Abstract
Afforestation is conducive to improving ecosystem service functions and ecosystem diversity in the Mu Us Sandy Land, however, the important attribute of biomass for Mongolian pine (Pinus sylvestris var. mongolica Litv.) plantations has yet to be accurately evaluated. This study aimed to develop additive allometric biomass equations for the species and evaluate biomass partitioning patterns within tree components. A total of 131 trees were measured for stem, branch, and leaf biomass by destructively sampling and tree climbing, with the latter as a supplement. For each biomass component, we tested three equations with the diameter at breast (D) alone, height (H) as additional, and diameter in combination with height (D2H) as predictors using the weighted least squared method. Weighted nonlinear seemingly unrelated regression was adopted to fit a system of additive allometric biomass equations utilizing the selected equations. A leave-one-out cross-validation method (the jackknife procedure) was used to assess the predictive ability. The biomass partitioning pattern was evaluated by calculating the ratios. The results revealed that the diameter alone is a good predictor for branches and foliage biomass estimates, while the stem requires H included to improve estimation accuracy. Mongolian pine allocates relatively more biomass to the crown (51.4%) compared to the stem (48.6%). Branch biomass fraction increased monotonously with increasing tree size while a reverse trend was observed for foliage. In conclusion, the additive models developed in this study provide a robust biomass estimation and can be extensively used to estimate Mongolian pine forests biomass in Mu Us Sandy Land.
1. Introduction
Desertification is one of the most serious global environmental problems and adversely affects ecosystem services and socio-economic development [1,2]. The Mu Us Sandy Land lies on the farming-pastoral ecotone [3] and in a transition zone from the Gobi Desert to forest-steppe [4], has been identified as a crucial ecological security barrier in northern China, but is very fragile and sensitive to ecological events [3]. Therefore, it is one of the important development areas in the Three-North Forest Shelterbelt program and artificial afforestation is the key to preventing degradation and stable sand [1,5]. Because Mongolian pine (Pinus sylvestris var. mongolica Litv.) has strong adaptability to cold, drought, and soil infertility, it has been one of the principal species in the program and has been introduced to the Mu Us Sandy land since the 1970s. Over four decades after plantation, the once sandy land with poor primary productivity is now covered by dense trees, implying a significant achievement in ecological restoration. Furthermore, the afforestation of Mongolian pine is still in progress. Hence, studying forest biomass is essential for comprehensively evaluating the effects of ecological restoration on biodiversity as well as for better understanding the contribution to carbon sinks in desertification regions. To our knowledge, however, little information is available regarding the biomass production of Mongolian pine plantations in arid regions, and relevant information is badly needed.
Harvesting trees and weighing all of their components is considered the most accurate method for determining biomass [6], but the destructive sampling method is time-consuming, labor-intensive, limited to small trees or small sample sizes, and is not appropriate for endangered tree species or in protected areas [7]. In contrast, the application of reliable allometric equations to infer dry mass from census data has become a preferred way and plays a key role in forest biomass estimation due to its advantages in efficiency, conciseness, and accuracy [8,9,10]. Allometric equations established based on sparse measurements from destructive sampling usually link tree biomass and its components, such as stem, branch, and foliage, with one or more easily measured biophysical metrics including diameter at breast height (D), total height (H), crown size, and the like [6,9,11]. Linear or nonlinear regression analyses are often used to test the validity of biological theories, of which, power-law functions are one of the most commonly used patterns in biology [12].
Determining a proper regression method is a major challenge when establishing allometric biomass models [13]. In most cases, allometric equations of biomass components and their aggregates are independently fitted by applying the ordinary least squares (OLS) technique [14]. However, this technique ignores the inherent correlations between different biomass components and the tree total [11]. Consequently, these biomass models were not additive, namely, the sum of the predictions obtained from independent models of the tree components may not equal that for the whole tree. Solving the additivity implies that the equations for different components and the total tree must be estimated concurrently [15]. Seemingly unrelated regression (SUR) and nonlinear seemingly unrelated regression (NSUR) methods utilizing simultaneous estimation have been considered preferred procedures to address this issue for linear and nonlinear equations, respectively [15,16,17,18]. The two procedures provide a set of contemporaneously correlated equations with cross-equation parameter constraints, thus ensuring additive predictions [19]. The NSUR procedure shows more efficient for parameter estimators because of its flexibility in applying a specific weighting function to stabilize variances [16,17], known as weighted nonlinear seemingly unrelated regression (WNSUR). The WNSUR procedure is therefore recommended to estimate nonlinear biomass equations and the heteroscedasticity in biomass data could be corrected as well [18].
Based on these facts, robust allometric equations are imperative for accurate forest biomass estimation. However, there is a general lack of allometric equations to predict the aboveground biomass for Mongolian pine trees in the Mu Us Sandy Land. To this end, we aimed to determine the best additive system of biomass equations using the appropriate variables for each component. The biomass equations system is validated through the leave-one-out cross-validation method and compared to previous models. In addition, we assessed the biomass partitioning pattern within the sampled trees.
2. Materials and Methods
2.1. Study Site Description
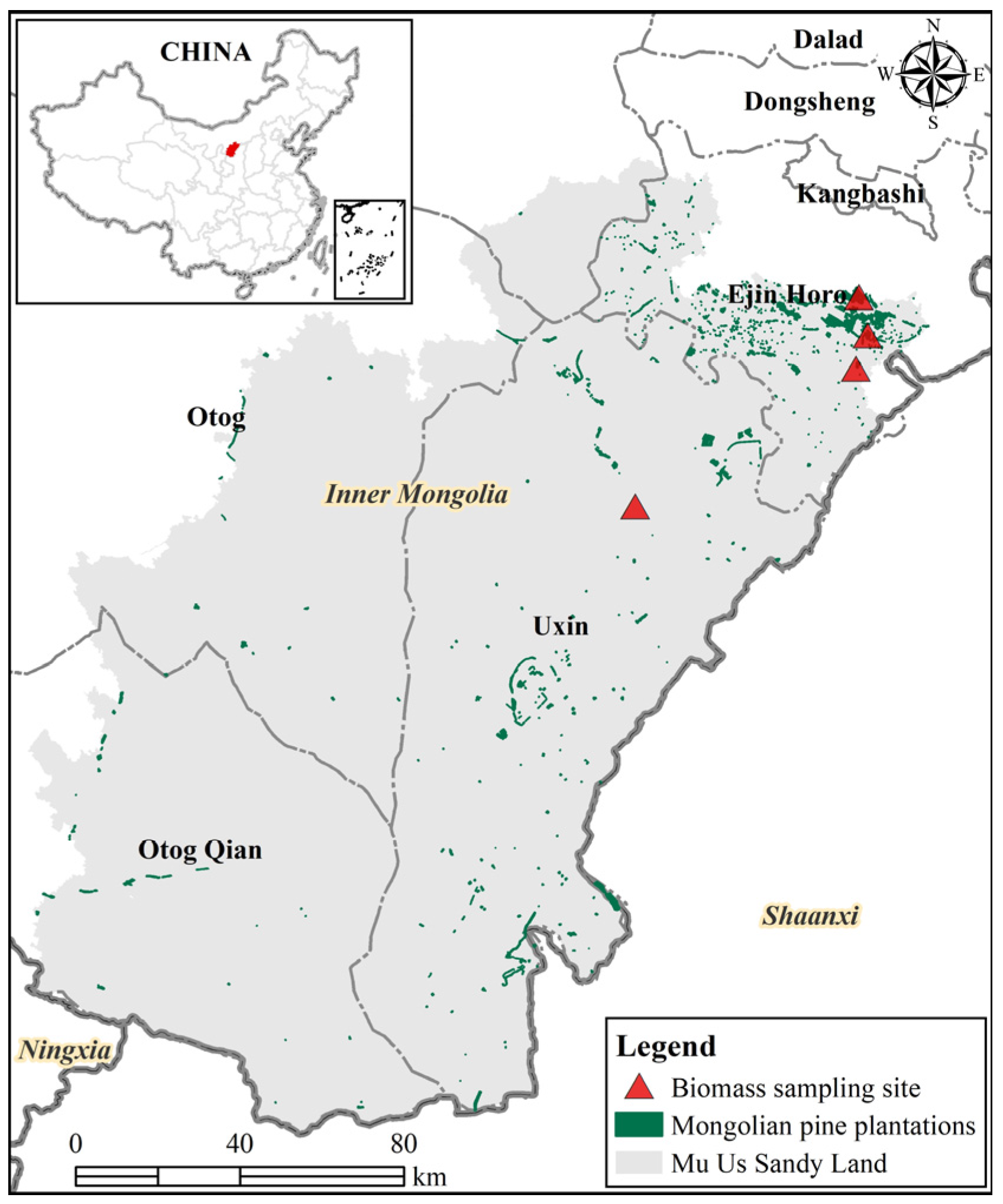
This study was conducted in the Mu Us Sandy Land (107°18′–110°12′ E, 37°37′–39°35′ N), located in the middle and southeastern part of the Ordos Plateau in central Inner Mongolia (Figure 1), with a total area of approximately 3.2 million hectares. The altitude varies between 1100–1500 m with a higher terrain in the northwest. This area is controlled by a distinct temperate continental monsoon climate, and the mean annual precipitation ranges from 250 mm in the west to 440 mm in the east. Precipitation is uneven among seasons, approximately 60%–75% happens from July to September. The annual average temperature ranges between 6.0 °C and 8.5 °C, with monthly mean temperatures of −9.5–12.0 °C in January and 22–24 °C in July. Soils are generally sandy and, according to the Chinese soil classification system, belong to the light chestnut soil, brown calcic soil, and sierozem, all are in the category calciorthid, as determined by the U.S. system. The landform of Mu Us Sandy Land is mainly divided into three subtypes: grasslands, cultivated lands, and sandy lands, and the sandy lands are subdivided into fixed, semi-fixed, and mobile dunes. The main life form in the sandy land is psammophytic vegetation. Dominant shrub and herb species in terms of abundance are Artemisia ordosica Krasch., Caragana intermedia Kuang et H. C. Fu, Juniperus sabina L., Salix cheilophila Schneid., Agropyron mongolicum Keng, Cleistogenes songorica (Roshev.) Ohwi, among others.
