scholar] [CrossRef]
- Pakkala, T.; Kouki, J.; Piha, M.; Tiainen, J. Phloem sap in damaged Scots pine trees provides instant foraging opportunities for Three-toed Woodpeckers Picoides tridactylus. Ornis Svec. 2017, 27, 144–149. [Google Scholar] [CrossRef]
- Versluijs, M.; Eggers, S.; Mikusiński, G.; Roberge, J.-M.; Hjältén, J. Foraging behavior of the Eurasian Three-toed Woodpecker (Picoides tridactylus) and its implications for ecological restoration and sustainable boreal forest management. Avian Conserv. Ecol. 2020, 15, 6. [Google Scholar] [CrossRef]
- Hogstad, O. On the ecology of the three-toed woodpecker Picoides tridactylus (L.) outside the breeding season. Nytt. Mag. Zool. 1970, 18, 221–227. [Google Scholar]
- Fayt, P. Available insect prey in bark patches selected by the three-toed woodpecker Picoides tridactylus prior to reproduction. Ornis Fenn. 1999, 76, 135–140. [Google Scholar]
- Pechacek, P.; Krištín, A. Comparative diets of adult and young three-toed woodpeckers in a European alpine community. J. Wildl. Manag. 2004, 68, 683–693. [Google Scholar] [CrossRef]
- Winkler, H.; Christie, D.A. Family Picidae (Woodpeckers). In Handbook of the Birds of the World; Del Hoyo, J., Elliott, A., Sargatal, J., Eds.; Jacamars to woodpeckers; Lynx Edicions: Barcelona, Spain, 2002; Volume 7, pp. 296–555. [Google Scholar]
- Pakkala, T.; Piiroinen, J.; Lakka, J.; Tiainen, J.; Piha, M.; Kouki, J. Tree sap as an important seasonal food resource for woodpeckers: The case of the Eurasian three-toed woodpecker (Picoides tridactylus) in southern Finland. Ann. Zool. Fenn. 2018, 55, 77–92. [Google Scholar] [CrossRef]
- Versluis, M.; Mikusiński, G.; Roberge, J.-M. Foraging behaviour of the Eurasian Three-toed Woodpecker Picoides tridactylus in its peak abundance after wildfire. Ardea 2020, 110, 75–88. [Google Scholar] [CrossRef]
- Jyske, T.; Hölttä, T. Comparison of phloem and xylem hydraulic architecture in Norway spruce stems. New Phytol. 2015, 205, 102–115. [Google Scholar] [CrossRef]
- Jyske, T.; Laakso, T.; Latva-Mäenpää, H.; Tapanila, T.; Saranpää, P. Yield of stilbene glucosides from the bark of young and old Norway spruce stems. Biomass Bioenergy 2014, 71, 216–227. [Google Scholar] [CrossRef]
- Jyske, T.; Kuroda, K.; Suuronen, J.-P.; Pranovich, A.; Roig Juan, S.; Aoki, D.; Fukushima, K. In planta localization of stilbenes within Picea abies phloem. Plant Physiol. 2016, 172, 913–928. [Google Scholar] [CrossRef]
- Jyske, T.; Kuroda, K.; Keriö, S.; Pranovich, A.; Linnakoski, R.; Hayashi, N.; Aoki, D.; Fukushima, K. Localization of (+)-Catechin in Picea abies Phloem: Responses to Wounding and Fungal Inoculation. Molecules 2020, 25, 2952. [Google Scholar] [CrossRef] [PubMed]
- Hölttä, T.; Vesala, T.; Sevanto, S.; Perämäki, M.; Nikinmaa, E. Modeling xylem and phloem water flows in trees according to cohesion theory and munch hypothesis. Trees 2006, 20, 67–78. [Google Scholar] [CrossRef]
- Paljakka, T.; Jyske, T.; Lintunen, A.; Aaltonen, H.; Nikinmaa, E.; Hölttä, T. Gradients and dynamics of inner bark and needle osmotic potentials in Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.). Plant Cell Environ. 2017, 40, 2160–2173. [Google Scholar] [CrossRef]
- Lintunen, A.; Paljakka, T.; Jyske, T.; Peltoniemi, M.; Sterck, F.; Von Arx, G.; Cochard, H.; Copini, P.; Caldeira, M.C.; Delzon, S.; et al. Osmolality and non-structural carbohydrate composition in the secondary phloem of trees across a latitudinal gradient in Europe. Front. Plant Sci. 2016, 7, 726. [Google Scholar] [CrossRef]
