Drought Shapes Photosynthetic Production Traits and Water Use Traits along with Their Relationships with Leaves of Typical Desert Shrubs in Qaidam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Survey and Sampling
2.3. Experimental Method
2.4. Processing of Climate Data
2.5. Data Analysis
3. Results
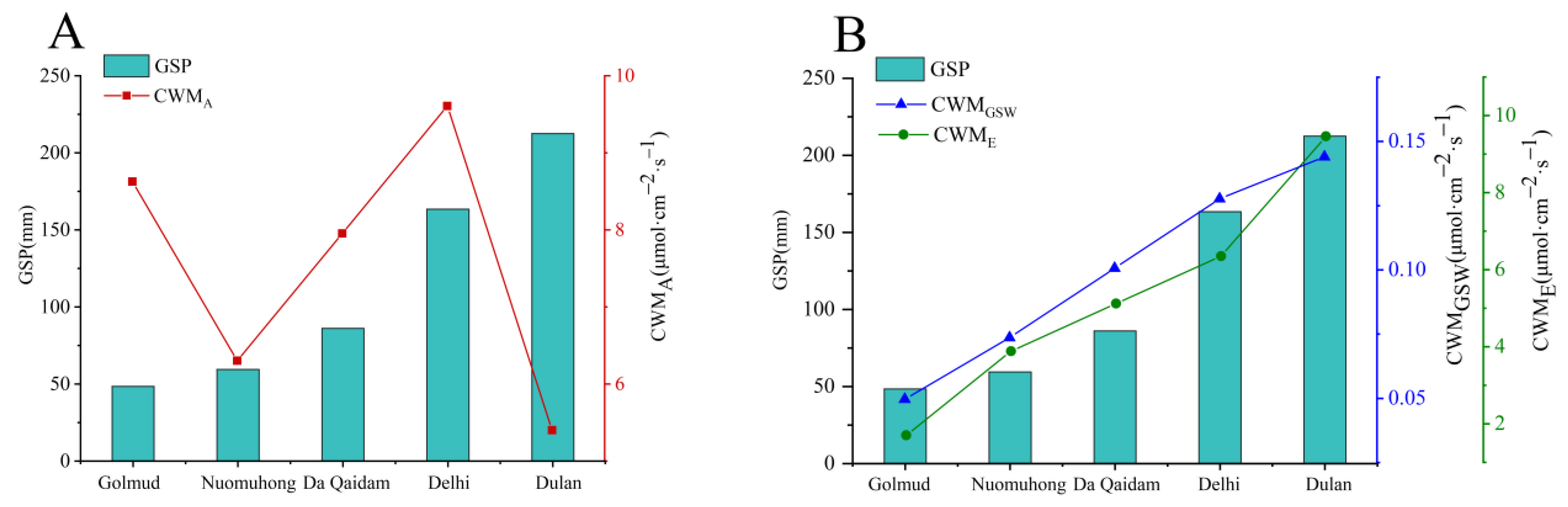
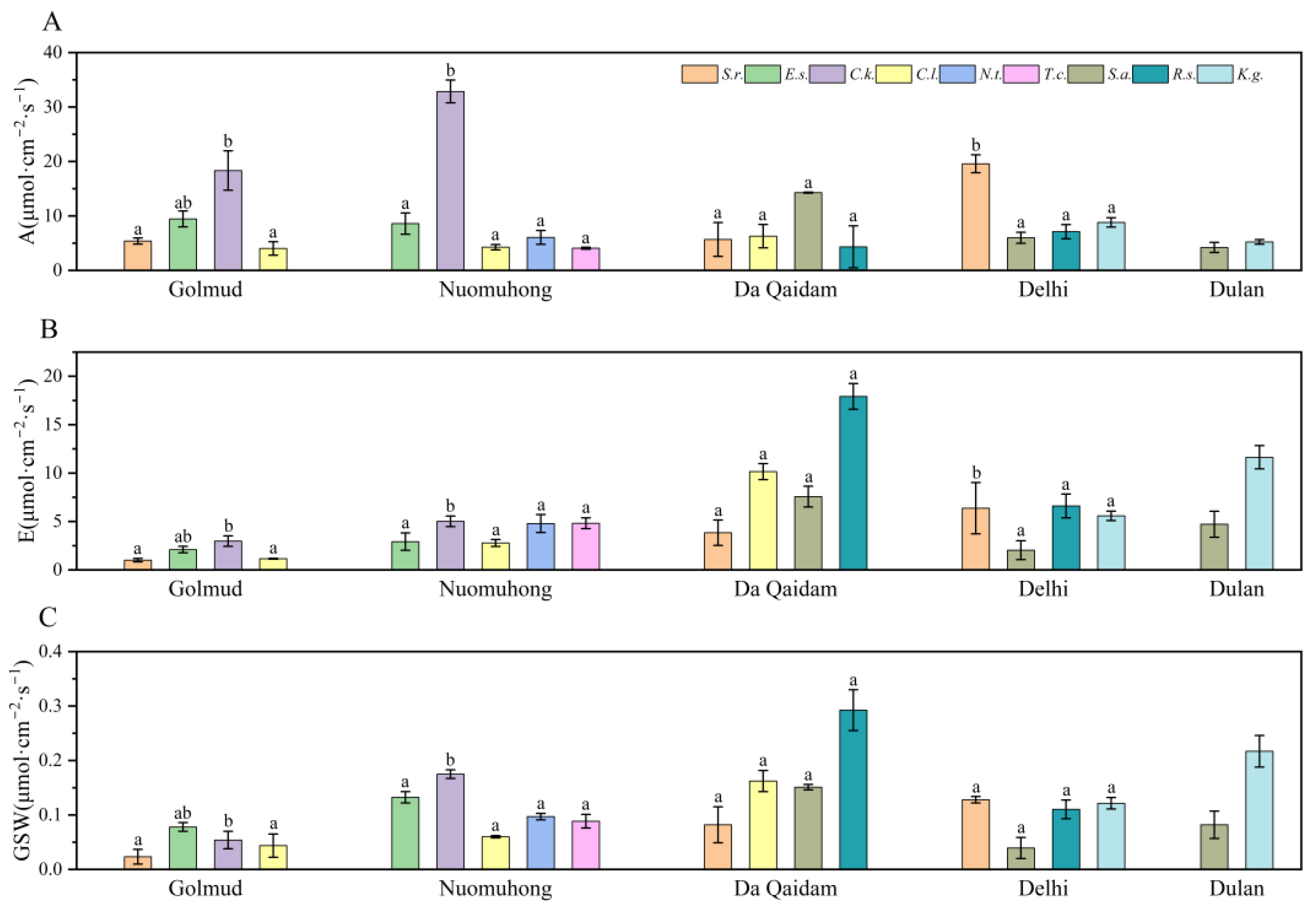
3.1. Changes of Photosynthetic Production Traits and Water Use Traits of Plants along Precipitation Gradient
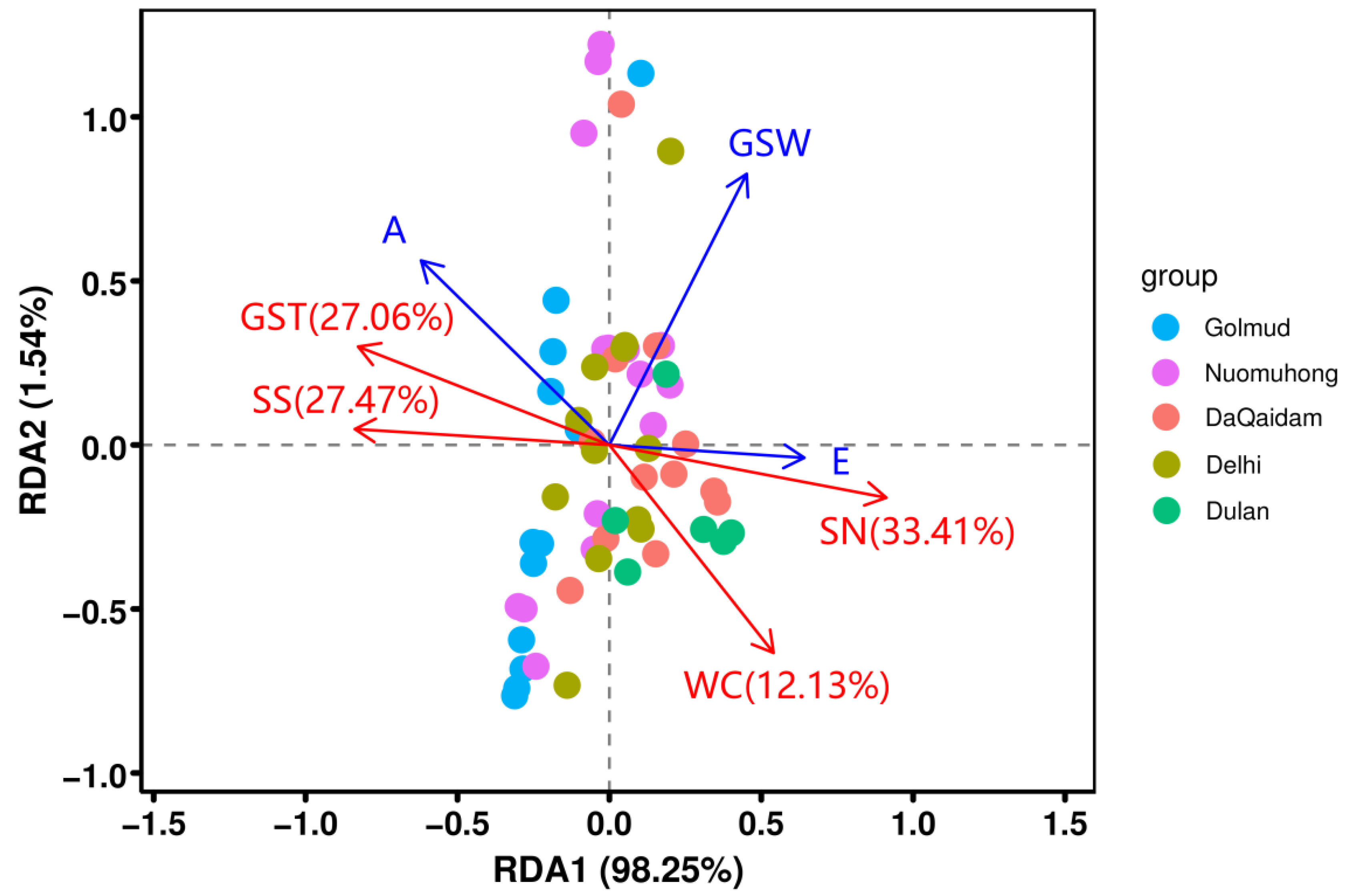
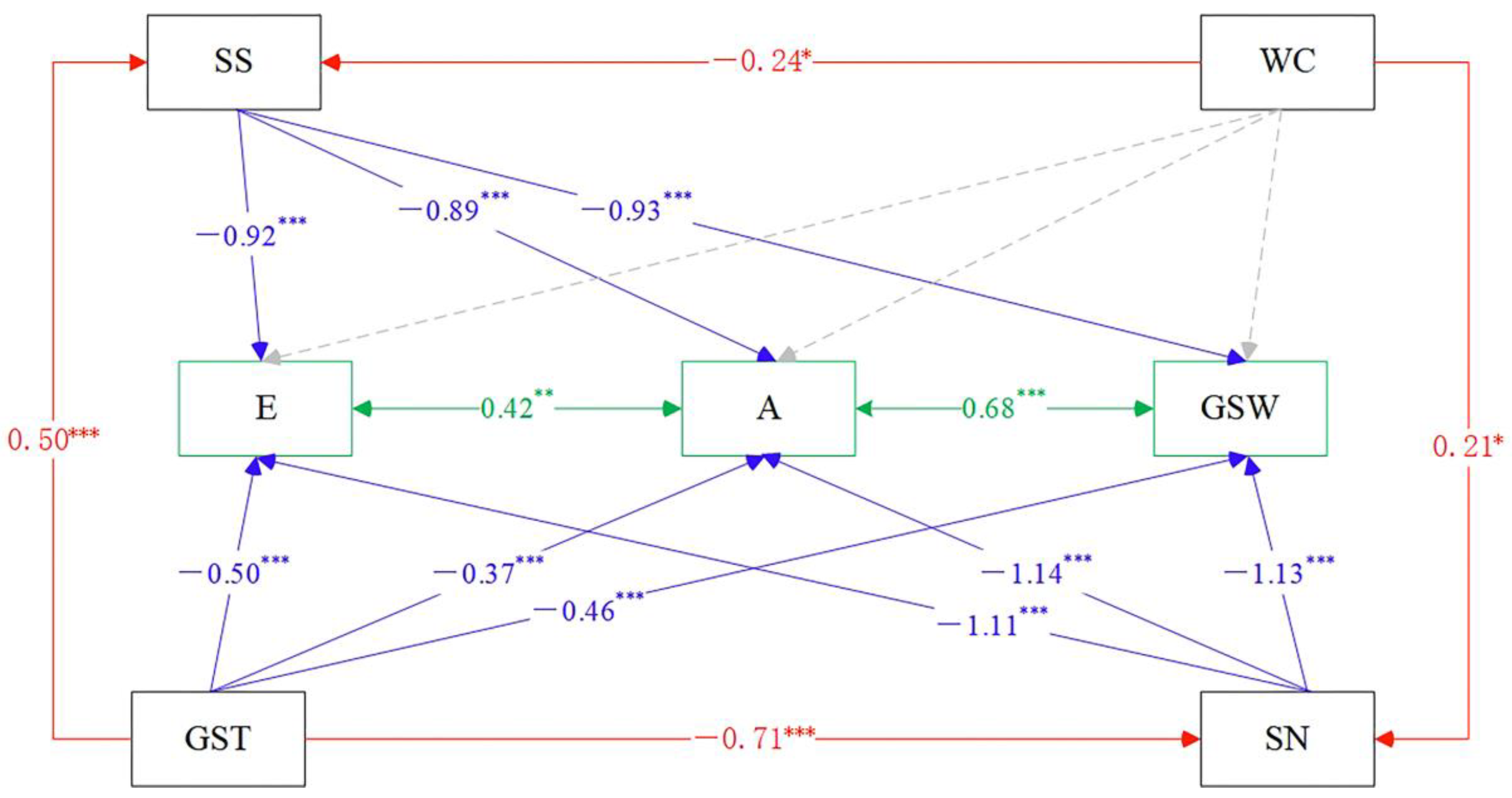
3.2. Relationship between Plant Functional Traits and Environmental Factors
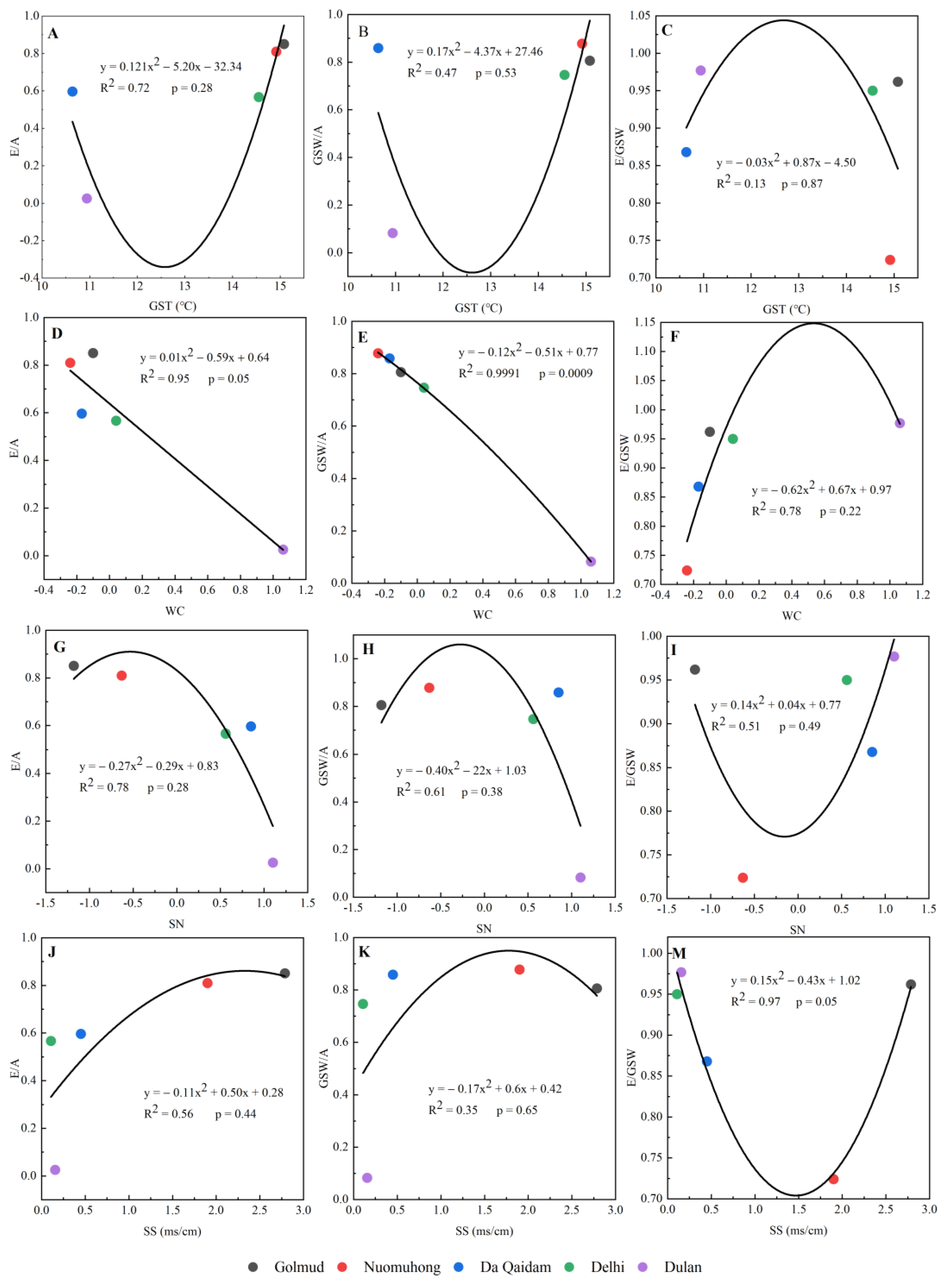
3.3. Changes of Relationships among Plant Functional Traits along the Drought Gradient
4. Discussion
4.1. Characteristics of Photosynthetic Production Traits and Water Use Traits of Plants and Their Influencing Factors
4.2. The Influence of Environmental Factors on the Relationship between Photosynthetic Production Traits and Water Use Traits of Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cayan, D.R.; Das, T.; Pierce, D.W.; Barnett, T.P.; Tyree, M.; Gershunov, A. Future dryness in the southwest US and the hydrology of the early 21st century drought. Proc. Natl. Acad. Sci. USA 2010, 107, 21271–21276. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.I.; Ault, T.R.; Smerdon, J.E. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Sci. Adv. 2015, 1, e1400082. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Leng, G.; Huang, Q.; Xie, Y.; Liu, S.; Meng, E.; Li, P. The asymmetric impact of global warming on US drought types and distributions in a large ensemble of 97 hydro-climatic simulations. Sci. Rep. 2017, 7, 5891. [Google Scholar] [CrossRef]