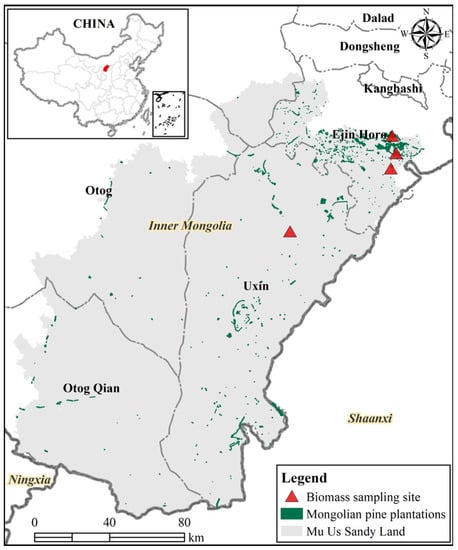
Figure 1.
The geographical location of the study area and biomass sampling sites in Mongolian pine plantations in the Mu Us Sandy Land.
2.2. Tree Sampling and Biomass Measurement
Developing accurate allometric biomass equations needs high sampling efforts across tree sizes and individuals [13]. However, destructive sampling is strictly restricted in this study area. In the present study, we took advantage of the action of clearing trees for use in construction executed by the local government to collect 116 felled trees. To expand the diameter range, we also collected biomass data by climbing 15 standing trees, with a diameter and height range of 29.1 to 44.2 cm and 9.62–15.25 m, respectively. The 131 trees were randomly picked according to occurring diameter classes. Only trees that had relatively intact bole and crown were selected for biomass measurements. These sampled trees were distributed in four separate locations (Figure 1) with stand density ranges between 250 and 1225 trees per hectare.
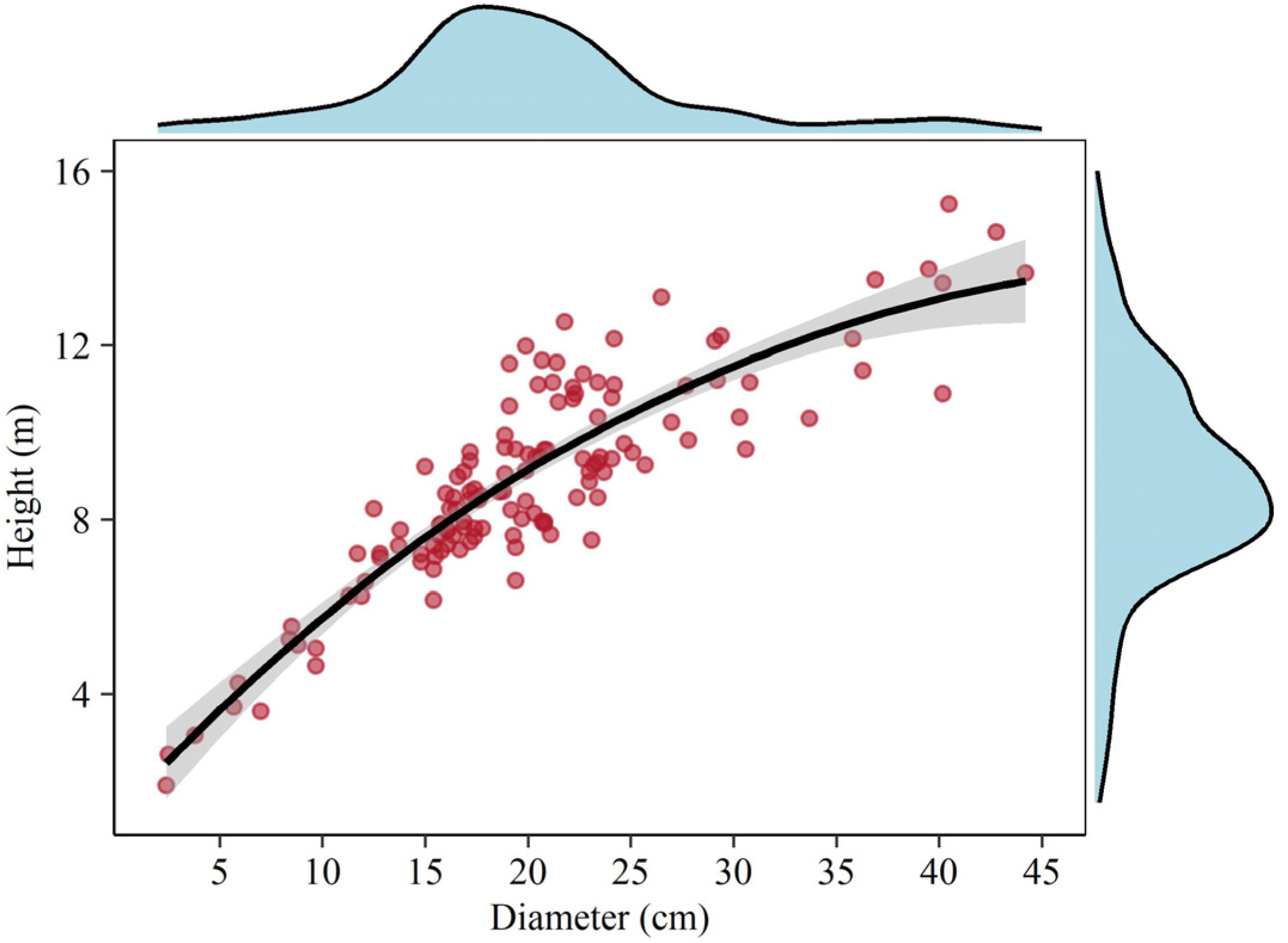
For each selected individual tree, dendrometric variables including diameter at breast height (1.30 m height) and total tree height (from the trunk base to the tip) were firstly measured and recorded with a tape graduated in cm and m, respectively. As displayed in Figure 2, a generally positive relationship between tree height and diameter was found. Thereafter, a nested regression method that treats the tree crown as a deducible system [20] was used to acquire branch and foliage biomass, and its reliability in biomass estimation has been validated many times [20,21,22]. Branches directly protruding from the trunk were defined as the first order, and those protruding from the first order were defined as the second order and so on. The branch without lateral branches was defined as the branch axis, which was treated as a cone-like minor tree bole for volume calculation using the sectional method. Consequently, the volume of all branches can be calculated by summing the branch axis and their lateral branches. Likewise, the number of leaves could be easily obtained.