- Raitanen, J.-E.; Järvenpää, E.; Korpinen, R.; Mäkinen, S.; Hellström, J.; Kilpeläinen, P.; Liimatainen, J.; Ora, A.; Tupasela, T.; Jyske, T. Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma? Molecules 2020, 25, 567. [Google Scholar] [CrossRef] [PubMed]
- Välimaa, A.L.; Raitanen, J.E.; Tienaho, J.; Sarjala, T.; Nakayama, E.; Korpinen, R.; Jyske, T. Enhancement of Norway spruce bark side-streams: Modification of bioactive and protective properties of stilbenoid-rich extracts by UVA-irradiation. Ind. Crops Prod. 2020, 145, 112150. [Google Scholar] [CrossRef]
- Hudgins, J.W.; Krekling, T.; Franceschi, V.R. Distribution of calcium oxalate crystals in the secondary phloem of conifers: A constitutive defense mechanism? New Phytol. 2003, 159, 677–690. [Google Scholar] [CrossRef]
- Krokene, P. Conifer defense and resistance to bark beetles. In Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2015; pp. 177–207. [Google Scholar]
- Sutinen, S.; Partanen, J.; Viherä-Aarnio, A.; Häkkinen, R. Development and growth of primordial shoots in Norway spruce buds before visible bud burst in relation to time and temperature in the field. Tree Physiol. 2012, 32, 987–997. [Google Scholar] [CrossRef]
- Jyske, T.; Mäkinen, H.; Kalliokoski, T.; Nöjd, P. Intra-annual xylem formation of Norway spruce and Scots pine across latitudinal gradient in Finland. Agric. For. Meteorol. 2014, 194, 241–254. [Google Scholar] [CrossRef]
- Jyske, T.; Suuronen, J.-P.; Pranovich, A.; Laakso, T.; Watanabe, U.; Kuroda, K.; Abe, H. Seasonal variation in formation, structure, and chemical properties of phloem in Picea abies as studied by novel microtechniques. Planta 2015, 242, 613–629. [Google Scholar] [CrossRef]
- Mäkinen, H.; Jyske, T.; Nöjd, P. Dynamics of diameter and height increment of Norway spruce and Scots pine in southern Finland. Ann. For. Sci. 2018, 75, 1–11. [Google Scholar] [CrossRef]
- Eberhardt, L.S. Use and selection of sap trees by yellow-bellied sapsuckers. Auk 2000, 117, 41–51. [Google Scholar] [CrossRef]
- Kozma, J.M. Characteristics of Trees Used by White-Headed Woodpeckers for Sap Feeding in Washington. Northwestern Nat. 2010, 91, 81–86. [Google Scholar] [CrossRef]
- Turček, F. The ringing of trees by some European woodpeckers. Ornis Fenn. 1954, 31, 33–41. [Google Scholar]
- Mancuso, K.; Nol, E.; Burke, D.; Elliot, K. Effect of selection logging on Yellow-bellied Sapsucker sap-feeding habits in Algonquin Provincial Park, Ontario. Can. J. For. Res. 2014, 44, 1236–1243. [Google Scholar] [CrossRef]
- Núñez Montellano, M.G.; Blendinger, P.G. Selection of plants for sap feeding by the white-fronted woodpecker Melanerpes cactorum in Chaco dry forest, Argentina. Acta Ornithol. 2016, 51, 105–122. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Winget, C.H. Diurnal and seasonal variation in radii of tree stems. Ecology 1964, 45, 149–155. [Google Scholar] [CrossRef]
- Gross, K.; Koch, W. Water relations of Picea abies I. Comparison of water relations parameters of spruce shoots examined at the end of the vegetation period and in winter. Physiol. Plant 1991, 83, 290–295. [Google Scholar] [CrossRef]
- Young-Robertson, J.M.; Bolton, R.; Bhatt, U.S.; Cristóbal, J.; Thoman, R. Deciduous trees are a large and overlooked sink for snowmelt water in the boreal forest. Sci. Rep. 2016, 6, 29504. [Google Scholar] [CrossRef]
- Pakkala, T.; Tiainen, J.; Piha, M.; Kouki, J. Nest tree characteristics of the old-growth specialist Three-toed Woodpecker Picoides tridactylus. Ornis Fenn. 2018, 95, 89–102. [Google Scholar]
- Cajander, A.K. Forest types and their significance. Acta For. Fennica 1949, 56, 1–71. [Google Scholar] [CrossRef]