- Huang, L.; He, B.; Chen, A.; Wang, H.; Liu, J.; Lű, A.; Chen, Z. Drought dominates the interannual variability in global terrestrial net primary production by controlling semi-arid ecosystems. Sci. Rep. 2016, 6, 24639. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Fan, J.; Yan, Y.; Wang, X. Responses of Soil CO2 Fluxes to Short-Term Experimental Warming in Alpine Steppe Ecosystem, Northern Tibet. PLoS ONE 2013, 8, e59054. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Wang, S.; Hu, Y.; Xu, G.; Luo, C.; Chang, X.; Duan, J.; Lin, Q.; Xu, B.; et al. Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan plateau. Agric. For. Meteorol. 2011, 151, 792–802. [Google Scholar] [CrossRef]
- Reich, A.; Ewel, J.J.; Nadkarni, N.M.; Dawson, T.; Evans, R.D. Nitrogen isotope ratios shift with plant size in tropical bromeliads. Oecologia 2003, 137, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-Y.; Chen, F.-H.; Xia, D.-S.; Sun, H.-L.; Duan, Z.-H.; Wang, G. Correlations between leaf delta13C and physiological parameters of desert plant Reaumuria soongorica. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2008, 19, 1166–1171. [Google Scholar]
- Lü, X.; Zhou, G.; Wang, Y.; Song, X. Effects of changing precipitation and warming on functional traits of zonal Stipa plants from Inner Mongolian grassland. J. Meteorol. Res. 2016, 30, 412–425. [Google Scholar] [CrossRef]
- Akram, M.A.; Wang, X.; Hu, W.; Xiong, J.; Zhang, Y.; Deng, Y.; Ran, J.; Deng, J. Convergent Variations in the Leaf Traits of Desert Plants. Plants 2020, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.A.; Zhang, Y.; Wang, X.; Shrestha, N.; Malik, K.; Khan, I.; Ma, W.; Sun, Y.; Li, F.; Ran, J.; et al. Phylogenetic independence in the variations in leaf functional traits among different plant life forms in an arid environment. J. Plant Physiol. 2022, 272, 153671. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, H.; Prentice, I.C.; Harrison, S.P.; Wright, I.J. Coordination of plant hydraulic and photosynthetic traits: Confronting optimality theory with field measurements. New Phytol. 2021, 232, 1286–1296. [Google Scholar] [CrossRef]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martínez-Vilalta, J. Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhao, S.; Cheng, H.; Hu, Y.; Wang, S.; Yue, P.; Liu, R.; Knapp, A.K.; Smith, M.D.; Yu, Q.; et al. Functional diversity response to geographic and experimental precipitation gradients varies with plant community type. Funct. Ecol. 2021, 35, 2119–2132. [Google Scholar] [CrossRef]
- Blackman, C.J.; Aspinwall, M.J.; de Dios, V.R.; Smith, R.A.; Tissue, D.T. Leaf photosynthetic, economics and hydraulic traits are decoupled among genotypes of a widespread species of eucalypt grown under ambient and elevated CO2. Funct. Ecol. 2016, 30, 1491–1500. [Google Scholar] [CrossRef]
- Li, L.; McCormack, M.L.; Ma, C.; Kong, D.; Zhang, Q.; Chen, X.-Y.; Zeng, H.; Niinemets, U.; Guo, D. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett. 2015, 18, 899–906. [Google Scholar] [CrossRef]
- Hedrich, R.; Neimanis, S.; Savchenko, G.; Felle, H.H.; Kaiser, W.M.; Heber, U. Changes in apoplastic pH and membrane potential in leaves in relation to stomatal responses to CO2, malate, abscisic acid or interruption of water supply. Planta 2001, 213, 594–601. [Google Scholar] [CrossRef]
- Schnabl, H. The effect of Cl- upon the sensitivity of starch-containing and starch-deficient stomata and guard cell protoplasts towards potassium ions, fusicoccin and abscisic acid. Planta 1978, 144, 95–100. [Google Scholar] [CrossRef]
- Stroock, A.D.; Pagay, V.V.; Zwieniecki, M.A.; Holbrook, N.M. The physicochemical hydrodynamics of vascular plants. In Annual Review of Fluid Mechanics; Davis, S.H., Moin, P., Eds.; 2014; Volume 46, pp. 615–642. [Google Scholar] [CrossRef]