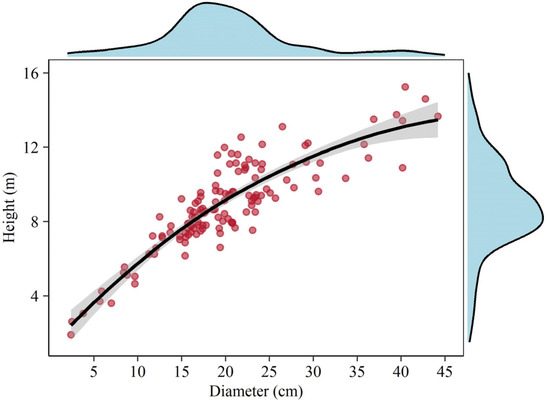
Figure 2.
Correlation between tree height and diameter for sampled trees. The density diagrams on the top and right side indicate the distribution of diameter and height, respectively. The solid line indicates loess smoothing with a 95% confidence interval.
In the actual measurement, we firstly measured the basal diameters of all the first-order branches, and then measured the basal diameters of the second-order branches, and the same for other orders. It is noteworthy that only the first-order branches were measured for the standing trees. Subsequently, over 200 branch axes were randomly collected according to size. These branch axes were sectioned into segments of 40 cm or 20 cm, and the diameters at both ends of a section were recorded. The leaf number of branch axes was concurrently counted. Finally, regression relationships between volume and leaf number, and diameter were established for branch axes. Based on the established relationships, we calculated the total volume of branches and the amounts of needle leaves synchronously.
A section length of 2 and 1 m for large and small trees, respectively, was applied to determine the stem volume. The diameter at each section position was measured and the top section, i.e., the tip, less than a segment was treated as a cone when estimating the volume. Finally, the total stem volume was obtained by summing the volumes of all sections [23].
Representative samples of branch and foliage, as well as stem discs, were collected and taken to the laboratory for basic characteristics determination. Three thin discs containing barks were taken from 1.30 m, 0.5, and 0.7 tree height of each felled trunk. Similarly, three branch discs were taken from the lower, middle, and upper canopy. Leaf fascicles of different sizes and ages were randomly taken from different trees and branches, amounting to over 1000. Sub-samples of twigs and leaves were stored in sealed bags to maintain moisture. In the laboratory, stem and branch samples were firstly saturated and then cut into moderate blocks to measure volume by the water displacement method. All samples including foliage were oven-dried at 75 °C to a constant weight. Basic density (g·cm−3) was then inferred by dividing dry weight from fresh volume. The total biomass of stems and branches was determined by multiplying each component volume based on their specific basic density. Similarly, we acquired total foliage biomass by multiplying the leaf amount (number of needles) by the average dry weight per needle leaf. Overall, tree aboveground biomass was expressed as the sum of all components, i.e., the stem, branch, and foliage (Table 1). The stump biomass was not included since the workload to dig it out was too great. The biomass partitioning was made by dividing the observed component biomass by aboveground biomass.

Table 1.
Statistics of dendrometric variables for developing biomass equations.
2.3. Allometric Biomass Model Establishment
Assembled data for dendrometric variables like D, H, and component biomass were used to develop biomass regression equations. The most common mathematical power function was adopted for biomass estimation. We preferred utilizing the nonlinear form with an additive error structure to avoid the system bias caused by log-transformed linear models [24,25]. Since biomass data always exhibit significant heteroscedasticity in model residuals [17,24], weightings were introduced to generate constant error variances over all observations in this study. A specific weighting function defined as w = 1/DQ was applied to correct each observation before parameter estimation. The power exponent Q was computed by the residuals of independent models fitted by OLS regression.
For each component, the nonlinear power model was tested to relate biomass with dendrometric variables including D, H, and D2H as defined in Equations (1)–(3).
where Wi represents the i-th observed component biomass, βi is the regression coefficient, and εi is the additive error term. The independent weighted least square (WLS) method was applied to each equation of each component. The “best model”, i.e., the most suitable predictor for each component was explored and determined by two fit statistics, including the adjusted coefficient of determination () and the root mean square error (RMSE). Only equations with statistically significant coefficients (p < 0.05) were eligible to be selected. The best models are those that have the highest as well as the lowest RMSE.
To realize the additive property between tree components, a system of equations for aboveground biomass and its components (stem, branch, and foliage) were simultaneously fitted utilizing the models selected above. The model for total aboveground biomass was equated by adding the equations for each specific component together [16,18]. Therefore, we defined the system of equations as displayed in Model (4):
where Wst, Wbr, Wfo, and Wag are estimates of the stem, branch, foliage, and aboveground biomass, respectively; f (Xi, βi) is the selected nonlinear equation of the i-th component; εi is the additive error term. The procedure of WNSUR was used to fit the system of equations [7,18], using the PROC MODEL procedure in SAS 9.3 (SAS Institute Inc., Cary, NC, USA). The WNSUR method considers the correlations between regressions residuals and set constraints on regression coefficients, resulting in lower variance and additive predictions [18].
2.4. Model Evaluation and Validation
The behavior of biomass estimators was evaluated by and RMSE, as described above. The formulas of the three goodness-of-statistics were presented as Equations (5) and (6). The predictive performance of the additive system of biomass equations was tested via Turkey’s jackknife procedure, a leave-one-out cross-validation method. Graphical analyses of predicted versus observed values of biomass were conducted and the linear fitting statistic of was achieved to evaluate the model goodness-of-fit. A paired t-test was utilized to check the consistency between predicted and observed values. And the cross-validation statistics of mean absolute error (MAE), mean prediction error (MPE), and total relative error (TRE) were used to diagnose the predictive ability. In addition, the measured biomass data in this study was used to compare with previous biomass equations. These statistics were displayed as follows:
where yj and represent the actual and estimated biomass, respectively; is the average value of the actual biomass; represents the j-th predicted value by the biomass equation fitted with the n-1 remaining observations; n and p are the number of observations and model coefficients, respectively.
3. Results
3.1. Allometric Biomass Models
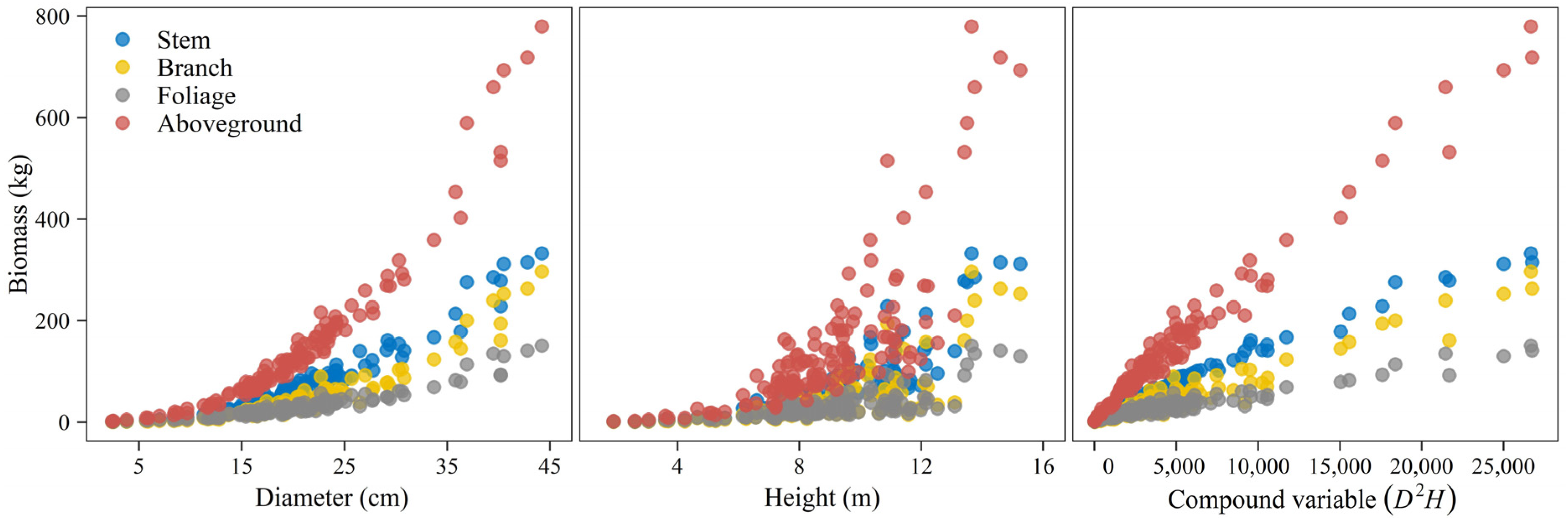
For the underlying estimation of biomass, allometric relationships were firstly examined between three size variables (D, H, D2H) and biomass of stem, branch, foliage, and aboveground total (Figure 3). Poorer correlations were found between biomass values and tree heights. The results of the biomass equations independently fitted through weighted regression are summarized in Table 2. In all, equations for total aboveground biomass and its constituents gave good fitting effects with greater than 0.91, and all model coefficients were significant (p < 0.05). For the stem component, the equation with D alone as its predictor already achieved a satisfactory goodness-of-fit performance statistic. Adding H as an additional predictor or combining it with D as a single compound predictor could improve the estimation with increased to over 0.99 and RMSE decreased by about half. Branch and foliage biomass equations, on the contrary, showed the highest and lowest RMSE when D was used as the sole predictor. The aboveground total was best estimated when using D and H as multiple variables, which had higher and lower RMSE values.