- Pakkala, T.; Tiainen, J.; Piha, M.; Kouki, J. Three-toed Woodpecker cavities in trees: A keystone structural feature in forests shows decadal persistence but only short-term benefit for secondary cavity-breeders. For. Ecol. Manag. 2018, 413, 70–75. [Google Scholar] [CrossRef]
- Devaux, M.; Ghashghaie, J.; Bert, D.; Lambrot, C.; Gessler, A.; Bathellier, C.; Ogee, J.; Loustau, D. Carbon stable isotope ratio of phloem sugars in mature pine trees throughout the growing season: Comparison of two extraction methods. Rapid Commun. Mass Spectrom. 2009, 23, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, A.; Sundberg, K.; Lillandt, C.; Holmbom, B.R. Determination of Hemicelluloses and Pectins in Wood and Pulp Fibres by Acid Methanolysis and Gas Chromatography. Nord. Pulp Pap. Res. J. 1996, 11, 216–219. [Google Scholar] [CrossRef]
- Pakkala, T.; Lakka, J.; Nurmi, T.; Piha, M.; Piiroinen, J.; Vepsäläinen, V. Pohjantikat Radioseurannassa A Radio-Tracking Project of Three-Toed Woodpeckers in southern Finland; Technical Report; University of Helsinki: Helsinki, Finland, 2005. [Google Scholar]
- Kilham, L. The relations of breeding yellow-bellied sapsuckers to wounded birches and other trees. Auk 1964, 81, 520–527. [Google Scholar] [CrossRef]
- De Kiriline, L. A Comparative Life-History Study of Four Species of Woodpeckers. Ornithol. Monogr. 1967, 5, 1–156. [Google Scholar] [CrossRef]
- Tate, J., Jr. Methods and Annual Sequence of Foraging by the Sapsucker. Auk 1973, 90, 840–856. [Google Scholar] [CrossRef]
- Schlatter, M.; Vergara, P. Magellanic Woodpecker (Campephilus magellanicus) sap feeding and its role in the Tierra del Fuego forest bird assemblage. J. Ornithol. 2005, 146, 188–190. [Google Scholar] [CrossRef]
- Howell, T.R. Natural history and differentiation in the Yellow-bellied Sapsucker. Condor 1952, 54, 237–282. [Google Scholar] [CrossRef]
- Oliver, W.W. Sapsucker damage to ponderosa pine. J. For. 1968, 66, 842–844. [Google Scholar]
- White, T.C.R. The cause of bark stripping of young plantation trees. Ann. For. Sci. 2019, 76, 105. [Google Scholar] [CrossRef]
- Ruiz-Peinado, R.; Pretzsch, H.; Löf, M.; Heym, M.; Bielak, K.; Aldea, J.; Barbeito, I.; Brazaitis, G.; Drössler, L.; Godvod, K.; et al. Mixing effects on Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.) productivity along a climatic gradient across Europe. For. Ecol. Manag. 2020, 482, 2021. [Google Scholar] [CrossRef]
- Temesgen, H.; LeMay, V.; Mitchell, S.J. Tree crown ratio models for multi-species and multi-layered stands of southeastern British Columbia. For. Chron. 2005, 81, 133–141. [Google Scholar] [CrossRef]
- Kantola, A.; Mäkelä, A. Development of biomass proportions in Norway spruce (Picea abies [L.] Karst.). Trees 2006, 20, 111–121. [Google Scholar] [CrossRef]
- Viiri, H.; Annila, A.; Kitunen, V. Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus Ceratocystis polonica. Trees 2001, 15, 112–122. [Google Scholar] [CrossRef]
- Lieutier, F.; Brignolas, F.; Sauvard, D.; Yart, A.; Galet, C.; Brunet, M.; van de Sype, H. Intra- and inter-provenance variability in phloem phenols of Picea abies and relationship to a bark beetle associated fungus. Tree Physiol. 2003, 23, 247–256. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef]