- Lampinen, M.J.; Noponen, T. Thermodynamic analysis of the interaction of the xylem water and phloem sugar solution and its significance for the cohesion theory. J. Theor. Biol. 2003, 224, 285–298. [Google Scholar] [CrossRef]
- Locke, A.M.; Ort, D.R. Leaf hydraulic conductance declines in coordination with photosynthesis, transpiration and leaf water status as soybean leaves age regardless of soil moisture. J. Exp. Bot. 2014, 65, 6617–6627. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.M.; Ort, D.R. Diurnal depression in leaf hydraulic conductance at ambient and elevated [CO2] reveals anisohydric water management in field-grown soybean and possible involvement of aquaporins. Environ. Exp. Bot. 2015, 116, 39–46. [Google Scholar] [CrossRef]
- Yin, Q.; He, J.; Tian, T.; Quan, J.; Zhao, P.; Chai, Y.; Wang, L.; Yue, M. Hydraulic and Photosynthetic Traits Vary with Successional Status of Woody Plants on the Loess Plateau. Forests 2019, 10, 327. [Google Scholar] [CrossRef]
- Niu, W.; Chen, H.; Wu, J. Soil Moisture and Soluble Salt Content Dominate Changes in Foliar δ13C and δ15N of Desert Communities in the Qaidam Basin, Qinghai-Tibetan Plateau. Front. Plant Sci. 2021, 12, 675817. [Google Scholar] [CrossRef]
- Yao, S.; Akram, M.; Hu, W.; Sun, Y.; Sun, Y.; Deng, Y.; Ran, J.; Deng, J. Effects of Water and Energy on Plant Diversity along the Aridity Gradient across Dryland in China. Plants 2021, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Rosseel, Y. Lavaan: AnRPackage for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Watt, M.S.; Buddenbaum, H.; Leonardo, E.M.C.; Estarija, H.J.; Bown, H.E.; Gomez-Gallego, M.; Hartley, R.J.; Pearse, G.D.; Massam, P.; Wright, L.; et al. Monitoring biochemical limitations to photosynthesis in N and P-limited radiata pine using plant functional traits quantified from hyperspectral imagery. Remote Sens. Environ. 2020, 248, 112003. [Google Scholar] [CrossRef]