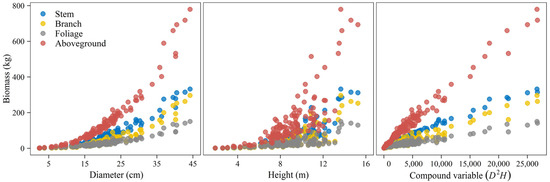
Figure 3.
Scatter plot of component-specific and total aboveground biomass against potential independent variables.

Table 2.
Regression coefficients with standard errors (in parentheses) and fitting statistics through the independently fitted procedure using weighted regression.
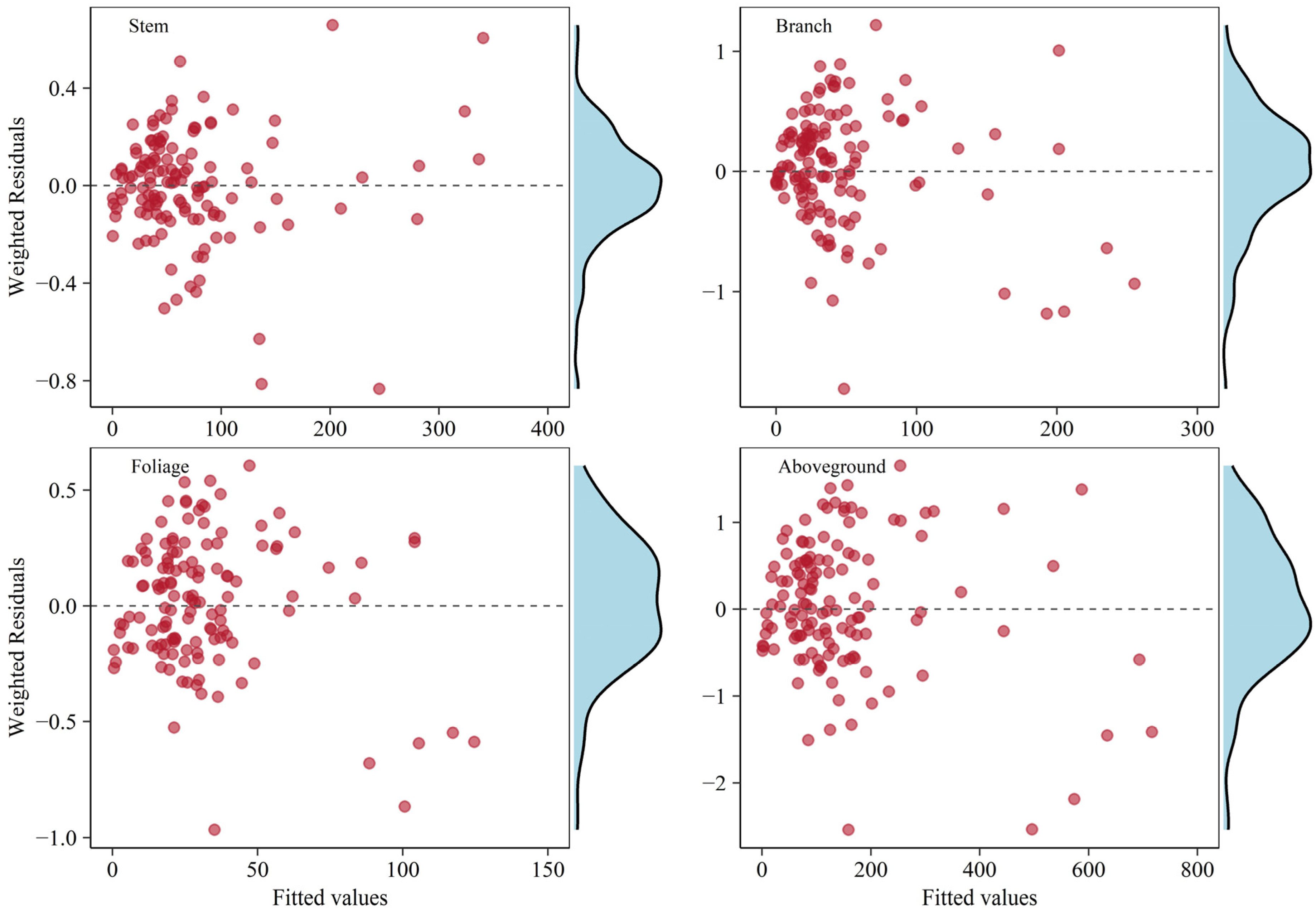
With the same weighting functions adopted in WLS regression, the additive model system based on the considered appropriate independently fitted biomass equations were presented in Table 3. The total aboveground biomass equation was obtained by combining the best equation of each component. The equations for the stem, foliage, and aboveground total showed statistics that fit a little better with higher and lower RMSE values compared to the independent fitting results. Simultaneously fitted allometric biomass equations for branch indicated the goodness-of-fit statistics were slightly poorer than that for independently fitted ones. Applying weightings to the additive system of equations could adequately result in homoscedasticity of the variance errors, as shown by the graphical analyses of the residuals (Figure 4).

Table 3.
Additive biomass models developed by simultaneously fitted (WNSUR) procedure based on the selected equations for each component.
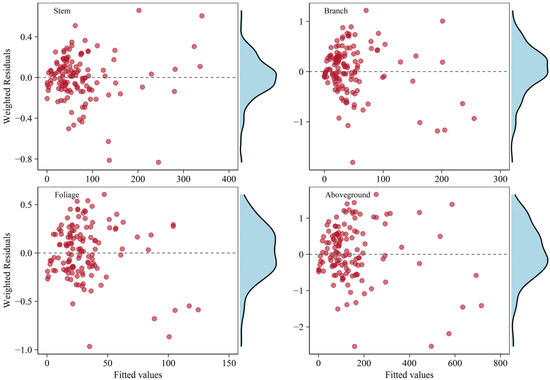
Figure 4.
Weighted residual plots for each biomass component in the system of equations fitted with the WNSUR procedure. The density diagram on the right side indicates residual distribution.