- Li, S.H.; Nagy, N.E.; Hammerbacher, A.; Krokene, P.; Niu, X.M.; Gershenzon, J.; Schneider, B. Localization of phenolics in phloem parenchyma cells of Norway spruce (Picea abies). ChemBioChem 2012, 13, 2707–2713. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Ralph, S.G.; Bohlmann, J.; Fenning, T.M.; Gershenzon, J.; Schmidt, A. Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol. 2011, 157, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Schmidt, A.; Wadke, N.; Wright, L.P.; Schneider, B.; Bohlmann, J.; Brand, W.A.; Fenning, T.M.; Gershenzon, J.; Paetz, C. A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol. 2013, 162, 1324–1336. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Paetz, C.; Wright, L.P.; Fischer, T.C.; Bohlmann, J.; Davis, A.J.; Fenning, T.M.; Gershenzon, J.; Schmidt, A. Flavan-3-ols in Norway spruce: Biosynthesis, accumulation, and function in response to attack by the bark beetle-associated fungus Ceratocystis polonica. Plant Physiol. 2014, 164, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Kandasamy, D.; Ullah, C.; Schmidt, A.; Wright, L.P.; Gershenzon, J. Flavanone-3-Hydroxylase Plays an Important Role in the Biosynthesis of Spruce Phenolic Defenses Against Bark Beetles and Their Fungal Associates. Front. Plant Sci. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- McKay, S.A.; Hunter, W.L.; Godard, K.A.; Wang, S.X.; Martin, D.M.; Bohlmann, J.; Plant, A.L. Insect Attack and Wounding Induce Traumatic Resin Duct Development and Gene Expression of (-)-Pinene Synthase in Sitka Spruce. Plant Physiol. 2003, 133, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, E. Ceratocystis polonica inoculated in Norway spruce: Blue-staining in relation to inoculum density, resinosis and tree growth. Eur. J. For. Pathol. 1985, 15, 160–167. [Google Scholar] [CrossRef]
- Knoblauch, M.; Mullendore, D.L. Sieve element occlusion. In “Phloem” Eds Thompson GA. Van. Bel. AJE 2012, 141–153. [Google Scholar] [CrossRef]
- Atkinks, C.A.; Smith, P.M.C.; Rodriguez-Medina, C. Macromolecules in phloem exudates—A review. Protoplasma 2011, 248, 165–172. [Google Scholar] [CrossRef]
- Douglas, A.E. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef]
- Short, L.L. Burdens of the picid hole-excavating habit. Wilson Bull. 1979, 91, 16–28. [Google Scholar]
- Schuppe, E.R.; Rutter, A.R.; Roberts, T.J.; Fuxjager, M.J. Evolutionary and Biomechanical Basis of Drumming Behavior in Woodpeckers. Front. Ecol. Evol. 2021, 9, 649146. [Google Scholar] [CrossRef]
- Gitzelmann, R.; Auriccio, S. The handling of soya alpha-galactosides by a normal and a galactosemic child. Pediatrics 1965, 36, 231–235. [Google Scholar] [CrossRef]
- Parsons, C.M.; Zhang, Y.; Araba, M. Nutritional evaluation of soybean meals varying in oligosaccharide content. Poult. Sci. 2000, 79, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Pechacek, P. Foraging behavior of Eurasian three-toed woodpeckers (Picoides tridactylus alpinus) in relation to sex and season in Germany. Auk 2006, 123, 235–246. [Google Scholar] [CrossRef]
- Hogstad, O. Sexual dimorphism and divergence in winter foraging behaviour of Three-toed Woodpeckers Picoides tridactylus. Ibis 1976, 118, 41–50. [Google Scholar] [CrossRef]
- Hogstad, O. Sexual dimorphism in relation to winter foraging and territorial behaviour of the Three-toed Woodpecker Picoides tridactylus and three Dendrocopos species. Ibis 1978, 120, 198–203. [Google Scholar] [CrossRef]
- Hogstad, O. Seasonal change in intersexual niche differentiation of the Three-toed Woodpecker. Ornis Scand 1977, 8, 101–111. [Google Scholar] [CrossRef]
- Hogstad, O. The effect of social dominance on foraging by the Three-toed Woodpecker Picoides tridactylus. Ibis 1991, 133, 271–276. [Google Scholar] [CrossRef]
- Zhu, Y.; Lü, N.; Pechacek, P.; Li, J.; Sun, Y.-H. Foraging behavior of the Eurasian Three-toed Woodpecker subspecies Picoides tridactylus funebris in southern Gansu, China. Chin. Birds 2012, 2012, 60–66. [Google Scholar] [CrossRef]