- Schreiber, J.B.; Nora, A.; Stage, F.K.; Barlow, E.A.; King, J. Reporting Structural Equation Modeling and Confirmatory Factor Analysis Results: A Review. J. Educ. Res. 2006, 99, 323–338. [Google Scholar] [CrossRef]
- Sage, R.F. The evolution of C4 photosynthesis. New Phytol. 2003, 161, 341–370. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.W.; Acosta-Martinez, V.; McIntyre, N.E.; Cox, S.; Tissue, D.T.; Zak, J.C. Linking Microbial Community Structure and Function to Seasonal Differences in Soil Moisture and Temperature in a Chihuahuan Desert Grassland. Microb. Ecol. 2009, 58, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Marion, G.; Verburg, P.; McDonald, E.; Arnone, J. Modeling salt movement through a Mojave Desert soil. J. Arid Environ. 2008, 72, 1012–1033. [Google Scholar] [CrossRef]
- Kopittke, P.M. Interactions between Ca, Mg, Na and K: Alleviation of toxicity in saline solutions. Plant Soil 2011, 352, 353–362. [Google Scholar] [CrossRef]
- Paradiso, R.; de Micco, V.; Buonomo, R.; Aronne, G.; Barbieri, G.; de Pascale, S. Soilless cultivation of soybean for Bioregenerative Life-Support Systems: A literature review and the experience of the MELiSSA Project—Food characterisation Phase I. Plant Biol. 2013, 16, 69–78. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Sperry, J.S.; Venturas, M.; Anderegg, W.R.L.; Mencuccini, M.; Mackay, D.; Wang, Y.; Love, D.M. Predicting stomatal responses to the environment from the optimization of photosynthetic gain and hydraulic cost. Plant Cell Environ. 2016, 40, 816–830. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Marôco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Buckley, T.N.; Mott, K.A. Modelling stomatal conductance in response to environmental factors. Plant Cell Environ. 2013, 36, 1691–1699. [Google Scholar] [CrossRef]