3.2. Model Validation by a Jackknife Procedure
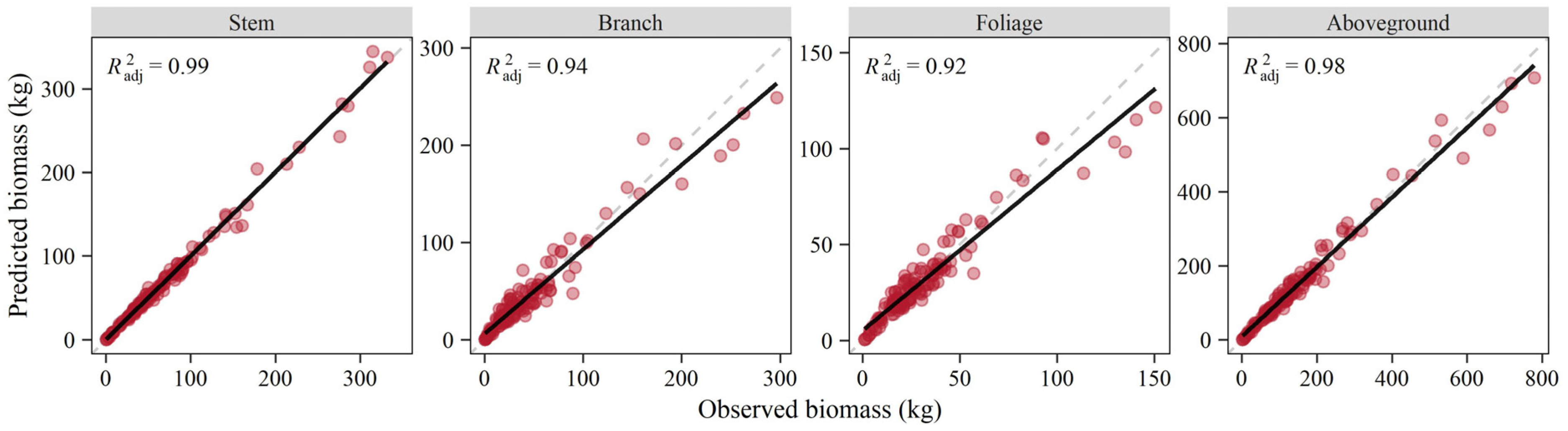
The additive system of biomass equations for total aboveground and its constituents was tested by the jackknife method, and relationships between the observed and predicted biomass values all showed a close correlation with larger than 0.92 (Figure 5). The linear fitting results identified the linear trend of y = x, especially for the stem component and aboveground total. The paired t-test demonstrated that the predicted and observed biomass were statistically consistent for all components (p > 0.05). The predictive ability was also supported by the cross-validation statistics which showed relatively little bias (Table 4). The corresponding TRE values were negative, resulting in conservative estimates.
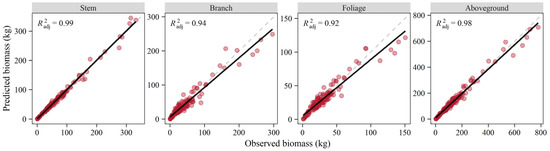
Figure 5.
Relationships between observed and predicted biomass values obtained by jackknife procedure.

Table 4.
Cross-validation statistics for component-specific and total aboveground biomass.
3.3. Aboveground Biomass Partitioning
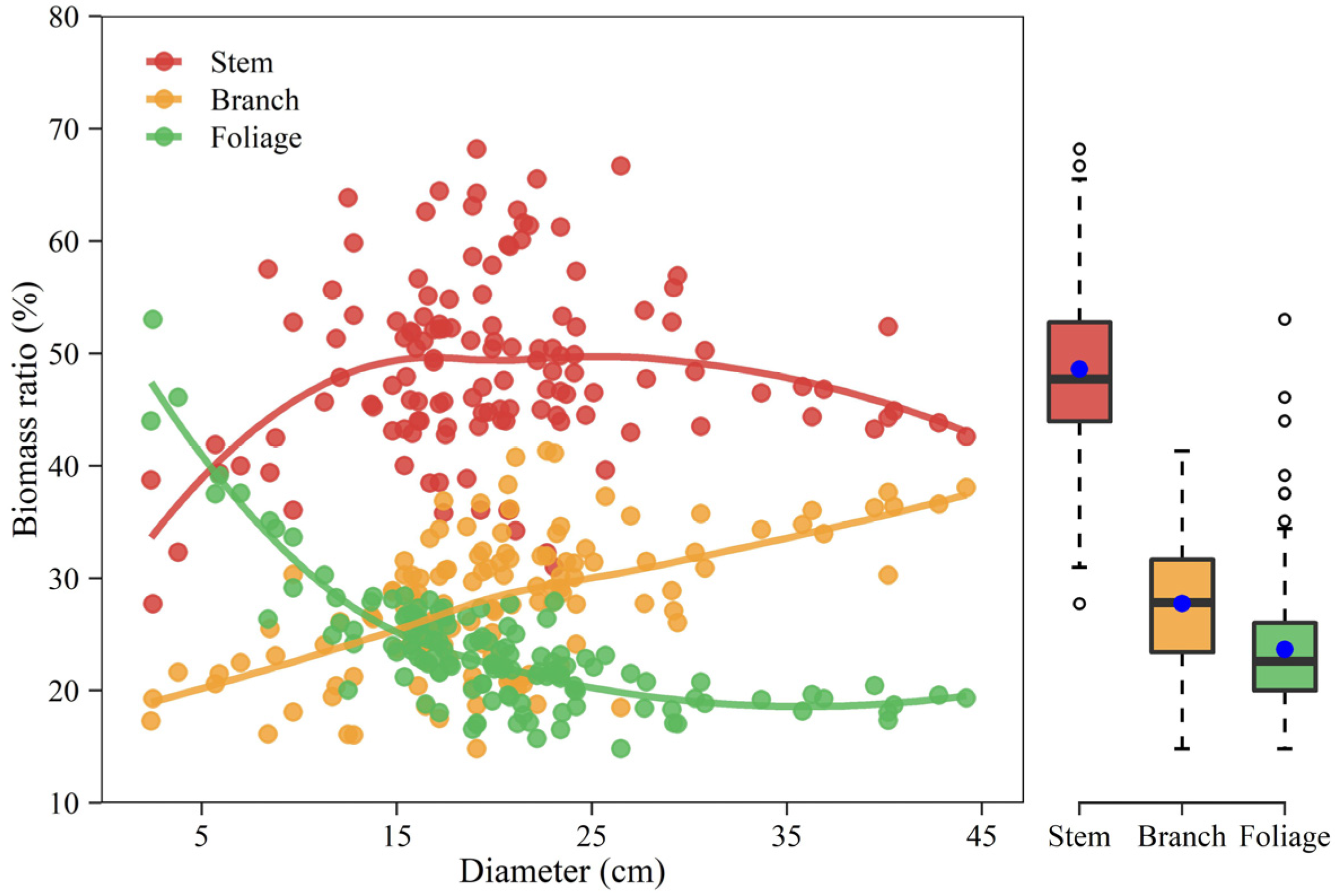
The relative proportion of component biomass varied against diameter in different ways (Figure 6). The results showed an increasing trend in the stem biomass fraction over small trees and a slightly declining trend over large trees. In contrast, the branch component showed a monotone increasing percentage while the foliage component had a monotone declining percentage with tree size. Aboveground biomass exhibited relatively larger biomass allocation to stems with a value of approximately 48.6% when compared with branches and foliage. The partitioning proportion was the lowest for foliage biomass (23.7%) and intermediate for branch biomass (27.7%).
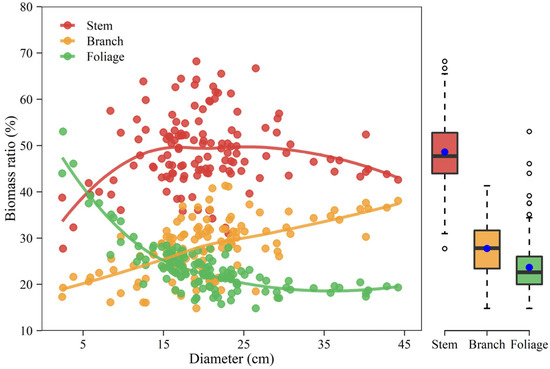
Figure 6.
Percentage of component biomass varies against diameter at breast height and the marginal boxplot shows the distribution of component biomass ratios. The solid line indicates loess smoothing. The length of the box indicates the 25th–75th percentile, the blue dot inside the box represents the mean value, the horizontal line inside the box represents the median, and outliers are marked as hollow circles.
4. Discussion
4.1. Aboveground Allometry Relationships
The best statistics of goodness-of-fit ( and RMSE) were found for the stem biomass equations, while relatively poor statistics were observed for branch and foliage components. Because of the influences of internal and external factors, branch and foliage biomass is naturally more variable than other parts like stems [15,26]. Despite this, the equations presented in this study for predicting the biomass of stem, branch, foliage, and their aggregates provided a satisfactory accuracy level.