- Imbeau, L.; Desrochers, A. Foraging ecology and use of drumming trees by Three-toed Woodpeckers. J. Wildl. Manag. 2002, 66, 222–231. [Google Scholar] [CrossRef]
- Pasinelli, G. Sexual dimorphism and foraging niche partitioning in the Middle Spotted Woodpecker Dendrocopos medius. Ibis 2000, 142, 635–644. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kara, K.; Kocaoğlu Güçlü, B.; Şentürk, M.; Konca, Y. Influence of catechin (flavan-3-ol) addition to breeder quail (Coturnix coturnix japonica) diets on productivity, reproductive performance, egg quality and yolk oxidative stability. J. Appl. Anim. Res. 2016, 44, 436–441. [Google Scholar] [CrossRef]
- Kara, K.; Guclu, B.K.; Senturk, M.; Eren, M.; Baytok, E. Effects of catechin and copper or their combination in diet on productive performance, egg quality, egg shelf-life, plasma 8-OHdG concentrations and oxidative status in laying quail (Coturnix coturnix japonica). J. Appl. Anim. Res. 2021, 49, 97–103. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; He, K.; Geng, Z.; Wan, X. Dietary green tea powder supplementation enriched egg nutrients and physicochemical property in an indigenous chicken breed. Poult. Sci. 2021, 100, 388–395. [Google Scholar] [CrossRef]
- Cherian, G. Essential fatty acids and early life programming in meat-type birds. World’s Poult. Sci. J. 2011, 67, 599–614. [Google Scholar] [CrossRef]
- Abdo, Z.M.; Hassan, R.A.; El-Salam, A.A.; Helmy, S.A. Effect of adding green tea and its aqueous extract as natural antioxidants to laying hen diet on productive, reproductive performance and egg quality during storage and its content of cholesterol. Egypt. Poult. Sci. J. 2010, 30, 1121–1149. [Google Scholar]


| Time | Nesting Cycle | Sap Feeding by TTWs | Norway Spruce Phenology |
|---|---|---|---|
| March | Pairs begin to settle into territories; territorial activity starting to increase | Ongoing, but usually occasional and restricted to warm days and afternoons | Ecodormancy, environmental factors preventing growth: (a) initial events in primordial bud development from mid-March; (b) soluble sugar levels in phloem decline and starch amount increases from mid-winter conditions. Especially raffinose content starts to decline. |
| 1–20 April | Pairs mostly at breeding territories; active territoriality, pair-bonding behavior increases | Ongoing, time used feeding on sap increases | Initial events in primordial bud development depleted with early development of vascular tissue and primordial needles by late-April. Starch and soluble sugar transformation active in phloem. Warm spells enhance tissue-level metabolism and growth. |
| 20 April to 15 May | Cavity excavation or preparation of old cavities for nesting; active territoriality, pair bonding finalized, copulations | Ongoing, peak period with decrease to the end of the period | Peak period for vascular tissue and needle development in primordial buds at the end of April. Peak period for cambial reactivation, first towards phloem. Phloem formation begins by later-April–early-May. Both soluble sugar and starch levels are high in phloem. |
| 15 May to 30 May | Nesting period starts; egg-laying, incubation; some late pairs may still prepare the cavity | Ongoing, turning occasional, clear decrease from the peak period | Bud break by late-May. Cambial divisions towards xylem begin by late-May–early-June. Soluble sugar levels begin to decline, starch content still high in phloem. |