- Damour, G.; Simonneau, T.; Cochard, H.; Urban, L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 2010, 33, 1419–1438. [Google Scholar] [CrossRef] [PubMed]
- Lombardozzi, D.L.; Zeppel, M.J.B.; Fisher, R.A.; Tawfik, A. Representing nighttime and minimum conductance in CLM4.5: Global hydrology and carbon sensitivity analysis using observational constraints. Geosci. Model Dev. 2017, 10, 321–331. [Google Scholar] [CrossRef]
- Lamour, J.; Davidson, K.J.; Ely, K.S.; Le Moguédec, G.; Leakey, A.D.B.; Li, Q.; Serbin, S.P.; Rogers, A. An improved representation of the relationship between photosynthesis and stomatal conductance leads to more stable estimation of conductance parameters and improves the goodness-of-fit across diverse data sets. Glob. Chang. Biol. 2022, 28, 3537–3556. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.H.; Ripley, B.S.; Martin, T.; De-Wet, L.A.; Woodward, F.I.; Osborne, C.P. Physiological advantages of C4 grasses in the field: A comparative experiment demonstrating the importance of drought. Glob. Chang. Biol. 2014, 20, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Ripley, B.; Woodward, F.I.; Osborne, C.P. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant Cell Environ. 2010, 34, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar] [CrossRef]
- Chen, W.; Cui, P.; Sun, H.; Guo, W.; Yang, C.; Jin, H.; Fang, B.; Shi, D. Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.). Ind. Crop. Prod. 2009, 30, 351–358. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous Proline Effects on Photosynthetic Performance and Antioxidant Defense System of Young Olive Tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef]