Allometric biomass equations with D as the sole explanatory variable are easy to build and employ, and usually provide satisfactory biomass estimates for many species and regions [27,28,29]. Our results also indicate that the regression models utilizing D could yield good fitting results for component biomass and their aggregates. Moreover, D is very easy to accurately measure in the field and is almost involved in all forest inventory data. The inclusion of H as an additional predictor improved equations for stem and aboveground biomass but led to a decline in the goodness-of-fit for branch and foliage biomass. Similar results have been found as well in previous studies [7,22]. This probably suggests that tree height may be essential for stem biomass but may not be a good predictor for crown biomass. In a way, tree heights capture the variation of volume among trees [30]. The model using single compound variables D2H showed less efficient estimations than the one based on the multiple variables of D and H.
Due to the absence of information about tree crown size, we developed biomass equations using D and H only. In general, crown size variables including crown length and crown diameter, have been found to further improve crown biomass estimations [11,17,31]. However, crown size measurements from the ground are a time-consuming and laborious task, and crown overlap may increase the difficulty in measurements, hence crown dimensions were rarely available in forest inventory data [24]. In practice, equations with easily measured metrics are more practical to estimate forest aboveground biomass.
As reported in published studies, the NSUR procedure results in greater statistical efficiency and lower variance by considering the contemporaneous correlations between different biomass components [18,32,33]. In this study, the simultaneous fitting of the system of biomass equations generated little improvement in terms of and RMSE for the stem, foliage, and aboveground total compared to the independently fitted equations. Similar results were demonstrated by previous researchers who found small differences in the fitting statistics between independent and simultaneous fitting procedures that applied to the total biomass and its constituent parts [15,34]. But actually, the system of equations fitted simultaneously ensures the additivity, which has long been considered a desirable property when modeling the biomass estimates of the total and its constituent parts [16,18]. The additivity property conforms to the biological point of view that the estimates of biomass components and total are consistent [34]. In addition, simultaneous fitting of equations usually provides narrower confidence intervals relative to the equations fitted independently, since the model for total biomass must be a combination of that for component biomass resulting in parameter restrictions [19,34]. Therefore, considering the model sensitivity, fitting efficiency, and biological consistency, the estimates are more robust by the simultaneous fitting procedure [16].
Heteroscedasticity is almost a certainty in allometric biomass model development, as already discussed in previous studies [14,17,18,35]. The fact that biomass variability around the equations increased with the size of the independent variables can be considered general and natural, resulting in the heterogeneity of variance of biomass models [16,24,36]. When developing biomass equations, the residuals should satisfy the normality, and as a result, heterogeneous variances cannot be ignored and must be dealt with to achieve stable and minimum variance. The direct application of the OLS technique to biomass data, in this case, does not remain efficient and reliable because of the unstable variance [16].
An alternative method to tackle this daunting issue is to log-transform both response and explanatory variables before model construction to comply with normality and homoscedasticity [7,37,38]. Approximately 66% of the biomass equations were analyzed using OLS log-linear regression [39]. However, the application of log-transformation may alter the relationship between predictor and response variables and may arouse substantial bias if the parameter estimation of an allometric equation on its original scale is estimated by linear fitting of logarithms [40]. A more efficient way is to model the error structure as a weighting function to correct heteroscedasticity in biomass data, as recommended by several studies [16,18,19]. The weighted nonlinear equations can achieve relatively low errors compared to OLS log-linear equations [25]. In this study, the variances were modeled as a power-law function of predictor D, stabilizing the model variances for all biomass components (Figure 4). Thereby, the WNSUR procedure is more suitable for fitting biomass equations since it can provide accurate estimates with lower uncertainty than those obtained from independently fitted unweighted equations, corresponding with previous analyses of the performance of the equations, the biological consistency, and statistical efficiency [16].
4.2. Model Comparison and Biomass Allocation
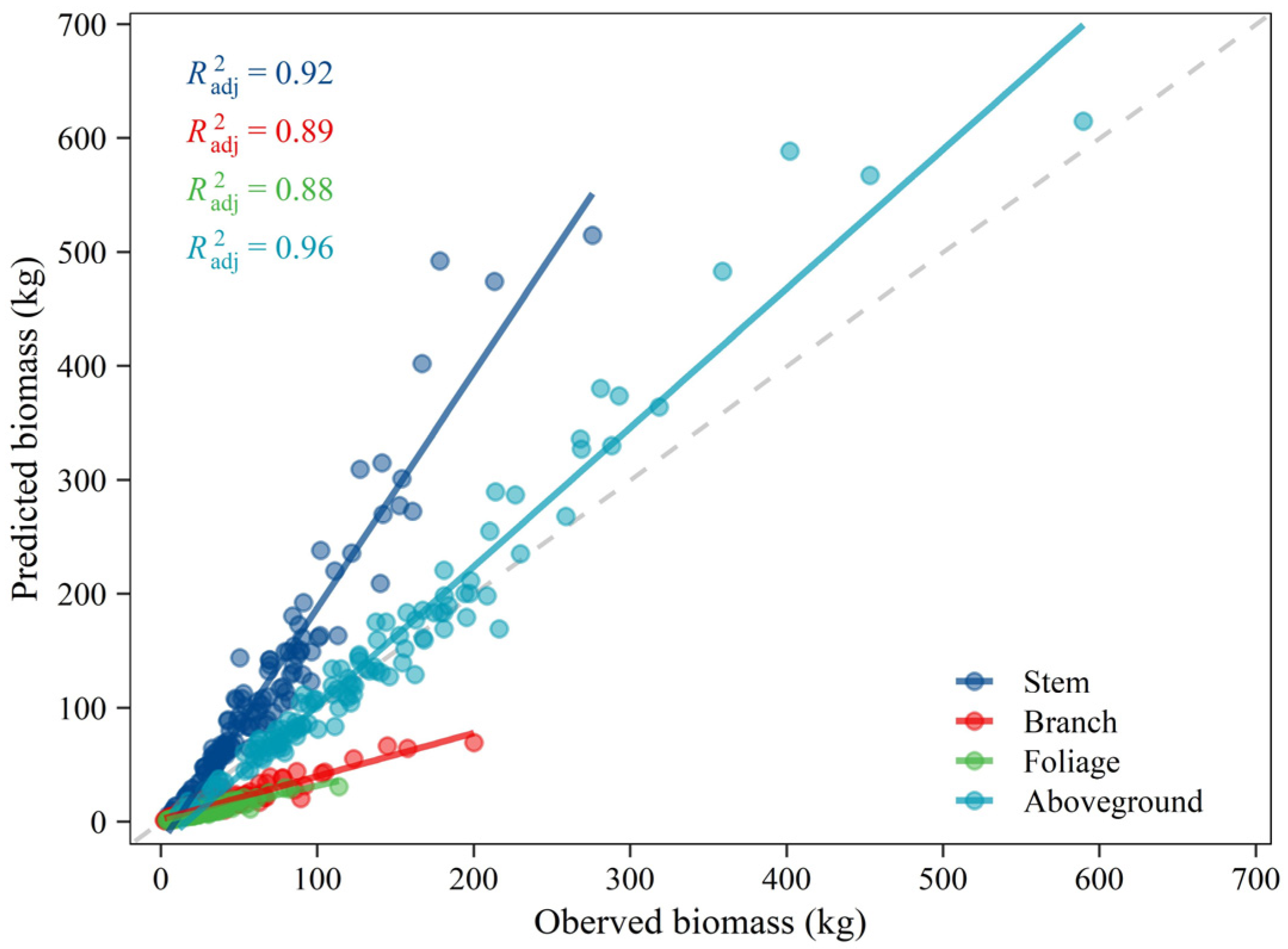
An additive system of equations was developed for estimating the aboveground biomass of Mongolian pine trees in Northeastern China [28]. The biomass measured during the present study was used to test the existing equations’ reliability on this study site. Since biomass models are valid only within the range of explanatory variables, the sampled trees that fell in the specific range were employed. Although the predicted and observed values showed good linear fitting results with larger than 0.88, the published equations did not provide accurate biomass estimates at the tree level, especially for individual components (Figure 7). By contrast with the 1:1 line, we found these equations overestimated the biomass for stem but underestimated for branches and foliage, resulting in relatively and seemingly effective biomass estimates for the aboveground total. The different biomass sampling methods (volume conversion versus direct weighing) may be the potential causes. But the primary reason may be that the biomass allocation pattern is driven by environmental conditions [41,42].