| June | Nesting period continues; nestlings hatch mostly from the 1st to the 10th, and leave the nest mostly from the 20th to the 30th | Infrequent by adult TTWs, nestlings are fed mostly by insect larvae, sap is not served to nestlings | Peak period of cambial growth. Xylem formation at maximal rate around mid-June. Phloem formation depletes by late June—early-July. Height increment decreases. Soluble sugar amounts at the lowest level and starch content begin to decline in phloem. |
| July and onwards | Latest nestlings leave the nest during 1st to 10th; nestlings move with adults first in the neighborhoods of the nest cavity, later wider in the nesting territory, brood often divided by the male and female | Infrequent, adults feed young with insects for some weeks, no observations of independent sap feeding of the young birds (this period is generally rather poorly known, occasional observations of sap feeding have been observed in later autumn) | Xylem formation and maturation ongoing. New bud set. Soluble sugar content in phloem increases from August towards winter. |
| Sample Tree Class | |||
|---|---|---|---|
| Old (O) | New (N) | Control (C) | |
| Diameter at BH (cm) | 35.4 (10.9) | 30.8 (7.4) | 31.9 (4.9) |
| Height (m) | 26.4 (6.8) | 25.6 (4.6) | 28.5 (2.3) |
| Slenderness index (height:diameter at BH) | 75.5 (5.9) | 84.5 (9.8) | 90.3 (9.6) |
| Sampling dates A (Phloem water content, osmolality and solute content) | |||
| 14 April, 30 April, 12 May | |||
| Sampling dates B (Chemical properties of phloem sap) | |||
| 12 May, 25 May, 29 May, 4 June | |||
| Sampling dates C (Chemical properties of phloem tissue) | |||
| 30 April, 12 May, 29 May, 4 June | |||
| Dependent Variable | Fixed Effect | F-Value | p-Value |
|---|---|---|---|
| RWC | T | 2.00 | 0.153 |
| S | 1.29 | 0.290 | |
| T*S | 0.23 | 0.919 | |
| WC | T | 1.53 | 0.232 |
| S | 3.65 | 0.038 | |
| T*S | 0.84 | 0.509 | |
| Osmolality | T | 0.29 | 0.752 |
| S | 4.84 | 0.015 | |
| T*S | 1.05 | 0.401 | |
| p | T | 2.13 | 0.138 |
| S | 2.16 | 0.134 | |
| T*S | 2.13 | 0.102 | |
| Slenderness | T | 2.97 | 0.082 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jyske, T.; Keinänen, S.; Hölttä, T.; Lintunen, A.; Pranovich, A.; Laakso, T.; Suuronen, J.-P.; da Silva Viana, G.; Pakkala, T. Pilot Study of Sap Properties of Norway Spruce (Picea abies (L.) Karst.) Trees Used and Not Used for Sap-Feeding by Three-Toed Woodpeckers (Picoides tridactylus). Forests 2022, 13, 1681. https://doi.org/10.3390/f13101681
Jyske T, Keinänen S, Hölttä T, Lintunen A, Pranovich A, Laakso T, Suuronen J-P, da Silva Viana G, Pakkala T. Pilot Study of Sap Properties of Norway Spruce (Picea abies (L.) Karst.) Trees Used and Not Used for Sap-Feeding by Three-Toed Woodpeckers (Picoides tridactylus). Forests. 2022; 13(10):1681. https://doi.org/10.3390/f13101681
Chicago/Turabian StyleJyske, Tuula, Sini Keinänen, Teemu Hölttä, Anna Lintunen, Andrey Pranovich, Tapio Laakso, Jussi-Petteri Suuronen, Gabriel da Silva Viana, and Timo Pakkala. 2022. "Pilot Study of Sap Properties of Norway Spruce (Picea abies (L.) Karst.) Trees Used and Not Used for Sap-Feeding by Three-Toed Woodpeckers (Picoides tridactylus)" Forests 13, no. 10: 1681. https://doi.org/10.3390/f13101681
APA StyleJyske, T., Keinänen, S., Hölttä, T., Lintunen, A., Pranovich, A., Laakso, T., Suuronen, J.-P., da Silva Viana, G., & Pakkala, T. (2022). Pilot Study of Sap Properties of Norway Spruce (Picea abies (L.) Karst.) Trees Used and Not Used for Sap-Feeding by Three-Toed Woodpeckers (Picoides tridactylus). Forests, 13(10), 1681. https://doi.org/10.3390/f13101681