- Subramanian, D. Role of Exogenous Proline in Ameliorating Salt Stress at Early Stage in Two Rice Cultivars. J. Stress Physiol. Biochem. 2011, 7, 157–174. [Google Scholar]
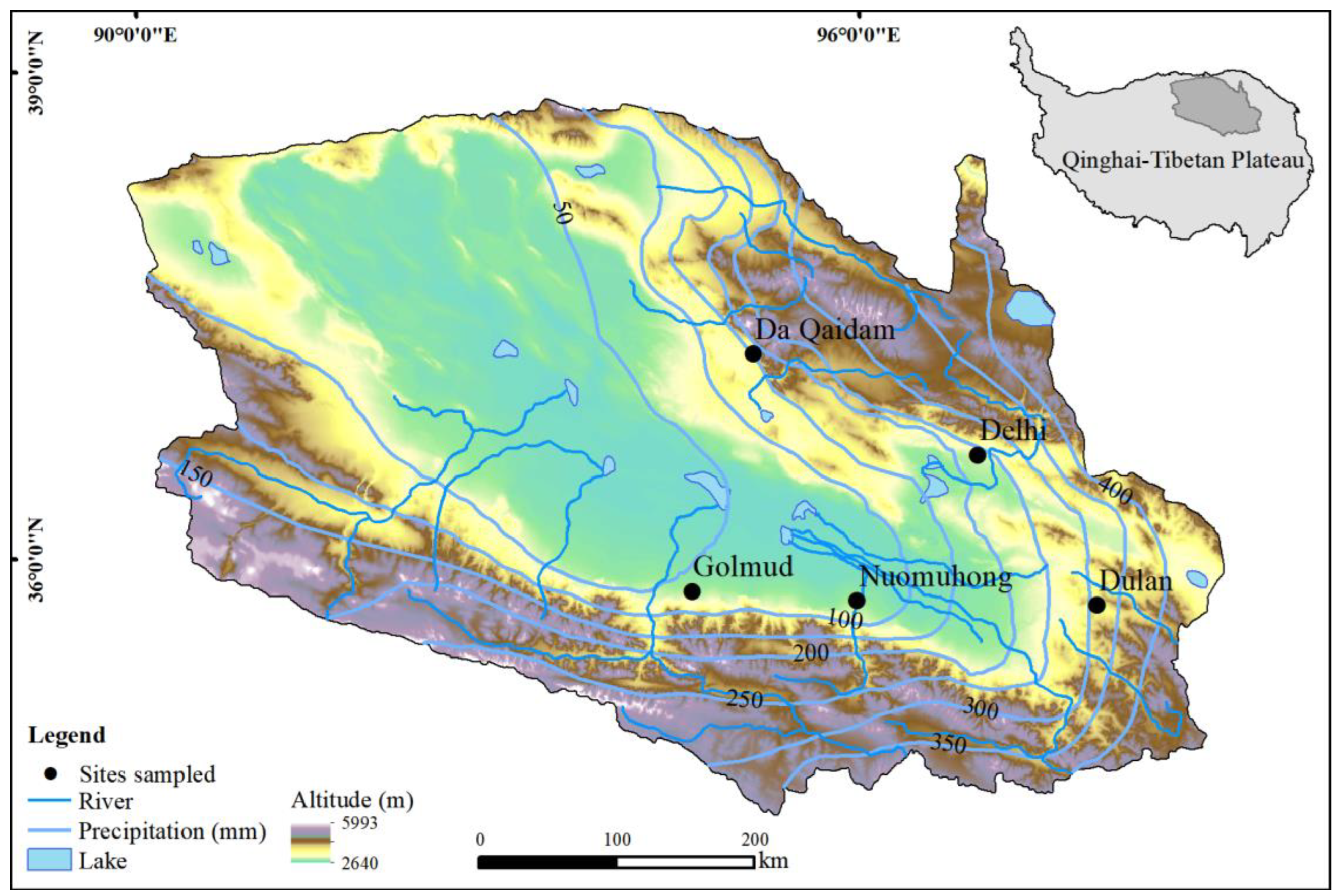
| Sites | Community Types | Dominant Species | Long | Lat | Alt | GST | GSP | AI | SWC | SS | STOC | STN | STP | STK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Total Cover: %) | (Relative Cover: %) | (°E) | (°N) | (m) | (°C) | (mm) | (%) | (EC25 ms/cm) | (g/kg) | (g/kg) | (g/kg) | (g/kg) | ||
| Golmud | C.k. (10%) | C.k. (40%); S.r. (30%); C.l. (18%); E.s. (12%) | 95.09 | 36.35 | 2880 | 15.08 | 48.49 | 28.98 | 0.11 | 2.79 | 2.52 | 0.35 | 0.30 | 9.27 |
| Nuomuhong | E.s. (20%) | E.s. (58%); C.k. (25%); C.l. (8%); T.c. (7%); N.t. (2%) | 96.41 | 36.38 | 2857 | 14.92 | 59.50 | 23.29 | 0.36 | 1.87 | 3.38 | 0.18 | 0.40 | 12.75 |
| Da Qaidam | S.r. (25%) | S.r. (35%); S.a. (25%); C.l. (20%); R.s. (20%) | 95.40 | 37.86 | 3315 | 10.64 | 86.12 | 13.76 | 5.18 | 0.54 | 6.74 | 0.57 | 0.39 | 20.96 |
| Delhi | S.r. (30%) | S.r. (50%); K.g. (40%); S.a. (5%); R.s. (5%) | 97.28 | 37.35 | 2972 | 14.55 | 163.53 | 8.17 | 4.29 | 0.11 | 15.25 | 0.56 | 0.36 | 19.19 |
| Dulan | K.g. (60%) | K.g. (90%); S.a. (10%) | 98.33 | 36.47 | 3323 | 10.94 | 212.44 | 4.92 | 19.21 | 0.16 | 10.25 | 0.18 | 0.41 | 21.30 |
| Comprehensive Index | Contribution Rate (%) | Cumulative Contribution Rate (%) | Index | Rotated Component Matrix | Weight (%) | ||
|---|---|---|---|---|---|---|---|
| Principal Component 1 | Principal Component 2 | ||||||
| Water content (WC) | Principal component 1 | 56.328 | 56.328 | SWC | 0.440 | 0.897 | 35.27 |
| Principal component 2 | 41.206 | 97.533 | GTP | 0.824 | −0.526 | 34.19 | |
| Principal component 3 | 2.467 | 100 | AI | −0.904 | −0.395 | 33.24 | |