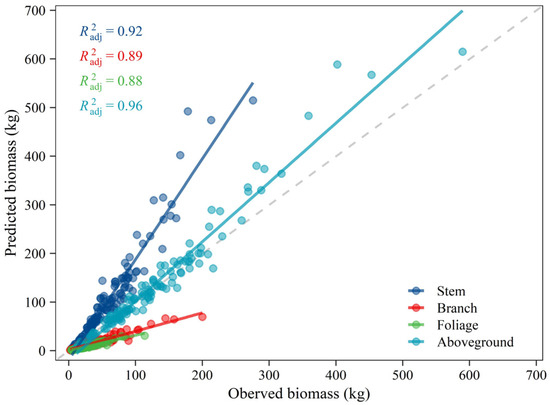
Figure 7.
Relationships between measured biomass of each component and total aboveground biomass in this study for comparison with published equations of Dong, et al. [28].
As displayed in Figure 6, the stem alone accounted for approximately 48.6% of the aboveground biomass on average, whereas branches accounted for 27.7% and the foliage for 23.7%. The partitioning pattern within the tree indicates that Mongolian pine in dryland allocates the greatest fraction of its resources to crown (branches and foliage included) development, then to the stem. Interestingly, the pattern of aboveground biomass allocation among components largely differed from that reported by Dong, et al. [28] who highlighted that stem biomass occupied over three-quarters of the aboveground total, while branches and foliage had only 13% and 9%, respectively. The discrepancies in partitioning pattern could be explained due to the site quality, climate, and competition among trees. Previous studies have demonstrated that many tree species develop broad crowns but short trunks in arid regions [43,44]. The hydraulic resistance is considered the most severe limitation to tree height growth [45]. The drier the site conditions the sooner upward growth will be stopped [44]. In this study area, the low precipitation makes it drier than northeastern China, Mongolian pine produces a rather shorter tree height. For example, a tree with a diameter of 38.3 cm has a height of 22.3 m in northeast China [28], but a tree with a 44.2 cm diameter only has a height of less than 14 m in Mu Us Sandy Land. Due to the low soil water availability in dryland, upper branches are limited by hydraulic resistance as well. The middle and lower lateral branches become the principal part of photosynthesis. However, the lower branches may be shaded and limited by light. In this issue, the middle branches may grow more and then develop a broad crown [43]. A spreading crown of a short tree could also reduce wind load and help to enhance wind resistance [43,44]. In addition, the relatively low stand density, i.e., the weak competition in this study, may promote crown growth as well.
As usual, the stem is always the largest biomass pool among different components [46]. Although the results in this study showed a similar allocation pattern, the stem holds a relatively small portion of aboveground biomass relative to other studies [11,46]. The proportional distribution of biomass among tree components changes with tree size, namely tree diameter has a strong effect on aboveground biomass allocation patterns. More specifically, foliage mass declined substantially while branches increased continuously as tree size increased, which is consistent with other studies [7,11,42]. This indicates that the accumulation of foliage biomass per unit of woody mass tends to decrease as trees grow larger, corresponding to the fact that woody biomass increases generally at the cost of foliage biomass [42,47]. When trees are small and at the early development stage, more carbohydrates are allocated to foliage to maintain efficient photosynthesis and promote vertical growth. As the trees grow big, lateral branches of different individuals contact each other, resulting in light competition between neighboring trees. Thus, more resources are allocated to stems allowing for primary and secondary growth to enable trees to compete with neighbors [42]. In the meantime, additional carbohydrates are invested for branch growth. Trees with increasing biomass investment in branches keep a competitive advantage that allows them to out-compete neighbors by developing broad crowns to fight for light and available water [7]. Indeed, the biomass partitioning patterns stress the plasticity of trees growing in different environmental conditions in nutrient acquisition and trait formation. Therefore, the application of site-specific and species-specific allometric biomass equations is recommended to the corresponding sites whenever possible [30].
5. Conclusions
This study recommends using the additive systems of equations to estimate the aboveground biomass of Mongolian pine forests in Mu Us Sandy Land. Diameter at breast height alone is a perfect predictor for branch and foliage biomass estimation, while the stem requires tree height as an additional predictor to improve estimation accuracy. Mongolian pine species in sandy land allocate relatively more biomass in the crown, as a possible mechanism to improve water absorption ability. Given the plasticity of trees under different environments, the allometric equations developed in this study can be applied to make accurate biomass estimates reliable to the corresponding sites. The applicability of these equations should restrict an independent variable to the range of the data used for model development in this study.
Author Contributions
Conceptualization, S.M. and Q.L.; methodology, S.M. and Z.X.; software, S.M. and G.Z.; validation, S.M. and J.Y.; formal analysis, B.S.; investigation, B.S. and L.L.; resources, B.S. and L.L.; data curation, B.S., S.M. and L.L.; writing—original draft preparation, B.S.; writing—review and editing, S.M. and Q.L.; visualization, S.M. and Z.X.; supervision, Q.L.; project administration, S.M. and Q.L.; funding acquisition, S.M. and Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2020YFA0608102, 2017YFC0506503) project.
Data Availability Statement
Not applicable.
Acknowledgments
Experimental conditions were guaranteed by The Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University. The authors are very grateful to the Ordos Forestry and Grassland Development Center for their support and encouragement in the research work. We also would like to express gratitude to the Ejin Horo banner state forest farm for the permission to collect biomass data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, L.; Zhu, J.; Zheng, X.; Wang, K.; Zhang, J.; Hao, G.; Wang, G.; Liu, J. Comparison of canopy transpiration between Pinus sylvestris var. mongolica and Pinus tabuliformis plantations in a semiarid sandy region of Northeast China. Agric. For. Meteorol. 2022, 314, 108784. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Fu, C.; Chen, F.; Fu, Q.; Dai, A.; Shinoda, M.; Ma, Z.; Guo, W.; Li, Z.; et al. Dryland climate change: Recent progress and challenges. Rev. Geophys. 2017, 55, 719–778. [Google Scholar] [CrossRef]
- Yan, F.; Wu, B.; Wang, Y. Estimating spatiotemporal patterns of aboveground biomass using Landsat TM and MODIS images in the Mu Us Sandy Land, China. Agric. For. Meteorol. 2015, 200, 119–128. [Google Scholar] [CrossRef]
- Teraminami, T.; Nakashima, A.; Ominami, M.; Yamamoto, M.; Sheng, Z.G.; Yoshikawa, K. Effects of sand burial depth on the root system of Salix cheilophila seedlings in Mu Us Sandy Land, Inner Mongolia, China. Landsc. Ecol. Eng. 2013, 9, 249–257. [Google Scholar] [CrossRef]
- Wang, F.; Letort, V.; Lu, Q.; Bai, X.; Guo, Y.; de Reffye, P.; Li, B. A functional and structural mongolian scots pine (Pinus sylvestris var. mongolica) model integrating architecture, biomass and effects of precipitation. PLoS ONE 2012, 7, e43531. [Google Scholar] [CrossRef] [PubMed]
- Basuki, T.M.; van Laake, P.E.; Skidmore, A.K.; Hussin, Y.A. Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forests. For. Ecol. Manag. 2009, 257, 1684–1694. [Google Scholar] [CrossRef]
- Dimobe, K.; Mensah, S.; Goetze, D.; Ouédraogo, A.; Kuyah, S.; Porembski, S.; Thiombiano, A. Aboveground biomass partitioning and additive models for Combretum glutinosum and Terminalia laxiflora in West Africa. Biomass Bioenergy 2018, 115, 151–159. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, M.; Liu, Z.; Li, P.; Xie, B.; Peng, C. Dynamic allometric scaling of tree biomass and size. Nat. Plants 2021, 7, 42–49. [Google Scholar] [CrossRef]
- Chave, J.; Rejou-Mechain, M.; Burquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Cui, Y.; Bi, H.; Liu, S.; Hou, G.; Wang, N.; Ma, X.; Zhao, D.; Wang, S.; Yun, H. Developing additive systems of biomass equations for Robinia pseudoacacia L. in the region of loess plateau of western Shanxi Province, China. Forests 2020, 11, 1332. [Google Scholar] [CrossRef]
- Meng, S.; Yang, F.; Hu, S.; Wang, H.; Wang, H. Generic additive allometric models and biomass allocation for two natural oak species in Northeastern China. Forests 2021, 12, 715. [Google Scholar] [CrossRef]
- Xiao, X.; White, E.P.; Hooten, M.B.; Durham, S.L. On the use of log-transformation vs. nonlinear regression for analyzing biological power laws. Ecology 2011, 92, 1887–1894. [Google Scholar] [CrossRef]
- Ganamé, M.; Bayen, P.; Ouédraogo, I.; Balima, L.H.; Thiombiano, A. Allometric models for improving aboveground biomass estimates in West African savanna ecosystems. Trees For. People 2021, 4, 100077. [Google Scholar] [CrossRef]
- Sanquetta, C.R.; Wojciechowski, J.; Dalla Corte, A.P.; Behling, A.; Netto, S.P.; Rodrigues, A.L.; Sanquetta, M.N.I. Comparison of data mining and allometric model in estimation of tree biomass. BMC Bioinform. 2015, 16, 247. [Google Scholar] [CrossRef]
- Sanquetta, C.R.; Behling, A.; Corte, A.P.D.; Péllico Netto, S.; Schikowski, A.B.; do Amaral, M.K. Simultaneous estimation as alternative to independent modeling of tree biomass. Ann. For. Sci. 2015, 72, 1099–1112. [Google Scholar] [CrossRef]
- Trautenmüller, J.W.; Péllico Netto, S.; Balbinot, R.; Watzlawick, L.F.; Dalla Corte, A.P.; Sanquetta, C.R.; Behling, A. Regression estimators for aboveground biomass and its constituent parts of trees in native southern Brazilian forests. Ecol. Indic. 2021, 130, 108025. [Google Scholar] [CrossRef]
- Zhao, D.; Kane, M.; Markewitz, D.; Teskey, R.; Clutter, M. Additive tree biomass equations for midrotation loblolly pine plantations. For. Sci. 2015, 61, 613–623. [Google Scholar] [CrossRef]
- Parresol, B.R. Additivity of nonlinear biomass equations. Can. J. For. Res. 2001, 31, 865–878. [Google Scholar] [CrossRef]
- Parresol, B.R. Assessing tree and stand biomass: A review with examples and critical comparisons. For. Sci. 1999, 45, 573–593. [Google Scholar]
- Liu, Q.J. Nested regression for establishing tree biomass equations. Chinese J. Plant Ecol. 2009, 33, 331–337. [Google Scholar]
- Xu, Z.; Du, W.; Zhou, G.; Qin, L.; Meng, S.; Yu, J.; Sun, Z.; SiQing, B.; Liu, Q. Aboveground biomass allocation and additive allometric models of fifteen tree species in northeast China based on improved investigation methods. For. Ecol. Manage. 2022, 505, 119918. [Google Scholar] [CrossRef]
- Meng, S.; Jia, Q.; Liu, Q.; Zhou, G.; Wang, H.; Yu, J. Aboveground biomass allocation and additive allometric models for natural Larix gmelinii in the western Daxing’anling mountains, Northeastern China. Forests 2019, 10, 150. [Google Scholar] [CrossRef]
- Laar, A.v.; Akça, A. Forest Mensuration; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Menéndez-Miguélez, M.; Calama, R.; Del Río, M.; Madrigal, G.; López-Senespleda, E.; Pardos, M.; Ruiz-Peinado, R. Species-specific and generalized biomass models for estimating carbon stocks of young reforestations. Biomass Bioenergy 2022, 161, 106453. [Google Scholar] [CrossRef]
- Huynh, T.; Lewis, T.; Applegate, G.; Pachas, A.N.A.; Lee, D.J. Allometric equations to estimate aboveground biomass in spotted gum (Corymbia citriodora subspecies variegata) plantations in Queensland. Forests 2022, 13, 486. [Google Scholar] [CrossRef]
- Antonio, N.; Tome, M.; Tome, J.; Soares, P.; Fontes, L. Effect of tree, stand, and site variables on the allometry of Eucalyptus globulus tree biomass. Can. J. For. Res. 2007, 37, 895–906. [Google Scholar] [CrossRef]
- Kebede, B.; Soromessa, T. Allometric equations for aboveground biomass estimation of Olea europaea L. subsp. cuspidata in Mana Angetu Forest. Ecosyst. Health Sustain. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, L.; Li, F. Developing two additive biomass equations for three coniferous plantation species in northeast China. Forests 2016, 7, 136. [Google Scholar] [CrossRef]
- Wang, C.K. Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For. Ecol. Manag. 2006, 222, 9–16. [Google Scholar] [CrossRef]
- Nogueira, F.C.B.; Dobe, E.K.; Silva Filho, J.B.; Rodrigues, L.S. Allometric equations to estimate aboveground biomass of Dalbergia cearensis species in the Brazilian seasonally dry tropical forest. For. Ecol. Manag. 2021, 484, 118920. [Google Scholar] [CrossRef]
- Carvalho, J.P.; Parresol, B.R. Additivity in tree biomass components of Pyrenean oak (Quercus pyrenaica Willd.). For. Ecol. Manag. 2003, 179, 269–276. [Google Scholar] [CrossRef]
- Huff, S.; Ritchie, M.; Temesgen, H. Allometric equations for estimating aboveground biomass for common shrubs in northeastern California. For. Ecol. Manag. 2017, 398, 48–63. [Google Scholar] [CrossRef]
- Riofrio, J.; Herrero, C.; Grijalva, J.; Bravo, F. Aboveground tree additive biomass models in Ecuadorian highland agroforestry systems. Biomass Bioenergy 2015, 80, 252–259. [Google Scholar] [CrossRef]
- Behling, A.; Netto, S.P.; Sanquetta, C.R.; Corte, A.P.D.; Affleck, D.L.R.; Rodrigues, A.L.; Behling, M. Critical analyses when modeling tree biomass to ensure additivity of its components. An. Acad. Bras. Cienc. 2018, 90, 1759–1774. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, L.; Chen, X.; Cheng, Z.; Ma, K.; Li, Z. Construction of compatible and additive individual-tree biomass models for Pinus tabulaeformis in China. Can. J. For. Res. 2017, 47, 467–475. [Google Scholar] [CrossRef]
- Cunia, T.; Briggs, R.D. Forcing additivity of biomass tables: Some empirical results. Can. J. For. Res. 1984, 14, 376–384. [Google Scholar] [CrossRef]
- Djomo, A.N.; Chimi, C.D. Tree allometric equations for estimation of above, below and total biomass in a tropical moist forest: Case study with application to remote sensing. For. Ecol. Manag. 2017, 391, 184–193. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Folster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Sileshi, G.W. A critical review of forest biomass estimation models, common mistakes and corrective measures. For. Ecol. Manage. 2014, 329, 237–254. [Google Scholar] [CrossRef]
- Packard, G.C. On the use of logarithmic transformations in allometric analyses. J. Theor. Biol. 2009, 257, 515–518. [Google Scholar] [CrossRef]
- Freschet, G.T.; Kichenin, E.; Wardle, D.A. Explaining within-community variation in plant biomass allocation: A balance between organ biomass and morphology above vs below ground? J. Veg. Sci. 2015, 26, 431–440. [Google Scholar] [CrossRef]
- Mensah, S.; Kakaï, R.G.; Seifert, T. Patterns of biomass allocation between foliage and woody structure: The effects of tree size and specific functional traits. Ann. For. Res. 2016, 59, 49–60. [Google Scholar] [CrossRef]
- Escoto-Rodríguez, M.; Facelli, J.M.; Watling, J.R. Do wide crowns in arid woodland trees reflect hydraulic limitation and reduction of self-shading? Funct. Plant Biol. 2014, 41, 1221. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Trees: Their Natural History; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Ryan, M.G.; Yoder, B.J. Hydraulic limits to tree height and tree growth. Bioscience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Xiang, W.; Li, L.; Ouyang, S.; Xiao, W.; Zeng, L.; Chen, L.; Lei, P.; Deng, X.; Zeng, Y.; Fang, J.; et al. Effects of stand age on tree biomass partitioning and allometric equations in Chinese fir (Cunninghamia lanceolata) plantations. Eur. J. For. Res. 2021, 140, 317–332. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Makkonen, K.; Kellomaki, S.; Valtonen, E.; Malkonen, E. Below- and above-ground biomass, production and nitrogen use in Scots pine stands in eastern Finland. For. Ecol. Manag. 2002, 165, 317–326. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).