| Soil nutrient (SN) | Principal component 1 | 66.743 | 66.743 | TN | 0.992 | −0.074 | 23.90 |
| Principal component 2 | 32.732 | 99.475 | TK | 0.874 | 0.482 | 28.49 | |
| Principal component 3 | 0.504 | 99.979 | TP | 0.130 | 0.990 | 20.59 | |
| Principal component 4 | 0.021 | 100 | TOC | 0.951 | 0.302 | 27.02 | |
| Sites | E/A | GSW/A | E/GSW | GSP (mm) | SWC (%) | AI |
|---|---|---|---|---|---|---|
| Golmud | 0.851 ** | 0.806 ** | 0.962 ** | 48.49 | 0.11 | 28.98 |
| Nuomuhong | 0.606 * | 0.861 ** | 0.724 ** | 59.50 | 0.36 | 23.29 |
| Da Qaidam | 0.597 * | 0.859 ** | 0.868 ** | 86.12 | 5.18 | 13.76 |
| Delhi | 0.567 | 0.747 ** | 0.933 ** | 163.53 | 4.29 | 8.17 |
| Dulan | 0.026 | 0.083 | 0.977 ** | 212.44 | 19.21 | 4.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Chen, H.; Chen, B.; Wang, Y.; Sun, H. Drought Shapes Photosynthetic Production Traits and Water Use Traits along with Their Relationships with Leaves of Typical Desert Shrubs in Qaidam. Forests 2022, 13, 1652. https://doi.org/10.3390/f13101652
Zhao L, Chen H, Chen B, Wang Y, Sun H. Drought Shapes Photosynthetic Production Traits and Water Use Traits along with Their Relationships with Leaves of Typical Desert Shrubs in Qaidam. Forests. 2022; 13(10):1652. https://doi.org/10.3390/f13101652
Chicago/Turabian StyleZhao, Liping, Hui Chen, Ben Chen, Yumeng Wang, and Hongyan Sun. 2022. "Drought Shapes Photosynthetic Production Traits and Water Use Traits along with Their Relationships with Leaves of Typical Desert Shrubs in Qaidam" Forests 13, no. 10: 1652. https://doi.org/10.3390/f13101652
APA StyleZhao, L., Chen, H., Chen, B., Wang, Y., & Sun, H. (2022). Drought Shapes Photosynthetic Production Traits and Water Use Traits along with Their Relationships with Leaves of Typical Desert Shrubs in Qaidam. Forests, 13(10), 1652. https://doi.org/10.3390/f13101652






